Systemic complement activation following human acute ischaemic stroke (original) (raw)
Abstract
The brain tissue damage after stroke is mediated partly by inflammation induced by ischaemia–reperfusion injury where the complement system plays a pivotal role. In the present study we investigated systemic complement activation and its relation to C-reactive protein (CRP), a known complement activator, and other inflammatory mediators after acute ischaemic stroke. Sequential plasma samples from 11 acute stroke patients were obtained from the time of admittance to hospital and for a follow-up period of 12 months. Nine healthy gender- and age-matched subjects served as controls. The terminal SC5b-9 complement complex (TCC), CRP, soluble adhesion molecules (L-, E- and P- selectin, ICAM, VCAM) and cytokines [tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-8] were analysed. All parameters were within normal values and similar to the controls the first hours after stroke. Terminal complement complex (TCC) increased significantly from 0·54 to 0·74 AU/ml at 72 h (P = 0·032), reached maximum at 7 days (0·90 AU/ml, P < 0·001), was still significantly increased at 12 days (0·70 AU/ml, P = 0·009) and thereafter normalized. CRP increased significantly from 1·02 to 2·11 mg/l at 24 h (P = 0·023), remained significantly increased for 1 week (2·53–2·94 mg/l, P = 0·012–0·017) and thereafter normalized. TCC and C-reactive protein (CRP) correlated significantly (r = 0·36, P < 0·001). The increase in TCC and CRP correlated to the size of infarction (r = 0·80 and P = 0·017 for TCC; r = 0·72 and P = 0·043 for CRP). No significant changes were seen for adhesion molecules and cytokines. In conclusion, transitory systemic complement activation takes place after stroke. The early rise in CRP and the following TCC increase suggest a possible role for CRP in complement activation, which may contribute to inflammation after stroke.
Keywords: complement, C-reactive protein, human ischaemic stroke
INTRODUCTION
There is growing evidence that inflammation plays an important role in the pathophysiology of stroke [1,2]. Brain tissue damage after acute ischaemic stroke is mediated partly by inflammation induced by ischaemia–reperfusion (I/R) injury [3]. One of the major systems that may be involved is complement [4,5].
A role for complement activation in myocardial I/R injury was suggested over 30 years ago [6]. Since Weisman et al., in 1990, documented substantial reduction in the size of experimental myocardial infarction in rats using the specific complement inhibitor sCR1 [7], a series of studies have confirmed their findings [8–10]. Numerous investigations have demonstrated that complement contributes to I/R injury in other organs such as intestine [11], skeletal muscle [12] and kidney [13]. In contrast, the role of complement in cerebral I/R injury has been a matter of controversy [14–18]. However, recent animal studies suggest a role for complement in neuronal damage after experimental stroke and show that inhibition of complement activation can reduce the size of brain infarction [19–21]. Furthermore, there is evidence that complement can also be activated locally in human stroke [22], but except for a preliminary report published in letter form [23] there is no information on systemic complement activation in stroke. In the present study we used the soluble terminal complement complex (TCC) as a marker of complement activation. TCC is a sensitive and reliable complement activation product for the evaluation of in vivo activation due to a low physiological concentration and a relatively long half-life (1 h) in contrast to, e.g. C5a, which binds rapidly to leucocytes and has a half-life of 1 min. Finally, in contrast to early activation products from, e.g. C4 and C3, TCC is relatively stable in vitro.
C-reactive protein (CRP) is a known activator of the complement system [24,25] and could also predict stroke [2,24]. A recent study showed that CRP enhanced inflammation in ischaemic myocardium by inducing local complement activation [26], and preliminary data suggest a possible role between CRP and complement activation in ischaemic stroke [23]. Furthermore, the complement system participate in inflammation in CNS in general [4], which could explain the local expression of adhesion molecules and cytokines seen as a part of the inflammatory reaction [27,28]. The relation between these inflammatory mediators and complement activation in the systemic circulation after stroke has not been studied previously. We therefore investigated systemic complement activation after acute ischaemic stroke with emphasis on its relation to CRP, soluble adhesions molecules and proinflammatory cytokines.
MATERIALS AND METHODS
Study design
Patients with acute ischaemic stroke and acute hemiparesis were included in this study.
