Transforming growth factor-β promotes ‘death by neglect’ in post-activated human T cells (original) (raw)
Abstract
Transforming growth factor-β (TGF-β) is central to the wound repair processes that follow local trauma and inflammation. In order to mimic the early events of wound-healing, we studied the effects of TGF-β on mitogen-stimulated peripheral blood cells. TGF-β added at the initiation of mitogenesis did not significantly alter T-cell activation, proliferation, CD45 isoform switching, or activation-induced cell death. By contrast, TGF-β added 72 hr post-activation (or later) enhanced the cumulative increase in apoptotic T cells. TGF-β had no effect on mitogen-induced up-regulation of Fas (CD95) or Fas ligand and did not enhance killing of the Fas-sensitive Jurkat cell line by activated T cells. Furthermore, TGF-β had no direct effect on levels of mRNA for members of the bcl family (bcl-X, bfl-1, bik, bak, bax, bcl-2 and mcl-1). These findings suggest that TGF-β does not directly induce apoptosis via the Fas system or by direct effects on bcl proteins. However, interleukin-2, which can ‘rescue’ lymphocytes from spontaneous apoptosis due to cytokine deprivation, abolished the pro-apoptotic effects of TGF-β on post-activated T cells, thus demonstrating that TGF-β increases the cytokine-dependence of T cells for survival. We propose a novel role for TGF-β in the suppression of inflammation by promoting the elimination of post-activated T cells once the initiating stimulus has been resolved.
Introduction
Local infection or injury provokes inflammation and a subsequent wound repair response, during which transforming growth factor-β (TGF-β) secretion is increased and the TGF-β zymogen is activated by the locally secreted proteases. TGF-β is a morphogenetic cytokine which is thought to be central to the tissue remodelling processes that occur during the wound-healing response, as well as being a major factor in scarring and fibrosis (recently reviewed in refs 1–3,7–9 However, there are numerous reports that it can promote, rather than inhibit, the primary response of antigen-naive T helper cells and the subsequent expansion of effector cell populations.10–17 Thus, it can be hypothesized that up-regulation of TGF-β activity during wound healing may suppress the preceding inflammatory response, without necessarily preventing the generation of effector or memory cells from their unprimed precursors.
The expansion of activated lymphocytes during inflammation is followed by elimination of most of these cells by apoptosis. The role of TGF-β in this process is not well understood and there are conflicting reports that TGF-β can either inhibit15–20 or promote21–24 T-cell apoptosis. Although it has been shown that TGF-β does not alter T-cell expression of bcl2 or Fas/CD95,18 there is disagreement in the reports concerning the effects of TGF-β on the expression of Fas ligand (FasL).20,25 In order to help resolve the literature discrepancies, our experiments were designed to determine the effects of TGF-β on T-cell activation and survival, such as to mimic the early events of a wound-healing response. Here, we report that TGF-β promotes apoptosis in T cells if added at, or after 72 hr post-activation, a situation which would mimic the local increase in active TGF-β that occurs following extravasation of peripheral T cells to the site of injury or infection and subsequent activation.
Materials and methods
Lymphocyte culture
Blood samples were taken by venepuncture from normal volunteer laboratory staff and mixed with preservative-free heparin at 10 U/ml of blood. Peripheral blood mononuclear cells (PBMC) were isolated by density barrier centrifugation over ‘Lymphoprep’ (Nycomed UK Ltd, Sheldon, UK), washed three times and cultured at 5 × 105 cells/ml in RPMI-1640 medium (Sigma, Poole, Dorset, UK) supplemented with 10% fetal bovine serum (FBS; Sera-Lab Ltd, Crawley Down, UK). Lymphocytes were activated with phytohaemagglutinin (PHA; Murex Diagnostics Ltd, Kent, UK) at a pretitred optimal concentration of 2·5 µg/ml added at the start of culture.26–28 Control cultures with no mitogen were always included. For flow cytometry determinations, 1-ml cultures were set up in duplicate in 48-well ‘tissue culture’ grade plates; for mitogenesis assays, six replicate cultures were set up in 0·2-ml volumes in 96-well plates (Corning Costar, High Wycombe, Bucks, UK). In each experiment, blood from at least two donors was used.
