Mannan-binding lectin modulates the response to HSV-2 infection (original) (raw)
Abstract
Viruses have developed numerous strategies to escape recognition by the immune system. However, some viruses such as herpes simplex virus-2 (HSV-2) are recognized by initiators of the complement system, e.g. mannan-binding lectin (MBL). To study the effects of MBL deficiency during viral infection we have chosen a model of generalized HSV-2 infection. We infected MBL-A and MBL-C double knock-out mice (DKO) with HSV-2 via the intraperitoneal (i.p.) route. DKO mice cleared HSV-2 from the liver less efficiently than the comparable wild-type animals. The impairment to effectively neutralize HSV-2 correlated with compromised liver function as measured by increased plasma levels of alanine-amino transferase. No differences in the viral burden were found in other organs such as spleen or brain. Thus, MBL-mediated protection was limited to the effects of preservation of liver homeostasis. Reconstitution with recombinant human MBL before and during the HSV-2 infection dramatically lowered the viral titres in the liver. Taken together, the data show that MBL modulates the response to HSV-2 in mice by affecting neutralization of the virus. To analyse if MBL plays a role in establishment and progression of human HSV-2 infection we analysed MBL levels in the serum samples from asymptomatic (virus-exposed people who have never displayed symptoms of HSV-2 infection) and symptomatic HSV-2 patients (people with recurrent HSV-2 infections). We found that the frequency of the MBL deficiency (<100 ng/ml) was higher in the symptomatic group and significantly different from that in the asymptomatic group (P = 0·0369). This suggests that lack of MBL-mediated complement activation increases susceptibility to viral infection.
Keywords: complement, HSV-2, double knock-out mice, mannan-binding lectin, MBL
INTRODUCTION
Mannan-binding lectin (MBL) recognizes a number of viruses, e.g. herpes simplex virus (HSV), human immunodeficiency virus (HIV) and influenza A virus (IAV) [1,2]. The interaction between MBL and virally infected cells is mediated through recognition by MBL of patterns of carbohydrates containing terminal non-reducing mannose or N-acetylglucoseamine [3]. The binding of MBL to viral enveloped proteins triggers complement activation, independently of C1q or antibodies, via the MBL pathway [4]. In plasma MBL is associated with MBL-associated serine proteases (MASPs), e.g. MASP-1 [5], MASP-2 [6] and MASP-3 [7]. After binding of MBL to its ligand (e.g. viral enveloped glycoprotein or bacterial surfaces), the conformational change in the MBL–MASP complex translates into auto-activation of MASP-2, followed by cleavage of complement C4 and complement C2, leading to the formation of C3 convertase and triggering of the complement cascade [8,9].
Complement activation provides an initial barrier for viral proliferation and viral spread via several mechanisms, and contributes to viral neutralization. Activated complement products can modulate humoral immunity to infectious HSV by facilitating immunoglobulin class-switching and promoting formation of HSV-2 neutralizing antibodies [10,11]. Alternatively, complement component C3 modulates CTL responses during other types of viral infections such as acute influenza virus infection [12]. Priming of CD8+ T cells and recruitment are severely impaired in infected complement C3-deficient animals. Because CD8+ T cells are responsible for regulation of establishment of HSV latency [13] it is likely that complement activation will modulate CD8+ T cell responses during HSV infection.
Unlike in humans, in rodents there are two different homologous forms of MBL, designated MBL-A and MBL-C, encoded by two different genes: MBL-A and _MBL-C_[14,15]. Human MBL is about 50% identical to both rodent forms, which in turn are about 60% identical [16]. In order to study the role of complement and, specifically, that of MBL, in the defence against HSV infections, we infected MBL-A and MBL-C double knock-out mice (MBL DKO) intraperitoneally with human clinical isolate of HSV-2. Following the initial HSV-2 replication in the liver and spleen, the virus was transported to the ganglia and proliferated rapidly in the brain. The infection model reflects a systemic infection, e.g. as seen in newborn children and elderly patients, where the cellular immune response is unable to control the infection, hence allowing spread to multiple organs [17,18].
Whereas systemic HSV-2 infections in humans are rare, the prevalence of genital HSV-2 infection is high [19]. To examine the role of MBL in the response to genital HSV-2 infection in humans we have analysed serum samples from HSV-2-infected patients for MBL levels.
MATERIALS AND METHODS
Animals
Homozygous MBL-A- and MBL-C-deficient mice were derived as described [20,21]. Briefly, homozygous MBL-A–/–/MBL-C–/– double-deficient mice (on mixed C57BL6 and 129 background) and wild-type (WT) littermates were derived by intercrossing heterozygous MBL-A+/– and MBL-C+/– and testing for MBL-A and MBL-C presence in the serum (see below) as well as by genotyping for presence of disrupted genes. Three to 4-week-old female mice were used throughout the experiments. All animals had free access to food and water and were housed according to the Danish animal health care regulations.
