Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo (original) (raw)
Abstract
Nitric oxide (NO) is an important regulator of blood flow, but its role in permeability is still challenged. We tested in vivo the hypotheses that: (a) endothelial nitric oxide synthase (eNOS) is not essential for regulation of baseline permeability; (b) eNOS is essential for hyperpermeability responses in inflammation; and (c) molecular inhibition of eNOS with caveolin-1 scaffolding domain (AP-Cav) reduces eNOS-regulated hyperpermeability. We used eNOS-deficient (eNOS−/−) mice and their wild-type control as experimental animals, platelet-activating factor (PAF) at 10−7 m as the test pro-inflammatory agent, and integrated optical intensity (IOI) as an index of microvascular permeability. PAF increased permeability in wild-type cremaster muscle from a baseline of 2.4 ± 2.2 to a peak net value of 84.4 ± 2.7 units, while the corresponding values in cremaster muscle of eNOS−/− mice were 1.0 ± 0.3 and 15.6 ± 7.7 units (P < 0.05). Similarly, PAF increased IOI in the mesentery of wild-type mice but much less in the mesentery of eNOS−/− mice. PAF increased IOI to comparable values in the mesenteries of wild-type mice and those lacking the gene for inducible NOS (iNOS). Administration of AP-Cav blocked the microvascular hyperpermeability responses to 10−7 m PAF. We conclude that: (1) baseline permeability does not depend on eNOS; (2) eNOS and NO are integral elements of the signalling pathway for the hyperpermeability response to PAF; (3) iNOS does not affect either baseline permeability or hyperpermeability responses to PAF; and (4) caveolin-1 inhibits eNOS regulation of microvascular permeability in vivo. Our results establish eNOS as an important regulator of microvascular permeability in inflammation.
Nitric oxide (NO) is a major second messenger in the control of cardiovascular function. The importance of NO in vasodilatation is supported by the hypertension developed in mice lacking the gene encoding for endothelial NO synthase (eNOS−/− mice)(Huang et al. 1995; Shesely et al. 1996). However, the role of eNOS and NO production in microvascular permeability is controversial. Inhibition of eNOS reduces the hyperpermeability caused by inflammatory agents (Boric et al. 1987; Dillon & Durán 1988; Durán & Dillon, 1990; Kobayashi et al. 1994; Mayhan, 1994; Ramírez et al. 1995; Huang & Yuan, 1997; Durán et al. 2000; Yuan, 2000; Lal et al. 2001; Breslin et al. 2003; Aramoto et al. 2004), but it may also increase microvascular permeability(Kubes & Granger, 1992; Kurose et al. 1994). The evidence for both of these is based on the ability of pharmacological agents to simultaneously block NO production by eNOS and inducible NOS (iNOS). Direct support for the role of eNOS-derived NO in Vascular endothelial growth factor (VEGF)-induced hyperpermeability was provided by studies in dorsal skin (a preparation involving several tissues) in eNOS−/− mice, in which transport of albumin was determined 20–40 min after the superfusion with VEGF (Fukumura et al. 2001). The authors of the study concluded that the influence of NO on permeability may depend on the tissue and the stimulus. Additional evidence supporting eNOS-derived NO in regulating VEGF-induced permeability comes from data showing reduced extravasation of Evans Blue-albumin after intradermal VEGF administration or tumours implanted into eNOS−/− mice (Gratton et al. 2003). To explore these issues further, we examined the involvement of eNOS in platelet-activating factor (PAF)-stimulated hyperpermeability in striated muscle of eNOS−/−, wild-type (eNOS+/+) and iNOS−/−mice.
eNOS colocalizes with caveolin-1 in the cell membrane, where the binding of caveolin-1 to eNOS inhibits eNOS activity (Feron et al. 1996; Garcia-Cardena et al. 1996; Shaul et al. 1996). This idea was supported in vivo in mice by the reduction of inflammation achieved by delivery of AP-Cav, a caveolin-1 scaffolding domain molecular probe that inhibits ACh-induced vasodilatation, NO production and acute inflammation (Bucci et al. 2000). Because previous evidence regarding permeability was obtained using either global or single vessel approaches (Bucci et al. 2000; Bernatchez et al. 2005), we chose to test the inhibitory capacity of AP-Cav on microvascular permeability using intravital microscopy and digital image analysis in tissues.
