Glucocorticoid-stimulated preadipocyte differentiation is mediated through acetylation of C/EBPβ by GCN5 (original) (raw)
Abstract
Preadipocyte differentiation in culture is driven by an insulin and cAMP dependant transcriptional cascade which induces the bzip transcription factors C/EBPβ and C/EBPδ. We have previously shown that glucocorticoid treatment, which strongly potentiates this differentiation pathway, stimulates the titration of the corepressor histone deacetylase 1 (HDAC1) from C/EBPβ. This results in a dramatic enhancement of C/EBPβ-dependent transcription from the C/EBPα promoter, concomitant with potentiation of preadipocyte differentiation. Here, we show that C/EBPβ is acetylated by GCN5 and PCAF within a cluster of lysine residues between amino acids 98–102 and that this acetylation is strongly induced by glucocorticoid treatment. Arginine substitution of the lysine residues within the acetylation motif of C/EBPβ prevented acetylation and blocked the ability of glucocorticoids to enhance C/EBPβ-directed transcription and to potentiate C/EBPβ-dependent preadipocyte differentiation. Moreover, acetylation of C/EBPβ appeared to directly interfere with the interaction of HDAC1 with C/EBPβ, suggesting that PCAF/GCN5-dependent acetylation of C/EBPβ serves as an important molecular switch in determining the transcriptional regulatory potential of this transcription factor.
Keywords: CCAAT/enhancer-binding protein β, transcription factor acetylation, p300/CBP-associated factor, HDAC1, adipogenesis
CCAAT/enhancer-binding protein β (C/EBPβ) is a member of the bzip family of transcription factors. C/EBPs are involved in coordinated transcriptional pathways that control preadipocyte differentiation, liver regeneration, and the inflammatory response, among others (1–3). There are six family members that vary widely in their transcriptional potential, among which C/EBPα, C/EBPβ, C/EBPδ, and C/EBPζ/CHOP are involved in preadipocyte differentiation. Because of their important roles in several differentiation pathways, the transcriptional activities of the C/EBPs are tightly regulated.
The differentiation of preadipocytes into adipocytes in response to adipogenic stimuli is mediated through the activation of a C/EBP and PPARγ transcription factor cascade (4). In culture, preadipocyte differentiation can be triggered by treatment of confluent cells with a mixture of insulin, 3-isobutyl-1-methylxanthine (MIX; a cAMP phosphodiesterase inhibitor) and glucocorticoids (5). The early response to insulin and MIX is up-regulation of expression of C/EBPβ and C/EBPδ (6), which subsequently induce the expression of C/EBPα and PPARγ to direct the differentiation program to completion (7, 8). Although glucocorticoids are not strictly required for differentiation, they act to strongly increase the transcriptional activation potential of C/EBPβ at the onset of preadipocyte differentiation (9, 10).
In preadipocytes after the induction of C/EBPβ, this factor initially interacts with an HDAC1-containing transcriptional corepressor complex that suppresses its transcriptional activation potential (10). Glucocorticoid treatment leads to the dissociation of C/EBPβ from the HDAC1-containing corepressor complex and promotes the degradation of the HDAC1 within the complex via the 26 S proteasome (10). This effect strongly potentiates C/EBPβ transcriptional activity at the C/EBPα promoter, leading to increased histone acetylation, enhanced recruitment of RNA polymerase II, and increased expression of C/EBPα (10). In the absence of steroid, occupation of the C/EBPα promoter by C/EBPβ in preadipocytes coincides with the corecruitment of HDAC1, a reduction in local histone acetylation and reduced level of transcription that correlates with a reduction in the number of preadipocytes that complete differentiation.
In addition to modulating acetylation of the C/EBPα promoter, the liberation of HDAC1 from C/EBPβ could also conceivably impact on C/EBPβ function through acetylation of the transcription factor itself. Indeed, regulation by acetylation is a recurrent theme for many proteins, including transcriptional coactivators such as p300/CBP and HDAC1, the structural protein tubulin, and transcription factors such as p53, MyoD, and GATA-1 (11–16). In the case of transcription factors, acetylation frequently modulates their ability to activate transcription, often by influencing protein-DNA association (12, 14, 15, 17). Alternatively, acetylation can modulate interactions with co-activators, as in the case for p53, where acetylation is required for interaction with CBP and TRRAP (18).
In this study, we have determined that glucocorticoid treatment promotes the acetylation of C/EBPβ by PCAF/GCN5 within a cluster of lysine resides from amino acids 98–102. Substitution of the lysine residues dramatically reduced the adipogenic potential of C/EBPβ in fibroblasts, prevented the potentiation of C/EBPβ transcriptional activation by glucocorticoids and extended the interaction of C/EBPβ with HDAC1. GCN5 was identified as a candidate acetyltransferase for C/EBPβ during preadipocyte differentiation and was shown to participate in the glucocorticoid-mediated stimulation of C/EBPα transcription by C/EBPβ.
