Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains (original) (raw)
Abstract
Stem cells are a valuable resource for treating disease, but limited access to stem cells from tissues such as brain restricts their utility. Here, we injected marrow stromal cells (MSCs) into the lateral ventricle of neonatal mice and asked whether these multipotential mesenchymal progenitors from bone marrow can adopt neural cell fates when exposed to the brain microenvironment. By 12 days postinjection, MSCs migrated throughout the forebrain and cerebellum without disruption to the host brain architecture. Some MSCs within the striatum and the molecular layer of the hippocampus expressed glial fibrillary acidic protein and, therefore, differentiated into mature astrocytes. MSCs also populated neuron rich regions including the Islands of Calleja, the olfactory bulb, and the internal granular layer of the cerebellum. A large number of MSCs also were found within the external granular layer of the cerebellum. In addition, neurofilament positive donor cells were found within the reticular formation of the brain stem, suggesting that MSCs also may have differentiated into neurons. Therefore, MSCs are capable of producing differentiated progeny of a different dermal origin after implantation into neonatal mouse brains. These results suggest that MSCs are potentially useful as vectors for treating a variety of central nervous system disorders.
Stem cells are characterized by a capacity to self-renew and to generate progeny capable of differentiating into multiple yet distinct cell lineages. Although stem cells derived from early embryos can differentiate into all somatic cell types (1–3), those derived from adult tissues are thought to produce only the cell lineages characteristic of the tissues wherein they reside. For example, hematopoietic stem cells resident in bone marrow give rise to only blood elements (4). Stem cells also have been identified in the gut, gonads, skin, and brain of adults (5, 6). Neural stem cells have been proposed as useful vectors for treating diseases of the central nervous system, but their lack of accessibility limits their utility.
Recently, several reports demonstrated that some stem cells may have greater plasticity than previously envisioned. Eglitis and Mezey reported that a few donor cells from bone marrow transfused into immunodeficient mice were recovered as macroglia in the host brain (7). However, the nature of the marrow cells that engrafted in brain was not determined. Azizi et al. (8) infused rat brains with human marrow stromal cells (MSCs) that are capable of expansion, self-renewal, and differentiation into multiple mesenchymal cell lineages (9, 10). The human MSCs migrated in brain in a manner similar to paraventricular astrocytes, but whether the cells adopted neural cell fates was not defined. In related experiments, Bjornson et al. showed that neural stem cells differentiated into myeloid and lymphoid cell lineages after transplantation into the hematopoietic system of irradiated hosts (11). Together, these findings suggest that it may be possible to reconstitute a tissue by using stem cells from a separate dermal origin. To determine whether mesenchymal stem cells can adopt neural cell fates, we injected a purified population of murine MSCs into the lateral ventricles of neonatal mice and examined the fate of these cells by immunohistochemistry.
MATERIALS AND METHODS
Isolation and Purification of MSCs.
Murine MSC cultures were established from the bone marrow of FVB/N mice (The Jackson Laboratory) and were cultured for 7 days (12). For in vivo transplantation experiments, MSC cultures grown for 5 days were pulsed for 48 hr with 5 μM 5-bromo-2-deoxyuridine (BrdUrd) (Sigma) in α-MEM (Eagle’s minimal essential medium) supplemented with 10% FBS or for 24 hr with 10 μg/ml bis-benzimide (Sigma) before harvest. Cells were harvested by incubating with 0.25% trypsin for 5 min at room temperature followed by gentle scraping. Cells then were incubated for 60 min at 4°C with biotinylated CD11b antibody (PharMingen) bound to avidin-coated paramagnetic beads (PGC Scientific, Gaithersburg, MD). The final concentration of the beads was 0.05 mg/ml, and the ratio of antibody to beads was 10 μg/mg. Cells not bound to the beads were harvested, washed, and resuspended in PBS or α-MEM and immediately were injected into brain or replated into single-well chamber slides, respectively.
Histology and Immunocytochemistry.