Cytokines
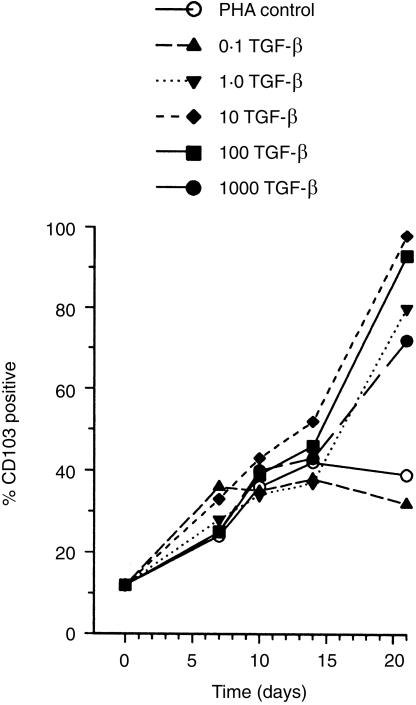
TGF-β (Genzyme, West Malling, Kent, UK) was added to the cultures either at the start of the experiments, or at time-points up to 8 days after initiation of culture. Following dose–response experiments (see Fig. 1), TGF-β was routinely used at a final concentration of 100 U/ml, consistent with previous observations.28,29 Both TGF-β1 and TGF-β2 isoforms were assessed. For some experiments, interleukin-2 (IL-2; R & D Systems, Abingdon, Oxfordshire, UK) was used at up to 1000 U/ml, added simultaneously with TGF-β at either the onset of mitogenesis or after 72 hr culture.
Figure 1.
Up-regulation of CD103 by TGF-β on activated T cells. Two-colour flow cytometry analysis showed that CD103 (integrin αE/β7, HML-1 antigen) was up-regulated by TGF-β on T cells in a dose-dependent manner following PHA stimulation of peripheral blood lymphocytes. Representative experiment illustrated; data are means of replicates ±SD.
Phenotypic analysis of lymphocyte activation
PBMC were analysed by ‘two-colour’ flow cytometry to assess expression of cell-surface markers using an (FACScan) analytical flow cytometer with cellquest software for acquisition and analysis (Becton Dickinson, Cowley, Oxord, UK). A direct-conjugated CD2 antibody was used to identify the T-cell fraction, together with a second direct-conjugated antibody of interest.26–28 Although expressed weakly by natural killer (NK) cells, CD2 was used in preference to CD3 to ‘gate’ the T-cell population as CD3 is down-regulated during T-cell activation. Both fluorescein isothiocyanate (FITC) and phycoerythrin (PE) conjugates were used, as appropriate. A panel of antibodies was used to study T-cell phenotype. FITC-conjugated CD4, CD5, CD8, CD25, CD28, CD45RA, CD45RB, CD69, CD95, CD45RO-PE, CD95-FITC and biotin-conjugated CD95L (FasL) were purchased from Dako (High Wycombe, Buckinghamshire, UK). Biotin-conjugated HML-1 antibody (CD103, integrin αE/β7) was obtained from Immunotech (Coulter Electronics, Luton, Bedfordshire, UK). A streptavidin-Quantum red second layer (Sigma) was used to detect biotin-conjugated antibodies. PE- and FITC-conjugated antibodies against Aspergillus niger glucose oxidase (Dako) were used as negative controls. Three thousand CD2+ cells were acquired per tube in duplicate and the amplifiers were set so that ≥95% of negative-control cells fell within the first decade. For analysis of CD4+ and CD8+ populations, results were expressed as ‘% positive’; expression of other surface markers was quantified as the median fluorescence channel.