Using eNOS−/−, iNOS−/− and wild-type (control) mice, we tested the hypotheses that: (a) eNOS does not contribute to regulation of baseline permeability; (b) eNOS is essential for hyperpermeability responses to pro-inflammatory agents (e.g. PAF); and (c) caveolin-1 scaffolding domain inhibits hyperpermeability responses in striated muscle and mesentery. Our results demonstrate unequivocally that eNOS is an important integral element in the signalling pathway for hyperpermeability responses in vivo, and that caveolin-1 inhibits modulation by eNOS of microvascular permeability.
Methods
Animals
Male wild-type (C57BL/6J, Jackson Laboratory, Bar Harbour, MA, USA), eNOS−/− (Jackson Laboratory, B6.129P2-Nos3 tm1Unc (C57BL/6J background)) and iNOS−/− mice (derived from C57BL/6Jx129v/Ev lines and obtained through donation from our School's Research Animal Facility) were used in this study. Mice were 12–15 weeks old and weighed 30–35 g at the time of the experiments. The study and procedures were approved by the Animal Care and Use Committee at the New Jersey Medical School and complied with the National Institutes of Health ‘Guide for the Care and Use of Laboratory Animals’. Mice were anaesthetized with sodium pentobarbital (50 mg kg−1; i.p.). A subcutaneous PE-10 tube was placed in the back for infusion of saline to compensate for surgical and ventilatory water loss. The right jugular vein was cannulated for infusion of fluorescein isothyocyanate (FITC)-dextran 77 and supplementary anaesthesia. At the end of the experiments, the animals were given a lethal dose of sodium pentobarbital (150 mg kg−1; i.v.), and pneumothorax was performed 3 min after the i.v. injection.
Cremaster muscle
After exposure, the cremaster muscle was placed in a Lucite chamber (Boric et al. 1987; Takenaka et al. 1998), which allowed for continuous perfusion of the muscle with bicarbonate buffer.
Mesentery
Following a midline abdominal incision, mice were placed in the prone position onto a silicone intestinal bath designed to fix the terminal ileum in the chamber and to maintain the intestine warm and moist.
Intravital microscopy and digital image analysis
An Olympus BHA microscope was used for intravital microscopy. The image was transferred through a silicon intensified TV camera (DAGE-MTI, Inc., Michigan City, IN, USA) to a computer with digital image analysis software Image1 or MetaMorph (Universal Imaging Co, Downingtown, PA, USA). Changes in microvascular permeability were measured using integrated optical intensity (IOI) (Bekker et al. 1989; Kim et al. 1990, 1993) as an index. Three or four interstitial areas, near postcapillary venules, were randomly selected for IOI analysis in cremaster muscle and mesentery.
Experimental protocol
Surgical preparation of either mesentery or cremaster muscle was followed by a 60-min stabilization period. The organ chamber was suffused with bicarbonate buffer, which was continuously equilibrated with 95% N2–5% CO2. Baseline data were obtained every 10 min, with collection starting 25 min before PAF application. PAF was topically applied for 3 min and data were obtained subsequently every 5 min for 60 min.
The cav-1 scaffolding domain (AP-Cav, 1 mg kg−1) and the scrambled control peptide (Cav-X, 1 mg kg−1) (Bucci et al. 2000) were injected intraperitoneally 24 h prior to the experiments to ensure their proper incorporation into the cell membranes. This approach labels the endothelium and adventitia (Bucci et al. 2000). Thus, these experiments should reflect directly the influence of endothelial caveolin-1 on eNOS function inasmuch as the cells of the adventitia lack eNOS. We tested the efficacy of injecting AP-Cav 2 h prior to IOI measurements. Because of variable results, we chose to evaluate the actions of AP-Cav only 24-h after an injection.