Virus preparation
We used the MS strain of HSV-2 [22]. The virus stock was produced by infecting Vero cells at a multiplicity of infection of 0·01. The cells were incubated at 37°C until a complete cytopathic effect was observed. After three rounds of freezing (−70°C) and thawing (room temperature) virus was separated from cell debris by centrifugation for 30 min at 4500 g (4°C) and pelleted from the supernatant by ultracentrifugation at 45 000 g for 1 h and resuspended in phosphate-buffered saline (PBS) supplemented with 0·1% (w/v) bovine serum albumin (BSA) by sonication for 30 s three times at 40 W. The virus load was quantified by plaque titration on Vero cells monolayer. The viral preparations were stored at −70°C.
Viral infection
The mice were infected intraperitoneally (i.p.) with 106 plaque-forming units (pfu) of HSV-2. Without intervention, compared to the experiments performed in the present study, the infected animals died on days 7–8 from the infection in the central nervous system. The experiments were performed with the permission of the Danish Ethics Committee not allowing for death as an end-point.
Plaque assay
Organs (liver, spleen and brain) were stored at −70°C. The organs were weighted and homogenized by sonication for 3 × 5 s in 1 ml modified Eagle's medium (MEM) (Gibco, Denmark) supplemented with 2% fetal calf serum (FCS) just before use in the plaque assay. After homogenization, the organ suspensions were centrifuged at 1600 g for 30 min, and the supernatants were used for plaque assay. The plaque assay was performed on Vero cells, seeded at 1 × 106 cells in 5% FCS, MEM, on 5 cm diameter plates (TPP, Switzerland) and left to settle overnight. The cells were infected by incubation with 100 _µ_l of fivefold serial dilutions of the organ suspensions for 1 h at 37°C. The organ suspensions were subsequently removed and 8 ml of medium with 0·2% (w/v) human immunoglobulins were added to each plate, the cells were incubated for 2 days, and stained with 0·03% (w/v) methylene blue for counting of plaques.
MBL-A and MBL-C binding to HSV-2
MBL-A and MBL-C binding to HSV-2 was analysed similarly to the manner described previously [22]. Briefly, microtitre plates (Maxisorb, Nunc, Denmark) were coated with 10 _µ_g/ml rabbit anti-HSV-2 antibody (Dako, Denmark), blocked with 1 mg/ml HAS in Tris-buffered saline (10 mm Tris-HCl, 140 mm NaCl, pH 7·4) (TBS) and incubated with. 1·5. 107 pfu/ml HSV-2 overnight. The plates were washed with TBS, 0·05% Tween 20 and then incubated with sera from WT DKO animals diluted in TBS containing 5 mm CaCl2 or 10 mm EDTA. The plates were developed with biotinylated anti-MBL-A and MBL-C MoAbs.
Neutralization assay
One thousand pfu of HSV-2 were incubated with 50 _µ_l 50% (w/v) mouse serum (final concentration) from DKO and WT animals at 37°C for different time-periods. For some experiments heat-inactivated serum at 56°C for 60 min was used. Virus titres were determined by plaque assay on Vero cells.
Quantification of MBL-A and MBL-C
Mouse MBL-A and MBL-C protein levels were determined as described previously [14] using a time-resolved immunofluorimetric assay (TRIFMA). Briefly, mannan-coated plates were incubated with mouse plasma diluted in a buffer containing high salt and CaCl2, followed by incubation with biotinylated anti-MBL-A or anti-MBL-C and then incubation with europium-labelled streptavidin.
Reconstitution with human recombinant MBL
Human recombinant MBL (rhMBL) (a gift from NatImmune A/S, Copenhagen, Denmark) was used for reconstitution of MBL DKO mice by injections of 150 _µ_g rhMBL 2 h before the infection and then every 8 h throughout the infection [23]. The recombinant protein was administrated i.p. in 150 _µ_l of Tris-buffered saline (10 mm Tris-HCl, 140 mm NaCl, pH 7·4) and the same buffer was used as a control in the placebo-treated group of animals. The rhMBL showed no presence of endotoxin contamination. The concentrations of rhMBL in plasma after reperfusion were measured on a mannan-coated microtitre plate and detected by a europium-labelled monoclonal antihuman MBL antibody (131-1 [24]) as described by Thiel et al. [25].
Quantification of plasma alanine amino transferase (ALAT)
Plasma ALAT was measured by standard technique, based on Thefeld's method, at Aarhus University Hospital [26].
Subjects
HSV-2 seropositive patients were recruited for the study from the Sahlgrenska University Hospital, Göteborg, Sweden and Uddevalla Hospital, Uddevalla, Sweden. The Ethics Committee at the Faculty of Medicine, Göteborg University, Sweden, approved these studies.