The examination procedures were in accordance with institutional guidelines and approved by the regional ethics committee. Acute ischaemic stroke was defined as a focal neurological deficit of sudden onset that persisted for 24 h, and with the presence of cerebral infarction on computed tomography (CT) or magnetic resonance imaging (MRI) scan. Patients with symptoms of ongoing cardiovascular, acute infection, acute inflammatory or malignant disease at stroke onset were excluded. Informed consent was obtained from all study participants or their legal representative.
Patients
The demographic data are listed in Table 1. Eleven patients with acute ischaemic stroke were included in this study. Mean age of the patients was 70 ranging from 54 to 79 years. Nine healthy subjects, matched for gender and age, with mean age of 71 years ranging from 67 to 75 years, served as controls. Two patients had a slightly elevated CRP level at the time of admission without any clinical signs of infection or inflammation. The patients with chronic diseases had no acute episodes of disease during the last year before the stroke onset. All patients survived during the study. None of the patients were treated with thrombolytic therapy. All patients were examined with a CT scan. Patients 8 and 10 had no lesions on CT scan and were investigated further with an MRI scan. Computer-assisted planimetry was used to calculate the volume of infarction 5 and 7 days after admission. Eight patients had infarction in the right hemisphere and three in the left. Complications and volume of infarction are presented in Table 1.
Table 1. Pre- and comorbidity at onset, complications during the course and volume of infarction.
| Patient | Pre- and co morbidity at onset | Complications and time from onset | Lesion volume (cm3) |
|---|---|---|---|
| 1 | Healthy | Fever (24 h), pneumonia (48 h) | 340 |
| 2 | Ankylosing spondylitis, mechanical aorta valve 12 years previously | Pacemaker (5 and 10 days) | 61 |
| 3 | Diverticulitis, stable last year before onset | GI-bleedings (13 days, 2, 5 and 5 months, 1 year) | 5 |
| 4 | Aortocoronary bypass 13 years previously, cholelithiasis, stable | Urinary infection (7 days), pneumonia (14 days, 2 months) | 150 |
| 5 | Osteoporosis, probable TIA several years previously | None | 18 |
| 6 | Ischaemic stroke 9 years previously, post-infarction epilepsy, stable | None | 34 |
| 7 | Healthy | Fever (48 h), urinary infection (72 h) | 45 |
| 8 | Hypertension, myocardial infarction 7 and 3 years previously,TIA 2 years before onset | Urinary infection (12 h) | 1 |
| 9 | Hypertension, kidney damage with high creatinine,myocardial infarction 5 years before onset | Pneumonia (48 h), post-infarction epilepsy (9 months) | 59 |
| 10 | Healthy | None | 1 |
| 11 | Mechanical heart-valve 11 years previously, osteoporosis | Fracture and infection (4 h, 20 days), urinary infection (19 days) | 64 |
Laboratory procedures
Plasma samples were obtained at the following time-points after stroke: <4, 4, 8, 12, 24, 48 and 72 h, 7 and 12 days, 3 months and 1 year. Samples from controls were obtained at one time-point in the same time-period as the patient samples were collected. Venous blood was drawn into pyrogen-free EDTA tubes, immersed immediately in melting ice. All samples were centrifuged and plasma was frozen at −80°C within 1 h after sampling. The soluble terminal SC5b-9 complement complex (TCC) was measured principally as described previously using an enzyme-linked immunosorbent assay (ELISA) developed in our laboratory [29]. This assay is based on a monoclonal antibody (aE11) specific for a neoepitope exposed when C9 is incorporated into the TCC. The results are given in arbitrary units (AU) per millilitre, related to a standard of zymosan activated serum defined to contain 1000 AU/ml. The original assay was modified slightly giving a reference range <1·0 AU/ml. The modification was made in the detection step with replacement of the polyclonal anti-C5 antibody with a monoclonal biotinylated anti-C6 antibody (clone 9C4 produced in our own laboratory) and subsequent enzyme-linked avidin, giving a substantially lower background than in the original assay. CRP was analysed by an immunonephelometric assay on a BN II analyser (Dade Behring, Dortmund, Germany). The soluble adhesion molecules ICAM, VCAM, P-, E- and L- selectin and the cytokines tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-8 were measured by ELISA (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol.
Statistical analyses
All data were analysed with the statistical program SPSS for Windows, version 11·0. Student's _t_-test was used to evaluate the differences between groups. Logarithmic transformation was made for data which were not normally distributed. Pearson's correlation test was used to evaluate linear relationship between variables. For the correlation coefficients the area under the curve (AUC) was computed. Eight patients had a sufficient number of observations for reliable calculation of AUC. The data in text and figures are expressed as a mean and standard error of the mean (s.e.m.). A _P_-value <0·05 was considered significant.
