NK cells, displaying early activation, cytotoxicity and adhesion molecules, are associated with mild dengue disease (original) (raw)
Abstract
During the innate immune response against infections, Natural Killer (NK) cells are as important effector cells as are Cytotoxic T lymphocytes (CTL) generated after antigenic stimulation in the adaptative response. NK cells increase in numbers, after viral infection or vaccination. We investigated the NK cell and CD8 T lymphocyte status in 55 dengue infected patients. The NK (CD56+CD3−) and CD56+ T cell (CD56+CD3+) rates rise during the acute phase of disease. The majority of NK cells from dengue patients display early markers for activation (CD69, HLA-DR, and CD38) and cell adhesion molecules (CD44, CD11a) during the acute phase of disease. The intracellular cytotoxic granule, TIA-1, is also up-regulated early in NK cells. Most of these markers appear also on CD8+ T lymphocytes but during the late acute phase. Circulating IL-15 is elevated in a significant number of patients during early acute infection and its values were statistically correlated with NK frequencies and cytotoxic markers on NKs. We have therefore shown that dengue virus infection is very likely stimulating a cytotoxic response that may be efficient in controlling the virus in synergism with CD8+ T lymphocytes. Interestingly, the heightened CD56+CD3−, CD56+CD3+, CD56+TIA-1+ and CD56+CD11a+ cell rates are associated with mild dengue clinical manifestations and might indicate a good prognosis of the disease.
Keywords: dengue, NK cells, patients, activation, cytotoxicity, adhesion molecules
Introduction
Dengue Virus (DENV) infection results in a spectrum of clinical disease ranging from an asymptomatic, acute self-limiting febrile illness (such as DF, Dengue fever) to a more severe potentially life threatening form of dengue infection, which is characterized by high fever, haemorrhagic phenomena and plasma leakage (such as DHF/DSS, Dengue Haemorrhagic Fever/Dengue Shock Syndrome). The first dengue epidemics in Brazil were reported during the 1980s, afterwards becoming endemic and spreading all over the country. During the last two decades incidence has been increasing gradually and has recently become alarming, mainly since the introduction of serotype 3 (DENV-3) in 2002 [1].
The immunological processes occurring during dengue infection are not yet completely defined, however, incidence of mild dengue manifestations and occasional progression to the more severe disease forms likely reflect a complex interplay between host and viral factors including cytokine production by inflammatory cells. Previous studies reported increased levels of circulating cytokines and soluble receptors in DHF patients when compared to those in DF, indicating that immune activation may quite possibly be related to disease severity [2,3]. In addition, the existence of DENV-specific cross-reactive CD4+ and CD8+ memory cytotoxic T lymphocyte (CTL) response has been described in individuals who were immunized with live-attenuated dengue vaccines as well as in children following natural infection [2,4–6]. Alterations in cell types related to the immune response have been observed, such as decreased CD4, CD8, γδ T subsets and Natural Killer (NK) cells in DHF in contrast to DF. Moreover, activated CD8 and NK cells, expressing CD69, were also detected in DHF [7].
The NK cell subset is an important component of innate immunity, enabling the limitation of viremia and tumour burden even before the adaptive immune system can be activated [8]. Advances in NK cell recognition indicate that NK cells display both activation and inhibitory receptors on their surface [8]. Engagement of activation receptors leads to directed exocytosis of granules containing perforin and granzymes, which in turn mediate target cell lysis and cytokine release, including interferon-γ (IFN-γ), tumour-necrosis factor (TNF), granulocyte-macrophage colony-stimulating factor (GM-CSF) and several chemokines [9–11]. NK cell functions are regulated by a balance between these activation and inhibition signals, generated by different receptors after engagement with their specific ligands [12].
We propose herein to study NK cells of dengue patients and to examine their association with the pathogenesis of dengue disease. We demonstrated a frequency increase in circulating NK cells during early dengue phase, which is associated with mild cases of disease. NK cells from patients displayed activation-related markers such as CD69, HLA-DR, CD38 and cytotoxic granule TIA-1, appearing earlier than on CD8+ T lymphocytes. Another interesting point concerns evidences of NK cells also displaying CD44, CD11a and CD16 surface expression molecules, which are characteristically exhibited not only in cells with the ability to migrate from the blood to inflammed tissues but in cells which have cytotoxic functions as well [10,13]. IL-15 is released in the early phase of dengue infection, probably contributing to NK cell activation. Taken all in consideration, our data indicate that during the innate immune response, NK cells likely contribute to a good prognosis of dengue disease mainly in the early phase of disease, prior to the adaptive immune response.
Materials and methods
Study population and blood samples
Heparinized peripheral blood samples were obtained from 56 dengue infected patients attended at two Health Centres in Niterói, Rio de Janeiro State (Posto de Saúde de Itaipú and Centro Previdenciário de Niterói). All patients presented clinical diagnosis of dengue infection according to World Health Organization criteria [14]. Blood samples were obtained from 14 healthy individuals (7 females, 6 males; age range 18–50 years), without history of any febrile or other illnesses in the previous 3 months were included as controls, and from 55 patients with dengue acute infection (29 females, 26 males; age range 15–73 years). We classified dengue patients according to early acute (1–5 days after disease onset), late acute (6–10 days) or convalescent (> 11 days) phases.
The diagnosis of DENV infection was confirmed by either antidengue enzyme-linked immunoassorbent assay (ELISA)-IgM, serotype specific reverse trancription-polymerase chain reaction (RT-PCR) [15], or virus isolation [16]. DENV isolations were attempted from serum samples of patients in the acute phase, using Aedes albopictus C6/36 cell line. Isolates were revealed by immunofluorescence assay. Alternatively, DENV was detected by flow cytometry in monocytes [17,18]. Dengue immune response was considered as primary or secondary by IgG ELISA according to previously established criteria [19].
A detailed physical examination was performed to detect haemorrhagic manifestations (positive tourniquet test for capillary fragility; skin haemorrhages; epistaxis; gingival, gastrointestinal, or urinary tract haemorrhage), signs of plasma leakage (pleural or pericardial effusion, ascites), signs of circulatory failure (cold extremities, cyanosis, hypotension, tachycardia) and hepatomegally. Informed and signed consent was obtained from all patients or their guardians prior to blood collection. The protocols followed were approved by the Ethics Committee of the Oswaldo Cruz Foundation, an organ of the Brazilian Health Ministry (recognized by the Brazilian National Ethics Committee) under Number 111/00.