Chemicals
The composition of bicarbonate buffer (pH 7.4), preparation of 10−7 m PAF and administration of FITC-dextran was as reported earlier (Dillon & Durán, 1988; Dillon et al. 1988; Kim et al. 1993). Briefly, the bicarbonate buffer contained (mm): NaCl 131.9, KCl 4.7, CaCl2 2.0, MgSO4 1.2 and NaHCO3 18.0. The buffer was adjusted to pH 7.4 and equilibrated with 95% N2–5% CO2. We used 10−7 m PAF as the test agonist because we have studied extensively the dose–response characteristics of this phospholipid and shown that PAF increases permeability by activation of protein kinase C and eNOS (Dillon & Durán, 1988; Durán & Dillon, 1990). The cav-1 scaffolding domain and its scrambled peptide Cav-X were prepared according to the methods of Bucci et al. (2000). PAF was from Sigma (St Louis, MO, USA).
Statistical analysis
The initial IOI value in each experiment was identified as baseline. This baseline value was subtracted from the subsequent experimental values to calculate net IOI and allow for comparison among animals. IOI data are presented as net mean values ± s.e.m. for each time point. Successful experiments were conducted in wild-type eNOS+/+ (n = 45, including experiments with AP-Cav and AP-Cav-X), eNOS−/− (n = 15) and iNOS−/− (n = 6) mice. Time related changes in IOI and differences of IOI between control wild-type mice and experimental mice were analysed with one-way ANOVA and Newman–Keuls post hoc test. Statistical significance was set at P < 0.05.
Results
Cremaster muscle
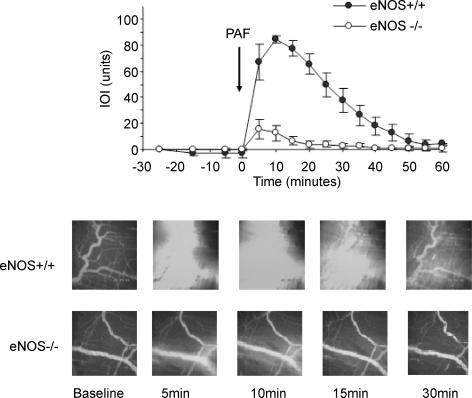
Wild-type and eNOS−/− mice did not present remarkable changes in arteriolar or venular diameter or in adhesion of leucocytes to endothelium in either baseline or after application of 10−7 m PAF. Figure 1 shows the time course of microvascular transport and the comparison between the elevation in IOI, an index of microvascular permeability, in response to 10−7 m PAF in the cremaster muscle of wild-type and eNOS−/− mice. The baseline IOI was similar in wild-type and eNOS−/− mice. In terms of permeability, a marked and significant difference was observed in response to challenge with 10−7 m PAF in wild-type versus eNOS−/− mice. The permeability index IOI increased from a baseline value of 2.4 ± 2.2 to a peak net value of 84.4 ± 2.7 units in the cremaster muscle postcapillary venules of wild-type mice in response to application of 10−7 m PAF. In contrast, a much smaller change in IOI was observed in the cremaster postcapillary venules of eNOS−/− mice wherein IOI increased from a baseline of 1.0 ± 0.3 to a maximum net value of 15.6 ± 7.7 units (P < 0.05 for maximum net value for wild-type versus eNOS−/− mice). The increase in IOI reached a peak at about 10 min following the topical application of PAF and slowly returned to baseline by the end of the 60-min observation period in wild-type mice. The postcapillary venules of eNOS−/− mice presented a small and short-lived increment in IOI after the topical application of PAF. The increased maximum response lasted about 10–15 minutes and had returned to baseline values 45 minutes after topical application of PAF. Figure 1 also shows a comparison of typical images captured in the microvasculature of the cremaster muscle of wild-type and eNOS−/− mice. The photographs show the extravasation of FITC-dextran 77 in wild-type mice in response to challenge with 10−7 m PAF. The corresponding images for eNOS−/− mice demonstrate the small changes in intensity in the interstitial space after application of PAF.
Figure 1. Microvascular permeability in mouse cremaster muscle.