RESULTS
TCC
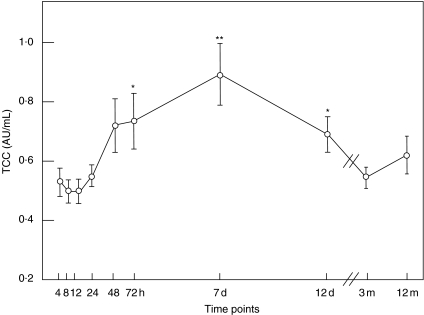
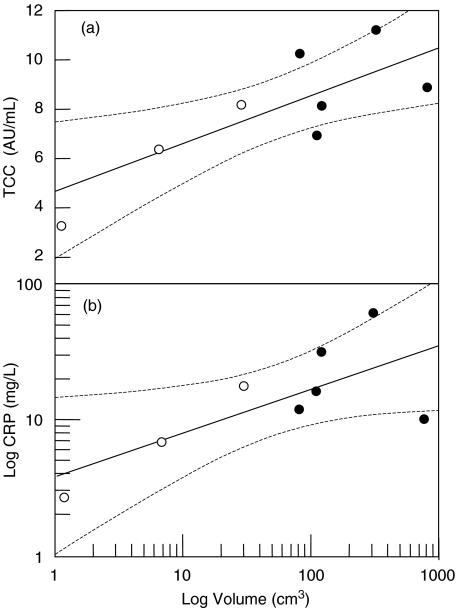
There was no difference in TCC concentration between stroke patients at the stroke onset and controls (mean 0·54 and 0·51 AU/ml, respectively; P = 0·73). TCC increased significantly from 0·54 to 0·74 AU/ml at 72 h (P = 0·032), reached maximum to 0·90 AU/ml at 7 days (P < 0·001), was still increased to 0·70 AU/ml at 12 days (P =0·009) and normalized thereafter to 0·64 AU/ml (Fig. 1). The TCC concentration correlated significantly with the CRP concentration (r = 0·36, P < 0·001). TCC also correlated closely with the size of infarction (r = 0·80, P = 0·017) (Fig. 2a), but not with infections.
Fig. 1.
The terminal complement complex (TCC) in plasma from patients with acute ischaemic stroke. Plasma concentration of TCC in 11 patients with acute ischaemic stroke at the following time-points from onset: 4, 8, 12, 24, 48 and 72 h, 7 days, 12 days, 3 months and 12 months. TCC increased significantly at 72 h, reached maximum at 7 days, was also significantly increased at 12 days and thereafter normalized. Note that the _x_-axis is broken between 12 days and 3 months. *P < 0·05, **P < 0·001. The data are shown as a mean ± standard error of the mean (s.e.m.).
Fig. 2.
TCC and volume of infarction (a). Correlation between TCC and volume of infarction in patients without infection (n = 3; open circles) and with infections (n = 5; closed circles). The correlation between increased TCC and volume of infarction was significant (r = 0·80; P = 0·017). CRP and volume of infarction (b). Correlation between CRP and volume of infarction in patients without infection (n = 3; open circles) and with infection (n = 5; closed circles). The correlation between increased CRP and volume of infarction was significant for the whole group (r = 0·72; P = 0·043) as well as for the group of patients without infections (r = 0·99; P = 0·024). The broken lines indicate 95% confidence interval.