Symptomatic HSV-2 infections
Forty-nine patients (29 females and 20 males; age range 23–79 years) had a typical history of recurrent genital herpes with more than six episodes per year and 15 of these received daily antiviral therapy to suppress the infection.
Asymptomatic HSV-2 infections
Twenty-one patients (age range 24–66 years, 11 females and 10 males) were recruited from an ongoing screening study of HSV-2 infection among the visitors and among partners to HSV-2-infected patients. All patients were provided with thorough information about the clinical spectrum of herpes infections and interviewed about genital symptoms. All patients expressed consent to the studies. Seropositive patients who, after information, admitted to having genital symptoms were excluded from the study. HSV-2 infection was confirmed serologically by Western blot analysis. A serum was considered to contain anti-HSV-2 antibodies if there was a reaction to one or more of three bands, representing the mgG-2, the precursor gG-2 or the high-mannose intermediate [27].
Statistical analysis
All data are presented as means ± standard deviation. The differences between the WT and DKO mice were analysed using one-way anova after confirming the normality of the distribution of the data. When multiple comparisons were to be made post-hoc tests were used (based on SPSS software). P < 0·05 was required for assumption of statistical significance. The _χ_2 analysis was used when patient samples were analysed for differences in the MBL levels.
RESULTS
Lectin pathway contributes to viral neutralization in vitro
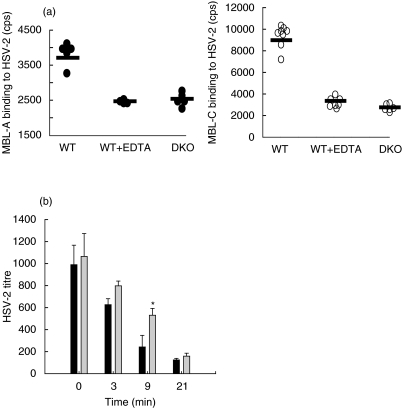
Previous reports showed that human MBL binds to HSV-2 [22]. We extended this observation to include mouse MBL-A and MBL-C (Fig. 1a,b). Both MBL-A and MBL-C recognized HSV-2 via the carbohydrate recognition domains (CRDs), as the binding of MBL-A and MBL-C to HSV-2 was dependent on the presence of Ca2+ in the buffer system of the reaction and the interaction could be inhibited by the presence chelating agent (e.g. 5 mm EDTA). To investigate the possible biological effect of recognition of HSV-2 by murine MBL, we analysed whether sera derived from DKO and WT animals differ in their ability to neutralize HSV-2 in vitro. The mouse sera were incubated with 103 pfu of HSV-2 and the viral titres were determined after different periods of incubation. When incubated with serum from WT animals, the viral titres dropped as early as after 3 min of incubation (Fig. 1c). Sera from DKO animals were less efficient in neutralizing the virus. The difference in neutralization ability of WT and DKO sera was statistically significant at 9 min of incubation (P = 0·01). After longer incubations (21–60 min) there was no difference between the neutralizing activities in sera from WT or DKO. Both sera showed comparable 90% neutralization (at periods of incubation longer than 21 min). Heat-inactivated serum had no neutralization activity (data not shown).
Fig. 1.
MBL binding to HSV-2 and MBL-mediated neutralization of HSV-2 in vitro. (a) MBL-A and MBL-C binding to HSV-2. MBL-A and MBL-C binding to HSV-2 was analysed in a TRIFMA assay, where HSV-2 viral proteins were captured by rabbit anti-HSV-2-coated plate and then exposed to sera from WT and DKO animals, followed by development with specific antimouse MBL-A and MBL-C antibodies. The animal sera were diluted in CaCl2-containing buffer to enable the MBL interaction with its ligands or in EDTA containing buffer. Individual animals are shown as either filled circles (in case of MBL-A) or open circles (in case of MBL-C). (b) Neutralization of HSV-2. HSV-2 virus loads were determined after incubation of 103 HSV-2 pfu with sera from WT (black bars) and DKO (grey bars) animals at 37°C for different periods of time. The viral titres were established by plaque assay on Vero cells. The presented data are mean values with standard deviations. The assay was performed in triplicate. The asterisk denotes significant difference in the neutralization ability between the MBL DKO sera and WT sera (P = 0·01).