MSCs cultured in single-well chamber slides (Becton Dickinson) were washed with PBS, were air dried, were fixed for 15 min in ice-cold methanol, and then were stained with Geimsa. For immunohistochemistry, slides were fixed in ice-cold acetone for 2 min, were air dried, and were blocked with 20% normal goat sera in blocking reagent (0.05% Tween 20 and 1% BSA in PBS). Slides then were incubated with a rat anti-mouse CD16/CD32, Fc block, (PharMingen) at a dilution of 1:50 for 30 min followed by a biotinylated CD11b antibody (PharMingen) at a dilution of 1:200 for 30 min. Primary antibody was detected with a FITC-conjugated anti-biotin antibody (Dako) at a dilution of 1:100. Slides were counterstained with 4′,6-diamidino-2-phenylindole.
Immunohistochemistry for BrdUrd on brain tissue was carried out by using a 1:100 dilution of anti-BrdUrd antibody (Dako) as described (13) except that the formic acid pretreatment was omitted. BrdUrd stained sections then were immunostained for various neural cell markers. For glial fibrillary acidic protein (GFAP), sections were treated for 10 min with 0.25% trypsin, followed by a 15-min blocking step with 20% normal goat serum in blocking reagent. Rabbit anti-GFAP at a 1:250 dilution (Dako) then was applied for 30 min at room temperature. Primary antibody was detected by gold-labeled goat anti-rabbit Ig at a 1:10 dilution followed by silver enhancement for 30 min (Polysciences).
Sections double stained for GFAP and BrdUrd also were stained for neurofilament. Double-labeled sections were rehydrated and heated in a microwave in 10 mM sodium citrate (pH 6.0) for 10 min. The sections then were left at room temperature in the hot citrate solution for an additional 15 min. Sections were rinsed in warm tap water to remove residual citrate buffer and were placed in 0.1% BSA in PBS for 5 min. Sections were incubated in normal serum for 15 min and then were incubated for 1 hr at room temperature with a 1:50 dilution of mouse monoclonal anti-human neurofilament (Dako). Sections then were washed and incubated with a biotinylated horse anti-mouse antibody at a 1:200 dilution for 30 min (Vector Laboratories). The antibody–enzyme complex then was visualized by using the chromagen AEC (Dako).
In Vitro Differentiation Assay.
Chondrogenesis. To induce MSCs to differentiate into chondrocytes, ≈200,000 immunodepleted MSCs were cultured in micromass (14) for ≈3 weeks in the presence of transforming growth factor β1 and an additional 3 weeks in hypertrophic media. Cell pellets then were fixed with 4% paraformaldehyde buffered in PBS and were embedded in glycol methacrylate (Technovit 8100, Energy Beam Sciences, Agawan, MA) according to the manufacturer’s instructions. Sections (1.5 μm) were stained with toluidine blue.
Adipogenesis.
To induce MSCs to differentiate into adipocytes, confluent MSC cultures were incubated in α-MEM supplemented with 10% FBS, 10% normal rabbit serum, 10−8 M dexamethasone, 5 μg/ml insulin, and 50 μM 5,8,11,14-eicosatetraynoic acid. After 2 days, the dexamethasone was removed from the media, and the cells were cultured an additional 5–7 days. Cells then were fixed with ice-cold methanol for 2 min or were stained with Oil Red O and counterstained with toluidine blue. All photographs were taken with a Optiphot 2 Nikon microscope.
Transplantation of MSCs into Brain.
Immunodepleted MSCs were resuspended in PBS at a concentration of 10,000 cells/μl. By using bregma as a landmark, ≈50,000 cells were slowly injected over a period of 5 min into the lateral ventricle of cryoanesthetized 3-day-old mice by using a stereotactic device. At 12 days posttransplant, mice were killed and perfused with 4% paraformaldehyde buffered in PBS. Brains were excised, were grossly dissected into four equal sized coronal sections, and then were processed for paraffin embedding. A total of three animals were analyzed histologically. All animal experiments were conducted under the guidelines of the Institutional Animal Care and Use Committee of MCP/Hahnemann University.