Results
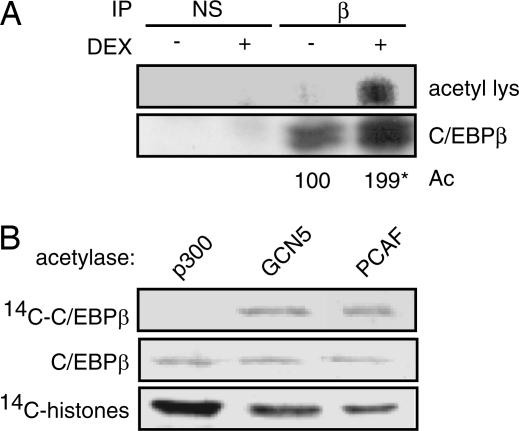
Because many transcription factors are direct targets of acetyltransferase activity, we tested whether glucocorticoid treatment affected C/EBPβ acetylation during the course of 3T3 L1 preadipocyte differentiation. C/EBPβ immunoprecipitates were prepared from 3T3 L1 cells stimulated for 24 h with MIX and insulin to up-regulate C/EBPβ expression or MIX, insulin, and the synthetic glucocorticoid dexamethasone (DEX) which both up-regulates expression and potentiates transcriptional activation by C/EBPβ. Under these conditions, acetylation of C/EBPβ was strongly enhanced in immunoprecipitates from DEX-treated cells (Fig. 1A).
Fig. 1.
Acetylated C/EBPβ is enriched in glucocorticoid-treated cells. (A) The 3T3 L1 preadipocytes were treated for 24 h with MIX and insulin in the presence or absence of DEX as indicated. Immunoprecipitations with anti-C/EBPβ antibody (β) or type matched nonspecific antibody (NS) were resolved by SDS/PAGE and probed with an antiacetyl lysine (acetyl lys) antibody or anti-C/EBPβ antibody (C/EBPβ). Relative acetylation (Ac) was quantified by PhosphorImager and normalized for the level of C/EBPβ over the course of four independent experiments (∗, P < 0.04). (B) In vitro acetylation of GST-C/EBPβ by recombinant p300, GCN5, or PCAF as indicated in the presence of [14C]acetyl CoA. C/EBPβ was resolved by SDS/PAGE, transferred to PVDF, and analyzed for C/EBPβ loading by Western blot. Membranes were then dried and visualized by PhosphorImager to detect acetylation. Acetylation of chicken histones was used as a control for acetylase activity.
To date, CBP/p300 are the only acetyltransferases known to interact with C/EBPβ (10, 19–21). Recombinant p300 was, however, unable to mediate acetylation of C/EBPβ in vitro, although it efficiently modified purified histones under identical reaction conditions (Fig. 1B). By contrast, incubation of C/EBPβ with recombinant PCAF, a HAT that associates strongly with p300/CBP in vivo, resulted in efficient acetylation of both C/EBPβ and histones (Fig. 1B).
PCAF is a member of the GNAT-family of acetyltransferases, which includes GCN5 (22). PCAF and GCN5 have extensive sequence similarity and share common functional properties including a preference for specific histone substrates and a common interaction with p300/CBP (23). As with PCAF, recombinant GCN5 was also able to acetylate C/EBPβ in vitro identifying both factors as candidate acetyltransferases for C/EBPβ (Fig. 1B).
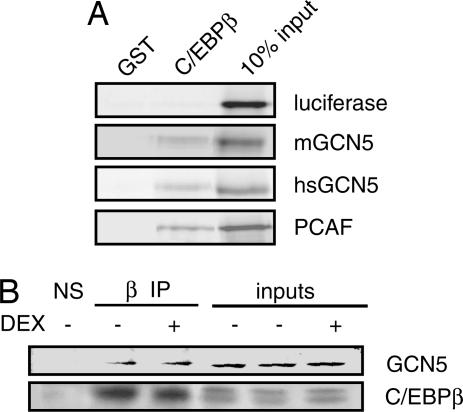
Consistent with our acetylation results, PCAF and both the long (mGCN5) and short (hsGCN5) isoforms of GCN5 interacted directly with C/EBPβ in vitro in a GST pulldown assay (Fig. 2A), with the interaction mapping to the conserved region located between the bromodomain and the HAT domain (data not shown). Endogenous GCN5 coimmunoprecipitated efficiently with endogenous C/EBPβ in 3T3 L1 preadipocyte extracts, providing in vivo confirmation of the interaction (Fig. 2B). Furthermore, this result established GCN5 as the candidate acetyltransferase in the preadipocytes as 3T3 L1 cells do not express endogenous PCAF (data not shown) (24). However, PCAF also coimmunoprecipitated with C/EBPβ in Cos7 cells (data not shown).
Fig. 2.
C/EBPβ interacts with GCN5 and PCAF. (A) Recombinant 35S-labeled mouse GCN5 (mGCN5), human GCN5 (hsGCN5), and human PCAF interact with GST-C/EBPβ in vitro. (B) Endogenous GCN5 interacts with C/EBPβ by coimmunoprecipitation. Endogenous C/EBPβ from 3T3 L1 preadipocytes treated with MIX and insulin in the presence or absence of DEX were immunoprecipitated by using anti-C/EBPβ antibody (β); NS, nonspecific. Resolved immunoprecipitates were probed for GCN5 and C/EBPβ. Inputs represent 10% of the extract used for immunoprecipitation.