MBL levels during systemic HSV-2 infection
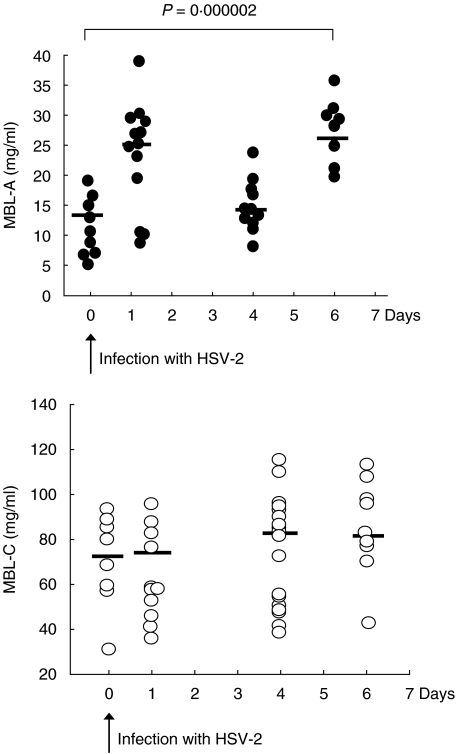
MBL is considered to be an acute-phase protein and therefore we determined the levels of MBL-A and MBL-C prior to and during the viral infection (Fig. 2). The MBL-A levels in the WT animals varied between 6 _µ_g/ml and 17 _µ_g/ml, mean value 11 _µ_g/ml. One day after infection the MBL-A levels rose, reaching a mean value of 25 _µ_g/ml (P = 0·0019). Six days after infection the MBL-A levels were also significantly different from the pre-infection levels, reaching a mean value of 26 _µ_g/ml (P = 0·008). No significant change in MBL-C levels was observed during the infection.
Fig. 2.
Circulating MBL-A and MBL-C levels in WT animals infected with 106 pfu HSV-2 i.p. at day 0. The MBL levels were determined in a TRIFMA assay where serum MBL-A and MBL-C proteins were detected with monoclonal antibodies. The individual animals tested are presented as filled circles for MBL-A or open circles for MBL-C. Mean values are presented as lines. The _P_-value denotes one-way anova analysis for significance between the groups. The data combine three independent experiments performed.
HSV-2 load in the organs of DKO
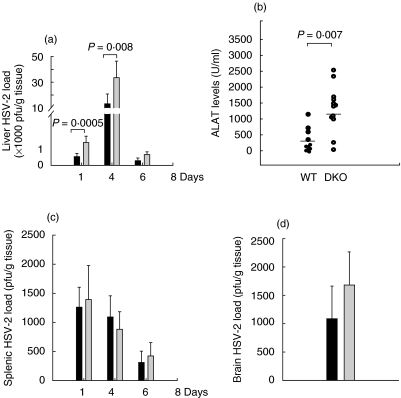
The HSV-2 infection spread to multiple organ systems including brain, liver and spleen. The progression of infection was followed by determining the viral load in those organs. Massive proliferation of HSV-2 was observed in the liver. The viral load peaked at day 4 in the livers of both the WT and DKO animals. The viruses were cleared from the livers by day 6 in both WT and DKO mice. However, the livers in the DKOs harboured significantly more HSV-2 than those from WT animals (Fig. 3a).
Fig. 3.
HSV-2 presence in the liver, spleen and brain of the infected animals. (a) Liver HSV-2 load at different time-points after infection. The HSV-2 load in WT (black bars) and DKO (grey bars) animals were determined by plaque assay. (b) Plasma ALAT levels were measured 4 days after infection in WT and DKO mice. Individual circles represent individual mice. (c) Splenic HSV-2 load in WT (black bars) and DKO (grey bars). (d) Brain HSV-2 load measured 6 days after the infection. In all the panels the bars represent mean values with standard deviations. At least 10 animals per group were used. The different groups of animals were compared by one-way anova. The presented data show one experiment of two performed.
Previous reports have shown that a severe acute focal necrotizing hepatitis is developed as a consequence of systemic murine HSV-2 infection, characterized with pronounced liver damage [28]. To determine liver function we have measured the plasma ALAT levels. The increase in the viral load in the liver correlated with increase in ALAT levels in both groups of animals (Fig. 3b). However, the DKO displayed a significantly higher increase in ALAT levels when compared to WT mice at day 4. By day 6, the ALAT levels were normalized (data not shown).
Virus proliferation was detected in the spleens during the infection (Fig. 3c). A comparable viral load was found in both DKO and WT animals. Virus presence in the brain could be detected as early as at day 6 after the infection. The viral burden in the brain in the DKO was no different when compared to the WT (P = 0·12) (Fig. 3d).