Time course of changes in net integrated optical intensity (IOI; an index of permeability) at baseline and in response to topically applied 10−7 m PAF (arrow) in wild-type and eNOS−/− mice. The data are means ± s.e.m. (P < 0.05). The lower panels show typical images of the extravasation of FITC-dextran 77 in wild-type and eNOS−/− mice during baseline and after application of 10−7 m PAF. Note the large increase in interstitial fluorescence (IOI) after application of PAF in wild-type mice. In contrast, PAF elicited a small and short-lived change in interstitial fluorescence in eNOS−/− mice. n = 8 for both eNOS+/+ and eNOS−/− mice.
Mesentery
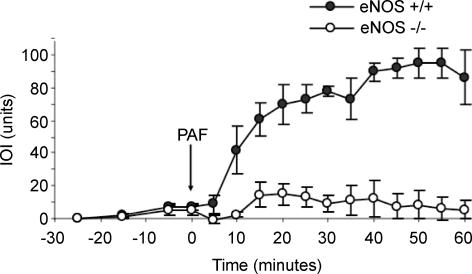
Figure 2 shows the time course of microvascular transport and the comparison between the elevation in IOI in response to 10−7 m PAF in the mesentery of wild-type and eNOS−/− mice. The baseline IOI was similar in wild-type and eNOS−/− mice. As in the cremaster muscle, the mesenteric microvasculature also presented a significant difference between wild-type and eNOS−/− mice in response to challenge with 10−7 m PAF. The IOI value increased from a baseline of 2.5 ± 1.0 to a mean net peak value of 94.8 ± 8.9 units in mesenteric postcapillary venules of wild-type mice in response to PAF. A small change in IOI was observed in mesenteric postcapillary venules of eNOS−/− mice where IOI increased from a mean baseline value of 1.0 ± 0.5 to a maximum net mean value of 15.1 ± 6.6 units (P < 0.05 for comparison between maximum net value for wild-type and eNOS−/− mice). The increase in IOI in wild-type mice was sustained for the 60-min observation period, in contrast to the results in the cremaster muscle. The increment in IOI in eNOS−/− mice mesentery was short in duration, lasting for approximately 10 min.
Figure 2. Microvascular permeability in mouse mesentery.
Time course of changes in net IOI at baseline and in response to topically applied 10−7 m PAF (arrow) in wild-type and eNOS−/− mice. The data are means ± s.e.m. (P < 0.05). n = 7 for both eNOS+/+ and eNOS−/− mice.
iNOS−/− mice
Deletion of the gene encoding for iNOS did not change significantly the magnitude of the microvascular permeability response to PAF in the mouse mesentery. PAF increased the mean IOI values from 2.7 ± 1.0 to 57.1 ± 7.1 units in the control wild-type mouse mesentery (n = 4), whereas the corresponding values in iNOS−/− mice (n = 6) were 2.2 ± 1.3 and 63.7 ± 5.9 units, with P > 0.05 for all comparisons.
Cav-1 scaffold domain
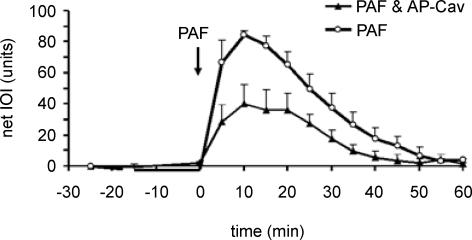
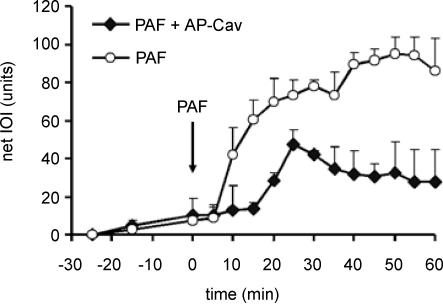
The administration of the scrambled Cav-X peptide did not alter the microvascular hyperpermeability response to PAF; for this reason the Cav-X data are incorporated into the control PAF group for the purpose of presentation (n = 3 each for cremaster muscle and mesentery). In marked contrast, administration of Cav-1 scaffold domain 24 h prior to the experiment led to a significant reduction in the hyperpermeability response to 10−7 m PAF in cremaster muscle (Fig. 3) and mesentery (Fig. 4).
Figure 3. Time course of microvascular permeability in cremaster muscle.
