Anchorage on fibronectin via VLA‐5 (α5β1 integrin) protects rheumatoid synovial cells from Fas‐induced apoptosis (original) (raw)
Abstract
Background
Rheumatoid synovial cells are resistant to apoptosis induction in vivo, whereas, fibroblast‐like synovial cells in rheumatoid arthritis (RA‐FLS) are vulnerable to Fas‐induced apoptosis in vitro.
Objective
To clarify this discrepancy by studying the contribution of the interaction between cellular integrin and matrix fibronectin (Fn), which is significantly increased in the rheumatoid joints, to the induction of apoptosis in RA‐FLS.
Methods
Integrin and Fas mRNAs were measured by reverse transcription‐polymerase chain reaction in RA‐FLS. Integrins expressed in rheumatoid synovial tissues were analysed by immunohistochemistry. RA‐FLS plated either on Fn or on control poly‐l‐lysine were incubated with agonistic anti‐Fas monoclonal antibodies (mAbs). Apoptosis induction was evaluated using terminal deoxynucleotidyl transferase mediated UTP nick end labelling (TUNEL) and immunoblotting for caspase‐3 and poly (ADP‐ribose) polymerase in the presence or absence of anti‐VLA‐5 mAb.
Results
VLA‐5 (α5β1 integrin), a major integrin expressed on RA‐FLS, was required for the adhesion of RA‐FLS on Fn. RA‐FLS plated on Fn were more resistant to Fas‐induced apoptosis than those plated on control poly‐l‐lysine. This protection by Fn was reversed by anti‐VLA‐5 mAb.
Conclusion
Anchorage of RA‐FLS on matrix Fn via VLA‐5 protects RA‐FLS from Fas‐induced apoptosis, and Fn abundantly present in rheumatoid synovium appears to afford RA‐FLS resistance against apoptosis induction in vivo.
Keywords: integrin, apoptosis, Fas, rheumatoid synovial cell, rheumatoid arthritis, fibronectin
Rheumatoid arthritis (RA) is a chronic polyarthritis characterised by extensive hyperplasia of synovia with villous projection and granulomatous tissue invasion termed pannus.1,2,3,4,5,6 Defects in cellular apoptosis may significantly contribute to the pathogenesis of synovial hyperplasia and joint destruction,7,8 but rheumatoid synovial cells are resistant to apoptosis induction in vivo, and the extent of resistance against apoptosis induction positively correlates with progression of joint destruction.9
Fas (CD95, APO‐1), a member of the tumour necrosis factor receptor superfamily, designated TNFRSF6, has been shown to be expressed on the fibroblast‐like type A synovial cells of patients with RA (RA‐FLS).7 Fas initiates a series of cellular events inducing apoptosis via cleavage of caspase‐3 (CPP32, apopain) and subsequent downstream poly (ADP‐ribose) polymerase (PARP), a substrate of activated caspase‐3.10,11,12 Previous studies indicate that RA‐FLS are susceptible to Fas‐induced apoptosis in vitro.7,13,14 They are, however, resistant to Fas‐induced apoptosis in vivo, which may contribute to the synovial cellular hyperplasia and subsequent joint destruction in vivo.15,16 Although several anti‐apoptotic molecules, including Bcl‐2,16 sentrin‐1,17 and Fas‐associated death domain‐like interleukin 1β‐converting enzyme inhibitory protein (FLIP),18 were identified in inflamed rheumatoid synovial fibroblasts, it remained unclear why rheumatoid synovial cells were so resistant to Fas‐induced apoptosis induction in vivo.
Many cells adhere to the extracellular matrix (ECM) via integrins.19 Certain cells undergo apoptosis, induced by inadequate cell‐ECM interaction,20,21 and cell adhesion via integrin molecules is important for cell survival.20,22 Previous studies have shown that fibronectin (Fn), one of the major constituents of the ECM and an important ligand for integrin, exists abundantly in synovial fluids and tissues22,23,24 and specifically on articular cartilage of patients with RA.25 Cell adhesion on Fn is mediated by integrins, including VLA‐5, and interaction between VLA‐5 and Fn transmits a potential survival signal to the cell.26,27 The contribution of integrin to Fas‐induced apoptosis induction remains unclear in RA.
We studied cell‐ECM interaction with particular reference to Fas‐induced apoptosis in RA. Our results showed that induction of Fas‐induced apoptosis of RA‐FLS was inhibited by the anchorage of RA‐FLS on matrix Fn via VLA‐5.