Reconstitution with rhMBL
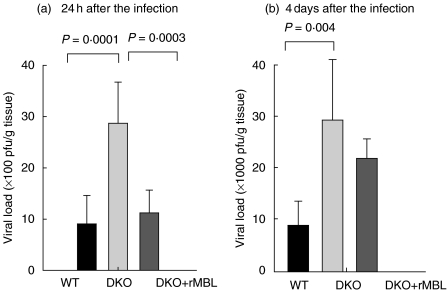
Reconstitution with 150 _µ_g of rhMBL 2 h before the infection and then every 8 h during the infection was required to maintain MBL levels in circulation and to reconstitute the MBL pathway activity (unpublished). Reconstitution resulted in a dramatic reduction of viral load in the liver at 24 h after the infection. (Fig. 4a). However, the protection was not as pronounced at day 4. Although the viral levels were reduced in the reconstituted animals at day 4, they were higher than the WT levels (Fig. 4b).
Fig. 4.
The effect of reconstitution with rhMBL. HSV-2 in the liver in WT, MBL DKO and MBL DKO mice reconstituted with rhMBL. Livers from WT, DKO and DKO reconstituted with rhMBL animals were harvested either at 24 h (a) or 4 days (b) after infection. The viral load was determined by plaque assay on Vero cells. Bars represent mean values with standard deviations of five animals per group. At 24 h the reconstituted animals regained the WT phenotype, whereas at 4 days after infection the reconstituted animals showed only partial protection. The different groups of animals were compared by one-way anova_post-hoc_ tests. Data from one experiment of two performed is shown.
MBL levels in patients with genital HSV-2 infection
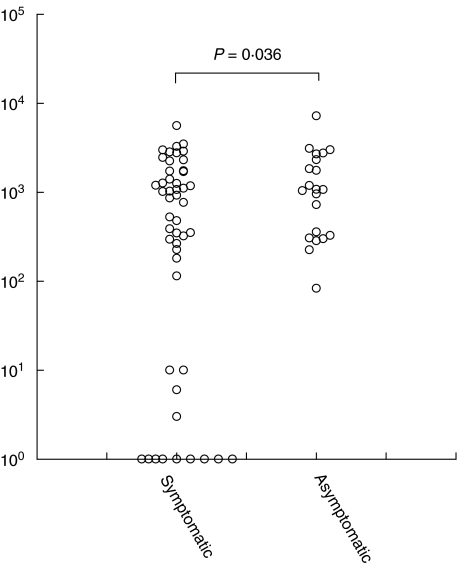
We have determined MBL levels in plasma samples from HSV-2 infected humans. HSV-2 carriers were divided into two groups: patients who had HSV-2 infection symptoms (symptomatic) and patients who had never presented with HSV-2 infection symptoms although having being exposed to HSV-2 (asymptomatic), as evidenced by the presence of anti-HSV-2 antibodies. The frequency of MBL deficiency was defined as MBL levels below 100 ng/ml and was higher among the sympomatic than among the asymptomatic group. We detected 13 MBL-deficient individuals of 49 tested symptomatic patients and only one MBL-deficient of 21 tested asymptomatics (P = 0·036 by _χ_2 analysis) (Fig. 5).
Fig. 5.
Serum MBL levels in symptomatic and asymptomatic HSV-2-infected patients. The MBL levels were determined by TRIFMA assay. Individual values are presented as circles. The two cohorts of samples are analysed by χ2 test.
DISCUSSION
The activation of complement against HSV is thought to be mediated mainly through natural antibodies that recognize envelope glycoproteins on the surface of HSV-1 or HSV-2 [29]. The natural antibodies trigger the classical pathway of complement activation and are directly responsible for limiting the initial HSV infection. The objective of this study was to investigate the importance of MBL in innate antiviral activity against HSV-2. MBL has been shown to provide a pathway for complement activation by other viruses such as HIV, HSV-1 gC null viruses and influenza virus [30–32]. Previous studies have shown that human MBL binds HSV-2 via the carbohydrate recognition domains [22]. Similarly to human MBL, both murine MBL-A and MBL-C recognized and bound HSV-2-containing Vero lysates. The binding triggered complement activation and increased neutralization of the virus (Fig. 1). The effect of MBL-mediated complement activation at 50% serum concentration was limited to a short period of time between 0 and 10 min, when the MBL-sufficient and MBL-deficient sera differed in their neutralization activity against 1000 pfu of HSV-2. This observation suggests that the MBL pathway is important at the initial stages of virally induced complement activation, where its presence modulates complement activation. Similar experiments with influenza A viruses (IAV) showed that MBL binding to IAV modulates virus infectivity and mediates opsonization and phagocytosis [33]. The fact that the MBL-facilitated increase in HSV-2 neutralization is pronounced at the early time-points of virus incubation with serum in the in vitro assay is comparable to the previous observations when other MBL functions were studied, e.g. MBL-mediated phagocytosis [34].