Purification and cryopreservation of human peripheral blood mononuclear cells (PBMCs)
PBMCs were obtained from 30 ml of heparinized venous blood. Blood samples were diluted 1 : 1 with RPMI 1640 (Sigma Chemical Co., St. Louis, MO, USA), layered onto Histopaque-1077 (Sigma Chemical Co.) and centrifuged at 400 g for 30 min. The PBMC layer was washed twice in RPMI 1640 medium and aliquots were cryopreserved for later study. The viability of PBMC was greater than 95% after Trypan blue exclusion. Approximately 107 PBMCs from patients and controls were re-suspended in 1 ml of solution destined for freezing (90% inactivated FBS (fetal bovine serum, Gibco, Invitrogen cell culture, Carlsbad, CA, USA) plus 10% DMSO (Sigma Chemical Co.) and stored initially at −70°C for 24 h before introduction into liquid nitrogen.
Reagents and monoclonal antibodies
The mouse anti-human surface antigen monoclonal antibodies (mAbs) used in this study included: FITC-, PE or Cy5-conjugated, anti-CD8 mAb (IgG1, clone DK25), anti-HLA-DP, DQ, DR mAb (IgG1, clone CR3/43), anti-CD44 mAb (IgG1, clone DF1485), anti-CD16 mAb (IgG2a, clone DJ130C) from DAKO (Glostrup, Denmark), anti-CD11a (LFA-1) mAb (IgG1, clone HI111), anti-CD3 mAb (IgG3, clone SP34), anti-CD56 (IgG1, clone B159) from Pharmingen (San Diego, CA, USA), anti-CD4 mAb (IgG2a, clone S3·5), anti-CD38 mAb (IgG1, clone HIT2) and anti-CD69 mAb (IgG1, clone 50) from Caltag Lab (Burlingame, CA, USA). Intracellular detection of TIA-1 proteins was performed using unconjugated anti-Tia-1 mAb (IgG1, clone 26gA10F5) from Coulter (Fullerton, CA, USA) followed by mouse FITC-conjugated anti-IgG from DAKO. Appropriate conjugated-isotype-matched controls were included and obtained from Pharmingen, Caltag and Beckman Coulter.
Extra- and intra-cellular staining by flow cytometry
Cryopreserved PBMCs (107 cells) were thawed and divided into aliquots, each containing 2 × 105 cells for flow cytometry analysis. Cells were washed in PBS-BSA-NaN3 (PBS pH 7·2, supplemented with 1% (w/v) BSA (Sigma Chemical Co.) and 0·1% NaN3 (Sigma Chemical Co.) and triple-stained for 30 min at 4 °C with the specific mAbs, previously described, at dilutions recommended by the manufacturer. Stained cells were then washed in PBS-BSA-NaN3 and fixed in PBS-BSA-NaN3 containing 1% paraformaldehyde (PFA) for 15 min at 4 °C. Fixed cells were applied to a FACScalibur (Becton-Dickinson).
The intracellular expression of the TIA-1 protein was analysed on thawed PBMCs. Briefly, cells were firstly stained with PE-conjugated anti-CD4 or PE-conjugated anti-CD8 and Cy5-conjugated anti-CD56 mAbs, washed twice with PBS-BSA-NaN3, and fixed with PBS-BSA-NaN3 containing 1% PFA. Fixed cells were washed twice in cold PBS before permeabilization with saponine 0·05% (w/v) (Sigma Chemical Co.) for 5 min at room temperature. PBMCs were stained with unconjugated anti-TIA-1 mAb in 0·05% saponine buffer for 45 min at 4 °C. Cells were then washed once in PBS, stained with FITC-conjugated anti-mouse and finally fixed in 1% PFA. For each sample 10 000–20 000 events were acquired and analyses were carried out with the WinMDI 2·8 and FlowJo (TreeStar, version 4·3) software.
Cytokine detection assays
Plasma samples were obtained on appointment from 43 dengue patients and 15 plasma controls and then stored in aliquots at −70°C awaiting application. Cytokine levels were determined by ELISA kits (R & D Systems, Minneapolis, MN, USA) in compliance with the manufacturer instruction. The lowest detectable IL-15 concentration was 3·9 pg/ml.
Statistical analyses
Statistical analyses were performed by Student's _t_-Distribution (t(_n_-1=14;α=0·025) = 2·145) calculating a referential limit value for positivity or negativity for circulating cytokine levels, according to the following formula:
Average of values from control samples + (Standard Deviation of values from control samples × t(_n_-1;α=0·025)).
Determinations above or below referential limit values were considered significantly altered.
The Fisher's exact test was applied to determine the significance of positive samples from patients among groups. The nonparametric Mann–Whitney _U_-test was used to evaluate differences in the expression of cell surface markers and cytokines between patients and control donors. _P_-values lower than 0·05 were considered to be associated with statistical significance. Correlation between cell surface markers expression and cytokine production was estimated by Spearman regression analysis. The statistics program Prism 4 was employed for analyses (GraphPad Softeware, San Diego, CA, USA).
Results
Clinical characterization of dengue disease in adult Brazilian patients
The clinical manifestations observed in Brazilian dengue patients reflected a spectrum of disease severity as indicated by haemorrhagic manifestations, signs of circulatory failure and varying degrees of thrombocytopenia. In most severe cases the only criteria of circulatory failure was hypotension. Hemoconcentration was not often detected since patients either received early hydration, or a recovery sample was not provided and no other plasma leakage parameter was evaluated. As other investigators have previously reported [20–22], we also were unable to meet WHO criteria [1] for severity classification. Indeed in Nicaragua, Harris et al. [22] defined a severe patient group with signs of shock that do not fit DHF/DSS classification therefore designating a new disease category: dengue with signs associated with shock. We considered all patients with thrombocytopenia (= 100 × 109/l) and hypotension (postural hypotension with decrease in systolic arterial pressure in 20 mmHg in supine position or systolic arterial pressure < 90 mmHg) as severe cases. Based on clinical grounds, we established that among dengue patients, 22 had platelet levels > 100 × 109/l, without hypotension and were classified as mild Dengue; 19 other patients had thrombocytopenia and hypotension, successively receiving parenteral hydration for at least 6 h and were classified as severe Dengue (due to not meeting classical DHF/DSS classification). Ten patients had thrombocytopenia with no other severe manifestation and another five patients exhibiting platelet levels > 100 × 109/l, had hypotension and received parenteral hydration.
Mild and severe Dengue patients were studied for prior incidence of infection, detected by serologic immune response (IgG antibodies for DENV). Subjects with mild Dengue/thrombocytopenia/hypotension (10 of 37) as opposed to severe Dengue (10 of 19) patients, were less likely to be experiencing a secondary dengue virus infection, although no statistical significance found in Fisher's exact test, P = 0·0797. Among 42 patients with DENV-1, 10 were classified as secondary infection and among 16 patients with DENV-3, 9 were considered secondary infection. Eighteen patients were studied in a longitudinal follow-up, where two or more samples were analysed.