Materials and methods
Synovial tissue and culture of synovial cells
Synovial tissue was obtained during joint surgery from patients with RA fulfilling the criteria of the American College of Rheumatology28 in accordance with the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. Tissues were minced and digested in Dulbecco's modified Eagle's medium (DMEM; Gibco BR, Grand Island, NY, USA) containing 0.2% of collagenase (Sigma, St Louis, MO, USA) at 37°C for 2 hours. Dissociated cells were cultured in DMEM supplemented with 10% fetal bovine serum (Biowhittaker, Walkersville, ML, USA) and 100 U/ml penicillin‐streptomycin. After overnight culture, non‐adherent cells were removed, and adherent cells were further incubated in fresh medium. All experiments were conducted using the cells of 3–4 passages.
Detection of integrins by reverse transcription‐polymerase chain reaction (RT‐PCR)
RA‐FLS (1.0×106) were cultured in 35 mm2 culture plates at 37°C for 24 hours. Total RNA was extracted using RNeasy kit (Qiagen, Hilden, Germany). Total RNA (1 μg) was reverse transcribed into complementary DNA (cDNA). A PCR was conducted in a 20 μl reaction containing 1 μl of cDNA, 10 mM Tris.HCl (pH 8.3), 50 mM KCl, 25 mM MgCl2, 2 mM dNTP, 0.5 U of Taq Gold (Applied Biosystems), and 4 μM of each oligonucleotide primer. The primer sequences were: α1, CATGCGGGGCTCGTTTTGGAA (forward) and CGGCCACATCTCGGGACCAGA (reverse), α2, GCATCTCAGAAGTCTGTTGCC and CCTGTTGTTACCTTCAGGGAG, α3, TACGTGCGAGGCAATGACCTA and TACGTGCGAGGCAATGACCTA, α4, TGGCGTGGTACAACTTGACTG and CATGCGCAACATTCTCATCCT, α5, CAGACCCTGCTCATCCAGAAT and GGCATTCTTGTCACCCAGGTAC, αV, ATGAAACAGGAGCGAGAGCC and CGACAGCCACAGAATAACCC, β1, GAGAGTGCGTCTGCGGACAG and TCTCACACGTTTGCCCTTG AA, β3, CGTGACGAGATTGAGTCAGT and CCCCGGTACGTGATATTGGT, β‐actin, ATCTGGCACCACACCTTCTACAATGAGCTGCG and CGTCATACTCCTGCTTGCTGATCCACATCTGC, Fas, GCGAAAGCCCATTTTTCTTCC and ATTTATTGCCACTGTTTCAGG. PCR products were analysed on 2.0% agarose gel electrophoresis.
Immunohistochemistry
Mouse antihuman VLA‐4 and VLA‐5 monoclonal antibody (mAb; Immunotech SA, Marseille Cedex, France) diluted 1:50 was applied to sections of rheumatoid synovial tissues cut on a cryostat (Bright Co, Huntington, UK) for 20 minutes. HistoFine Simple Stain Kit (Nichirei, Tokyo, Japan) with peroxidase labelled biotin and 3,3′‐diaminobenzidine and alkaline phosphatase labelled biotin and FastBlue (Nichirei) was used for double colour staining according to the avidin‐biotin complex method. Before applying primary Abs, endogenous peroxidase was inactivated with 3% hydrogen peroxide for 30 minutes. As a control, specimens were treated with purified mouse IgG followed by the avidin‐biotin complex method and stained with haematoxylin and eosin.
RA‐FLS culture on Fn coated plates
Culture plates (35 mm2) were coated overnight at 4°C with 30 μg/ml Fn obtained from human plasma (Sigma), as previously described.29 After washing with phosphate buffered saline (PBS), plates were incubated with 1% bovine serum albumin for 2 hours. RA‐FLS (1.0×105) were cultured in serum‐free DMEM on the Fn coated 35 mm2 plates. Cells were incubated either with 20 μg/ml of mouse antihuman VLA‐5 mAb (JBS5; Chemicon International, Temecula, CA, USA) or 20 μg/ml of control IgG for 2 hours. Plates were then washed gently with PBS three times, and non‐adherent cells were removed. Adherent cells in five microscope fields/well were counted under phase contrast microscopy.30 All experiments were performed in triplicate.
TUNEL assay
Eight‐well chamber slides were coated with Fn or poly‐l‐lysine (pLL; Sigma) as control. RA‐FLS (1.0×105) were incubated with 1.0 μg/ml of anti‐Fas mAb (Ab‐2; Oncogene Research Product, San Diego, CA, USA) for 24 hours to induce apoptosis. For experiments using anti‐VLA‐5 mAb, RA‐FLS were treated with 20 μg/ml of anti‐VLA‐5 mAb or control IgG for 1 hour before anti‐Fas mAb treatment. The concentration of each Ab was as used in previous studies.31,32 After incubation for 24 hours, RA‐FLS were fixed in 4% formalin for 10 minutes and then apoptotic cells were determined using a terminal deoxynucleotidyl transferase (TdT) mediated dUTP nick end labelling (TUNEL ) assay kit (Wako, Osaka, Japan), according to the manufacturer's instructions. Apoptotic synovial cells were also quantified by a modified TUNEL method using an apoptosis screening kit (Wako, Osaka, Japan): after incubation, cells in 96‐well microplates were collected by centrifugation and treated with 14 μM TdT at 37°C for 30 minutes. Peroxidase reaction was carried out with horseradish peroxidase and _o_‐phenylenediamine as substrates and TUNEL positive cells were measured at 490 nm.