RESULTS
Purification of MSCs.
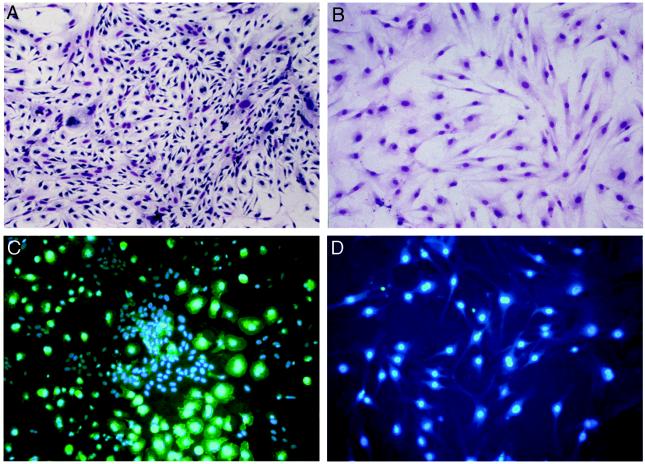
Typically, MSCs are isolated from bone marrow by their adherence to plastic (15). We previously showed that murine MSC cultures established from various inbred mouse strains represent a heterogeneous population dominated by myelopoietic cells that express the cell surface receptor CD11b (Fig. 1 A and C) (12). Based on these results, we developed a reliable method to eliminate these unwanted cells from the cultures via immunodepletion using an anti-CD11b antibody. After immunodepletion, MSCs appear as a uniform population of fibroblastoid-shaped cells (Fig. 1B) that are devoid of CD11b (Fig. 1D)- or CD45 (data not shown)-positive cells. Lack of these antigens also was confirmed by flow cytometry (data not shown).
Figure 1.
Murine MSC cultures before and after immunodepletion. Seven-day-old murine MSC cultures before (A and C) and after (B and D) immunodepletion were stained with Geimsa (A and B) or with a rat anti-mouse CD11b antibody, detected with an FITC-conjugated anti-rat secondary, and counterstained with 4′,6-diamidino-2-phenylindole (C and D).
Differentiation of MSCs into Chondrocytes and Adipocytes.
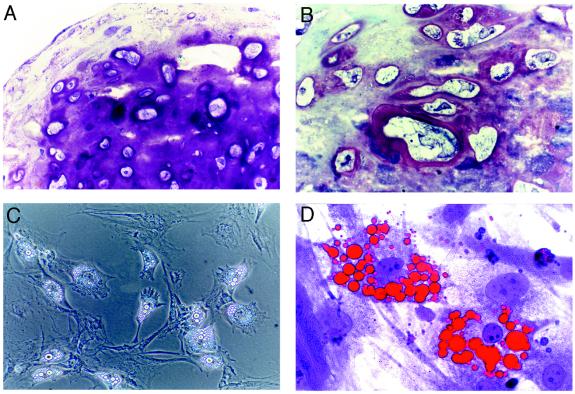
To verify that immunodepleted MSCs retain a multipotential phenotype, we subjected the cells to in vitro differentiation assays. When the cells were cultured in micromass to induce chondrogenesis, histological analysis of the pellets revealed strong staining with toluidine blue, indicating an abundance of glycosaminoglycans within the extracellular matrix (Fig. 2A). In addition, many large cells embedded within lacunae were evident that assumed the morphology of hypertrophic chondrocytes, characteristic of mineralizing cartilage (Fig. 2B). Immunohistochemistry revealed expression of both type I and type II collagens whereas ossification of the micromass was detected by strong staining with Alizarin Red S (data not shown). MSCs also differentiated into adipocytes after exposure to the peroxisome proliferator activating receptor-γ agonist 5,8,11,14-eicosatetraynoic acid. Cells adopted a round morphology and accumulated large, cytoplasmic vacuoles (Fig. 2C). Accumulation of lipid in these vacuoles was assayed histologically by Oil Red O staining (Fig. 2D). Collectively, these results demonstrate that CD11b depletion of plastic adherent murine bone marrow cultures results in a cell population that is morphologically and functionally characteristic of multipotential mesenchymal progenitors.