CD56+CD3−/CD56+CD16+NK and CD56+ T lymphocyte high frequency during Dengue
Human NK cells comprise approximately 5–10% of all lymphocytes and are defined phenotypically by their CD56 expression, lacking CD3 [23,24]. The NK cell (CD56+CD3−) frequency was higher in the dengue-infected patients as compared to healthy individuals (Fig. 1a,b). This effect was more striking in the early acute phase (1–5 days after disease onset with 15 ± 8·9%versus 7·8 ± 2·7% on control) but also present in later phases of the disease (11 ± 7·1% at days 6–10 and 16 ± 8·5% after 11 days). CD16 is also a marker for NK cells [10] presnt this molecule is also increased in CD56+ cells (Fig. 1b,d) at early dengue infection. Interestingly, 10 of 38 dengue-infected patients evaluated displayed elevated CD56+ cell rates, exhibiting also CD3 surface expression during early infection (1–5 days of disease with 11.7 ± 8.8%versus controls-5·8 ± 3·01%; Fig. 1a,c). CD56+ T cell subset was recently defined as T cells bearing a unique invariant T cell receptor (TCR) plus CD56 which account for a small percentage (∼5%) among PBMCs [25,26].
Fig. 1.
NK CD56+CD3−, CD56+CD3+ and CD56+CD16+ cell frequencies during dengue. Representative dot plots for NK CD56+CD3−, CD56+CD3+ (a) and CD56+CD16+ (b) cell labellings from a healthy donor and a dengue patient at 5 days after the disease onset (early acute phase) are shown. Numbers in each quadrant indicate the percentage for NK CD56+CD3−, CD56+CD3+ or CD56+CD16+ within the lymphocyte gate. PBMCs from healthy subjects (n = 13) or from dengue patients in early (days 1–5), late acute (days 6–10) and convalescent (after 11 days) phases were labelled as described in Materials and methods and analysed by flow cytometry within lymphocyte gate. Individual and mean percentages for (c) CD56+CD3− (d) CD56+CD3+ or (e) CD56+CD16+ cells among lymphocytes are shown in all groups. Statistical significance was assessed by the Mann–Whitney _U_-test. ***P < 0·001, **P < 0·01 and *P < 0·05.
With respect to other peripheral lymphocyte subsets, most dengue patients analysed presented a reduced frequency of CD4+ T lymphocytes during both early and late acute phases of infection. We also observed a modest but significant decrease in CD8+ T lymphocytes among PBMC during the dengue infection course. However, normal cell subset rates were gradually recovered in convalescence (data not shown).
Activation-related marker, CD69, and intracellular cytotoxic granule, TIA-1, are earlier up regulated in NK cells than in CD8+ T lymphocytes during dengue
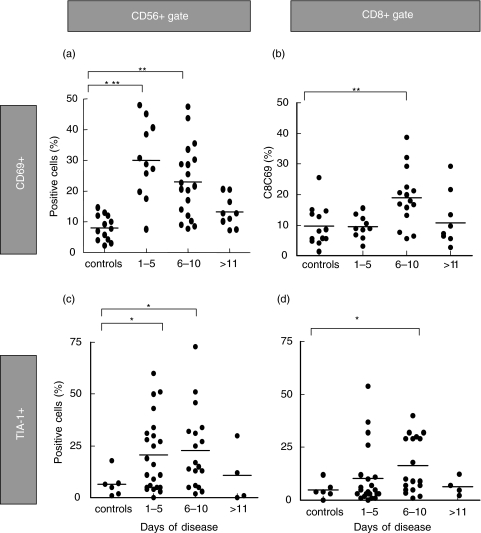
NK cells can be stimulated by direct engagement of activating receptors on the NK cell surface with ligands that are expressed on the target cell surface, so they are poised to respond rapidly to infection [27]. We focused on the immunophenotypic changes occurring in vivo in NK cells and compared them to those detected on CD8+ T lymphocytes during disease. We observed a significant increase in the CD69+ cell percentage among NK (CD56+) cells in dengue patients at early acute phase (days 1–5 with 29·2 ± 13·3%). This increase is maintained at days 6–10 (23·9 ± 17·7%), decreasing after 11 days (13·2 ± 5·0%). (Figs 2a and 3a) Differently, CD69 expression was significantly increased on CD8+ T cells only during the late acute phase (days 6–10, with 18 ± 9·2%) (Fig. 3b).
Fig. 2.
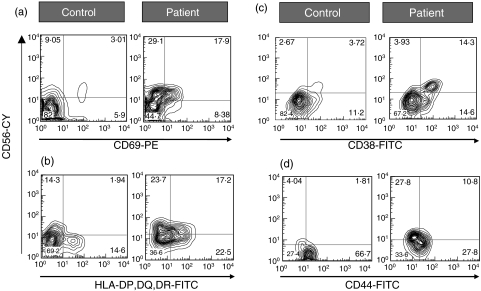
Representative contour plots for increased expression of activation cell markers during dengue. PBMC from healthy subjects or from patients with acute dengue were labelled as described in Materials and methods and analysed by flow cytometry within lymphocyte gate. Ex vivo (a) CD69, (b) HLA-DR, (c) CD38 and (d) CD44 coexpression on CD56+ NK cells from a healthy control and a dengue patient with early acute infection was illustrated. Numbers in quadrants indicate the percentage of cells within the corresponding cell subset.
Fig. 3.
CD69 and Tia-1 expression on NK cells and CD8+ T lymphocytes during dengue. PBMC from healthy subjects samples or from dengue patients in early (days 1–5), late acute (days 6–10) and convalescent (11 days after disease onset) phase were labelled as described in Materials and methods and analysed by double-colour flow cytometry for particular activation marker expression in CD56+ and CD8+ T lymphocytes. Mean percentages of (a,b) CD69 and (c,d) Tia-1+ among (a,c) CD56+ and (b,d) CD8+ T lymphocytes are shown in all groups. Statistical significance was assessed by the Mann–Whitney _U_-test. ***P < 0·001, **P < 0·01, *P < 0·05.
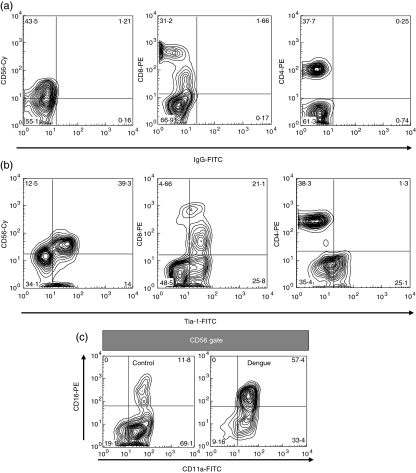
The cytotoxic cell characterization may be evaluated by intracellular protein expression, such as granule cytotoxic T-cell intracellular antigen (TIA)-1 [28]. Unlike healthy individuals that presented low levels of TIA-1 among total CD56+ cells (6·5 ± 6·0%), dengue-infected patients exhibited a marked increase in TIA-1+ cells (21 ± 18% at days 1–5 and 22·8 ± 19% at days 6–10) (Fig. 3c). Among CD8+ T lymphocytes, TIA-1+ cells were significantly higher in the majority of dengue patients during late acute phase (16·0 ± 13·1% at days 6–10) as compared to controls (Fig. 3d).