Immunoblot analysis of cleaved and uncleaved forms of caspase‐3 and PARP
After incubation, cells were lysed in a buffer containing 25 mM Tris.HCl (pH 7.5), 150 mM NaCl, 1.0% Nonidet P‐40, 1.0 mM phenylmethylsulphonyl fluoride, 0.15 U/ml aprotinin, 20 mg/ml leupeptine, and 1 mM sodium vanadate. Detergent soluble proteins (10 μg/lane) were separated by 10% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis and transferred to Immobilon‐P transfer membrane (Millipore, Bedford, CA, USA). After blocking with 1.0% bovine serum albumin and 5.0% skimmed milk in PBS containing 0.1% Triton X‐100, the membranes were incubated overnight with the following Abs: anti‐caspase‐3 rabbit polyclonal Ab (Cell Signaling, Beverly, MA, USA); anti‐cleaved caspase‐3 rabbit polyclonal Ab (Cell Signaling); anti‐PARP rabbit polyclonal Ab (Cell Signaling); and anti‐actin goat polyclonal Ab (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing, the membrane was incubated for 60 minutes with horseradish peroxidase‐conjugated secondary Ab (Amersham, Piscataway, NJ, USA) and visualised by enhanced chemiluminescence (ECL Plus, Amersham, Arlington Heights, IL, USA).
Data analysis
All data are expressed as mean (standard deviation) unless otherwise indicated. For normally distributed data, a two tailed Student's t test was used for comparisons between the two groups. When data were not normally distributed, Wilcoxon's rank sum test was used for comparisons between the two groups.
Results
VLA‐5 is required for adhesion of RA‐FLS
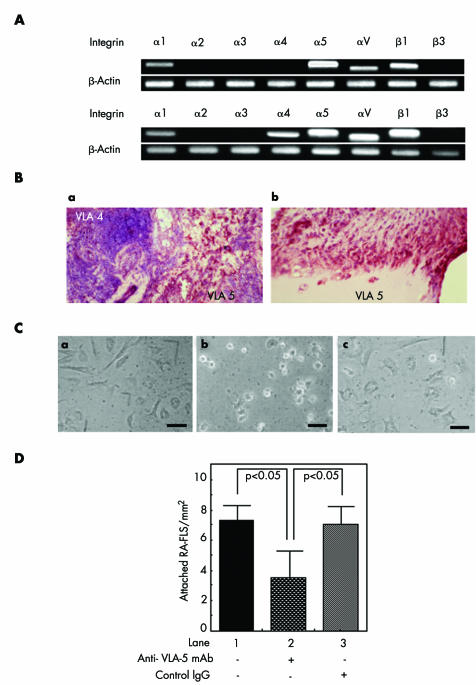
RT‐PCR showed that mRNA of α1, α5, αV, and β1 integrins was expressed in RA‐FLS of all five patients examined, whereas α4 integrin mRNA was expressed in two out of five patients (fig 1A). Immunohistochemical study showed that rheumatoid synovial tissues expressed integrins VLA‐4 and VLA‐5. VLA‐4 was, however, expressed almost exclusively in the aggregated lymphocytes (fig 1Ba), while VLA‐5 was mainly expressed in the fibroblast‐like cells in the apical portion of synovia and pannus (fig 1Bb). An adhesion study showed that RA‐FLS plated on Fn coated dishes displayed a spindle shaped morphology when incubated for 2 hours in the absence or presence of control IgG (fig 1Cac), whereas they displayed a round shape in the presence of anti‐VLA‐5 mAb (fig 1Cb). When enumerated, the number of RA‐FLS attached on Fn coated plates was significantly decreased by the treatment with anti‐VLA‐5 mAb (fig 1D), indicating that VLA‐5 is required for adhesion of RA‐FLS to Fn coated plates.
Figure 1 (A) Integrin mRNA expression on RA‐FLS as detected by RT‐PCR. (a) A representative result of three out of five samples. (b) A representative result of two out of five samples. (B) Double colour immunohistochemistry of RA‐FLS for VLA‐4 (blue) and VLA‐5 (dark red). (a) Rheumatoid synovial tissue with lymphoid aggregates. (b) Apical portion of rheumatoid synovial tissue. (C) RA‐FLS culture on Fn plates. Shape of RA‐FLS plated on Fn and cultured for 2 hours without anti‐VLA5 mAb (a), with anti‐VLA5 mAb (b) or with control IgG (c) (bars = 25 μm). (D) The number of RA‐FLS attached on Fn coated plates after 2 hours' culture. Statistical analyses were performed for five independent experiments each derived from different patients (p<0.05).