T-cell activation was assessed by up-regulation of CD69 at 24 hr and CD25 at 72 hr, as described previously.26–28 The biological effects of TGF-β were monitored by assessing the TGF-β-induced up-regulation of the integrin αE/β7 on post-activated T cells.13
Assessment of apoptosis
Apoptosis was routinely assessed by flow cytometry by using Annexin V (FITC conjugate, BioWhittaker, Wokingham, Bucks, UK)30 in conjunction with propidium iodide, and by criteria of forward and side scatter.31 To confirm nuclear fragmentation, a characteristic of the late stages of apoptosis, preparations were stained with acridine orange and examined by fluorescence microscopy (Zeiss Axioplan) using a ×63 oil-immersion objective.
T-cell mitogenesis assays
Cell cultures were established at 5 × 105 lymphocytes/ml in 96-well microtitre plates in 0·2 ml volumes as described previously.26,27 PHA was used as the mitogen and TGF-β was added either at the onset of culture or after 72 hr, as above. Positive and negative controls consisted of PHA alone and no mitogen, respectively. Six replicates were used for each condition. [3H]Thymidine (0·5 µCi; Amersham, Bucks, UK) was added to each well after 72 hr culture and cells were harvested onto glass-fibre filter mats after a further 18-hr incubation. Precursor incorporation was measured by liquid scintillation spectrometry and results were expressed as a stimulation index (SI) where: SI = (test counts per minute)/(control counts per minute) after subtraction of background counts.
Apoptosis assay of Jurkat cells
In addition to flow cytometric analysis (above), the ‘JAM’ test of DNA fragmentation32 was used to determine if TGF-β functionally promoted the ability of activated T cells to kill the Fas-sensitive Jurkat T lymphoblastoid cell line. Jurkat cells [obtained from the European Collection of Cell Cultures (ECACC), Porton Down, Wiltshire, UK] were maintained in suspension culture in RPMI-1640 medium with 10% FBS. Cells in log-phase growth were prelabelled overnight with [3H]thymidine, washed and incubated for 24 hr with lymphocytes taken at various stages after activation, using a ratio of 1:1 effector:target cells. Cultures were harvested onto glass-fibre filter mats and the amount of intact DNA retained was estimated by liquid scintillation spectrometry.
Ribonuclease protection assays
Multitemplate ribonuclease protection assays were used to examine mRNA from lymphocytes cultured with and without TGF-β, using the template set hAOP-2 containing probes for bcl-X, bfl-1, bik, bak, bax, bcl-2 and mcl-1 (PharMingen, Becton Dickinson, Cowley, Oxford, UK). Control probes for L32 and GAPDH were included. Cells were harvested 24 hr after addition of TGF-β and the assay was performed using reagents and the protocol provided by Ambion (AMS Biotechnology, Abingdon, Oxford, UK). Protected fragments were separated and visualized by electrophoresis through 5% (w/v) denaturing polyacrylamide gels. Dried gels were analysed on a phosphorimager.
Statistics
Means and standard deviations were used as descriptive statistics. For determination of statistical significance, analysis of variance (anova) was performed using the instat software package (GraphPAD, San Diego) with Bonferroni correction for multiple comparisons.
Results
Effects of TGF-β on T-cell activation and mitogenesis
The bioactivity of TGF-β was confirmed by the expected up-regulation of the CD103 integrin αE/β7 (Fig. 1) (cf. ref. 13). The effects of TGF-β1 and TGF-β2 isoforms on both cell activation and cell-surface phenotype were closely comparable (Fig. 2).
Figure 2.
Effects of TGF-β on T-cell activation and proliferation. T-cell activation was determined by flow cytometry by gating on the CD2+ population following PHA stimulation. Neither TGF-β1 nor TGF-β2 isoforms had any significant effect on either the kinetics or magnitude of up-regulation of CD69 (a) or CD25 (b). Irrespective of whether added at 0 hr or 72 hr, TGF-β did not affect proliferation, relative to mitogen-only controls, as determined by [3H]thymidine incorporation (c). Results are expressed as a Stimulation Index (ratios of counts of PHA-stimulated to unstimulated cultures after background subtraction). Representative experiment illustrated; data are means of replicates ±SD.