Administration of AP-Cav significantly reduces the hyperpermeability response to 10−7 m PAF in the mouse cremaster muscle (PAF applied at arrow). The data are means ± s.e.m. (P < 0.05). PAF group, n = 7; PAF + AP-Cav, n = 6.
Figure 4. Time course of microvascular permeability in mesentery.
Administration of AP-Cav significantly reduces the hyperpermeability response to 10−7 m PAF in the mouse mesentery (PAF applied at arrow). The data are means ± s.e.m. (P < 0.05). PAF group, n = 7; PAF + AP-Cav, n = 6.
Discussion
Our results demonstrate that: (1) baseline permeability is not compromised by deletion of the gene encoding for eNOS; (2) eNOS and eNOS-derived NO are integral elements of the signalling pathway for the hyperpermeability response to PAF; (3) deletion of the gene encoding for iNOS does not impact baseline permeability nor does it significantly influence the hyperpermeability response to PAF; and (4) caveolin-1 inhibits regulation by eNOS of microvascular permeability in striated muscle and mesentery in vivo.
PAF induced large increases in permeability in the mouse cremaster muscle and mesentery. It is interesting that the response to PAF reached a peak and returned to baseline during the observation period while the response was much longer in the mesenteric postcapillary venules. We are not aware of reports describing these different patterns of response. Because we did not explore experimentally the phenomenon, we assume that the permeability alteration subsides and transport returns to baseline as a function of time. We can only speculate that the different patterns are related to the different microvascular density in the mesentery and the cremaster muscle. An alternative possibility is that, in addition to structural differences, the biochemical signal stopping the action of PAF in the mesentery is much slower than the stop signal in the cremaster muscle.
That baseline permeability is not affected by the lack of eNOS is not surprising. Deletion of the gene encoding for eNOS is not lethal to mice, so it stands to reason that some of the redundant adaptive mechanisms are present to maintain the integrity of the barrier responsible for homeostasis between vascular and extravascular compartments. Our data confirm our earlier work (Ramírez et al. 1995) and support the work of other investigators regarding the function of eNOS in the regulation of microvascular permeability. eNOS is important in regulating junctional integrity (Schubert et al. 2002; Predescu et al. 2005), and contributes to the enhanced permeability observed in acute peritonitis (Ni et al. 2003).
It is believed that neuronal NOS (nNOS) is over-expressed in some organs of eNOS−/− mice and serves to compensate for the lack of eNOS (Lee & Stull, 1998). Protein levels were not measured as an integral element of this study; however, we have previously verified by Western blotting that eNOS is not present in eNOS−/− mice (cremaster, mesentery and lung were tested), whereas eNOS, nNOS, iNOS and caveolin-1 are present in the eNOS+/+ mice. The impact of lack of eNOS is, however, most impressively demonstrated when the organs are challenged with an inflammatory agent such as PAF. Our data support the hypotheses that (a) NO mediates hyperpermeability responses, and (b) eNOS plays a pivotal role in the signalling for hyperpermeability (Mayhan, 1994; Ramírez et al. 1995; Ramírez et al. 1996; Huang & Yuan, 1997; Yuan, 2000; Fukumura et al. 2001; Lal et al. 2001; Breslin et al. 2003; Aramoto et al. 2004; Bernatchez et al. 2005). Our results also strongly indicate that potential over-expression of nNOS, if present in the cremaster muscle and mesentery of eNOS−/− mice, is insufficient and incapable of mounting an appropriate hyperpermeability response to PAF. It is possible that nNOS and other undetermined factors may account for the residual microvascular hyperpermeability observed following challenge with PAF in eNOS−/− mice.
The lack of impact of deletion of iNOS on PAF-induced hyperpermeability confirms the greater relevance of eNOS-derived NO in the immediate regulation of microvascular permeability. It is conceivable that iNOS-derived NO plays a more significant role in microvascular processes that evolve over long periods of time, which would involve induction and synthesis of iNOS and subsequent production of NO, such as permeability alterations associated with sustained angiogenesis (Fukumura et al. 2001).