Fn protects RA‐FLS from Fas‐induced apoptosis
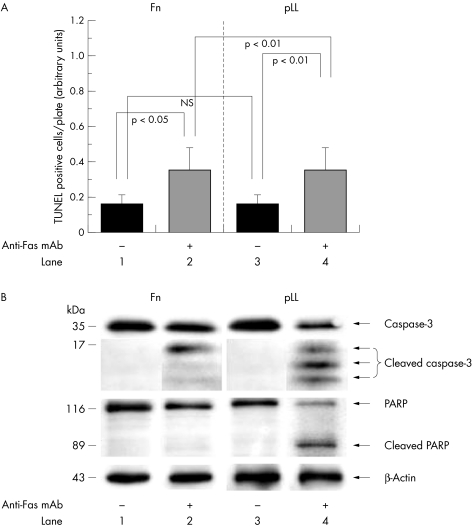
To study the contribution of cell adhesion to Fas‐induced apoptosis induction in RA‐FLS, RA‐FLS plated either on Fn or on control pLL were cultured in the presence of anti‐Fas mAb: pLL was used as a control because it does not transmit an intracellular signal but supports cell attachment.33 We found that RA‐FLS plated on Fn for 2 hours displayed a spindle shaped morphology and a greater extent of spreading than those plated on pLL. After treatment with anti‐Fas mAb for 24 hours, most RA‐FLS plated on pLL were detached or displayed a round shape, whereas RA‐FLS cultured on Fn still remained attached (data not shown). Measurement showed that the TUNEL positive cells cultured on Fn were significantly decreased compared with those plated on pLL after treatment with anti‐Fas mAb (fig 2A).
Figure 2 Effect of anti‐Fas treatment. (A) Measurement of TUNEL positive cells plated on Fn or on pLL after anti‐Fas mAb treatment for 24 hours. Statistical analyses (mean (SD) were performed for five independent experiments (NS, not significant). (B) Immunoblotting for caspase‐3, cleaved caspase‐3, PARP, cleaved PARP, and β‐actin 6 hours after anti‐Fas treatment. A representative result of three independent experiments is shown.
Immunoblotting showed that, 6 hours after treatment with anti‐Fas mAb, caspase‐3 and PARP were significantly cleaved in RA‐FLS plated on pLL (fig 2B, lane 4). In contrast, cleavage of PARP was completely inhibited and cleavage of caspase‐3 was partially but significantly inhibited in RA‐FLS plated on Fn (fig 2B, lane 2), indicating that Fn protected RA‐FLS from Fas‐induced apoptosis. Further, by RT‐PCR we confirmed that Fas expression on RA‐FLS was constant or marginally increased by the incubation on Fn (data not shown).
VLA‐5 is required for resistance against Fas‐induced apoptosis
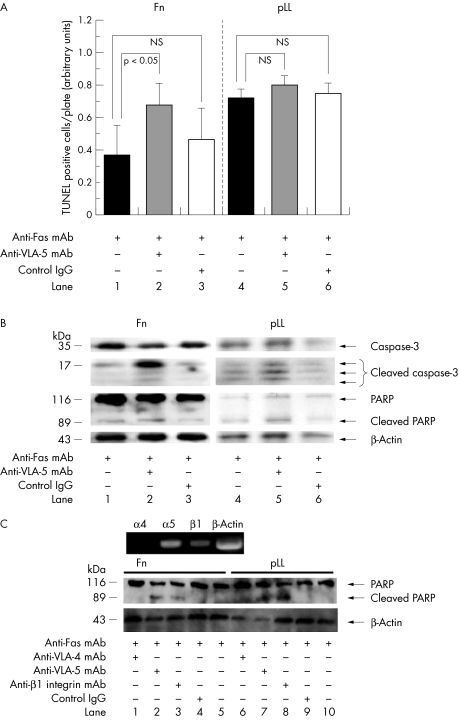
When RA‐FLS plated on Fn were first treated with anti‐VLA‐5 mAb for 2 hours and then cultured with anti‐Fas mAb for 24 hours, most cells formed a round shape and the number of attached cells with a spindle shape significantly decreased (data not shown). Measurement showed that TUNEL positive apoptotic cells significantly increased in RA‐FLS cultured on Fn coated plates after treatment with anti‐VLA‐5 mAb (p<0.05) (fig 3A, left panel). The effect of anti‐VLA‐5 treatment was minimal for RA‐FLS plated on pLL (fig 3A, right panel).