Irrespective of whether TGF-β was added at the initiation of culture or at 72 hr, there was no significant effect on T-cell activation or mitogenic responses (Fig. 2). TGF-β also had no significant effect on the subsequent development of a CD45R0hi CD45Ralo CD45RBlo ‘memory’ T-cell phenotype (Fig. 3). Furthermore, TGF-β did not preferentially affect the percentages of CD4+ (49 ± 9·6 versus 52 ± 2·7 and 51 ± 7·8%, in PHA only controls versus PHA with TGF-β at 0 hr and 72 hr, respectively, n = 10 donors) and CD8+ cells (37 ± 12·9 versus 36 ± 11·8 and 38 ± 13·6%, respectively, n = 10 donors) at 7 days. There was no significant effect of TGF-β on the expression of CD28 (median fluorescence values of 67 ± 26·6 versus 73 ± 31·2 and 75 ± 35·3, respectively, n = 6 donors) and TGF-β had no significant effect on the activation-induced up-regulation of CD5 (median fluorescence values of 238 ± 133 versus 296 ± 143 and 263 ± 159, respectively, compared with 105 ± 18·7 in unstimulated controls, n = 10 donors) at 7 days.
Figure 3.
Effects of TGF-β on development of an antigen-committed phenotype. The development of the CD45R0hi CD45Ralo CD45RBlo phenotype of antigen-committed T cells following PHA stimulation was unaffected by the presence of TGF-β. Note the superimposition of some data points. Representative experiment illustrated; data are means of replicates ±SD.
Effects of TGF-β on T-cell survival and apoptosis
When added at the same time as mitogen, TGF-β induced a slight (typically 4–5%) but consistent increase in the percentage of apoptotic cells during extended culture (up to 21 days), compared to PHA-activated T cells cultured in the absence of TGF-β. However, the difference was not statistically significant. (Fig. 4)
Figure 4.
TGF-β promotes apoptosis in post-activated T cells. Apoptosis was assessed by flow cytometry following incubation with Annexin V and propidium iodide (a,b). The negative cells in the lower left quadrant were considered as viable (non-apoptotic), whereas apoptotic cells were Annexin V-positive, with the late apoptotic/dead cells showing the highest levels of propidium iodide staining. In the representative experiment illustrated, the percentage viable cells in the control culture (a) at 10 days was 46%, compared to 26% in the treated culture (b). (c) shows that the percentage of viable T cells decreased more rapidly following PHA stimulation if TGF-β was added at 72 hr post-activation, compared to TGF-β added at the onset of mitogenesis or omitted. Data represent mean values (± SEM) from 15 independent experiments.*P < 0·05; **P < 0·01.
By contrast, when TGF-β was added at 72 hr or later post-mitogenesis, there was a profound slow, cumulative increase in the percentages of apoptotic T cells (Fig. 4). The effect became statistically significant within 2 days of culture after addition of TGF-β (P < 0·05) and increased steadily with time. By the end of 14 days from the onset of mitogenesis, the percentage of T cells surviving in cultures where TGF-β had been added 72 hr post-mitogenesis was less than 50% of mitogen-only controls or cells where TGF-β had been added at the onset of mitogenesis (Fig. 4).
Effects of TGF-β on the Fas/FasL system
In order to determine any involvement of the Fas/FasL system, expression of Fas (CD95) and FasL (CD95L) was determined on T cells activated in the presence or absence of TGF-β. However, no significant changes were observed in either Fas or FasL up-regulation attributable to TGF-β (Fig. 5).
Figure 5.