To analyse whether MBL is capable of mediating viral neutralization in vivo we infected MBL-deficient and MBL-sufficient animals with HSV-2. During the course of the infection MBL-A levels changed significantly, increasing at days 1 and 6 after the infection, whereas MBL-C levels remained unaffected, suggesting that MBL-A and MBL-C differ in their expression. The observation is consistent with previously noted differences in MBL-A mRNA regulation, showing that MBL-A is regulated as an acute phase response protein, whereas MBL-C mRNA is not regulated above the baseline level [35].
We hypothesized that MBL-mediated functions synergize with cellular immunity to determine viral protection during HSV-2 infection. In both neonatal and young mice (as are the mice used in this study) the cellular immunity is not fully developed and those animals are susceptible to HSV-2 infection [28,36]. The introduction of MBL deficiency further exacerbated the sensitivity of the mice towards the infection spread. Infection with 106 pfu of HSV-2 caused systemic infection characterized by spread of the virus to different organs such as liver, spleen and brain in both MBL-deficient and MBL-sufficient mice. The effect of MBL deficiency was primarily to modulate the viral load in the liver (Fig. 3a, b). MBL-deficient animals demonstrated higher viral loads during HSV-2 infection when compared with MBL-sufficient animals. Reconstitution with rhMBL caused a decrease in the liver viral loads, suggesting that rhMBL contributes to viral clearance in the liver (Fig. 4). The decrease was significant at the earlier time-points of the infection (at 24 h after the HSV-2 challenge). Levels of rhMBL in circulation did not reach the WT levels (less than 50% reconstitution) and this perhaps accounted for the lack of full reconstitution of viral titres at the later stages of infection.
HSV hepatitis is a common manifestation of the systemic infection in mice, unlike in humans [28,37]. However, certain rare cases of human HSV hepatitis have been documented [38,39]. Those cases are usually lethal, with an 81% mortality rate [40,41]. It is thought that they occur primarily in patients with impaired immunity, such as compromised cellular immunity. In those cases MBL deficiency will exacerbate the hepatitis further, therefore it may be profitable to determine the MBL levels in such patients.
Marked increases in transaminase levels are seen frequently in those patients. Similarly, a dramatic increase in ALAT levels was observed in infected mice (Fig. 3c). The ALAT levels in DKOs were significantly higher than those in WT mice, suggesting that virally mediated liver destruction can be limited by MBL presence. It is likely that MBL deficiency may predispose to higher susceptibility and dramatic progression of fulminant hepatitis in humans.
Although MBL facilitated viral clearance in the liver, it failed to affect dramatically the viral propagation in the spleen. The lack of MBL effect in the spleen may be explained by the sensitivity of viral proliferation towards interferon (IFN)-mediated effects. In the splenic environment those effects will be dominant and responsible for viral clearance [22,42]. Because a slight increase was found in the viral load in the brain in the MBL-deficient animals when compared to MBL-sufficient animals, it may be concluded that MBL presence may have an effect on animal survival during the course of infection. In this systemic HSV-2 infection model the presence of virus in the brain correlates well with mortality. As only a slight difference was found in the viral load in the brain when MBL-sufficient and MBL-deficient animals were compared, it may be concluded that MBL presence had no effect on the viral evasion pattern and will not affect animal survival during the course of infection.
Other viruses, such as hepatitis B virus, can also cause chronic hepatitis, fulminant hepatitis or cirrhosis. Serum MBL levels could be used as a marker for progression of viral mediated fulminant hepatitis [43]. Unfortunately, we did not have access to patients suffering from generalized HSV-2 infection to perform similar correlative studies. However, we analysed a cohort of 70 patients with genital HSV-2 infection (symptomatic and asymptomatic). A higher frequency of MBL-deficient patients was established in the symptomatic group when compared to the asymptomatic group, suggesting that MBL may be involved in the clearance of HSV-2 infection.
Taken together, our data suggest that MBL can modulate the spread and severity of viral infections that target liver and that MBL reconstitution therapy may ameliorate the recovery from severe hepatitis in MBL-deficient individuals.
Acknowledgments
The authors wish to acknowledge the technical assistance of Kirsten Petersen.