To delimit the lysine residues modified by GCN5/PCAF, we acetylated C/EBPβ in vitro with recombinant GCN5 and analyzed the product by mass spectrometry. Despite achieving sequence coverage of >50%, we were unable to detect any acetylation modification, including modification that had been described at K215/216 (25). The unsequenced regions were rich in basic amino acids, and incomplete sequence coverage is not uncommon in such situations; these results suggested the acetylation was occurring within lysines in one of these basic regions.
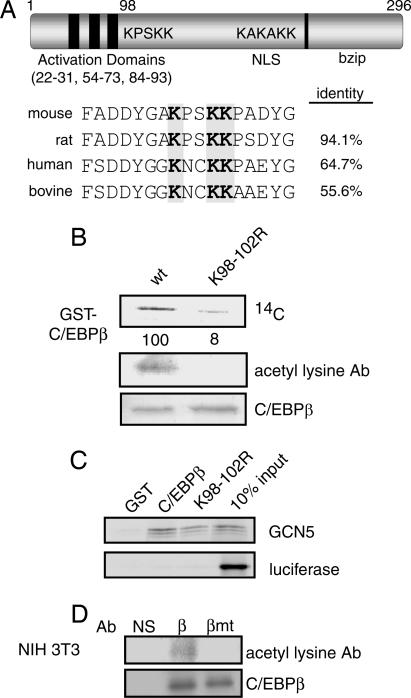
Two lysine clusters occur within C/EBPβ (Fig. 3A). Substitution of K98/101/102 with arginine within the cluster lysines immediately C-terminal to the C/EBPβ activation domain reduced the acetylation of C/EBPβ by GCN5 in vitro by >90% as measured by 14C-incorporation from [14C]acetyl-CoA. It similarly abrogated the detection of acetylation by the acetyl lysine antibody (Fig. 3B). Notably, the arginine substitutions, which preserve the side chain charge but are not able to be acetylated, did not affect the physical association of the GCN5 with GST-C/EBPβ in vitro (Fig. 3C).
Fig. 3.
C/EBPβ is acetylated within lysines 98, 101, and 102. (A) Schematic representation of mouse C/EBPβ and the location of its lysine clusters. (B) In vitro acetylation of GST-C/EBPβ and GST-C/EBPβK98/101/102R by recombinant PCAF. Acetylation reactions were resolved by SDS/PAGE, and blots were probed for acetylation by using an anti-acetyl lysine antibody and for C/EBPβ. Dried membranes were analyzed by PhosphorImager for incorporation of 14C from [14C]acetyl CoA and normalized for C/EBPβ levels. (C) Binding of GST-C/EBPβ and GST-C/EBPβK98/101/102R to 35S-labeled GCN5. (D) NIH 3T3 cells retrovirally transduced to express WT C/EBPβ (β), C/EBPβK98/101/102R (βmt), or with empty virus (pLX) were treated with DEX for 24 h after which C/EBPβ was immunoprecipitated. Resolved immunoprecipitates were probed for acetylation by using the anti-acetyl lysine antibody and for C/EBPβ.
To determine whether the same C/EBPβ residues were subject to glucocorticoid-stimulated acetylation in vivo, we explored the potential of WT C/EBPβ and C/EBPβK98/101/102R to become acetylated during the induction of preadipocyte differentiation. For these experiments, we used NIH 3T3 fibroblasts, which, like 3T3 L1 cells, are derived from disaggregated day 17 Swiss NIH mouse embryos but require ectopic expression of C/EBPβ to differentiate into adipocytes because their endogenous C/EBPβ is not induced by insulin/MIX treatment. In the presence of C/EBPβ the course of differentiation of NIH 3T3 cells closely mimics that of 3T3 L1 cells. NIH 3T3 cells like the 3T3 L1, express GCN5 but not PCAF.
C/EBPβ and C/EBPβK98/101/102R expressed in NIH 3T3 cells by retroviral transduction were immunoprecipitated from cultures 24 h after adipogenic stimulation in the presence of DEX and probed for acetylation with the acetyl lysine antibody (Fig. 3D). Both the level of expression and efficiency of immunoprecipitation of C/EBPβ and C/EBPβK98/101/102R were the same in these experiments (see also Fig. 5A). However, acetylation was only observed for the WT C/EBPβ. Thus, our results indicated that steroid-induced acetylation of C/EBPβ occurred within the K98/101/102 cluster.
Fig. 5.