CRP
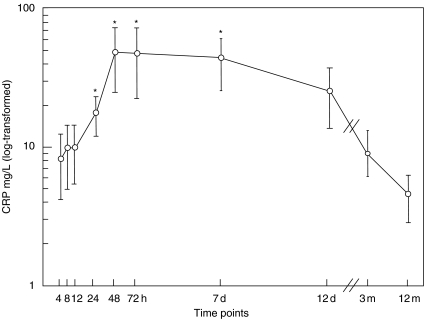
There was no significant difference in CRP concentration between stroke patients at the stroke onset and controls (mean 5·4 and 3·1 mg/l, respectively; P = 0·49). CRP increased significantly from 6·2 to 14·4 mg/l (P = 0·023) at 24 h, remained significantly increased to 41·4 mg/l (P = 0·012) at 48 h, 40·7 mg/l (P = 0·007) at 72 h and 38·0 mg/l (P = 0·017) at 7 days (Fig. 3). Thereafter CRP decreased to 24·5 mg/l (P = 0·07) at 12 days and normalized to 9·1 mg/l (P = 0·26) at 3 months. In contrast to TCC, CRP was markedly elevated in patients who developed infection (n = 6) with mean 30·3 mg/l compared to patients without infection (n = 5) with mean 6·1 mg/l. The difference between these to groups was significant (P < 0·001). There was a significant correlation between CRP and volume of infarction in the whole patient group (r = 0·72 and P = 0·043). In the group of patients without infection there was a highly significant correlation between CRP and size of infarction (r = 0·99 and P = 0·024) in contrast to the group of patients who developed infections (r = −0·18 and 0·88) (Fig. 2b).
Fig. 3.
C-reactive protein (CRP) in plasma from patients with acute ischaemic stroke. Plasma concentration of CRP in 11 patients with acute ischaemic stroke at the following time-points from onset: 4, 8, 12, 24, 48 and 72 h, 7 days, 12 days, 3 months and 12 months. CRP increased significantly at 24 h, remained significantly increased during 1 week and then normalized at 12 days. Note that the _x_-axis is broken between 12 days and 3 months. *P < 0·05. The data are shown as a mean ± standard error of the mean (s.e.m.).
Soluble adhesion molecules and cytokines
There were no significant changes for soluble adhesion molecules (L-, P-, E-selectin, ICAM and VCAM) or cytokines (TNF-α, IL-1β, IL-8) during the course.
DISCUSSION
Activation of complement leads to inflammatory reactions with potentially harmful effects in numerous diseases [5]. As a major defence mechanism against infection, the complement system has the unique property of discriminating between self and non-self [30]. However, complement may also be activated in the absence of microbes, e.g. in autoimmune conditions where immune complexes may cause activation in a self-destructive manner [31]. Furthermore, complement activation contributes to tissue damage in ischaemic- and trauma-related conditions [32]. Inflammation, induced by the I/R injury, is an important mechanism of the brain tissue damage after acute ischaemic stroke [33], where complement plays a central role [3]. Experimental animal studies indicate that the complement system plays a role in tissue damage after cerebral infarction and inhibition of complement can reduce cerebral infarct volume [19–21].
In the present study we show for the first time that systemic complement activation occurs after ischaemic stroke. A previous preliminary report suggested that such activation might take place [23]. These data were, however, based on quantification of C3 and C4 and thus valid only as an indirect indication of in vivo activation, as lowered concentrations of C3 and C4 may be due to decreased synthesis and do not necessarily imply increased activation. The authors suggested classical pathway activation based on the C4 results. It should be emphasized, however, that C4 also participates in the mannose-binding lectin (MBL) pathway activation and that this pathway was found to be activated in major human I/R injury [34].
The activation we observed in the present study took place within the first week after the stroke. It is reasonable to suggest that this activation might have harmful effects not only on the brain, but on remote organs as well. Thus, unfavourable effects on remote organs after I/R injury has been demonstrated previously [35,36]. This may be due to the effects of biologically highly active products such as C5a, which are released during fluid-phase complement activation. Because TCC is the final activation product in the complement cascade, detection of TCC implies that all upstream activation products are formed. It should be noted that TCC, like most biological parameters, shows interindividual variation. The fact that TCC was not increased immediately after the infarction makes it possible to obtain a baseline reference value for calculation of the post-infarction increase for each individual patient.
In our study we found no correlation between increased TCC and development of infections after stroke. This indicates that the infections were localized and not disseminated, as complement would be activated systemically only in the latter case [37]. However, we found a significant correlation between TCC and volume of infarction, suggesting that the local necrosis after some days leads to a systemic activation of complement, induced possibly by release of complement-activating substances from the lesion. An alternative candidate for the complement activation we observed is CRP, which is a known activator of the classical complement pathway [24]. Complement plays a crucial role for tissue damage in myocardial infarction. Co-deposition of complement and CRP has been documented in myocardial infarction [26]. There is a well-known association between increased CRP and ischaemic stroke [38–40], and a role for CRP in initiation of inflammation in ischaemic stroke as well as for long-term prognosis has been postulated [41–43]. In the present study we found a significant correlation between increases in TCC and CRP. The early rise in CRP and its correlation with subsequent TCC increase suggests a possible role for CRP in complement activation after stroke, which may be similar to that described for myocardial infarction. Complement is activated when CRP is bound to a surface or substrate and not by soluble CRP only [24]. This may explain that TCC did not increase in the patients with infection who had high levels of circulating CRP. The close correlation between the size of infarction and TCC in all patients and between CRP and size of infarction in patients without infection suggests that both TCC and CRP mirror the cerebral tissue damage systemically in acute stroke.