TGF-β does not promote Fas-mediated killing. Flow cytometry analysis (a) revealed that TGF-β had no significant effect on either the kinetics or the magnitude of PHA-induced up-regulation of CD95 (Fas) (open symbols) or FasL (solid symbols), whether added at the onset of mitogenesis or after 72 hr. The JAM test of DNA fragmentation (b) showed that activated T cells at day 3 (dark bars) induced apotosis in the Fas-sensitive Jurkat T cell line, as measured by the amount of [3H]thymidine retained on filter mats. However, 5 days after activation, T cells had lost the ability to induce apoptosis in Jurkat cells (shaded bars), compared to the day 0 control (open bar). The effects were unaltered whether TGF-β was absent (Cont), added at the time of mitogenesis (0 hr) or added 72 hr after the onset of stimulation.
To confirm these data, the functional ability of activated T cells to kill the Fas-sensitive Jurkat cell line was investigated. Activated T cells showed a potent ability to kill Jurkat cells at 72 hr post-activation, which was largely lost by 120 hr (Fig. 5). This closely mirrored the kinetics of FasL expression. Irrespective of whether TGF-β was added at the same time as mitogen or after 72 hr, there was no functional difference in the ability of activated cells to induce apoptosis in Jurkat cells (Fig. 5).
Bcl family transcipt expression
Ribonuclease protection assays showed that TGF-β did not directly affect levels of bcl family transcripts. Cultures of lymphocytes without TGF-β, with TGF-β added at 0 hr and with TGF-β added at 72 hr all showed comparable amounts of message after a further 24 hr for bcl-X, bfl-1, bik, bak, bax, bcl-2 and mcl-1 when normalized against mRNA for the ‘housekeeping’ genes L32 and GAPDH (not shown).
Effects of exogenous IL-2
Although several cytokines have been reported to ‘rescue’ post-activated T cells from spontaneous apoptosis due to cytokine deprivation, the archetypal cytokine in this respect is IL-2. Addition of IL-2 completely abolished the pro-apoptotic affect of TGF-β. In the presence of exogenous IL-2, there was no increase in the percentages of apoptotic cells with time in cultures treated with TGF-β either at the onset of mitogenesis or after 72 hr (Fig. 6).
Figure 6.
IL-2 overcomes TGF-β-mediated apoptosis in post-activated T cells. Addition of IL-2 together with TGF-β either at the onset of mitogenesis (0 hr) or at 72 hr abolished the ability of TGF-β to promote apoptosis in post-activated T cells. Representative experiment illustrated; data are means of replicates ±SD.
Discussion
Our observations suggest that TGF-β may play a hitherto unsuspected role in the elimination of activated T cells at the resolution of an immune response. The two major pathways involved are ‘activation-induced cell death’ (AICD), an active process mainly mediated by the Fas/FasL system, and ‘death by neglect’, where the default apoptotic machinery is engaged in the absence of sufficient rescue factors, such as IL-2 (recently reviewed in refs 33–39). AICD alone cannot account for our observations, as TGF-β had no effect on the Fas/FasL system and did not directly alter the balance of the pro- and anti-apoptotic members of the bcl family. Conversely, the ability of exogenous IL-2 to abrogate the pro-apoptotic effects of TGF-β suggests that post-activated T cells exposed to TGF-β become more vulnerable to cytokine deprivation. This may be due to premature exit from the cell cycle, with consequent impairment of IL-2 production. This view is supported by the relatively slow kinetics of the effect, as increased cell death did not occur immediately, but rather was a cumulative effect, increasing steadily with time. Another contributory factor may be inhibition of IL-2-induced phosphorylation and activation of the JAK/STAT pathway of T cells.23 This, together with reduced expression of the α- and β-chains of the IL-2 receptor,23 could also account for the increased requirement for IL-2 to permit T-cell survival.