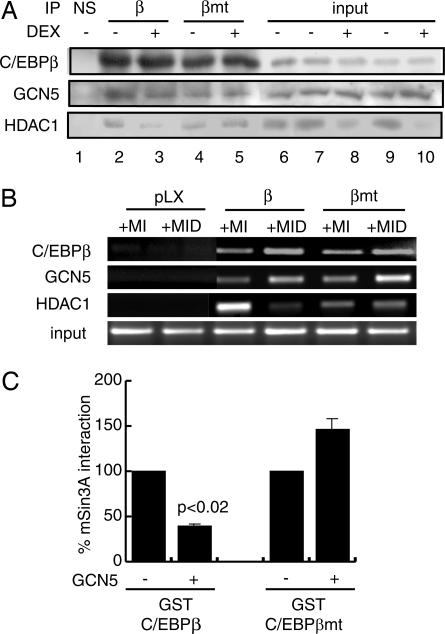
Glucocorticoid-dependant acetylation of C/EBPβ modulates interactions with corepressors. (A) Interaction of GCN5 and HDAC1 with retrovirally expressed C/EBPβ and C/EBPβK98/101/102R by coimmunoprecipitation. NIH 3T3 cells expressing the C/EBPβ constructs were induced to differentiate (MIX, insulin) in the presence or absence of DEX as indicated for 24 h after which C/EBPβ was immunoprecipitated (β); NS, nonspecific. Resolved immunoprecipitates were probed for GCN5, HDAC1, and C/EBPβ. Inputs represent 10% of the extract used for immunoprecipitation. (B) Occupancy of the endogenous C/EBPα promoter in NIH 3T3 cells transduced to express WT (β), mutant C/EBPβ (βmt), or with empty vector (pLX) and induced to differentiate as in A. Antibodies used for ChIP are indicated on the left. Input represents 25% of the material used for ChIP. (C) Acetylation of C/EBPβ modulates the interaction with mSin3A. In vitro acetylation of GST-C/EBPβ by recombinant GCN5 was followed by incubation with 35S-labeled mSin3A. The interaction between GST-C/EBPβ and GST-C/EBPβK98/101/102R (βmt) and mSin3A was assessed by PhosphorImager analysis and compared with mock-acetylated GST-C/EBPβ or GST-C/EBPβK98/101/102R. Data are representative of three independent experiments.
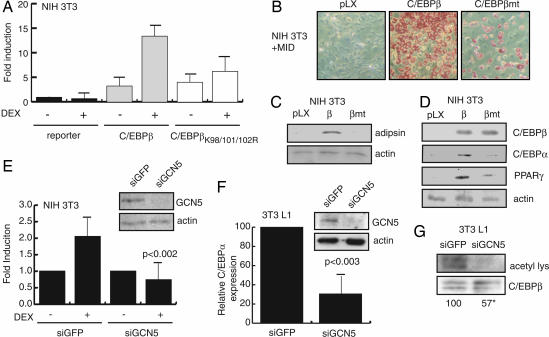
In the absence of steroid treatment, C/EBPβ is a weak activator of the C/EBPα promoter, a key target gene in the adipogenic program. Treatment with DEX greatly enhances activation of this promoter by C/EBPβ, and the targeted degradation of the C/EBPβ-associated HDAC1 underlies this outcome, at least in part (10). To test whether the acetylation of K98/101/102 in C/EBPβ is important for the stimulation of the C/EBPα promoter during preadipocyte differentiation, we compared the ability of WT and K98/101/102R substituted C/EBPβ to stimulate C/EBPα transcription and to promote differentiation of NIH 3T3 fibroblasts. Both the WT and mutant C/EBPβ activated transcription from the C/EBPα promoter in a transient transcription assay similarly (≈3-fold, Fig. 4A). When cells were treated with DEX, WT C/EBPβ became a more potent activator of transcription, triggering an additional 4-fold induction of transcription over vehicle-treated cells cotransfected with C/EBPβ (Fig. 4A). By contrast, the transcription elicited by C/EBPβK98/101/102R was not significantly affected by DEX treatment.
Fig. 4.
Lysines 98, 101, and 102 are critical for potentiation of C/EBPβ activity by DEX. (A) WT C/EBPβ (β) and C/EBPβK98/101/102R (βmt) were transiently expressed in NIH 3T3 cells and transcription from the −350/+7 C/EBPα promoter–luciferase reporter was measured in the presence or absence of DEX. Luciferase activity was corrected for transfection efficiency by using a cotransfected RSV-βgal expression plasmid. Data represent the fold induction of luciferase activity by C/EBPβ construct expression from three independent experiments performed in duplicate. Error bars are the standard error of the means. (B) Oil red O staining of NIH 3T3 cells retrovirally transduced with empty virus (pLX) or to express WT C/EBPβ or C/EBPβK98/101/102R (βmt) 8 days after induction to differentiate in the presence of DEX. (C) Western blot analysis of adipsin and actin levels from cells differentiated as in B. (D) Western blot analysis of early preadipocyte differentiation markers in NIH 3T3 cells retrovirally transduced as in B 24 h after induction to differentiate in the presence of DEX. (E) NIH 3T3 cells were cotransfected with siRNA targeting GCN5 (siGCN5) or GFP (siGFP), C/EBPβ expression plasmid and the C/EBPα promoter-luciferase reporter. Luciferase activity was measured as in A. Data are normalized for reporter activity in the absence of DEX for each siRNA construct. Western blot analysis of GCN5 protein levels 24 h after transfection with siRNA constructs (Inset). Data are representative of three independent experiments performed in duplicate. (F) Quantitative PCR analysis of endogenous C/EBPα mRNA expression in 3T3 L1 cells transfected with siRNA directed against GFP (siGFP) or GCN5 (siGCN5) and induced to differentiate for 24 h in the presence of DEX. C/EBPα expression was normalized against GAPDH over four independent experiments. Western blot analysis of GCN5 expression at harvesting (Inset). (G) Immunoprecipitations of C/EBPβ from 3T3 L1 cells transfected with siRNA and induced to differentiate as in F were probed for acetylation by using the anti-acetyl lysine antibody (acetyl lys) and anti-C/EBPβ. Relative acetylation was quantified by PhosphorImager and normalized for the level of C/EBPβ over the course of four independent experiments (∗, P < 0.003).