The mechanisms by which eNOS, and its derived NO, regulates or induces microvascular hyperpermeability remain to be further elucidated. The signalling mechanism may involve phosphorylation of eNOS, inasmuch as PAF-induced hyperpermeability is associated with eNOS phosphorylation and production of NO in the hamster cheek pouch (Durán et al. 2000). Phosphorylation of eNOS in association with NO production has also been documented in vitro (Dimmeler et al. 1999; Fulton et al. 1999; Wu et al. 1999; Yuan, 2000) and in endothelial cells in response to permeability modulating agents.
In summary, our results demonstrate in vivo that eNOS and eNOS-derived NO are essential elements in the regulation of hyperpermeability. In particular, eNOS and eNOS-derived NO are fundamental to activate a robust hyperpermeability response to challenge with PAF, a well-known pro-inflammatory agent. In addition, we demonstrate that iNOS- and nNOS-derived NO are insufficient to activate or mediate the signalling cascade responsible for hyperpermeability in response to PAF. We have also confirmed the inhibitory role of caveolin-1 on NO function in the regulation of microvascular permeability. Our data strengthen our previous work demonstrating in vivo and in vitro the significance of eNOS in the regulation of postcapillary venular permeability and support an impact of eNOS, NO and caveolin-1 on the barrier function of endothelial cell junctions in health and disease.
Acknowledgments
This work was supported in part by the National Institutes of Health grant RO1 HL 70634, a Biomedical Research Support grant from the University of Medicine and Dentistry of New Jersey (UMDNJ)-New Jersey Medical School, and grant no. 1040816 from National Fund for Scientific and Technological Research (FONDECYT)-Chile.
References
- Aramoto H, Breslin JW, Pappas PJ, Hobson RW, 2nd, Durán WN. Vascular endothelial growth factor stimulates differential signaling pathways in in vivo microcirculation. Am J Physiol Heart Circ Physiol. 2004;287:H1590–H1598. doi: 10.1152/ajpheart.00767.2003. [DOI] [PubMed] [Google Scholar]
- Bekker AY, Ritter AB, Durán WN. Analysis of microvascular permeability to macromolecules by video-image digital processing. Microvasc Res. 1989;38:200–216. doi: 10.1016/0026-2862(89)90028-9. [DOI] [PubMed] [Google Scholar]
- Bernatchez PN, Bauer PM, Yu J, Prendergast JS, He P, Sessa WC. Dissecting the molecular control of endothelial NO synthase by caveolin-1 using cell-permeable peptides. Proc Natl Acad Sci U S A. 2005;102:761–766. doi: 10.1073/pnas.0407224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boric MP, Roblero JS, Durán WN. Quantitation of bradykinin-induced microvascular leakage of FITC-dextran in rat cremaster muscle. Microvasc Res. 1987;33:397–412. doi: 10.1016/0026-2862(87)90030-6. [DOI] [PubMed] [Google Scholar]
- Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW, 2nd, Durán WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol. 2003;284:H92–H100. doi: 10.1152/ajpheart.00330.2002. [DOI] [PubMed] [Google Scholar]
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- Dillon PK, Durán WN. Effect of platelet-activating factor on microvascular permselectivity: dose–response relations and pathways of action in the hamster cheek pouch microcirculation. Circ Res. 1988;62:732–740. doi: 10.1161/01.res.62.4.732. [DOI] [PubMed] [Google Scholar]
- Dillon PK, Ritter AB, Durán WN. Vasoconstrictor effects of platelet-activating factor in the hamster cheek pouch microcirculation: dose-related relations and pathways of action. Circ Res. 1988;62:722–731. doi: 10.1161/01.res.62.4.722. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Durán WN, Dillon PK. Acute microcirculatory effects of platelet-activating factor. J Lipid Med. 1990;2(Suppl.):S215–S227. [PubMed] [Google Scholar]
- Durán WN, Seyama A, Yoshimura K, Gonzalez DR, Jara PI, Figueroa XF, Boric MP. Stimulation of NO production and of eNOS phosphorylation in the microcirculation in vivo. Microvasc Res. 2000;60:104–111. doi: 10.1006/mvre.2000.2250. [DOI] [PubMed] [Google Scholar]