Figure 2.
Differentiation of immunodepleted MSCs into chondrocytes and adipocytes in vitro. (A) Photomicrograph of immunodepleted MSCs cultured in micromass for 6 weeks and stained with toluidine blue. (B) Higher magnification image of A reveals rich glycosaminoglycan deposition pericellular to hypertrophic chondrocytes embedded within lacunae. (C) Phase contrast image of immunodepleted MSCs cultured for 1 week in adipogenic medium. (D) Photomicrograph of cells in C stained with Oil Red O and counterstained with toluidine blue. (A and D, ×400; B, ×1,000; C, ×100.)
Transplantation of MSCs into Neonatal Mouse Brain.
To determine whether MSCs can engraft into brain, we initially injected ≈50,000 bis-benzimide-labeled cells into the lateral ventricle of neonatal mice. The use of bis-benzimide, a fluorescent DNA binding dye, provided a quick and convenient method to track the fate of the cells in brain. Examination of cryostat sections by fluorescent microscopy revealed that 17 of 18 neonates that received injections contained labeled MSCs at 12 or 45 days postinjection. Moreover, fluorescent nuclei were observed at varying distances away from the injection site, suggesting that some of the MSCs migrated throughout the brain (data not shown). Importantly, in >40 intraventricular injections performed to date, only 2 mice failed to survive. We attribute this loss, however, to prolonged exposure to the anesthesia.
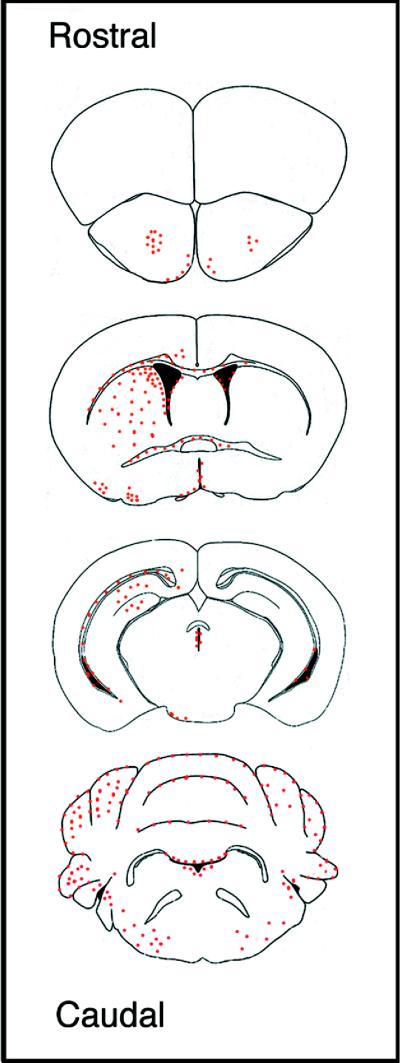
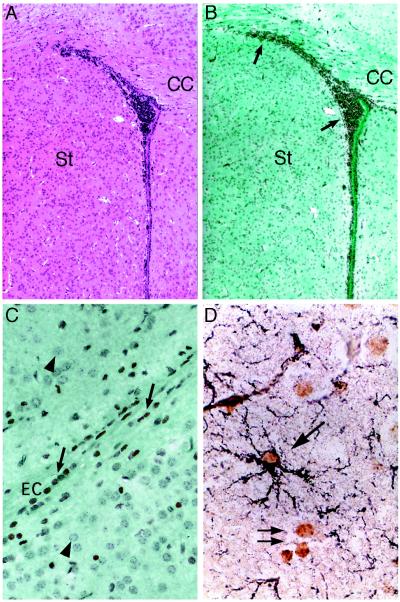
To examine the engraftment of these cells in brain in greater detail, we repeated the injections by using BrdUrd-labeled MSCs. At 12 days postinjection, the overall distribution of MSCs throughout brain was essentially identical in all three animals examined histologically (Fig. 3). In the forebrain, a large number of donor cells were found ipsilateral to the injection site throughout the striatum, spanning from the anterior commissure to the cingulate cortex (Fig. 4 A and B). MSCs also were found lining white matter tracts, including the corpus callosum (Fig. 4B) and the external capsule (Fig. 4C), suggesting that their spaciotemperal distribution throughout the forebrain was mediated, in part, by an ordered process of migration. Many MSCs, however, also were detected lining the ependyma throughout the ventricles. Localization of donor cells within the ventricles may have provided an alternative route by which the cells gained access to various structures along the neuraxis.