NIH 3T3 cells expressing C/EBPβK98/101/102R by viral transduction also differentiated less efficiently than cells expressing WT C/EBPβ, as reflected by a large reduction in lipid accumulation and the absence of adipsin expression (Fig. 4 B and C). C/EBPβK98/101/102R was also less effective than WT C/EBPβ in inducing the early accumulation of C/EBPα and PPARγ in NIH 3T3 cells treated with adipogenic mixture including DEX (Fig. 4D). Differentiation of mutant or WT C/EBPβ-expressing NIH 3T3 cultures in the absence of DEX (MIX and insulin alone) did not result in significant lipid accumulation or adipocyte marker expression (data not shown). Further, C/EBPβK98/101/102R expression in 3T3 L1 preadipocytes also resulted in decrease in both expression of adipogenic markers and lipid accumulation compared with expression of WT C/EBPβ (data not shown).
To test for a specific role for GCN5 in the K98/101/102-dependent potentiation of C/EBPα expression, we examined the effect of ablation of GCN5 on the induction of C/EBPα reporter gene transcription by C/EBPβ and DEX (Fig. 4E). Here, C/EBPβ-dependent transcription from the C/EBPα promoter was enhanced 2-fold by DEX treatment in cells cotransfected with the control anti-GFP siRNA. However, siRNA directed toward GCN5, which efficiently titrated GCN5 (Fig. 4E Inset) completely prevented the glucocorticoid-stimulated enhancement of transcription. Similarly, siRNA-mediated ablation of GCN5 strongly reduced the induction of C/EBPα mRNA in 3T3 L1 preadipocytes (Fig. 4F) and substantially reduced the in vivo acetylation of endogenous C/EBPβ in 3T3 L1 cells induced to differentiate in the presence of DEX (Fig. 4G).
Our results to this point suggested that DEX treatment potentiated C/EBPβ transcriptional activation by stimulating the titration of HDAC1 from C/EBPβ, thus allowing for the accumulation of C/EBPβ acetylation at K98/101/102. However, although DEX treatment caused a reduction in cellular HDAC1 levels (Fig. 5A, lanes 8 and 10) and abrogated the association of HDAC1 with WT C/EBPβ (lane 2 versus lane 3), it failed to reduce the association of HDAC1 with C/EBPβK98/101/102R (lane 4 versus lane 5). Furthermore, HDAC1 was also found to persist at the C/EBPα promoter in C/EBPβK98/101/102R transduced NIH 3T3 cells after adipogenic stimulation including DEX, a treatment which reduced the HDAC1 occupancy in cells expressing the WT C/EBPβ (Fig. 5B). By contrast, the interaction of GCN5 with the C/EBPα promoter depended on C/EBPβ expression but was unaffected by the K98/101/102R substitution in C/EBPβ and DEX treatment.
These results suggested that dissociation of HDAC1 from C/EBPβ depended on K98/101/102 acetylation. Previously we demonstrated that the interaction of HDAC1 with C/EBPβ is mediated through mSin3A (10). We compared the interaction of GST-C/EBPβ and GST-C/EBPβK98/101/102R to _in vitro_-translated mSin3A after acetylation of the C/EBPβ constructs with GCN5 (Fig. 5C). Acetylation of WT GST-C/EBPβ reduced mSin3A binding by 60% (Fig. 5C). By contrast the interaction between C/EBPβK98/101/102R and mSin3A was slightly enhanced after the incubation with GCN5 (P > 0.05). Taken together, these data indicate that the stimulatory effect of DEX treatment on preadipocyte differentiation depends on a combination of the stimulation of HDAC1 turnover through the 26S proteasome and the accumulation of GCN5/PCAF-mediated acetylation of C/EBPβ at K98/101/102.
Discussion
C/EBPβ acts a commitment factor involved in the first steps of the transcriptional cascades that determine differentiation of a diverse group of cell types including hepatocytes, keratinocytes, mammary epithelial cells, macrophages and neurons (2, 26–29). In many cases, including preadipocyte and hepatocyte differentiation, C/EBPα is among the key target genes regulated by C/EBPβ (2, 10). The importance of C/EBPβ in these differentiation processes is reflected by the multiple regulatory inputs that impact on its activity, which include regulation of its expression, the expression of positive and negative heterodimerization partners such as C/EBPδ and CHOP, its phosphorylation, and the modulation of transactivation potential through regulation of interactions with coregulators including a steroid-sensitive HDAC1-containing corepressor complex (6, 10, 30, 31).