Other immunophenotypic profiles associated with the NK cell activation kinetics were studied. Thus, NK cells displayed alterations in HLA-DP,DQ,DR+ cells expression that ranged from 4·7 ± 2·7% in controls to 16·6 ± 5·6% in dengue patients during early acute phase of disease (Fig. 2b). Another activation marker, CD38, detected on 20·4 ± 17·2% of NK cells in controls, increased among CD56+cells to 49·9 ± 27% in dengue patients during the disease course (Fig. 2c). No difference was observed in the HLA-DP, DQ, DR and CD38 detection kinetics on NK and CD8+ gated T cells, originating from patient samples. Therefore, NK cells from dengue-infected patients display simultaneously high CD69, TIA-1, HLA- DP, DQ, DR and CD38 levels, supporting the concept that NK cells from dengue-infected patients correspond to a subset of activated circulating mononuclear leucocytes.
NK cells from dengue-infected patients display markers for tissue migration and for cytotoxicity
Most activation-related molecules proved to play a role in NK cell migration into tissues, as well as in NK cell proliferation regulation, target cell recognition, conjugate formation and effective killing [13]. CD44 is an adhesion molecule for extracellular matrix proteins and has been implicated mainly in NK cell homing and binding to mucosal High Endothelial Venules [29,30]. Additionally, CD44 is a cytotoxicity marker molecule that may be present on activated NK cells [31]. We observed a significant increase in the CD44+ rate among CD56+ cells in dengue patients already from days 1–5 (22· 2 ± 12·6%), decreasing gradually from days 6–10 (17·7 ± 6·2%) and after 11 days (15·5 ± 13·5%) of disease onset, when compared to controls (Figs 2d and 4a).
Fig. 4.
CD44 and CD11a expression on CD56+ NK cells during acute dengue.PBMC from healthy phases subjects or from dengue patients in early (days 1–5), late acute (days 6–10) and convalescent (after 11 days after disease onset) phases were labelled as described in Materials and methods and analysed by double-colour flow cytometry for particular adhesion molecule expression in CD56+. Mean percentages for (a) CD44 and (b) CD11a expression among gated CD56+ NK cells are shown in all groups. Statistical significance was assessed by the Mann–Whitney _U_-test. ***P < 0·001, **P < 0·01 and *P < 0·05.
Another adhesion molecule, CD11a, has also been involved in cell adhesion to vascular endothelium and transmigration across the endothelial cell barrier [32,33], in CTL adhesion [34] and NK-mediated cytotoxicity [35,36]. There was a significant increase in CD56+ cells presenting CD11a in the early acute phase as compared to controls (Figs 4b and 5c). Figure 5c is a representative contour plot from gated CD56+ cells that coexpress CD11a and CD16, other surface molecules expressed characteristically in cytotoxic cells [10,13]. CD8+ cells expressed as well, a significant CD11a+ cell increase at early (P = 0·0039) and late (P = 0·0185) dengue phases. The CD44+ and CD11a+ increase on CD8+ T cells from dengue patient samples followed similar patterns presented by NK cells (data not shown).
Fig. 5.
Representative contour plots for increased expression of cytotoxic cell markers during dengue. PBMC from patients with acute dengue were labelled as described in Materials and methods and analysed by flow cytometry within lymphocyte gate. Ex vivo (a) Isotype labelling for (b) TIA-1 in double stained CD56+, CD8+ or CD4+ cells (c)Ex vivo CD11a and CD16 coexpression on gated CD56+ cells. Numbers in quadrants indicate the percentage of cells within the corresponding cell subset.
Elevated IL-15 levels during early dengue phase are associated with elevated NK rates and with cytotoxic marker increase on NK cells
IL-15 can induce the in vivo and in vitro NK cell development, phenotypically and functionally cytotoxic, resulting in a mature IFN-γ producer cell [37,38]. Figure 6a shows that IL-15 was significantly elevated in the plasma of most dengue patients during the early acute phase (11·4 ± 3·9 pg/ml) as compared to healthy donors (4·8 ± 1·06 pg/ml). IL-15 levels directly correlated with NK cell percentages (Fig. 6b) as well as with TIA-1 expression among NK cells (Fig. 6c). These observations reinforce the IL-15 role as a crucial factor for human NK cell development [37].
Fig. 6.
(a) Alteration in IL-15 plasma levels during the dengue time course and association with NK cells. IL-15 levels in dengue patients increase in early acute phase returning to normal on convalescence. Statistical significance was assessed by the Mann–Whitney _U_-test with P = 0·0001. Positive correlation between IL-15 and (b) CD56+CD3− or (c) CD56+Tia-1+ cells was performed by Spearman regression analysis. PBMCs from dengue patients were analysed by flow cytometry as described in Figs 1 and 2. IL-15 levels were measured by ELISA. ***P < 0·001, **P < 0·01, *P < 0·05.
Mild Dengue is associated with increased cell rates and maintained absolute counts in CD56+ subsets in contrast to severe Dengue reduced cell rates and counts
As mentioned previously severe Dengue cases were defined as presenting hypotension and platelet counts = 100 × 109/l, while mild Dengue had platelet counts > 100 × 109/l and no circulatory failure signs. Absolute mononuclear leucocyte counts decrease in dengue patients regardless of whether or not they are affected with severe or mild Dengue (controls 1662 ± 329·4 × 106/l versus dengue 912·1 ± 541·6 × 106/l). CD56+CD3− and CD56+CD3+ absolute cell counts are maintained in mild Dengue and cell frequency is significantly increased as compared to healthy individuals (Table 1). In severe Dengue, relative rates and absolute cell counts are significantly reduced in both cell subsets as compared to mild Dengue and, in relation to controls, absolute counts are lowered for CD56+CD3+ cells. Similarly CD56+CD16+ cell rates are significantly increased in mild (11·8 ± 10·5%) as compared to severe (5·8 ± 5·1%) Dengue (Mann–Whitney, P = 0·0052).
Table 1.