Figure 3.
Distribution of immunodepleted MSCs throughout brain at 12 days postinjection. Red dots indicate the regions of BrdUrd-labeled MSCs in coronal sections. Similar results were obtained with three mice.
Figure 4.
Immunohistochemical localization of BrdUrd-labeled MSCs in forebrain. Hematoxylin/eosin (A)- or anti-BrdUrd (B)-stained serial sections of striatum and lateral ventricle, ipsilateral to the injection site at bregma. (C) High power magnification of BrdUrd-labeled cells in the external capsule. Photomicrograph is from same section as B but shows a more lateral field. (D) MSC-derived astrocyte in the molecular layer of the hippocampus double labeled with anti-BrdUrd and anti-GFAP (black). Arrows, BrdUrd-labeled nuclei; arrow-heads, nuclei negative for BrdUrd-labeling. (A and B, ×40; C, ×400; D, ×1,000.)
Double labeling for BrdUrd and GFAP revealed that some of the MSCs within the corpus striatum_,_ the molecular layer of the hippocampus, and the cerebellum differentiated into astrocytes (Fig. 4D). In contrast, none of the cells in these regions stained positive for neurofilament. Therefore, MSCs migrated along established routes and differentiated into macroglia in a manner consistent with ongoing developmental events in neonatal forebrain.
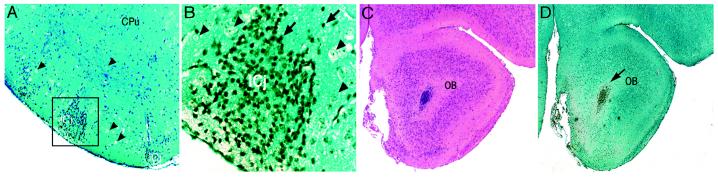
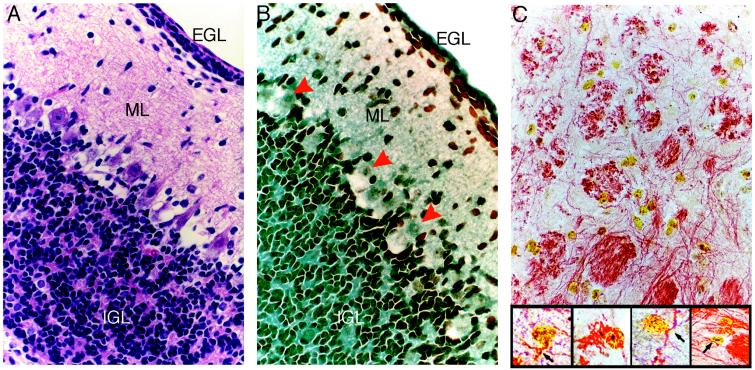
MSCs also were localized to areas undergoing active postnatal neurogenesis (Fig. 5), including the Islands of Calleja (16) in the ventral forebrain and the subependyma of the olfactory bulb (17, 18). A large number of BrdUrd-labeled MSCs integrated within the folia of the cerebellum, as well (Fig. 6 A and B); the majority of the cells were localized to the external granular layer (EGL), the internal granular layer (IGL), and, to a lesser extent, the molecular layer. In contrast, Purkinje cells in the cerebellum adjacent to the IGL were not labeled with BrdUrd. This is consistent with the fact that this neuronal population matures during embryogenesis and is nonmitotic in postnatal brain (19, 20). Most of the BrdUrd-labeled cells in cerebellum were on the side ipsilateral to the injection, but some also were found on the contralateral side. In addition, many BrdUrd-labeled MSCs uniformly lined the fourth ventricle whereas small foci were seen in the white matter tracts adjacent to the dorsal horns of the fourth ventricle. This distribution of the MSCs raised the possibility that they gained access to the EGL and the reticular formation of the brain stem by following a pathway similar to that used by neural progenitors at the time when they emigrate out of the rhombic lip into the primordial EGL during embryogenesis (21).