Our results show that C/EBPβ becomes acetylated at K98/101/102 by GCN5/PCAF and that this acetylation functions to increase the transcriptional activation potential of C/EBPβ by decreasing its interaction with an mSin3A/HDAC1-containing transcriptional corepressor complex. Interestingly, the balance between acetylation and interaction with HDAC1 is determined, at least in preadipocytes and fibroblasts, by the action of glucocorticoid hormones. In the absence of steroid treatment, K98/101/102 are primarily deacetylated, and C/EBPβ appears to associate equally with GCN5/PCAF and mSin3A/HDAC1, resulting in only a modest transcriptional activation potential that is sufficient to engage the C/EBPα promoter and activate transcription at relatively low levels. For 3T3 L1 preadipocytes and NIH 3T3 fibroblasts this level of engagement leads to a modest level of differentiation to mature adipocytes. When K98/101/102 become acetylated through the action of GCN5, the interaction of C/EBPβ mSin3A/HDAC1 is markedly decreased, allowing C/EBPβ and its associated factors to potently activate transcription in a manner that strongly stimulates the formation of mature adipocytes. It will be interesting to determine whether acetylation of C/EBPβ K98/101/102 holds a similar importance for the differentiation of hepatocytes, mammary epithelial cells, and other C/EBPβ target tissues.
Of note, acetylation of C/EBPβ at other residues (K215/216) has been reported and appears to reduce C/EBPβ's affinity for DNA and thereby its transcriptional activation potential. Thus, deacetylation by HDAC1 increases C/EBPβ's affinity for the Id-1 promoter (25). Our present work does not preclude a role for HDAC1 in the regulation DNA binding affinity but suggests that efficient transcriptional activation by C/EBPβ depends on subsequent dissociation of mSin3A/HDAC1 from C/EBPβ.
Glucocorticoid treatment has been shown to promote the degradation of HDAC1 within corepressor complexes that interact with C/EBPβ. This effect does not require physical interaction between GR and C/EBPβ, and indeed the presence of C/EBPβ is dispensable for the effect on HDAC1. Rather, preliminary results suggest that physical degradation may require physical association between GR and HDAC1 (J.J.T., D.W., and R.J.G.H., unpublished work). Furthermore, although steroid appears to promote acetylation of C/EBPβ, it is not sufficient to promote the dissociation of mSin3A/HDAC1 from C/EBPβ because C/EBPβK98,101,102R maintained a stable interaction with HDAC1 even in the presence of glucocorticoids. One possibility suggested by these results is that the decrease in HDAC1-levels mediated by steroid treatment alters the equilibrium between the interaction of GCN5/PCAF and mSin3A/HDAC1 that allows for a gradual accumulation of C/EBPβ acetylation that excludes further corepressor association. Recently, it has been shown that physical interaction between GR and HDAC1 promotes the acetylation of HDAC1 in a manner that inhibits of its deacetylase activity (13). Thus, the combination of decreasing the enzymatic activity of HDAC1 in concert with its increased turnover could easily allow for the accumulation of C/EBPβ acetylation.
Our results with NIH 3T3 and 3T3 L1 cells, which are derived from similar embryonic sources have identified GCN5 as a salient effector of C/EBPβ acetylation in these cells. PCAF and GCN5 share extensive sequence and functional similarity and are known to act to regulate transcription in a number of systems. However, GCN5 is expressed predominantly in the embryo and newborn and is essential for normal embryonic development, whereas PCAF is expressed primarily in adult tissues and is dispensable for development (24). Therefore, at least with respect to the adipocyte, whereas GCN5 appears to be important for differentiation of embryonic-derived preadipocytes, PCAF may prove to be more relevant to the differentiation of preadipocytes derived from adult sources. Understanding the interplay between GCN5 and PCAF, and the extent to which they complement each other in promoting the action of C/EBPβ in its target tissues will require additional investigation.
Methods
Constructs.
C/EBPβ, GST-C/EBPβ, GCN5 constructs, human PCAF, mSin3A, and the WT C/EBPα-luciferase reporter gene (−350/+7) have been described (10, 32, 33). Mutation of K98/101/102 of C/EBPβ and GST-C/EBPβ was accomplished by site-directed mutagenesis by using the QuikChange kit (Stratagene, La Jolla, CA). For expression of C/EBPβ and C/EBPβK98/101/102R by viral infection, cDNAs encoding the full-length proteins cloned into the pLXSN vector (Clontech, Palo Alto, CA).
Retroviral Infection and Cellular Differentiation.
NIH 3T3 cells were maintained in DMEM containing 4.5 g/L glucose supplemented with 10% calf serum (CS) and 3T3 L1 preadipocytes in DMEM with 1.5 g/liter glucose and 10% CS. Replication-incompetent pLXSN-based (Clontech) retroviruses were generated in Phoenix Ampho packaging cells (from G. Nolan, American Type Culture Collection, Manassas, VA). Ten-centimeter dishes of 50% confluent 3T3 L1 and NIH 3T3 cells were infected and selected as described (10).
For differentiation, 2-day postconfluent cells (designated day 0) were treated with 100 nM insulin, 500 μM MIX, and 250 nM DEX) for 48 h. Cells were subsequently incubated in DMEM supplemented with 10% CS and 100 nM insulin for 8 days.
Oil red O staining was performed as described (34, 35). Phase-contrast photomicrographs are representative of a minimum of three experiments performed in duplicate. To assess expression of preadipocyte differentiation markers, Western blot analysis was performed with antibodies to: C/EBPβ C-19, C/EBPα 14AA, PPARγ H-100, adipsin P-16 (all Santa Cruz Biotechnology, Santa Cruz, CA), and actin (Sigma, St. Louis, MO).