Association between Dengue fever severity and CD56+ cell subsets from adult Brazilian patients.
| Control | Mild | Thrombocytopenia | Hypotension | Severe* | Mild versus severe (_P_-value) |
|---|---|---|---|---|---|
| No. of patients | 12† | 22 | 10 | 5 | 19 |
| ″Platelets | |||||
| ″″Counts (×109/l) | 336·0 ± 41·9 | 187·4 ± 63·3 | 88·9 ± 8·5 | 158·4 ± 60·6 | 66·4 ± 22·7 |
| ″″_P_-value (versus control) | < 0·0001‡ | < 0·0001 | 0·0007 | < 0·0001 | < 0·0001§ |
| ″CD56+CD3– | |||||
| ″″% | 7·4 ± 2·4¶ | 19·2 ± 9·3 | 11·5 ± 2·8 | 14·2 ± 8·2 | 8·0 ± 5·2 |
| ″″_P_-value (versus control) | 0·0001 | 0·0022 | 0·0001 | ||
| ″″Counts (×106/l) | 78·2 ± 32·5 | 101·3 ± 68·4 | 51·4 ± 30·8 | 77·7 ± 45·4 | 63·2 ± 62·8 |
| ″″_P_-value (versus control) | 0·0455 | ||||
| ″CD56+CD3+ (%) | |||||
| ″″% | 5·9 ± 3·0 | 11·1 ± 9·4 | 17·3 ± 7·8 | 11·9 ± 9·0 | 4·1 ± 3·5 |
| ″″_P_-value (versus control) | 0·0451 | 0·0007 | 0·0448 | 0·0001 | |
| ″″Counts (×106/l) | 62·4 ± 31·5 | 63·6 ± 74·5 | 82·3 ± 64·6 | 57·0 ± 39·4 | 26·0 ± 23·9 |
| ″″_P_-value (versus control) | 0·0016 | 0·0460 | |||
| No. of patients | 10 | 13 | 3 | 4 | 16 |
| ″CD56+TIA-1+ | |||||
| ″″% | 3·7 ± 3·1 | 15·1 ± 8·6 | 9·1 ± 5·5 | 11·2 ± 10·3 | 8·0 ± 4·5 |
| ″″_P_-value (versus control) | 0·0010 | 0·0078 | 0·0226 | ||
| ″″Counts (×106/l) | 41·8 ± 35·4 | 102·3 ± 89·7 | 37·6 ± 378·6 | 60·1 ± 44·2 | 72·5 ± 562·4 |
| ″″_P_-value (versus control) | 0·0376 |
Additionally, we investigated whether other cell markers on CD56+ cells, such as activation (CD69; HLA-DP,DQ,DR), adhesion molecules (CD11a; CD44) or the TIA-1 protein are exhibited differentially or not in severe (n = 16) and mild Dengue (n = 13). We found that patients with mild Dengue have a significant increase in CD56+ cells expressing the CD11a molecule (82·3 ± 10%, P = 0·0401) in comparison with severe Dengue (70 ± 17%). The frequency of circulating CD56+TIA-1+ cells was also increased among mild Dengue patient lymphocytes from when compared to severe Dengue and to controls. In addition CD56+TIA-1+ absolute counts in mild Dengue were increased in relation to controls (Table 1). Few patients presented only thrombocytopenia or only hypotension and displayed intermediate values between mild and severe Dengue in different cell subsets.
Hence, our data indicated that during dengue infection, mainly at the disease early phase, the presence of NK cell component within the innate immune system, could provide a good prognosis of disease.
Discussion
The innate immune response, and particularly the NK cell play a key role during the early infection events, due to the ability to rapidly limit infectious pathogen dissemination and to prepare the antigen specific/adaptative immune response to effectively clear the pathogen [39]. CD56+ T cells can expand rapidly in response to various stimuli [40–42]. Humans with selective NK cell deficiencies have a tendencies towards recurrent severe infection, especially from herpes viruses, such as cytomegalovirus (CMV) [43]. In our study, we demonstrated a significant increase in circulating NK (CD56+CD3−) cell frequencies in patients during acute dengue disease. Accordingly, mice infected with DENV had increased NK cell levels after experimental infection [44]. In addition, patients with mild Dengue have elevated NK and CD56+ T cell rates when compared to those with severe Dengue. This fact is in agreement with previous studies where a decrease in NK percentages was observed during DHF in contrast to DF [7,45]. However, other investigations demonstrated a reduction in NK absolute cell counts in DF patients [7,45,46]. In our Brazilian adult patients, NK and CD56+T cell counts were maintained in mild Dengue presenting higher levels than in severe Dengue. Asian children with DF had decreased NK counts, although count decrease in DHF was more pronounced [7,45,46]. Other authors discribed similar results with respect to the maintenance of circulating NK cells during DF [46,47]. The relative increase in NK rates we encountered may be explained by the decrease of CD4 and CD8 absolute counts as well as relative counts among the lymphocyte population. The differences observed between the present study and others might be attributed to various factors, including patient genetic variations and/or different virus genotypes associated with the geografical incidence. Alternatively, it could quite possible be age dependent, since most of the other studies were in children while ours was performed in adult Brazilian patients.
It is important to mention that dengue incidence in Brazil occurs mainly in adults while in Asia it is predominantly a paediatric disease. The definition of dengue severity in Latin Americas has often been a matter of debate as severe cases did not satisfy WHO criteria for DHF/DSS. Mainly in Latin American adults, but also in patients from Asian countries, this issue has become striking [21,22,48]. Circulatory collapse is frequently associated with dengue without thrombocytopenia or haemorrhagic manifestations. Concerning our patient cohort epidemics in Rio de Janeiro in 2002, most severe cases were associated with shock but without consistent haemorrhagic manifestations. Shock has been considered as high risk even without thrombocytopenia [49], as registered during several fatal cases in this same Brazilian epidemics (unpublished observation). Harris et al. [22] classified their patients in Nicaragua in accordance with criteria that did not meet WHO definitions, considering that severe cases with signs of shock need not include thrombocytopenia or haemoconcentration.
Severity in Brazilian adults is either related with Dengue-3 incidence or secondary disease [50] We suspect that in general secondary infections may be more serious than primary and also that DENV-3 infection was more severe than DENV-1. However our analysis did not confirm this hypothesis. Either our small sampling did not favour a statistical analysis or the less frequent incidence of severe cases in primary infection/DENV-1 would hide this effect. We believe that genetic differences among patients may also contribute to severity besides serotype or the previous infection. Therefore our aim here was to compare clinical manifestations with the NK cell subsets regardless the origin of these manifestations.
Phenotypic changes have been noted to occur after in vitro NK cell stimulation, including the acquisition of new molecules and receptors not expressed on resting NK cells [13]. These molecules have an important role in determining NK cell migration to tissues as well as in NK cell proliferation. In addition to rising NK cell frequencies in dengue patients, we observed early increased expression of the CD69 activation molecule on NK cells and later on CD8+ T lymphocytes, which is supported by a previous study from Green and collaborators [7]. Moreover, early CD69 expression on mouse NK cells was observed in response to DENV infection [44]. Hence, mouse and human observations imply a potential role for NK cells during early stages of disease.