Figure 3 Anti‐VLA‐5 and anti‐Fas treatment. (A) Measurement of TUNEL positive cells cultured either on Fn or on pLL with anti‐Fas treatment for 24 hours in the presence of anti‐VLA‐5 mAb or control IgG. Statistical analyses (mean (SD)) were performed for five independent experiments (NS, not significant). (B) Immunoblot of RA‐FLS for caspase‐3, cleaved caspase‐3, PARP, cleaved PARP, and β‐actin 12 hours after treatment with either anti‐Fas mAb, anti‐VLA‐5 mAb, or control IgG. The result of three independent experiments is shown. (C) Upper panel: integrin mRNA expression on the prototype of RA‐FLS used in the present study. Lower panel: immunoblot of the RA‐FLS for PARP, cleaved PARP, and β‐actin 24 hours after treatment with anti‐Fas mAb following incubation for 1 hour with either anti‐VLA‐4 mAb, anti‐VLA‐5 mAb, anti‐β1 integrin mAb, or control IgG. The result of three independent experiments is shown.
Immunoblotting showed that Fas‐induced cleavage of caspase‐3 was significantly enhanced in the presence of anti‐VLA‐5 mAb in the RA‐FLS cultured on Fn (fig 3B, lane 2). Cleavage of downstream PARP was marginally enhanced (lane 2). In contrast, Fas‐induced cleavage of caspase‐3 was less enhanced by anti‐VLA‐5 mAb when plated on pLL (lanes 4–6) than on Fn, suggesting that anchorage of RA‐FLS on matrix Fn via VLA‐5 protected RA‐FLS from Fas‐induced apoptosis in a process significantly interfering with receptor‐induced intracellular upstream apoptosis signalling. We also showed that a prototype of RA‐FLS, mainly expressing α5 and β1 integrins, was induced to apoptosis as verified by the cleavage of PARP by Fas when the cell was incubated with either anti‐VLA‐5 or β1 integrin Ab, but not with anti‐VLA‐4 Ab (fig 3C).
Discussion
Previous studies have shown that cells easily undergo apoptosis in the absence of integrin mediated signalling.28,30 Zhang et al showed that CHO cells expressing VLA‐5, a ligand for Fn, are resistant to apoptosis induction during serum starvation.26 α4β1 Integrin (VLA‐4) engagement protects certain B cells from apoptosis induction during serum deprivation,33 indicating that cell anchorage on matrix Fn via VLA protects the cells from apoptosis. Although adhesion to Fn is mediated mainly via VLA‐4 and VLA‐5, several other integrins such as α3β1, αVβ1, αIIβ3, αVβ6, α4β7, α8β1, and αVβ3 can also behave similarly.34 In rheumatoid joints, matrix Fn is significantly increased in synovial fluids and tissues and articular cartilage surfaces,22,23,24,25 and synovial tissues significantly express α3, α4, α5, and β1 integrins.35,36,37 Among β1 integrins, VLA‐5 is dominantly expressed in rheumatoid synovial tissues.38 Studies also indicated that adhesion of RA‐FLS to Fn is mediated by β1 integrin receptors,39,40 which is consistent with the present finding that α5β1 integrin (VLA‐5) is consistently expressed in rheumatoid synovium and anti‐VLA‐5 mAb blocks adhesion of RA‐FLS onto Fn.
Fas antigen is readily and constantly demonstrable on cultured RA‐FLS,7,41 and RA‐FLS are particularly vulnerable to Fas‐induced apoptosis in vitro. In contrast, while comparable levels of Fas antigen were expressed on the surface of the synovial cells of patients with RA and osteoarthritis,13,14 the frequency of rheumatoid synovial cells that actually went into the late stages of apoptosis was surprisingly small in vivo. Studies also indicated that RA‐FLS survived in vivo far longer than expected.16,42 There are several explanations for the discrepancy between in vitro and in vivo results. Firstly, the interaction between soluble Fas and soluble Fas ligand might have interfered with in vivo interaction between RA synovial cells and T cells, and hence down regulated the in vivo Fas‐induced apoptosis signalling.13,40 Secondly, apoptosis induction in vivo might have been inhibited by Bcl‐2, an anti‐apoptotic protein overexpressed in rheumatoid synovium as compared with osteoarthritis.16,43
In this study we confirmed that RA‐FLS expressing Fas persistently were sensitive to Fas‐induced apoptosis in vitro, irrespective of whether the cells were plated on Fn or not. However, we also found that RA‐FLS plated on Fn became more resistant to Fas‐induced apoptosis than those plated on pLL. Importantly, this protection was reversed by pretreatment of RA‐FLS with anti‐VLA‐5 mAb and anti‐β1 integrin mAb, suggesting that Fn protects RA‐FLS from Fas‐induced apoptosis via VLA‐5.