Cytokines and soluble adhesion molecules are well-known mediators of inflammation as well as indicators thereof. Several studies have shown changes both in adhesion molecules [44–46] and proinflammatory cytokines after acute ischaemic stroke [47–50]. In our study we found no changes in adhesions molecules or cytokines. This does not exclude local production of these mediators as large amounts are required to be detected systemically. Furthermore, the relative small sample size of the patient population might have led to statistical Type II errors, as modest changes were observed for several of the products. Our data indicate that both CRP and TCC are more sensitive systemic markers of the inflammatory reaction taking place after acute ischaemic stroke than are the adhesion molecules and cytokines.
In conclusion, we have demonstrated a transitory systemic complement activation which takes place after acute ischaemic stroke in humans. The early increase in CRP and correlation with subsequent TCC increase suggest a possible role for CRP in inducing complement activation. This activation may contribute to the inflammatory process and tissue damage, both in brain and in remote organs, after acute ischaemic stroke.
Acknowledgments
We kindly thank Gunni Ulvund for excellent technical assistance. The project was supported financially by the Research Council of Rikshospitalet, Medinnova SF, the Norwegian Council on Cardiovascular Disease, the Norwegian Foundation for Health and Rehabilitation and the Family Blix and Odd Fellow legacies.
REFERENCES
- 1.Danton GH, Dietrich WD. Inflammatory mechanisms after ischaemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–36. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 2.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischaemic stroke. Stroke. 2003;34:2518–32. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 3.D'Ambrosio AL, Pinsky DJ, Connolly ES. The role of the complement cascade in ischaemia/reperfusion injury: implications for neuroprotection. Mol Med. 2001;7:367–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Barnum SR. Complement in central nervous system inflammation. Immunol Res. 2002;26:7–13. doi: 10.1385/IR:26:1-3:007. [DOI] [PubMed] [Google Scholar]
- 5.Mollnes TE, Song WC, Lambris JD. Complement in inflammatory tissue damage and disease. Trends Immunol. 2002;23:61–4. doi: 10.1016/s1471-4906(01)02129-9. [DOI] [PubMed] [Google Scholar]
- 6.Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971;133:885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisman HF, Bartow T, Leppo MK, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischaemic myocardial inflammation and necrosis. Science. 1990;249:146–51. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 8.Wang QD, Pernow J, Sjoquist PO, Ryden L. Pharmacological possibilities for protection against myocardial reperfusion injury. Cardiovasc Res. 2002;55:25–37. doi: 10.1016/s0008-6363(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 9.Shernan SK, Collard CD. Role of the complement system in ischaemic heart disease: potential for pharmacological intervention. Biodrugs. 2001;15:595–607. doi: 10.2165/00063030-200115090-00004. [DOI] [PubMed] [Google Scholar]
- 10.Monsinjon T, Richard V, Fontaine M. Complement and its implications in cardiac ischaemia/reperfusion: strategies to inhibit complement. Fundam Clin Pharmacol. 2001;15:293–306. doi: 10.1046/j.1472-8206.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 11.Williams JP, Pechet TTV, Weiser MR, et al. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol. 1999;86:938–42. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 12.Weiser MR, Williams JP, Moore FD, et al. Reperfusion injury of ischaemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–8. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou W, Farrar CA, Abe K, et al. Predominant role for C5b-9 in renal ischaemia/reperfusion injury. J Clin Invest. 2000;105:1363–71. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowell RM, Plane JM, Silverstein FS. Complement activation contributes to hypoxic-ischaemic brain injury in neonatal rats. J Neurosci. 2003;23:9459–68. doi: 10.1523/JNEUROSCI.23-28-09459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imm MD, Feldhoff PW, Feldhoff RC, Lassiter HA. The administration of complement component C9 augments post-ischaemic cerebral infarction volume in neonatal rats. Neurosci Lett. 2002;325:175–8. doi: 10.1016/s0304-3940(02)00271-9. [DOI] [PubMed] [Google Scholar]