Another function that is also apparently enhanced in dengue infected patients is the expression of protein TIA-1, that is restricted to NK cells and cytotoxic T lymphocytes (CTLs) [51]. The correlation of circulating IL-15 with NK cell frequencies or with NK cells expressing TIA-1+ supports the concept that IL-15 is activating cytotoxic cells and sharing many biological activities with IL-2, such as activated T cell growth factors, as well as NK and CTLs activation [52]. In fact, IL-15 has an NK cell-dependent antiviral activity against Herpes [53], HHV-6 [54] and HIV [55–57]. We suggest that IL-15 secretion following DENV infection may therefore represent an important host innate defence mechanism by NK cells involved in restricting viral growth and in limiting the spread of the infectious agent.
Furthermore, herein NK cells isolated from dengue patients were found to express cell activation markers other than CD69 such as human leucocyte antigens grouped as class II MHC genes (HLA-DP, DQ, DR) and CD38. In fact, activated NK cells express de novo CD69 synthesis [58], HLA- DP, DQ, DR [13,59] and CD38 [60,61]. Interestingly, significantly higher levels of these activation cell molecules on NK cells are commonly present during other viral infections [13]. CD38 can also trigger lytic and secretory responses in IL-2-activated human NK cells, mediating therefore natural cytotoxicity [60,61].
Our results exhibited that adhesion molecules such as CD44 and LFA-1 (CD11a/CD18), are frequently highly expressed in both NK and CD8+ T cells during acute dengue disease. We propose that activated NK cells and CTL presenting these molecules may contribute to the NK cell sequestration within inflammed tissues and play a role in local processes, as NK adhesion molecules such as LFA-1 and CD44 usually establish firm bindings to target cells [62,63] and have a crucial role in the IL-2 activated killer cell cytotoxicity on target cells such as tumours [63].
According to our data, the DENV induced NK-mediated response might occur during early infection, since the DENV was antigenically detected in monocytes up to 10 days after disease onset (but not later). In this period CD8+TIA-1+ cells are already present, indicating that the adaptative response mediated by CTLs is also likely participating in the efficient virus elimination in the late acute phase. Theoretically, the complete clearance of intracellular viruses, during the innate and adaptative responses, depends on infected cell elimination by effector cells, NK cells and CTLs acting in concert [64]. Moreover, DENV replication occurs in dendritic cells that produce cytokines, such as IFN-α and TNF-α, which are important for infection control [65,66]. Also, dendritic cells may be able to produce IL-15 in response to DENV and consequently induce proliferation, activation and antiviral activity in NK cells. In turn, NK cells may mediate apoptosis in infected dendritic cells. Therefore, a complex interaction occurs between NK cells and dendritic cells contributing to the regulation of adaptative immunity against infection [67]. NK cells may produce cytokines that favour infectious agent elimination during the adaptive response [26,40,68]. In fact, CD56+ T cells have been reported to produce rapidly proinflammatory Th1 and Th2 cytokines, suggesting roles for these cells both in the innate and in the regulation of the adaptative immune responses [69–71]. We studied these cells in detail in dengue patients. Accordingly, previous investigations in patients presented significantly elevated plasmatic cytokine levels [47,72–74] of which a variety may be directly or indirectly produced after NK activation.
In conclusion, results presented here are the first to substantiate that DENV during human infection enhances NK cell markers for both activation and cytotoxicity. The circulating NK cell activity may contribute to mildness during dengue disease and to an efficient viral clearance in collaboration with CD8+ T cells. Viral infections induce cytokine production, such as IL-12, IL-15 and interferons, which consequently stimulates NK cells to produce other antiviral cytokines [75]. However one should be aware that expressive effector response may be as well responsible for pathology development as well.
Acknowledgments
Oswaldo Cruz Foundation (FIOCRUZ), Brazilian National Council for Research (CNPq) and Rio de Janeiro State Research Support Foundation (FAPERJ) supported financially this work. D.I.S. Cerqueira was a fellow from CNPq and E L Azeredo was a predoctoral fellow from FIOCRUZ. We acknowledge Dr Marize Miagostovich and Ms. Eliana Saraiva for the help during laboratorial diagnosis and the technical support from Mr Allan Alvarenga, Ms. Mariana Lopes and Ms. Maryrose Lavatori. The manuscript was reviewed by Mr Mitchell Raymond Lishen.
References
- 1.World Health Organisation. [December, 2005];Communicable Disease Surveillance and Response (CSR) Disease Outbreaks Reported 8 May Dengue/dengue haemorrhagic fever in Brazil – Update 2. 2002 [WWW document]. URL http://www.who.int/crs/don/2002_05_08/index.html/
- 2.Kurane I, Brinton MA, Samson AL, Ennis FA. Dengue virus-specific, human CD4+ CD8- cytotoxic T-cell clones: multiple patterns of virus cross-reactivity recognized by NS3-specific T-cell clones. J Virol. 1991;65:1823–8. doi: 10.1128/jvi.65.4.1823-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green S, Vaughn DW, Kalayanarooj S, et al. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179:755–62. doi: 10.1086/314680. [DOI] [PubMed] [Google Scholar]
- 4.Green SI, Kurane R, Edelman CO, Tacket KH, Eckels DW, Vaughn CH, Hoke FA., Jr Ennis Dengue virus-specific human CD4+ T-lymphocyte responses in a recipient of an experimental live-attenuated dengue virus type 1 vaccine: bulk culture proliferation, clonal analysis, and precursor frequency determination. J Virol. 1993;67:5962–7. doi: 10.1128/jvi.67.10.5962-5967.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston PG, Kurane I, Dai LC, et al. Dengue virus-specific, HLA-B35-restricted, human CD8+ cytotoxic T lymphocyte (CTL) clones. Recognition of NS3 amino acids 500–508 by CTL clones of two different serotype specificities. J Immunol. 1995;154:1287–95. [PubMed] [Google Scholar]
- 6.Mathew A, Kurane I, Green S, Vaughn DW, Kalayanarooj S, Suntayakorn S, Ennis FA, Rothman AL. Impaired T cell proliferation in acute dengue infection. J Immunol. 1999;162:5609–15. [PubMed] [Google Scholar]
- 7.Green S, Pichyangkul S, Vaughn DW, et al. Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. J Infect Dis. 1999;180:1429–35. doi: 10.1086/315072. [DOI] [PubMed] [Google Scholar]
- 8.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanier LL. Turning on natural killer cells. J Exp Med. 2000;191:1259–62. doi: 10.1084/jem.191.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. [Google Scholar]