Figure 5.
Localization of BrdUrd-labeled MSCs in neural rich regions of forebrain. (A) Low magnification of forebrain showing BrdUrd-labeled cells in the Islands of Calleja that are rich in granule neurons. (B) Higher magnification of boxed region in A. Arrows indicate typical positive cells; arrowheads indicate typical negative cells. (C) Hematoxylin/eosin-stained section of the subependyma of the olfactory bulb. (D) Adjacent section stained for BrdUrd. (A, C, and D, ×40; B, ×400.)
Figure 6.
Localization of BrdUrd-labeled MSCs in cerebellum. Hematoxylin/eosin (A) or anti-BrdUrd (B) staining of serial sections reveal MSCs within the EGL, molecular layer (ML), and IGL of the cerebellum. Red arrowheads indicate negative staining of Purkinje cells. (C) MSCs in the reticular formation of the brain stem triple-labeled with anti-BrdUrd, anti-GFAP, and anti-neurofilament. (×400.) (Inset) Higher magnification reveals neurofilament staining (red reaction product) in the cytoplasmic processes of numerous BrdUrd (yellow reaction product)-labeled MSCs. (×1,000.)
Some of the brain sections doubly stained for BrdUrd and GFAP also were stained for neurofilament. Sections from the reticular formation of the brain stem contained cells that were negative for GFAP but positive for both BrdUrd and neurofilament (Fig. 6C); thus, some of the cells may have differentiated into neurons. As also indicated in Fig. 6C, some of the BrdUrd-positive cells did not stain for either GFAP or neurofilament.
DISCUSSION
In this report, we demonstrate that MSCs migrate throughout the forebrain and cerebellum after transplantation into neonatal mouse brains. These events occur in a manner consistent with the ongoing developmental processes that occur in early postnatal life, and they suggest that MSCs mimic the behavior of neural progenitor cells. This is supported further by the finding that some MSCs differentiated into astrocytes whereas others may have differentiated into neurons, as indicated by expression of neurofilament. Therefore, MSCs can produce differentiated progeny of a different dermal origin.
We believe the most significant factor contributing to this behavior of MSCs is their exposure to the neonatal brain microenvironment. Early in postnatal life, the brain continues to undergo extensive development. For example, in forebrain, the process of gliogenesis coincides with a dramatic expansion of the subventricular zone. This germinal zone forms adjacent to the lateral ventricle during embryogenesis and is known to contain multipotent neural stem cells (22–24). During a period of several weeks after birth, highly proliferative neural progenitors emigrate out of the subventricular zone along lateral white matter tracts and colonize the cortex and striatum, differentiating into macroglia (25–27). Remarkably, the spaciotemperal distribution of MSCs throughout forebrain, coupled with their differentiation into astrocytes, suggests that cells injected into the lateral ventricle can participate in the normal events of postnatal development. Localization of MSCs within the olfactory bulb, an alternative destination of neural progenitors born in the subventricular zone (17, 18), is consistent with these findings.