Anchorage of cells onto the matrix is important for regulating cell growth.44 Dike et al have shown that adhesion of cells to Fn induces expression of several growth associated genes in fibroblasts.45 Furthermore, recent reports show that collagen type I, an ECM, inhibits Fas‐induced apoptosis in T lymphocytes through α2β1 integrin signalling,46 that synovial fibroblasts bind to fibronectin through α5β1 integrin, and that FLS proliferation requires a signal provided by the ECM.47 Our results indicated that interaction between Fn and VLA‐5 exerted anti‐apoptotic effects against Fas. However, Nakayamada et al have shown that crosslinking of β1 integrins and ligation of Fn induce the expression of intracellular adhesion molecule 1 (ICAM‐1) and Fas on RA‐FLS, and that crosslinking of β1 integrins enhances Fas‐induced apoptosis, suggesting that Fn‐integrin interaction may up regulate apoptosis on RA‐FLS.48 In their experiments, the induced expression of Fas and ICAM‐1 by crosslinking of β1 integrins continues after 24 hours, while the induction by Fn diminishes after 24 hours. This suggests that the effects of crosslinking β1 integrins may be very different from the effects of blocking the Fn‐integrin interaction and should not be generalised. Receptor conformations transformed by crosslinking may influence the efficacy of integrin signalling.49
Although our results do not directly suggest that Fn‐integrin interaction is disease‐specific for RA, by taking the findings together, in the present study we hypothesise that RA‐FLS are particularly resistant to ligand‐induced apoptosis induction because Fn and its ligand integrin molecules are abundantly expressed in rheumatoid synovium.50,51,52 Down regulation of Fas‐induced apoptosis in vivo through interaction between VLA‐5 and matrix Fn may be important for the pathogenesis of joint destruction and a therapeutic intervention. Further studies are necessary to identify the signalling target molecules responsible for Fn‐integrin interaction.
Abbreviations
DMEM - Dulbecco's modified Eagle's medium
ECM - extracellular matrix
Fn - fibronectin
mAb - monoclonal antibody
PARP - poly (ADP‐ribose) polymerase
PBS - phosphate buffered saline
pLL - poly‐l‐lysine
RA - rheumatoid arthritis
RA‐FLS - fibroblast‐like synovial cells in rheumatoid arthritis
RT‐PCR - reverse transcription‐polymerase chain reaction
TdT - terminal deoxynucleotidyl transferase
TUNEL - terminal deoxynucleotidyl transferase mediated UTP nick end labelling
References
- 1.Hirohata K, Kobayashi I. Fine structures of the synovial tissues in rheumatoid arthritis. Kobe J Med Sci 196410195–225. [PubMed] [Google Scholar]
- 2.Barland P, Novikoff A B, Hamerman D. Fine structure and cytochemistry of the rheumatoid synovial membrane, with special reference to lysosomes. Am J Pathol 196444853–866. [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi I, Ziff M. Electron microscopic studies of the cartilage‐pannus junction in rheumatoid arthritis. Arthritis Rheum 197518475–483. [DOI] [PubMed] [Google Scholar]
- 4.Shiozawa S, Jasin H E, Ziff M. Absence of immunoglobulins in rheumatoid cartilage‐pannus junctions. Arthritis Rheum 198023816–821. [DOI] [PubMed] [Google Scholar]
- 5.Gay S, Gay R E, Koopman W J. Molecular and cellular mechanisms of joint destruction in rheumatoid arthritis: two cellular mechanisms explain joint destruction? Ann Rheum Dis 199352(suppl 1)S39–S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiozawa S, Shiozawa K, Fujita T. Morphologic observations in the early phase of the cartilage‐pannus junction. Light and electron microscopic studies of active cellular pannus. Arthritis Rheum 198326472–478. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima T, Aono H, Hasunuma T, Yamamoto K, Shirai T, Hirohata K.et al Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum 199538485–491. [DOI] [PubMed] [Google Scholar]
- 8.Baier A, Meineckel I, Gay S, Pap T. Apoptosis in rheumatoid arthritis. Curr Opin Rheumatol 200315274–279. [DOI] [PubMed] [Google Scholar]
- 9.Ceponis A, Hietanen J, Tamulaitiene M, Partsch G, Patiala H, Konttinen Y T. A comparative quantitative morphometric study of cell apoptosis in synovial membranes in psoriatic, reactive and rheumatoid arthritis. Rheumatology (Oxford) 199938431–440. [DOI] [PubMed] [Google Scholar]