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci U S A. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton JP, Lin MI, Yu J, Weiss ED, Jiang ZL, Fairchild TA, Iwakiri Y, Groszmann R, Claffey KP, Cheng YC, Sessa WC. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer Cell. 2003;4:31–39. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Huang Q, Yuan Y. Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. Am J Physiol. 1997;273:H2442–H2451. doi: 10.1152/ajpheart.1997.273.5.H2442. [DOI] [PubMed] [Google Scholar]
- Kim D, Armenante PM, Durán WN. Mathematical modeling of mass transfer in microvascular wall and interstitial space. Microvasc Res. 1990;40:358–378. doi: 10.1016/0026-2862(90)90033-n. [DOI] [PubMed] [Google Scholar]
- Kim D, Armenante PM, Durán WN. Transient analysis of macromolecular transport across microvascular wall and into interstitium. Am J Physiol. 1993;265:H993–H999. doi: 10.1152/ajpheart.1993.265.3.H993. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Kim D, Hobson RW, 2nd, Durán WN. Platelet-activating factor modulates microvascular transport by stimulation of protein kinase C. Am J Physiol. 1994;266:H1214–H1220. doi: 10.1152/ajpheart.1994.266.3.H1214. [DOI] [PubMed] [Google Scholar]
- Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992;262:H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- Kurose I, Wolf R, Grisham MB, Granger DN. Modulation of ischemia/reperfusion-induced microvascular dysfunction by nitric oxide. Circ Res. 1994;74:376–382. doi: 10.1161/01.res.74.3.376. [DOI] [PubMed] [Google Scholar]
- Lal BK, Varma S, Pappas PJ, Hobson RW, 2nd, Durán WN. VEGF increases permeability of the endothelial cell monolayer by activation of PKB/akt, endothelial nitric-oxide synthase, and MAP kinase pathways. Microvasc Res. 2001;62:252–262. doi: 10.1006/mvre.2001.2338. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Stull JT. Calmodulin-dependent regulation of inducible and neuronal nitric-oxide synthase. J Biol Chem. 1998;273:27430–27437. doi: 10.1074/jbc.273.42.27430. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Nitric oxide accounts for histamine-induced increases in macromolecular extravasation. Am J Physiol. 1994;266:H2369–H2373. doi: 10.1152/ajpheart.1994.266.6.H2369. [DOI] [PubMed] [Google Scholar]
- Ni J, Moulin P, Gianello P, Feron O, Balligand JL, Devuyst O. Mice that lack endothelial nitric oxide synthase are protected against functional and structural modifications induced by acute peritonitis. J Am Soc Nephrol. 2003;14:3205–3216. doi: 10.1097/01.asn.0000099382.18284.57. [DOI] [PubMed] [Google Scholar]
- Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289:L371–L381. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- Ramírez MM, Kim DD, Durán WN. Protein kinase C modulates microvascular permeability through nitric oxide synthase. Am J Physiol. 1996;271:H1702–H1705. doi: 10.1152/ajpheart.1996.271.4.H1702. [DOI] [PubMed] [Google Scholar]
- Ramírez MM, Quardt SM, Kim D, Oshiro H, Minnicozzi M, Durán WN. Platelet activating factor modulates microvascular permeability through nitric oxide synthesis. Microvasc Res. 1995;50:223–234. doi: 10.1006/mvre.1995.1055. [DOI] [PubMed] [Google Scholar]
- Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka H, Oshiro H, Kim DD, Thompson PN, Seyama A, Hobson RW, 2nd, Durán WN. Microvascular transport is associated with TNF plasma levels and protein synthesis in postischemic muscle. Am J Physiol. 1998;274:H1914–H1919. doi: 10.1152/ajpheart.1998.274.6.H1914. [DOI] [PubMed] [Google Scholar]
- Wu HM, Yuan Y, Zawieja DC, Tinsley J, Granger HJ. Role of phospholipase C, protein kinase C, and calcium in VEGF-induced venular hyperpermeability. Am J Physiol. 1999;276:H535–H542. doi: 10.1152/ajpheart.1999.276.2.H535. [DOI] [PubMed] [Google Scholar]
- Yuan SY. Signal transduction pathways in enhanced microvascular permeability. Microcirculation. 2000;7:395–403. [PubMed] [Google Scholar]