Although we found that TGF-β added after mitogenic stimulation promoted apoptosis, it had minimal effects if present throughout the activation response. These observations provide direct confirmation of a previous report13 and are consistent with the notion that TGF-β does not necessarily suppress T-cell activation or proliferation.10–12,15,19,40–43
Our observations imply that the timing of TGF-β signalling is an important determinant of cell fate. This hypothesis is in keeping with previous observations, which suggest that TGF-β has different effects on cells undergoing initial stimulation or a priming response, compared to cells undergoing re-stimulation. Thus, TGF-β can reduce apoptosis during re-stimulation, either if present in the initial phase18 or if present during the restimulation phase.20 Furthermore, we and others;18,25 have shown that FasL expression is unaffected by TGF-β during first-round stimulation, whereas in cells undergoing restimulation, TGF-β can inhibit up-regulation of FasL.20
On the basis of the above, we propose that TGF-β plays a biphasic role in the modulation of local inflammatory responses and the subsequent fate of post-activated lymphocytes during wound-healing. The published data suggest that during the initial phase of the inflammatory response, TGF-β enhances the recruitment of naive cells10–12,16 and, during the ongoing reaction, promotes effector cell expansion and the generation of memory cells,13–15,17,18 perhaps by down-regulation of FasL.20 However, once the initiating stimulus has been eliminated and local production of IL-2 has ceased, our findings show that TGF-β promotes the removal of post-activated T cells by increasing the survival requirement for rescue factors. This is a novel role for TGF-β in the resolution of inflammatory responses and the promotion of wound-healing and failure of this mechanism may be a contributory factor in the perpetuation of chronic inflammatory conditions.
Acknowledgments
This work was funded in part by the Defence Evaluation and Research Agency. We are grateful to Lynette Steele for assistance with flow cytometry analysis.
References
- 1.Letterio JJ, Roberts AB. Transforming growth factor-beta1-deficient mice: identification of isoform-specific activities in vivo. J Leukocyte Biol. 1996;59:769–74. doi: 10.1002/jlb.59.6.769. [DOI] [PubMed] [Google Scholar]
- 2.Pignatelli M, Gilligan CJ. Transforming growth factor-beta in GI neoplasia, wound healing and immune response. Baillieres Clin Gastroenterol. 1996;10:65–81. doi: 10.1016/s0950-3528(96)90040-8. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence DA. Transforming growth factor-β: a general review. Eur Cytokine Netw. 1996;7:363–74. [PubMed] [Google Scholar]
- 4.O'Kane S, Ferguson MWJ. Transforming growth factor βs and wound healing. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- 5.Grande JP. Role of transforming growth factor-β in tissue injury and repair. Proc Soc Exp Biol Med. 1997;214:27–40. doi: 10.3181/00379727-214-44066. [DOI] [PubMed] [Google Scholar]
- 6.Roberts AB, Sporn MB. Transforming growth factor-β. In: Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. 2. New York and London: Plenum Press; 1998. pp. 275–308. [Google Scholar]
- 7.Fontana A, Constam DB, Frei K, Malipiero U, Pfister HW. Modulation of the immune response by transforming growth factor beta. Int Arch Allergy Immunol. 1992;99:1–7. doi: 10.1159/000236328. [DOI] [PubMed] [Google Scholar]
- 8.Letterio JJ, Roberts AB. TGF-β: a critical modulator of immune cell function. Clin Immunol Immunopathol. 1997;84:244–50. doi: 10.1006/clin.1997.4409. 10.1006/clin.1997.4409. [DOI] [PubMed] [Google Scholar]