- 16.Lassiter HA, Feldhoff RC, Dabhia N, Parker JC, Jr, Feldhoff PW. Complement inhibition does not reduce post-hypoxic-ischaemic cerebral injury in 21-day-old rats. Neurosci Lett. 2001;302:37–40. doi: 10.1016/s0304-3940(01)01653-6. [DOI] [PubMed] [Google Scholar]
- 17.Lew SM, Gross CE, Bednar MM, et al. Complement depletion does not reduce brain injury in a rabbit model of thromboembolic stroke. Brain Res Bull. 1999;48:325–31. doi: 10.1016/s0361-9230(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 18.Vasthare US, Barone FC, Sarau HM, et al. Complement depletion improves neurological function in cerebral ischaemia. Brain Res Bull. 1998;45:413–9. doi: 10.1016/s0361-9230(97)00408-5. [DOI] [PubMed] [Google Scholar]
- 19.Akita N, Nakase H, Kaido T, Kanemoto Y, Sakaki T. Protective effect of C1 esterase inhibitor on reperfusion injury in the rat middle cerebral artery occlusion model. Neurosurgery. 2003;52:395–400. doi: 10.1227/01.neu.0000043710.61233.b4. [DOI] [PubMed] [Google Scholar]
- 20.De Simoni MG, Storini C, Barba M, et al. Neuroprotection by complement (C1) inhibitor in mouse transient brain ischaemia. J Cerebral Blood Flow Metabolism. 2003;23:232–9. doi: 10.1097/01.WCB.0000046146.31247.A1. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Kim LJ, Mealey R, et al. Neuronal protection in stroke by an sLex-glycosylated complement inhibitory protein. Science. 1999;285:595–9. doi: 10.1126/science.285.5427.595. [DOI] [PubMed] [Google Scholar]
- 22.Lindsberg PJ, Ohman J, Lehto T, et al. Complement activation in the central nervous system following blood–brain barrier damage in man. Ann Neurol. 1996;40:587–96. doi: 10.1002/ana.410400408. [DOI] [PubMed] [Google Scholar]
- 23.Di Napoli M. Systemic complement activation in ischaemic stroke. Stroke. 2001;32:1444. doi: 10.1161/01.str.32.6.1443-a. [DOI] [PubMed] [Google Scholar]
- 24.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38:189–97. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 26.Nijmeijer R, Lagrand WK, Lubbers YT, et al. C-reactive protein activates complement in infarcted human myocardium. Am J Pathol. 2003;163:269–75. doi: 10.1016/S0002-9440(10)63650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arvin B, Neville LF, Barone FC, Feuerstein GZ. The role of inflammation and cytokines in brain injury. Neurosci Biobehav Rev. 1996;20:445–52. doi: 10.1016/0149-7634(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 28.Kozuka K, Kohriyama T, Nomura E, Ikeda J, Kajikawa H, Nakamura S. Endothelial markers and adhesion molecules in acute ischaemic stroke − sequential change and differences in stroke subtype. Atherosclerosis. 2002;161:161–8. doi: 10.1016/s0021-9150(01)00635-9. [DOI] [PubMed] [Google Scholar]
- 29.Mollnes TE, Lea T, Frøland SS, Harboe M. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol. 1985;22:197–202. doi: 10.1111/j.1365-3083.1985.tb01871.x. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson JP, Farries T. Separation of self from non-self in the complement system. Immunol Today. 1987;8:212–5. doi: 10.1016/0167-5699(87)90167-8. [DOI] [PubMed] [Google Scholar]
- 31.Lachmann PJ. Complement and disease. Recent Prog Med. 1988;79:293–9. [PubMed] [Google Scholar]
- 32.Mollnes TE, Fosse E. The complement system in trauma-related and ischaemic tissue damage: a brief review. Shock. 1994;2:301–10. doi: 10.1097/00024382-199410000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Hou ST, MacManus JP. Molecular mechanisms of cerebral ischaemia-induced neuronal death. Int Rev Cytol. 2002;221:93–148. doi: 10.1016/s0074-7696(02)21011-6. [DOI] [PubMed] [Google Scholar]