- 11.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–83. [PubMed] [Google Scholar]
- 12.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–64. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 13.Lima M, Almeida J, dos Anjos Teixeira M, Queiros ML, Justica B, Orfao A. The ‘ex vivo’ patterns of CD2/CD7, CD57/CD11c, CD38/CD11b, CD45RA/CD45RO and CD11a/HLA-DR expression identify acute/early and chronic/late NK-cell activation states. Blood Cells Mol Dis. 2002;28:181–90. doi: 10.1006/bcmd.2002.0506. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organisation. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2. Geneva: World Health Organization; 1997. Clinical diagnosis; pp. 13–23. [WWW document]. URL http://www.who.int/csr/resources/publications/dengue/Denguepublication/en/ accessed December, 2005. [Google Scholar]
- 15.Miagostovich MP, dos Santos FB, de Araujo ES, Dias J, Schatzmayr HG, Nogueira RM. Diagnosis of dengue by using reverse transcriptase-polymerase chain reaction. Mem Inst Oswaldo Cruz. 1997;92:595–9. doi: 10.1590/s0074-02761997000500006. [DOI] [PubMed] [Google Scholar]
- 16.Miagostovich MP, Nogueira RM, Cavalcanti SM, Marzochi KB, Schatzmayr HG. Dengue epidemic in the state of Rio de Janeiro, Brazil: virological and epidemiological aspects. Rev Inst Med Trop Sao Paulo. 1993;35:149–54. doi: 10.1590/s0036-46651993000200006. [DOI] [PubMed] [Google Scholar]
- 17.Sydow FF, Santiago MA, Neves-Souza PC, Cerqueira DI, Gouvea AS, Lavatori MF, Bertho AL, Kubelka CF. Comparison of dengue infection in human mononuclear leukocytes with mosquito C6/36 and mammalian Vero cells using flow cytometry to detect virus antigen. Mem Inst Oswaldo Cruz. 2000;95:483–9. doi: 10.1590/s0074-02762000000400007. [DOI] [PubMed] [Google Scholar]
- 18.Neves-Souza PC, Azeredo EL, Zagne SM, Valls-de-Souza R, Reis SR, Cerqueira DI, Nogueira RM, Kubelka CF. Inducible nitric oxide synthase (iNOS) expression in monocytes during acute Dengue Fever in patients and during in vitro infection. BMC Infect Dis. 2005;5:64. doi: 10.1186/1471-2334-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miagostovich MP, Nogueira RM, dos Santos FB, Schatzmayr HG, Araujo ES, Vorndam V. Evaluation of an IgG enzyme-linked immunosorbent assay for dengue diagnosis. J Clin Virol. 1999;14:183–9. doi: 10.1016/s1386-6532(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 20.Wilder A, -Smith Schwartz E. Dengue in Travelers. N Engl J Med. 2005;353:924–32. doi: 10.1056/NEJMra041927. [DOI] [PubMed] [Google Scholar]
- 21.Phuong CX, Nhan NT, Kneen R, et al. Clinical diagnosis and assessment of severity of confirmed dengue infections in Vietnamese children: is the world health organization classification system helpful? Am J Trop Med Hyg. 2004;70:172–9. [PubMed] [Google Scholar]
- 22.Harris E, Videa E, Perez L, et al. Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am J Trop Med Hyg. 2000;63:5–11. doi: 10.4269/ajtmh.2000.63.5. [DOI] [PubMed] [Google Scholar]
- 23.Robertson MJ, Caligiuri MA, Manley TJ, Levine H, Ritz J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J Immunol. 1990;145:3194–201. [PubMed] [Google Scholar]
- 24.Lanier LL, Chang C, Azuma M, Ruitenberg JJ, Hemperly JJ, Phillips JH. Molecular and functional analysis of human natural killer cell-associated neural cell adhesion molecule (N-CAM/CD56) J Immunol. 1991;146:4421–6. [PubMed] [Google Scholar]
- 25.Schmidt-Wolf IG, Lefterova P, Johnston V, Huhn D, Blume KG, Negrin RS. Propagation of large numbers of T cells with natural killer cell markers. Br J Haematol. 1994;87:453–8. doi: 10.1111/j.1365-2141.1994.tb08297.x. [DOI] [PubMed] [Google Scholar]
- 26.Dunne J, Lynch S, O'Farrelly C, Todryk S, Hegarty JE, Feighery C, Doherty DG. Selective expansion and partial activation of human NK cells and NK receptor-positive T cells by IL-2 and IL-15. J Immunol. 2001;167:3129–38. doi: 10.4049/jimmunol.167.6.3129. [DOI] [PubMed] [Google Scholar]
- 27.Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nat Rev Microbiol. 2005;3:59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
- 28.Anderson P, Nagler-Anderson C, O'Brien C, Levine H, Watkins S, Slayter HS, Blue ML, Schlossman SF. A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol. 1990;144:574–82. [PubMed] [Google Scholar]
- 29.Uksila J, Salmi M, Butcher EC, Tarkkanen J, Jalkanen S. Function of lymphocyte homing-associated adhesion molecules on human natural killer and lymphokine-activated killer cells. J Immunol. 1997;158:1610–7. [PubMed] [Google Scholar]
- 30.Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189–96. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sconocchia G, Titus JA, Segal DM. Signaling pathways regulating CD44-dependent cytolysis in natural killer cells. Blood. 1997;90:716–25. [PubMed] [Google Scholar]
- 32.Staunton DE, Dustin ML, Springer TA. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989;339:61–4. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- 33.Allavena P, Paganin C, Martin-Padura I, Peri G, Gaboli M, Dejana E, Marchisio PC, Mantovani A. Molecules and structures involved in the adhesion of natural killer cells to vascular endothelium. J Exp Med. 1991;173:439–48. doi: 10.1084/jem.173.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krensky AM, Sanchez-Madrid F, Robbins E, Nagy JA, Springer TA, Burakoff SJ. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL–target interactions. J Immunol. 1983;131:611–6. [PubMed] [Google Scholar]
- 35.Hildreth JE, Gotch FM, Hildreth PD, McMichael AJ. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983;13:202–8. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- 36.Chen BP, Malkovsky M, Hank JA, Sondel PM. Nonrestricted cytotoxicity mediated by interleukin 2-expanded leukocytes is inhibited by anti-LFA-1 monoclonal antibodies (MoAb) but potentiated by anti-CD3 MoAb. Cell Immunol. 1987;110:282–93. doi: 10.1016/0008-8749(87)90123-7. [DOI] [PubMed] [Google Scholar]
- 37.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–40. [PubMed] [Google Scholar]
- 38.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity. differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188:2375–80. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delves PJ, Roitt IM. The immune system. Second of two parts. N Engl J Med. 2000;343:108–17. doi: 10.1056/NEJM200007133430207. [DOI] [PubMed] [Google Scholar]
- 40.Mingari MC, Vitale C, Cambiaggi A, Schiavetti F, Melioli G, Ferrini S, Poggi A. Cytolytic T lymphocytes displaying natural killer (NK)-like activity. expression of NK-related functional receptors for HLA class I molecules (p58 and CD94) and inhibitory effect on the TCR-mediated target cell lysis or lymphokine production. Int Immunol. 1995;7:697–703. doi: 10.1093/intimm/7.4.697. [DOI] [PubMed] [Google Scholar]