- 10.Casciola‐Rosen L, Nicholson D W, Chong T, Rowan K R, Thornberry N A, Miller D K.et al Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med 19961831957–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy N J, Whyte M K, Gilbert C S, Evan G I. Inhibition of Ced‐3/ICE‐related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl‐2 homologue Bak. J Cell Biol 1997136215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M.et al The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 199166233–243. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto H, Tanaka M, Suda T, Tomita T, Hayashida K, Takeuchi E.et al Soluble Fas ligand in the joints of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum 199841657–662. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T, Okamoto K, Kobata T, Hasunuma T, Sumida T, Nishioka K. Tumor necrosis factor alpha regulation of the FAS‐mediated apoptosis‐signaling pathway in synovial cells. Arthritis Rheum 199942519–526. [DOI] [PubMed] [Google Scholar]
- 15.Firestein G S, Yeo M, Zvaifler N J. Apoptosis in rheumatoid arthritis synovium. J Clin Invest 1995961631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto S, Muller‐Ladner U, Gay R E, Nishioka K, Gay S. Ultrastructural demonstration of apoptosis, Fas and Bcl‐2 expression of rheumatoid synovial fibroblasts. J Rheumatol 1996231345–1352. [PubMed] [Google Scholar]
- 17.Franz J K, Pap T, Hummel K M, Nawrath M, Aicher W K, Shigeyama Y.et al Expression of sentrin, a novel antiapoptotic molecule, at sites of synovial invasion in rheumatoid arthritis. Arthritis Rheum 200043599–607. [DOI] [PubMed] [Google Scholar]
- 18.Perlman H, Liu H, Georganas C, Koch A E, Shamiyeh E, Haines G K., 3rd_et al_ ifferential expression pattern of the antiapoptotic proteins, Bcl‐2 and FLIP, in experimental arthritis. Arthritis Rheum 2001442899–2908. [DOI] [PubMed] [Google Scholar]
- 19.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 19926911–25. [DOI] [PubMed] [Google Scholar]
- 20.Meredith J E, Jr, Fazeli B, Schwartz M A. The extracellular matrix as a cell survival factor. Mol Biol Cell 19934953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frisch S M, Francis H. Disruption of epithelial cell‐matrix interactions induces apoptosis. J Cell Biol 1994124619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiozawa S, Ziff M. Immunoelectron microscopic demonstration of fibronectin in rheumatoid pannus and at the cartilage‐pannus junction. Ann Rheum Dis 198342254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott D L, Wainwright A C, Walton K W, Williamson N. Significance of fibronectin in rheumatoid arthritis and osteoarthrosis. Ann Rheum Dis 198140142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carnemolla B, Cutolo M, Castellani P, Balza E, Raffanti S, Zardi L. Characterization of synovial fluid fibronectin from patients with rheumatic inflammatory diseases and healthy subjects. Arthritis Rheum 198427913–921. [DOI] [PubMed] [Google Scholar]
- 25.Shiozawa K, Shiozawa S, Shimizu S, Fujita T. Fibronectin on the surface of articular cartilage in rheumatoid arthritis. Arthritis Rheum 198427615–622. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Vuori K, Reed J C, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up‐regulates Bcl‐2 expression. Proc Natl Acad Sci USA 1995926161–6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien V, Frisch S M, Juliano R L. Expression of the integrin alpha 5 subunit in HT29 colon carcinoma cells suppresses apoptosis triggered by serum deprivation. Exp Cell Res 1996224208–213. [DOI] [PubMed] [Google Scholar]
- 28.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 29.Barillari G, Albonici L, Incerpi S, Bogetto L, Pistritto G, Volpi A.et al Inflammatory cytokines stimulate vascular smooth muscle cells locomotion and growth by enhancing alpha5beta1 integrin expression and function. Atherosclerosis 2001154377–385. [DOI] [PubMed] [Google Scholar]
- 30.Barillari G, Sgadari C, Palladino C, Gendelman R, Caputo A, Morris C B.et al Inflammatory cytokines synergize with the HIV‐1 Tat protein to promote angiogenesis and Kaposi's sarcoma via induction of basic fibroblast growth factor and the alpha v beta 3 integrin. J Immunol 19991631929–1935. [PubMed] [Google Scholar]
- 31.Cifone M G, De Maria R, Roncaioli P, Rippo M R, Azuma M, Lanier L L.et al Apoptotic signaling through CD95 (Fas/Apo‐1) activates an acidic sphingomyelinase. J Exp Med 19941801547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bao W, Stromblad S. Use of an immobilized monoclonal antibody to examine integrin alpha5beta1 signaling independent of cell spreading. Biol Proced Online 2002481–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia‐Gila M, Lopez‐Martin E M, Garcia‐Pardo A. Adhesion to fibronectin via alpha4 integrin (CD49d) protects B cells from apoptosis induced by serum deprivation but not via IgM or Fas/Apo‐1 receptors. Clin Exp Immunol 2002127455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson S, Svineng G, Wennerberg K, Armulik A, Lohikangas L. Fibronectin‐integrin interactions. Front Biosci 19972d126–d146. [DOI] [PubMed] [Google Scholar]