- 9.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 10.Swain SL, Huston G, Tonkonogy S, Weinberg A. Transforming growth factor-beta and IL-4 cause helper T cell precursors to develop into distinct effector helper cells that differ in lymphokine secretion pattern and cell surface phenotype. J Immunol. 1991;147:2991–3000. [PubMed] [Google Scholar]
- 11.Lee HM, Rich S. Co-stimulation of T cell proliferation by transforming growth factor-beta. J Immunol. 1991;147:1127–33. [PubMed] [Google Scholar]
- 12.de Jong J, Van Lier RAW, Ruscetti FR, Schmitt C, Debre P, Mossalayi MD. Differential effect of transforming growth factor-β1 on the activation of human naive and memory CD4+T lymphocytes. Int Immunol. 1994;6:631–8. doi: 10.1093/intimm/6.4.631. [DOI] [PubMed] [Google Scholar]
- 13.Cerwenka A, Bevec D, Majdic O, Knapp W, Holter W. TGF-β1 is a potent inducer of human effector T cells. J Immunol. 1994;153:4367–77. [PubMed] [Google Scholar]
- 14.Swain SL. CD4 T cell development and cytokine polarization: an overview. J Leukoc Biol. 1995;57:795–8. doi: 10.1002/jlb.57.5.795. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Giangreco L, Broome HE, Dargan CM, Swain SL. Control of CD4 effector fate: transforming growth factor beta 1 and interleukin 2 synergize to prevent apoptosis and promote effector expansion. J Exp Med. 1995;182:699–709. doi: 10.1084/jem.182.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rich S, Van Nood N, Lee HM. Role of alpha 5 beta 1 integrin in TGF-β1-costimulated CD8+ T cell growth and apoptosis. J Immunol. 1996;157:2916–23. [PubMed] [Google Scholar]
- 17.Carter LL, Zhang X, Dubey C, Rogers P, Tsui L, Swain SL. Regulation of T cell subsets from naive to memory. J Immunother. 1998;21:181–7. doi: 10.1097/00002371-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Cerwenka A, Kovar H, Majdic O, Holter W. Fas- and activation-induced apoptosis are reduced in human T cells preactivated in the presence of TGF-β. J Immunol. 1996;156:459–64. [PubMed] [Google Scholar]
- 19.Schiott A, Sjogren HO, Lindvall M. The three isoforms of transforming growth factor-beta co-stimulate rat T cells and inhibit lymphocyte apoptosis. Scand J Immunol. 1998;48:371–8. doi: 10.1046/j.1365-3083.1998.00405.x. 10.1046/j.1365-3083.1998.00405.x. [DOI] [PubMed] [Google Scholar]
- 20.Genestier L, Kasibhatla S, Brunner K, Green DR. Transforming growth factor β1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc. J Exp Med. 1999;189:231–9. doi: 10.1084/jem.189.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weller M, Malipiero U, Groscurth P, Fontana A. T cell apoptosis induced by interleukin-2 deprivation or transforming growth factor-β2: Modulation by the phosphatase inhibitors okadaic acid and calyculin A. Exp Cell Res. 1995;221:395–403. doi: 10.1006/excr.1995.1390. 10.1006/excr.1995.1390. [DOI] [PubMed] [Google Scholar]
- 22.Andjelic S, Khanna A, Suthanthiran M, Nikolic-Zugic J. Intracellular Ca2+ elevation and Cyclosporin A synergistically induce TGF-β1-mediated apoptosis in lymphocytes. J Immunol. 1997;158:2527–34. [PubMed] [Google Scholar]
- 23.Bright JJ, Kerr LD, Sriram S. TGF-β inhibits IL-2-induced tyrosine phosphorylation and activation of Jak-1 and Stat 5 in T lymphocytes. J Immunol. 1997;159:175–83. [PubMed] [Google Scholar]
- 24.Bright JJ, Sriram S. TGF-β inhibits IL-12-induced activation of Jak-STAT pathway in T lymphocytes. J Immunol. 1998;161:1772–7. [PubMed] [Google Scholar]
- 25.Asselin-Paturel C, Pardoux C, Gay F, Chouaib S. Failure of TGFβ1 and IL-12 to regulate human FasL and mTNF alloreactive cytotoxic T-cell pathways. Tissue Antigens. 1998;51:242–9. doi: 10.1111/j.1399-0039.1998.tb03098.x. [DOI] [PubMed] [Google Scholar]