- 41.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 42.Satoh M, Seki S, Hashimoto W, Ogasawara K, Kobayashi T, Kumagai K, Matsuno S, Takeda K. Cytotoxic gammadelta or alphabeta T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. J Immunol. 1996;157:3886–92. [PubMed] [Google Scholar]
- 43.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–5. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 44.Shresta S, Kyle JL, Robert Beatty P, Harris E. Early activation of natural killer and B cells in response to primary dengue virus infection in A/J mice. Virology. 2004;319:262–73. doi: 10.1016/j.virol.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 45.Wahid SF, Sanusi S, Zawawi MM, Ali RA. A comparison of the pattern of liver involvement in dengue hemorrhagic fever with classic dengue fever. Southeast Asian J Trop Med Public Health. 2000;31:259–63. [PubMed] [Google Scholar]
- 46.Fadilah SA, Sahrir S, Raymond AA, Cheong SK, Aziz JA, Sivagengei K. Quantitation of T lymphocyte subsets helps to distinguish dengue hemorrhagic fever from classic dengue fever during the acute febrile stage. Southeast Asian J Trop Med Public Health. 1999;30:710–7. [PubMed] [Google Scholar]
- 47.Azeredo EL, Zagne SM, Santiago MA, et al. Characterisation of lymphocyte response and cytokine patterns in patients with dengue fever. Immunobiology. 2001;204:494–507. doi: 10.1078/0171-2985-00058. [DOI] [PubMed] [Google Scholar]
- 48.Guzman MG, Kouri G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol. 2003;27:1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 49.Lum LC, Goh AY, Chan PW, El-Amin AL, Lam SK. Risk factors for hemorrhage in severe dengue infections. J Pediatr. 2002;140:629–31. doi: 10.1067/mpd.2002.123665. [DOI] [PubMed] [Google Scholar]
- 50.Siqueira JB, Jr, Martelli CM, Coelho GE, Simplicio AC, Hatch DL. Dengue and dengue hemorrhagic fever, Brazil, 1981–2002. Emerg Infect Dis. 2005;11:48–53. doi: 10.3201/eid1101.031091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian Q, Streuli M, Saito H, Schlossman SF, Anderson P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell. 1991;67:629–39. doi: 10.1016/0092-8674(91)90536-8. [DOI] [PubMed] [Google Scholar]
- 52.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gosselin J, Tomolu A, Gallo RC, Flamand L. Interleukin-15 as an activator of natural killer cell-mediated antiviral response. Blood. 1999;94:4210–9. [PubMed] [Google Scholar]
- 54.Flamand L, Stefanescu I, Menezes J. Human herpesvirus-6 enhances natural killer cell cytotoxicity via IL-15. J Clin Invest. 1996;97:1373–81. doi: 10.1172/JCI118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.d’Ettorre G, Forcina G, Lichtner M, Mengoni F, D’Agostino C, Massetti AP, Mastroianni CM, Vullo V. Interleukin-15 in HIV infection: immunological and virological interactions in antiretroviral-naive and – treated patients. Aids. 2002;16:181–8. doi: 10.1097/00002030-200201250-00006. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lalezari JP, Beal JA, Ruane PJ, et al. Low-dose daily subcutaneous interleukin-2 in combination with highly active antiretroviral therapy in HIV+ patients: a randomized controlled trial. HIV Clin Trials. 2000;1:1–15. doi: 10.1310/T5FR-8JPX-0NEF-XDKD. [DOI] [PubMed] [Google Scholar]
- 58.Rabinowich H, Herberman RB, Whiteside TL. Differential effects of IL12 and IL2 on expression and function of cellular adhesion molecules on purified human natural killer cells. Cell Immunol. 1993;152:481–98. doi: 10.1006/cimm.1993.1306. [DOI] [PubMed] [Google Scholar]
- 59.Rabinowich H, Sedlmayr P, Herberman RB, Whiteside TL. Response of human NK cells to IL-6 alterations of the cell surface phenotype, adhesion to fibronectin and laminin, and tumor necrosis factor-alpha/beta secretion. J Immunol. 1993;150:4844–55. [PubMed] [Google Scholar]
- 60.Sconocchia G, Titus JA, Mazzoni A, Visintin A, Pericle F, Hicks SW, Malavasi F, Segal DM. CD38 triggers cytotoxic responses in activated human natural killer cells. Blood. 1999;94:3864–71. [PubMed] [Google Scholar]
- 61.Mallone R, Funaro A, Zubiaur M, et al. Signaling through CD38 induces NK cell activation. Int Immunol. 2001;13:397–409. doi: 10.1093/intimm/13.4.397. [DOI] [PubMed] [Google Scholar]
- 62.Storkus WJ, Dawson JR. Target structures involved in natural killing (NK): characteristics, distribution, and candidate molecules. Crit Rev Immunol. 1991;10:393–416. [PubMed] [Google Scholar]
- 63.Matsumoto G, Nghiem MP, Nozaki N, Schmits R, Penninger JM. Cooperation between CD44 and LFA-1/CD11a adhesion receptors in lymphokine-activated killer cell cytotoxicity. J Immunol. 1998;160:5781–9. [PubMed] [Google Scholar]
- 64.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 65.Ho LJ, Wang JJ, Shaio MF, Kao CL, Chang DM, Han SW, Lai JH. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J Immunol. 2001;166:1499–506. doi: 10.4049/jimmunol.166.3.1499. [DOI] [PubMed] [Google Scholar]
- 66.Libraty DH, Pichyangkul S, Ajariyakhajorn C, Endy TP, Ennis FA. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J Virol. 2001;75:3501–8. doi: 10.1128/JVI.75.8.3501-3508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferlazzo G, Pack M, Thomas D, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–11. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bancroft GJ. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–10. doi: 10.1016/0952-7915(93)90030-v. [DOI] [PubMed] [Google Scholar]
- 69.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–21. [PubMed] [Google Scholar]
- 70.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med. 1997;186:109–20. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prussin C, Foster B. TCR V alpha 24 and V beta 11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J Immunol. 1997;159:5862–70. [PubMed] [Google Scholar]
- 72.Hober D, Poli L, Roblin B, et al. Serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), and interleukin-1 beta (IL-1 beta) in dengue-infected patients. Am J Trop Med Hyg. 1993;48:324–31. doi: 10.4269/ajtmh.1993.48.324. [DOI] [PubMed] [Google Scholar]
- 73.Pinto LM, Oliveira SA, Braga EL, Nogueira RM, Kubelka CF. Increased pro-inflammatory cytokines (TNF-alpha and IL-6) and anti-inflammatory compounds (sTNFRp55 and sTNFRp75) in Brazilian patients during exanthematic dengue fever. Mem Inst Oswaldo Cruz. 1999;94:387–94. doi: 10.1590/s0074-02761999000300019. [DOI] [PubMed] [Google Scholar]
- 74.Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Rothman AL, Ennis FA. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J Med Virol. 1999;59:329–34. [PubMed] [Google Scholar]
- 75.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]