- 34.Fiane AE, Videm V, Lingaas PS, et al. Mechanism of complement activation and its role in the inflammatory response after thoracoabdominal aortic aneurysm repair. Circulation. 2003;108:849–56. doi: 10.1161/01.CIR.0000084550.16565.01. [DOI] [PubMed] [Google Scholar]
- 35.Kelly KJ. Distant effects of experimental renal ischaemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1549–58. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 36.Fleming SD, Mastellos D, Karpel-Massler G, Shea-Donohue T, Lambris JD, Tsokos GC. C5a causes limited, polymorphonuclear cell-independent, mesenteric ischaemia/reperfusion-induced injury. Clin Immunol. 2003;108:263–73. doi: 10.1016/s1521-6616(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 37.Brandtzaeg P, Høgåsen K, Kierulf P, Mollnes TE. The excessive complement activation in fulminant meningococcal septicemia is predominantly caused by alternative pathway activation. J Infect Dis. 1996;173:647–55. doi: 10.1093/infdis/173.3.647. [DOI] [PubMed] [Google Scholar]
- 38.Di Napoli M, Papa F, Bocola V. C-reactive protein in ischaemic stroke: an independent prognostic factor. Stroke. 2001;32:917–24. doi: 10.1161/01.str.32.4.917. [DOI] [PubMed] [Google Scholar]
- 39.Di Napoli M, Papa F, Bocola V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischaemic stroke. Stroke. 2001;32:133–8. doi: 10.1161/01.str.32.1.133. [DOI] [PubMed] [Google Scholar]
- 40.Di Napoli M, Papa F. Clinical use of C-reactive protein for prognostic stratification in ischaemic stroke: has the time come for including it in the patient risk profile? Stroke. 2003;34:375–6. doi: 10.1161/01.str.0000055021.73548.6c. [DOI] [PubMed] [Google Scholar]
- 41.Curb JD, Abbott RD, Rodriguez BL, et al. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation. 2003;107:2016–20. doi: 10.1161/01.CIR.0000065228.20100.F7. [DOI] [PubMed] [Google Scholar]
- 42.Muir KW, Weir CJ, Alwan W, Squire IB, Lees KR. C-reactive protein and outcome after ischaemic stroke. Stroke. 1999;30:981–5. doi: 10.1161/01.str.30.5.981. [DOI] [PubMed] [Google Scholar]
- 43.Winbeck K, Poppert H, Etgen T, Conrad B, Sander D. Prognostic relevance of early serial C-reactive protein measurements after first ischaemic stroke. Stroke. 2002;33:2459–64. doi: 10.1161/01.str.0000029828.51413.82. [DOI] [PubMed] [Google Scholar]
- 44.Blann AD, Ridker PM, Lip GY. Inflammation, cell adhesion molecules, and stroke: tools in pathophysiology and epidemiology? Stroke. 2002;33:2141–3. doi: 10.1161/01.str.0000029008.00497.d3. [DOI] [PubMed] [Google Scholar]
- 45.Cha JK, Jeong MH, Kim EK, et al. Surface expression of P-selectin on platelets is related with clinical worsening in acute ischemic stroke. J Korean Med. 2002;17:811–6. doi: 10.3346/jkms.2002.17.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frijns CJ, Kappelle LJ, van Gijn J, Nieuwenhuis HK, Sixma JJ, Fijnheer R. Soluble adhesion molecules reflect endothelial cell activation in ischaemic stroke and in carotid atherosclerosis. Stroke. 1997;28:2214–8. doi: 10.1161/01.str.28.11.2214. [DOI] [PubMed] [Google Scholar]
- 47.Grau AJ, Reis A, Buggle F, et al. Monocyte function and plasma levels of interleukin-8 in acute ischaemic stroke. J Neurol Sci. 2001;192:41–7. doi: 10.1016/s0022-510x(01)00590-1. [DOI] [PubMed] [Google Scholar]
- 48.Hopkins SJ. The pathophysiological role of cytokines. Leg Med (Tokyo) 2003;5(Suppl. 1):S45–57. doi: 10.1016/s1344-6223(02)00088-3. [DOI] [PubMed] [Google Scholar]
- 49.Kumar K. Overview: pro-inflammatory cytokines in cerebrovascular ischaemia. Curr Opin Invest Drugs. 2001;2:1748–50. [PubMed] [Google Scholar]
- 50.Stoll G. Inflammatory cytokines in the nervous system: multifunctional mediators in autoimmunity and cerebral ischaemia. Rev Neurol (Paris) 2002;158:887–91. [PubMed] [Google Scholar]