Analysis of Reporter Gene Expression.
NIH 3T3 cells were transfected by using ExGen 500 (MBI Fermentas, St. Leon Rot, Germany). Two hundred nanograms of reporter DNA and 200–400 ng of C/EBPβ expression vector were used for transfection. For siRNA experiments, NIH 3T3 cells were cotransfected with a 300 nM of SMARTPool (Dharmacon, Lafayette, CO) against murine GCN5 or GFP by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After transfection, cells were cultured in phenol red-free DMEM supplemented with 10% charcoal-stripped FBS (Wisent, St-Bruno, QC, Canada) and treated with DEX (10−6 M) or vehicle, as indicated, for 20 h. Luciferase assays were performed according to standard protocol. Error bars represent the standard error of the mean of a minimum of three experiments performed in duplicate and is corrected for transfection efficiency by using cotransfected β-gal expression plasmid.
siRNA-Mediated Knockdown of GCN5.
The 3T3 L1 cells were transfected with 600 pmol of SMARTPool (Dharmacon) designed to target murine GCN5 or GFP by using Lipofectamine 2000 (Invitrogen). Media was changed the following day. At confluency, cultures were induced to differentiate and harvested for RNA analysis or immunoprecipitation. GCN5 levels were monitored by Western blotting.
Quantitative PCR Analysis.
Total RNA was reverse transcribed according to standard protocol. cDNA was amplified in a real-time PCR System (Applied Biosystems, Foster City, CA) by using Power SYBR green PCR Master Mix (Applied Biosystems) with primers that amplify the mouse C/EBPα mRNA transcript (+835/+1065). Amplification was normalized to glyceraldehyde-3-phosphate dehydrogenase expression.
Analysis of Protein–Protein Interactions.
Preparation of whole cell lysates, and coimmunoprecipitations were performed as described (10). Blots were probed with antibodies directed against C/EBPβ C-19, GCN5 N-18, (all Santa Cruz Biotechnology), and HDAC1 (Affinity Bioreagents, Golden, CO).
To analyze protein–protein interactions with C/EBPβ in vitro, GST, GST-C/EBPβ, and GST-C/EBPβK98/101/102R were prepared in Escherichia coli BL21 (36). _In vitro_-translated 35S-labeled PCAF, GCN5, or mSin3A constructs were tested for binding to 1 μg of GST proteins by incubation for 2 h in 0.6× lysis buffer (25 mM Hepes, pH 7.9, /100 mM KCl/2 mM EDTA/20% glycerol/2 mM DTT) containing 0.1% Nonidet P-40. After extensive washing, proteins were resolved by 10% SDS/PAGE and binding visualized by PhosphorImager analysis (Molecular Dynamics, Sunnyvale, CA). Results were reproduced consistently over a minimum of three independent experiments.
Detection of Acetylation in Vivo.
Five milligrams of 3T3 L1 or NIH 3T3 whole-cell lysate prepared in buffers containing 5 μM TSA to inhibit deacetylation during handling were immunoprecipitated with anti-C/EBPβ protein A Sepharose beads for 2 h at 4°C. Precipitates were washed three times, resolved on a 10% SDS/PAGE and C/EBPβ acetylation was assessed by using the panacetyl lysine antibody ab193 (Abcam, Cambridge, MA).
Acetylation of C/EBPβ in Vitro.
GST fusion proteins (5–10 μg) or core histones (10 μg, Upstate Biotechnology, Lake Placid, NY) were incubated with 500 ng of recombinant PCAF (Upstate Biotechnology), recombinant p300 (Novagen, San Diego, CA) or His-GCN5, produced as described (37), in 1× HAT buffer (50 mM Tris, pH 8.0/0.1 mM EDTA/1 mM DTT/10% glycerol) with 0.2 μCi (1 Ci = 37 GBq) of [14C]acetyl CoA at 30°C for 1 h. Reactions were stopped by addition of SDS/PAGE loading buffer, and samples were resolved by electrophoresis. Blots were probed for acetylated products by using the ab193 antibody (Abcam) or anti-C/EBPβ. Membranes were then dried, and radiolabeled proteins were visualized by PhosphorImager analysis.
ChIP Assay.
3T3 L1 or NIH 3T3 cells transduced with retrovirus expressing WT C/EBPβ or C/EBPβK98/101/102R were treated with vehicle, MIX/insulin, or MIX/insulin and DEX, as indicated, for 24 h. Cells were then washed twice in serum-free media and treated with 1% formaldehyde at room temperature for 10 min. ChIP was performed essentially as described (10) by using the following antibodies for precipitation: C/EBPβ C-19, HDAC1 C-19, and GCN5 H-75 (all from Santa Cruz Biotechnology) at 4°C overnight. DNA was purified by using the Qiaquick PCR purification kit (Qiagen, Valencia, CA) and amplified by PCR using the primers for positions −334 and −118 within the mouse C/EBPα promoter (10). Results shown are representative of a minimum of three independent experiments.