- 26.Rowbotham DS, Howdle PD, Trejdosiewicz LK. Peripheral cell-mediated immune response to mycobacterial antigens in inflammatory bowel disease. Clin Exp Immunol. 1995;102:456–61. doi: 10.1111/j.1365-2249.1995.tb03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sillett HK, Whicher JT, Trejdosiewicz LK. Effects of resuscitation fluids on T cell immune responses. Br J Anaesthes. 1998;81:242–3. doi: 10.1093/bja/81.2.242. [DOI] [PubMed] [Google Scholar]
- 28.Cruickshank SM, Southgate J, Selby PJ, Trejdosiewicz LK. Inhibition of T cell activation by normal human biliary epithelial cells. J Hepatol. 1999;31:1026–33. doi: 10.1016/s0168-8278(99)80315-8. [DOI] [PubMed] [Google Scholar]
- 29.Cruickshank SM, Southgate J, Selby PJ, Trejdosiewicz LK. Expression and cytokine regulation of immune recognition elements by normal human biliary epithelial and established liver cell lines in vitro. J Hepatol. 1998;29:550–8. doi: 10.1016/s0168-8278(98)80149-9. [DOI] [PubMed] [Google Scholar]
- 30.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, van Pals ST, Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–20. [PubMed] [Google Scholar]
- 31.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. 10.1002/(sici)1097-0320(19970101)27:1<1::aid-cyto2>3.0.co;2-l. [PubMed] [Google Scholar]
- 32.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–92. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 33.Podack ER. Execution and suicide: cytotoxic lymphocytes enforce Draconian laws through separate molecular pathways. Curr Opin Immunol. 1995;7:11–16. doi: 10.1016/0952-7915(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 34.Strasser A. Life and death during lymphocyte development and function: evidence for two distinct killing mechanisms. Curr Opin Immunol. 1995;7:228–34. doi: 10.1016/0952-7915(95)80007-7. [DOI] [PubMed] [Google Scholar]
- 35.Lenardo MJ. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183:721–4. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott DW, Grdina T, Shi Y. T cells commit suicide, but B cells are murdered. J Immunol. 1996;156:2352–6. [PubMed] [Google Scholar]
- 37.Akbar AN, Salmon M. Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol Today. 1997;18:72–6. doi: 10.1016/s0167-5699(97)01003-7. 10.1016/s0167-5699(97)01003-7. [DOI] [PubMed] [Google Scholar]
- 38.Lincz LF. Deciphering the apoptotic pathway: all roads lead to death. Immunol Cell Biol. 1998;76:1–19. doi: 10.1046/j.1440-1711.1998.00712.x. [DOI] [PubMed] [Google Scholar]
- 39.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis – immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 40.Weinberg AD, Whitham R, Swain SL, Morrison WJ, Wyrick G, Hoy C, Vandenbark AA, Offner H. Transforming growth factor-beta enhances the in vivo effector function and memory phenotype of antigen-specific T helper cells in experimental autoimmune encephalomyelitis. J Immunol. 1992;148:2109–17. [PubMed] [Google Scholar]
- 41.Schiott A, Sjogren HO, Lindvall M. Monocyte-dependent costimulatory effect of TGF-beta 1 on rat T-cell activation. Scand J Immunol. 1996;44:252–60. doi: 10.1046/j.1365-3083.1996.d01-308.x. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Rizzo LV, Silver PB, Caspi RR. Uveitogenicity is associated with a Th1-like lymphokine profile: cytokine-dependent modulation of early and committed effector T cells in experimental autoimmune uveitis. Cell Immunol. 1997;178:69–78. doi: 10.1006/cimm.1997.1121. 10.1006/cimm.1997.1121. [DOI] [PubMed] [Google Scholar]
- 43.Schiott A, Widegren B, Sjogren HO, Lindvall M. Transforming growth factor-beta1, a strong costimulator of rat T-cell activation promoting a shift towards a Th2-like cytokine profile. Immunol Lett. 1999;67:131–9. doi: 10.1016/s0165-2478(99)00005-x. 10.1016/s0165-2478(99)00005-x. [DOI] [PubMed] [Google Scholar]