Injection of MSCs into the lateral ventricle may have enabled cells to gain access to various regions of the brain, including the EGL of the cerebellum, without having to migrate from the subventricular zone. At birth, neuronal precursors that comprise the EGL, which represents a displaced germinal zone, enter a phase of rapid proliferation and undergo an inward migration through the molecular layer to form the IGL. This prolonged period of clonal expansion of EGL precursors generates a large population of interneurons, which outnumber Purkinje cells by 250:1 (23). The large number of BrdUrd-labeled cells found throughout the folia of the cerebellum suggest that MSCs also participate in this prolonged period of rapid cell proliferation.
How can cells from bone marrow exhibit nondisruptive integration and adopt neural cell fates after transplantation into brain? Several factors known to induce mesenchymal progenitors to differentiate into osteoblasts are also expressed in brain and affect the maturation of neural cell lineages. For example, levels of fibroblast growth factor 2 expression increase dramatically during late embryonic and early postnatal stages of development in rodent brain (28), and this polypeptide growth factor also has been shown to promote self-renewal in vitro of neural stem cells derived from the neuroepithelium of the developing cortex (29) and the adult subventricular zone (30). More recently, a number of fibroblast growth factors and their receptors have been shown to be transiently up-regulated within the IGL during early postnatal cerebellar development (31). Because fibroblast growth factor 2 is mitogenic for MSCs and also prevents their differentiation in vitro (12, 32, 33), high levels of this protein in the brain may induce MSCs to proliferate. Other neurotrophic factors for which MSCs express receptors, such as nerve growth factor (34), may facilitate their differentiation into neural cell lineages. Finally, migration of MSCs along white matter tracts in brain may result from the up-regulation of adhesion molecules, such as neural cell adhesion molecule, which is expressed by embryonic mesenchymal cells (35).
An important aspect of this work is that a purified population of MSCs were injected into brain. Although various methods to purify rodent MSCs have been reported, including limiting dilution (36), exposure to the metabolic poison potassium thiocyanate (37), and cell sorting using anti-Sca-1 antibodies (38), in each case, the multipotentiality of the purified MSCs was not examined. In contrast, we have shown that immunodepleted MSCs differentiate into different mesenchymal cell lineages. More importantly, these cells also have the potential to adopt neural cell fates after transplantation into brain.
In summary, our results demonstrate that, after injection into developing neonatal mouse brains, immunodepleted MSCs mimicked the behavior of neural progenitors by participating in many aspects of normal brain development, including proliferation, migration along established routes, nondisruptive integration within striatal, cortical, and cerebellar regions, and differentiation into astrocytes and perhaps neurons. These events occurred without significant morbidity or mortality. Because MSCs can be isolated from small volume aspirates from the iliac crest and, therefore, provide an easily accessible and replenishable source of autologous cells for transplantation, they may be useful vehicles for treating a variety of central nervous system disorders.
ABBREVIATIONS
MSC
marrow stromal cell
GFAP
glial fibrillary acidic protein
EGL
external granular layer
IGL
internal granular layer
References
- 1.Evans M J, Kaufman M H. Nature (London) 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin G R. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen R A. Reprod Fertil Dev. 1994;6:543–552. doi: 10.1071/rd9940543. [DOI] [PubMed] [Google Scholar]