REFERENCES
- 1.Lubinski J, Nagashunmugam T, Friedman HM. Viral interference with antibody and complement. Semin Cell Dev Biol. 1998;9:329–37. doi: 10.1006/scdb.1998.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thielens NM, Tacnet-Delorme P, Arlaud GJ. Interaction of C1q and mannan-binding lectin with viruses. Immunobiology. 2002;205:563–74. doi: 10.1078/0171-2985-00155. [DOI] [PubMed] [Google Scholar]
- 3.Hart ML, Saifuddin M, Spear GT. Glycosylation inhibitors and neuraminidase enhance human immunodeficiency virus type 1 binding and neutralization by mannose-binding lectin. J Gen Virol. 2003;84:353–60. doi: 10.1099/vir.0.18734-0. [DOI] [PubMed] [Google Scholar]
- 4.Friedman HM, Wang L, Pangburn MK, Lambris JD, Lubinski J. Novel mechanism of antibody-independent complement neutralization of herpes simplex virus type 1. J Immunol. 2000;165:4528–36. doi: 10.4049/jimmunol.165.8.4528. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiel ST, Vorup-Jensen CM, Stover W, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–10. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 7.Dahl MR, Thiel S, Matsushita M, et al. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity. 2001;15:127–35. doi: 10.1016/s1074-7613(01)00161-3. [DOI] [PubMed] [Google Scholar]
- 8.Holmskov U, Thiel S, Jensenius JC. Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–78. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 9.Vorup-Jensen T, Petersen SV, Hansen AG, et al. Distinct pathways of mannan-binding lectin (MBL)- and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J Immunol. 2000;165:2093–100. doi: 10.4049/jimmunol.165.4.2093. [DOI] [PubMed] [Google Scholar]
- 10.Verschoor A, Brockman MA, Gadjeva M, Knipe DM, Carroll MC. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. J Immunol. 2003;171:5363–71. doi: 10.4049/jimmunol.171.10.5363. [DOI] [PubMed] [Google Scholar]
- 11.Zheng ZM, Hsiung GD. Complement-requiring neutralizing antibody in guinea pigs with primary and recurrent genital herpes. Proc Soc Exp Biol Med. 1984;177:332–6. doi: 10.3181/00379727-177-41952. [DOI] [PubMed] [Google Scholar]
- 12.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–8. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 13.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Jensen L, Hansen S, et al. Characterization and quantification of mouse mannan-binding lectins (MBL-A and MBL-C) and study of acute phase responses. Scand J Immunol. 2001;53:489–97. doi: 10.1046/j.1365-3083.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 15.White RA, Dowler LL, Adkison LR, Ezekowitz RA, Sastry KN. The murine mannose-binding protein genes (Mbl 1 and Mbl 2) localize to chromosomes 14 and 19. Mamm Genome. 1994;5:807–9. doi: 10.1007/BF00292020. [DOI] [PubMed] [Google Scholar]
- 16.Sastry R, Wang JS, Brown DC, Ezekowitz RA, Tauber AI, Sastry KN. Characterization of murine mannose-binding protein genes Mbl1 and Mbl2 reveals features common to other collectin genes. Mamm Genome. 1995;6:103–10. doi: 10.1007/BF00303252. [DOI] [PubMed] [Google Scholar]
- 17.Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247–52. doi: 10.1056/NEJM199105023241804. [DOI] [PubMed] [Google Scholar]
- 18.Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337:509–15. doi: 10.1056/NEJM199708213370801. [DOI] [PubMed] [Google Scholar]
- 19.Langenberg AG, Burke RL, Adair SF, et al. A recombinant glycoprotein vaccine for herpes simplex virus type 2: safety and immunogenicity [corrected] Ann Intern Med. 1995;122:889–98. doi: 10.7326/0003-4819-122-12-199506150-00001. [DOI] [PubMed] [Google Scholar]
- 20.Shi L, Takahashi K, Dundee J, et al. Mannose-binding lectin-deficient mice are susceptible to Infection with Staphylococcus aureus. J Exp Med. 2004;199:1379–90. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi K, Gordon J, Liu H, et al. Lack of mannose-binding lectin-A enhances survival in a mouse model of acute septic peritonitis. Microbes Infect. 2002;4:773–84. doi: 10.1016/s1286-4579(02)01597-6. [DOI] [PubMed] [Google Scholar]
- 22.Fischer PB, Ellermann-Eriksen S, Thiel S, Jensenius JC, Mogensen SC. Mannan-binding protein and bovine conglutinin mediate enhancement of herpes simplex virus type 2 infection in mice. Scand J Immunol. 1994;39:439–45. doi: 10.1111/j.1365-3083.1994.tb03398.x. [DOI] [PubMed] [Google Scholar]
- 23.Jensenius JC, Jensen PH, McGuire K, Larsen JL, Thiel S. Recombinant mannan-binding lectin (MBL) for therapy. Biochem Soc Trans. 2003;31:763–7. doi: 10.1042/bst0310763. [DOI] [PubMed] [Google Scholar]