- 35.Nikkari L, Aho H, Yli‐Jama T, Larjava H, Jalkanen M, Heino J. Expression of integrin family of cell adhesion receptors in rheumatoid synovium. Alpha 6 integrin subunit in normal and hyperplastic synovial lining cell layer. Am J Pathol 19931421019–1027. [PMC free article] [PubMed] [Google Scholar]
- 36.el Gabalawy H, Wilkins J. Beta 1 (CD29) integrin expression in rheumatoid synovial membranes: an immunohistologic study of distribution patterns. J Rheumatol 199320231–237. [PubMed] [Google Scholar]
- 37.Ishikawa H, Hirata S, Nishibayashi Y, Imura S, Kubo H, Ohno O. The role of adhesion molecules in synovial pannus formation in rheumatoid arthritis. Clin Orthop 1994297–303. [PubMed]
- 38.Ishikawa H, Hirata S, Andoh Y, Kubo H, Nakagawa N, Nishibayashi Y.et al An immunohistochemical and immunoelectron microscopic study of adhesion molecules in synovial pannus formation in rheumatoid arthritis. Rheumatol Int 19961653–60. [DOI] [PubMed] [Google Scholar]
- 39.Rinaldi N, Schwarz‐Eywill M, Weis D, Leppelmann‐Jansen P, Lukoschek M, Keilholz U.et al Increased expression of integrins on fibroblast‐like synoviocytes from rheumatoid arthritis in vitro correlates with enhanced binding to extracellular matrix proteins. Ann Rheum Dis 19975645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasunuma T, Kayagaki N, Asahara H, Motokawa S, Kobata T, Yagita H.et al Accumulation of soluble Fas in inflamed joints of patients with rheumatoid arthritis. Arthritis Rheum 19974080–86. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto K, Kobayashi T, Kobata T, Hasunuma T, Kato T, Sumida T.et al Fas‐associated death domain protein is a Fas‐mediated apoptosis modulator in synoviocytes. Rheumatology (Oxford) 200039471–480. [DOI] [PubMed] [Google Scholar]
- 42.Catrina A I, Ulfgren A K, Lindblad S, Grondal L, Klareskog L. Low levels of apoptosis and high FLIP expression in early rheumatoid arthritis synovium. Ann Rheum Dis 200261934–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perlman H, Georganas C, Pagliari L J, Koch A E, Haines K, 3rd, Pope R M. Bcl‐2 expression in synovial fibroblasts is essential for maintaining mitochondrial homeostasis and cell viability. J Immunol 20001645227–5235. [DOI] [PubMed] [Google Scholar]
- 44.Stoker M, O'Neill C, Berryman S, Waxman V. Anchorage and growth regulation in normal and virus‐transformed cells. Int J Cancer 19683683–693. [DOI] [PubMed] [Google Scholar]
- 45.Dike L E, Farmer S R. Cell adhesion induces expression of growth‐associated genes in suspension‐arrested fibroblasts. Proc Natl Acad Sci USA 1988856792–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gendron S, Couture J, Aoudjit F. Integrin alpha2beta1 inhibits Fas‐mediated apoptosis in T lymphocytes by protein phosphatase 2A‐dependent activation of the MAPK/ERK pathway. J Biol Chem. 2003;278: 48633–43, Epub 2003 Sep 17. [DOI] [PubMed]
- 47.Sarkissian M, Lafyatis R. Integrin engagement regulates proliferation and collagenase expression of rheumatoid synovial fibroblasts. J Immunol 19991621772–1779. [PubMed] [Google Scholar]
- 48.Nakayamada S, Saito K, Fujii K, Yasuda M, Tamura M, Tanaka Y. Beta1 integrin‐mediated signaling induces intercellular adhesion molecule 1 and Fas on rheumatoid synovial cells and Fas‐mediated apoptosis. Arthritis Rheum 2003481239–1248. [DOI] [PubMed] [Google Scholar]
- 49.Mould A P, Humphries M J. Regulation of integrin function through conformational complexity: not simply a knee‐jerk reaction? Curr Opin Cell Biol 200416544–551. [DOI] [PubMed] [Google Scholar]
- 50.Hino K, Shiozawa S, Kuroki Y, Ishikawa H, Shiozawa K, Sekiguchi K.et al EDA‐containing fibronectin is synthesized from rheumatoid synovial fibroblast‐like cells. Arthritis Rheum 199538678–683. [DOI] [PubMed] [Google Scholar]
- 51.Hino K, Maeda T, Sekiguchi K, Shiozawa K, Hirano H, Sakashita E.et al Adherence of synovial cells on EDA‐containing fibronectin. Arthritis Rheum 1996391685–1692. [DOI] [PubMed] [Google Scholar]
- 52.Shiozawa S, Yoshihara R, Kuroki Y, Fujita T, Shiozawa K, Imura S. Pathogenic importance of fibronectin in the superficial region of articular cartilage as a local factor for the induction of pannus extension on rheumatoid articular cartilage. Ann Rheum Dis 199251869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]