Acknowledgments
We thank Dr. David Lohnes for critical review of this manuscript; Drs. S. L. McKnight (University of Texas Southwestern Medical Center, Dallas, TX), X.-J. Yang (McGill University, Montreal, QC, Canada), P. Antonson (Karolinska Institutet, Stockholm, Sweden), S. Khochbin (Institut National de la Santé et de la Recherche Médicale, Institut Albert Bonniot, Grenoble, France), and S. Y. R. Dent (University of Texas M. D. Anderson Cancer Center, Houston, TX) for plasmids; and G. Nolan for the Phoenix Ampho cells. This work was supported by a grant from the Canadian Institutes of Health Research (to R.J.G.H.). R.J.G.H. holds a University of Ottawa Health Research Chair.
Abbreviations
C/EBP
CCAAT/enhancer-binding protein
PCAF
p300/CBP-associated factor
DEX
dexamethasone.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Poli V. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- 2.Diehl AM. J Biol Chem. 1998;273:30843–30846. doi: 10.1074/jbc.273.47.30843. [DOI] [PubMed] [Google Scholar]
- 3.MacDougald OA, Lane MD. Annu Rev Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 4.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin CS, Hirsch A, Fung C, Rosen OM. J Biol Chem. 1978;253:7570–7578. [PubMed] [Google Scholar]
- 6.Cao Z, Umek RM, McKnight SL. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 7.Lin FT, Lane MD. Proc Natl Acad Sci USA. 1994;91:8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 9.Green H, Kehinde O. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 10.Wiper-Bergeron N, Wu D, Pope L, Schild-Poulter C, Hache RJ. EMBO J. 2003;22:2135–2145. doi: 10.1093/emboj/cdg218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glozak MA, Sengupta N, Zhang X, Seto E. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Lamonica JM, Vakoc CR, Blobel GA. Blood. 2006;108:3736–3738. doi: 10.1182/blood-2006-07-032847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu Y, Zhao Y, Becker M, John S, Parekh BS, Huang S, Hendarwanto A, Martinez ED, Chen Y, Lu H, et al. Mol Cell. 2006;22:669–679. doi: 10.1016/j.molcel.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Gu W, Roeder RG. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 15.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 16.Mal A, Sturniolo M, Schiltz RL, Ghosh MK, Harter ML. EMBO J. 2001;20:1739–1753. doi: 10.1093/emboj/20.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Scolnick DM, Trievel RC, Zhang HB, Marmorstein R, Halazonetis TD, Berger SL. Mol Cell Biol. 1999;19:1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, Berger SL. Mol Cell. 2001;8:1243–1254. doi: 10.1016/s1097-2765(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 19.Mink S, Haenig B, Klempnauer KH. Mol Cell Biol. 1997;17:6609–6617. doi: 10.1128/mcb.17.11.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacs KA, Steinmann M, Magistretti PJ, Halfon O, Cardinaux JR. J Biol Chem. 2003;278:36959–36965. doi: 10.1074/jbc.M303147200. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz C, Beck K, Mink S, Schmolke M, Budde B, Wenning D, Klempnauer KH. EMBO J. 2003;22:882–892. doi: 10.1093/emboj/cdg076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterner DE, Berger SL. Microbiol Mol Biol Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth SY, Denu JM, Allis CD. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 24.Xu W, Edmondson DG, Evrard YA, Wakamiya M, Behringer RR, Roth SY. Nat Genet. 2000;26:229–232. doi: 10.1038/79973. [DOI] [PubMed] [Google Scholar]
- 25.Xu M, Nie L, Kim SH, Sun XH. EMBO J. 2003;22:893–904. doi: 10.1093/emboj/cdg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes-Canteli M, Pignatelli M, Santos A, Perez-Castillo A. J Biol Chem. 2002;277:5460–5467. doi: 10.1074/jbc.M108761200. [DOI] [PubMed] [Google Scholar]
- 27.Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM. Mol Endocrinol. 2000;14:359–368. doi: 10.1210/mend.14.3.0434. [DOI] [PubMed] [Google Scholar]
- 28.Xie H, Ye M, Feng R, Graf T. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 29.Zhu S, Oh HS, Shim M, Sterneck E, Johnson PF, Smart RC. Mol Cell Biol. 1999;19:7181–7190. doi: 10.1128/mcb.19.10.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD. Proc Natl Acad Sci USA. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang QQ, Lane MD. Proc Natl Acad Sci USA. 2000;97:12446–12450. doi: 10.1073/pnas.220425597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu W, Edmondson DG, Roth SY. Mol Cell Biol. 1998;18:5659–5669. doi: 10.1128/mcb.18.10.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 34.Wise LS, Green H. J Biol Chem. 1979;254:273–275. [PubMed] [Google Scholar]
- 35.Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Mol Cell Biol. 1997;17:1552–1561. doi: 10.1128/mcb.17.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boruk M, Savory JG, Hache RJ. Mol Endocrinol. 1998;12:1749–1763. doi: 10.1210/mend.12.11.0191. [DOI] [PubMed] [Google Scholar]
- 37.Col E, Caron C, Seigneurin-Berny D, Gracia J, Favier A, Khochbin S. J Biol Chem. 2001;276:28179–28184. doi: 10.1074/jbc.M101385200. [DOI] [PubMed] [Google Scholar]