- 24.Thiel S, Bjerke T, Hansen D, Poulsen LK, Schiotz PO, Jensenius JC. Ontogeny of human mannan-binding protein, a lectin of the innate immune system. Pediatr Allergy Immunol. 1995;6:20–3. doi: 10.1111/j.1399-3038.1995.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 25.Thiel S, Moller-Kristensen M, Jensen L, Jensenius JC. Assays for the functional activity of the mannan-binding lectin pathway of complement activation. Immunobiology. 2002;205:446–54. doi: 10.1078/0171-2985-00145. [DOI] [PubMed] [Google Scholar]
- 26.Thefeld W, Hoffmeister H, Busch EW, Koller PU, Vollmar J. [Reference values for the determination of GOT, GPT, and alkaline phosphatase in serum with optimal standard methods (author's translation)] Dtsch Med Wochenschr. 1974;99:343–4. doi: 10.1055/s-0028-1107760. passim. [DOI] [PubMed] [Google Scholar]
- 27.Liljeqvist JA, Trybala E, Hoebeke J, Svennerholm B, Bergstrom T. Monoclonal antibodies and human sera directed to the secreted glycoprotein G of herpes simplex virus type 2 recognize type-specific antigenic determinants. J Gen Virol. 2002;83:157–65. doi: 10.1099/0022-1317-83-1-157. [DOI] [PubMed] [Google Scholar]
- 28.Mogensen S. Role of macrophages in hepatitis induced by herpes simplex virus types 1 and 2 in mice. Infect Immun. 1977;15:686–91. doi: 10.1128/iai.15.3.686-691.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-gamma-secreting CD4(+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001;82:845–53. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- 30.Anders EM, Hartley CA, Jackson DC. Bovine and mouse serum beta inhibitors of influenza A viruses are mannose-binding lectins. Proc Natl Acad Sci USA. 1990;87:4485–9. doi: 10.1073/pnas.87.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubinski JM, Jiang M, Hook L, et al. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J Virol. 2002;76:9232–41. doi: 10.1128/JVI.76.18.9232-9241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saifuddin M, Hart ML, Gewurz H, Zhang Y, Spear GT. Interaction of mannose-binding lectin with primary isolates of human immunodeficiency virus type 1. J Gen Virol. 2000;81:949–55. doi: 10.1099/0022-1317-81-4-949. [DOI] [PubMed] [Google Scholar]
- 33.Hartshorn KL, Sastry K, White MR, et al. Human mannose-binding protein functions as an opsonin for influenza A viruses. J Clin Invest. 1993;91:1414–20. doi: 10.1172/JCI116345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neth O, Jack DL, Johnson M, Klein NJ, Turner MW. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–6. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- 35.Sastry K, Zahedi K, Lelias JM, Whitehead AS, Ezekowitz RA. Molecular characterization of the mouse mannose-binding proteins. The mannose-binding protein A but not C is an acute phase reactant. J Immunol. 1991;147:692–7. [PubMed] [Google Scholar]
- 36.Ramsburg E, Tigelaar R, Craft J, Hayday A. Age-dependent requirement for gammadelta T cells in the primary but not secondary protective immune response against an intestinal parasite. J Exp Med. 2003;198:1403–14. doi: 10.1084/jem.20030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mogensen SC, Teisner B, Andersen HK. Focal necrotic hepatitis in mice as a biological marker for differentiation of herpes virus hominis type 1 and type 2. J Gen Virol. 1974;25:151–5. doi: 10.1099/0022-1317-25-1-151. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman B, Gandhi SA, Louie E, Rizzi R, Illei P. Herpes simplex virus hepatitis: case report and review. Clin Infect Dis. 1997;24:334–8. doi: 10.1093/clinids/24.3.334. [DOI] [PubMed] [Google Scholar]
- 39.Shanley CJ, Braun DK, Brown K, et al. Fulminant hepatic failure secondary to herpes simplex virus hepatitis. Successful outcome after orthotopic liver transplantation. Transplantation. 1995;59:145–9. doi: 10.1097/00007890-199501150-00028. [DOI] [PubMed] [Google Scholar]
- 40.Pellise M, Miquel R. Liver failure due to herpes simplex virus. J Hepatol. 2000;32:170. doi: 10.1016/s0168-8278(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 41.Tan G, Frankel WL, Suster S. Pathologic quiz case: multiple foci of necrosis in the liver in a patient with T-cell lymphoma. Herpes simplex virus hepatitis. Arch Pathol Lab Med. 2003;127:1049–50. doi: 10.5858/2003-127-1049-PQCMFO. [DOI] [PubMed] [Google Scholar]
- 42.Paludan SR, Melchjorsen J, Malmgaard L, Mogensen SC. Expression of genes for cytokines and cytokine-related functions in leukocytes infected with herpes simplex virus: comparison between resistant and susceptible mouse strains. Eur Cytokine Netw. 2002;13:306–16. [PubMed] [Google Scholar]
- 43.Hakozaki Y, Yoshiba M, Sekiyama K, et al. Mannose-binding lectin and the prognosis of fulminant hepatic failure caused by HBV infection. Liver. 2002;22:29–34. doi: 10.1046/j.0106-9543.2001.01516.x. [DOI] [PubMed] [Google Scholar]