- 4.Morrison S J, Uchida N, Weissman I L. Annu Rev Cell Dev Biol. 1994;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 5.Hall P A, Watt F M. Development (Cambridge, UK) 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- 6.Morrison S J, Shah N M, Anderson D J. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 7.Eglitis M A, Mezey E. Proc Natl Acad Sci USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azizi S A, Stokes D, Augelli B J, DiGirolamo C, Prockop D J. Proc Natl Acad Sci USA. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owen M. J Cell Sci Suppl. 1988;10:63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 10.Aubin J E. J Cell Biochem Suppl. 1998;30–31:73–82. [PubMed] [Google Scholar]
- 11.Bjornson C R R, Rietze R L, Reynolds B A, Magli M C, Vescovi A L. Science. 1999;238:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 12.Phinney D G, Kopen G, Issacson R L, Prockop D J. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 13.Gage F H, Coates P W, Palmer T D, Kuhn H G, Fisher L J, Suhonen J O, Peterson D A, Suhr S T, Ray J. Proc Natl Acad Sci USA. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnstone B, Hering T M, Caplan A I, Goldberg V M, Yoo J U. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 15.Prockop D J. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 16.Talbot K, Woolf N J, Butcher L L. J Comp Neurol. 1988;275:533–579. doi: 10.1002/cne.902750407. [DOI] [PubMed] [Google Scholar]
- 17.Altman J. J Comp Neurol. 1969;137:433–458. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 18.Lois C, Alvarez-Buylla A. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 19.Altman J, Bayer S A. J Comp Neurol. 1978;179:23–48. doi: 10.1002/cne.901790104. [DOI] [PubMed] [Google Scholar]
- 20.Altman J, Bayer S A. J Comp Neurol. 1985;231:42–65. doi: 10.1002/cne.902310105. [DOI] [PubMed] [Google Scholar]
- 21.Hatten M E, Heintz N. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- 22.Doetsch F, Garcia-Verdugo J, Avarez-Buylla A. J Neurosci. 1997;17:5046–5062. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lois C, Avarez-Buylla A. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss S, Reynolds B A, Vescovi A L, Morshead C, Craig C G, Van der Kooy D. Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 25.Halliday A L, Cepko C L. Neuron. 1992;9:15–26. doi: 10.1016/0896-6273(92)90216-z. [DOI] [PubMed] [Google Scholar]
- 26.Zerlan M, Levison S W, Goldman J E. J Neurosci. 1995;15:7238–7249. doi: 10.1523/JNEUROSCI.15-11-07238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig C G, Tropepe V, Morshead C M, Reynolds B A, Weiss S, Van der Kooy D. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caday C G, Klagsbrun M, Fanning P J, Mirzabegian A, Finklestein S P. Brain Res Dev Brain Res. 1990;52:241–246. doi: 10.1016/0165-3806(90)90240-y. [DOI] [PubMed] [Google Scholar]
- 29.Gritti A, Parati E A, Cova L, Frolichsthal P, Galli R, Wanke E, Faravelli L, Morassutti D J, Roisen F, Nickel D D, Vescovi A L. J Neurosci. 1996;16:1091–1100. doi: 10.1523/JNEUROSCI.16-03-01091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartlett P F, Richards L R, Kilpatrick T J, Talman P T, Bailey K A, Brooker G J, Dutton R, Koblar S A, Nurcombe V, Ford M O. Ciba Found Symp. 1995;193:85–99. doi: 10.1002/9780470514795.ch5. [DOI] [PubMed] [Google Scholar]
- 31.Hattori Y, Miyake A, Ohta M, Itoh N. Brain Res Mol Brain Res. 1997;47:262–266. doi: 10.1016/s0169-328x(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 32.Oliver L J, Rifkin D B, Gabrilove J, Hannocks M J, Wilson E L. Growth Factors. 1990;3:231–236. doi: 10.3109/08977199009043907. [DOI] [PubMed] [Google Scholar]
- 33.Locklin R M, Williamson M C, Beresford J N, Triffitt J T, Owen M E. Clin Orthop Relat Res. 1995;313:27–35. [PubMed] [Google Scholar]
- 34.Caneva L, Soligo D, Cattoretti G, DeHarven E, Deliliers G L. Blood Cells Mol Dis. 1995;21:738–785. doi: 10.1006/bcmd.1995.0011. [DOI] [PubMed] [Google Scholar]
- 35.Fang J, Hall B K. Int J Dev Biol. 1995;39:519–528. [PubMed] [Google Scholar]
- 36.Wang Q-R, Wolf N S. Exp Hematol. 1990;18:355–359. [PubMed] [Google Scholar]
- 37.Modderman W E, Vrijheid-Lammers T, Lowik C W, Nijweide P J. Exp Hematol. 1994;22:194–201. [PubMed] [Google Scholar]
- 38.Van Vlasselaer N, Falla H, Smoeck H, Mathieu E. Blood. 1994;84:753–763. [PubMed] [Google Scholar]