MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway (original) (raw)
Abstract
MT1-matrix metalloproteinase (MT1-MMP) is one of the most critical factors in the invasion machinery of tumor cells. Subcellular localization to invasive structures is key for MT1-MMP proinvasive activity. However, the mechanism driving this polarized distribution remains obscure. We now report that polarized exocytosis of MT1-MMP occurs during MDA-MB-231 adenocarcinoma cell migration into collagen type I three-dimensional matrices. Polarized trafficking of MT1-MMP is triggered by β1 integrin-mediated adhesion to collagen, and is required for protease localization at invasive structures. Localization of MT1-MMP within VSV-G/Rab8-positive vesicles, but not in Rab11/Tf/TfRc-positive compartment in invasive cells, suggests the involvement of the exocytic traffic pathway. Furthermore, constitutively active Rab8 mutants induce MT1-MMP exocytic traffic, collagen degradation and invasion, whereas Rab8- but not Rab11-knockdown inhibited these processes. Altogether, these data reveal a novel pathway of MT1-MMP redistribution to invasive structures, exocytic vesicle trafficking, which is crucial for its role in tumor cell invasiveness. Mechanistically, MT1-MMP delivery to invasive structures, and therefore its proinvasive activity, is regulated by Rab8 GTPase.
Keywords: matrix metalloproteinases, membrane traffic, MT1-MMP, Rab8, tumor invasion
Introduction
Key processes for tumor progression such as angiogenesis, cell growth, invasion and metastasis are based on the ability of endothelial and tumor cells to invade the surrounding tissue. Focused degradation of tissue barriers by matrix metalloproteinases (MMPs) plays a critical role in invasion (Egeblad and Werb, 2002; Sato et al, 2005). MMPs are either secreted from the cell or anchored to the plasma membrane (PM) as integral proteins (membrane-type MMPs). Of these, MT1-MMP has been widely studied as its expression is closely associated with invasiveness and malignancy of tumors (Egeblad and Werb, 2002). Moreover, MT1-MMP overexpression enhances invasive ability of cells and silencing MT1-MMP suppresses cell migration and invasion, demonstrating that this enzyme is one of the most critical factors of the invasion machinery (Sato et al, 2005; Itoh and Seiki, 2006).
MT1-MMP is produced as an inactive precursor and is proteolytically cleaved intracellularly by furin, being delivered to the PM in the active form as a type I transmembrane protein (Osenkowski et al, 2004). However, the exact mechanism by which active MT1-MMP traffics to the PM is not known. Once in the PM, MT1-MMP can degrade a number of ECM macromolecules including type I, II and VI collagens, gelatin, laminins 1 and 5, fibronectin, vitronectin, aggrecan, fibrin and lumican. It also activates other proteases like pro-MMP2 and pro-MMP13 and cleaves several cell surface proteins such as CD44, transglutaminase, low-density lipoprotein receptor-related protein, αv integrin and syndecan (Sato et al, 2005; Itoh and Seiki, 2006). Given the wide array of substrates that can be irreversibly processed by MT1-MMP and the fact that the enzyme is expressed at the PM as an active enzyme (Sato et al, 1994; Mazzone et al, 2004), it seems clear that MT1-MMP is a potentially harmful enzyme and needs to be tightly regulated. Classical regulatory mechanisms of MT1-MMP include transcriptional regulation, intracellular processing of the inactive zymogen (Sato et al, 1994; Mazzone et al, 2004) and inhibition by endogenous tissue inhibitors (TIMP-2, RECK or testican) (Will et al, 1996; Nakada et al, 2001; Oh et al, 2001). Recently, more precise means of regulating MT1-MMP activity on the cell surface, like internalization (Jiang et al, 2001; Uekita et al, 2001; Galvez et al, 2002; Wang et al, 2004), recycling (Remacle et al, 2003; Wang et al, 2004), autocatalytic processing to an inactive degradation product (Stanton et al, 1998; Lehti et al, 2000; Tam et al, 2002; Toth et al, 2002), oligomerization (Itoh et al, 2001; Rozanov et al, 2001; Lehti et al, 2002; Galvez et al, 2005) and post-trasductional regulation (Wu et al, 2004) have been described. Subcellular localization of MT1-MMP to invasive structures is another important aspect of MT1-MMP regulation and constitutes a prerequisite for exerting its proinvasive activity (Nakahara et al, 1997; Lehti et al, 2000; Mori et al, 2002), although the mechanism driving this polarized distribution remains to be elucidated.
Expression of PM proteins is controlled by the ubiquitous process of constitutive secretion, and can be slowly up- or downregulated by synthesis de novo or degradation of existing protein. Additionally, cells can rapidly modulate the levels of surface expression of some receptors, channels and transporters by having a pool of ready synthesized molecules available for their rapid insertion into and retrieval from the PM in a process called constitutive cycling (Royle and Murrell-Lagnado, 2003). This process involves regulated exocytosis, which is the translocation of membrane proteins from intracellular compartments to the PM as a consequence of cell stimulation (Chieregatti and Meldolesi, 2005). Polarized exocytosis towards the leading edge of migrating cells has been suggested as a mechanism causing membrane extension and recycling of integrin molecules endocytosed at the rear of the cell (Lawson and Maxfield, 1995; Sesaki and Ogihara, 1997). Leading edge-directed exocytosis seems to transport both secretion and endocytic recycling membranes (Bretscher and Aguado-Velasco, 1998). MT1-MMP has been shown to reside intracellularly (Jiang et al, 2001; Uekita et al, 2001; Galvez et al, 2002; Remacle et al, 2003; Wang et al, 2004) and its expression at the cell surface is usually very weak in most cell types. There are clear evidences showing that this protein undergoes endocytosis (Jiang et al, 2001; Uekita et al, 2001; Galvez et al, 2002) and recycling to the surface (Remacle et al, 2003; Wang et al, 2004) in stationary cells. However, there are no evidences so far describing regulation of MT1-MMP by regulated exocytic processes.
Rab8 was initially isolated as a transforming gene from a melanoma cell line (Nimmo et al, 1991). It belongs to the Rab family of Ras-related GTPases that play a crucial role in membrane traffic by determining the specificity of vesicle transport (Zerial and McBride, 2001). Although the traffic route regulated by Rab8 is not established, it is known to regulate polarized membrane transport of newly synthesized proteins to PM protrusions, participating in remodelling the cell shape in response to different signals (Huber et al, 1993; Peranen et al, 1996; Hattula et al, 2002; Ang et al, 2003).
We herein report a novel regulatory mechanism of MT1-MMP activity involving regulated exocytosis to the cell surface at invasive structures driven by integrin-mediated adhesive events that is controlled by Rab8 GTPase. The importance of this regulatory mechanism is highlighted by the complete functional blockade of MT1-MMP-dependent collagen degradation and invasion when Rab8 protein levels are knocked down.
Results
Live cell confocal imaging of MT1-MMP dynamic redistribution and activity at invasive structures
To understand better MT1-MMP regulation during tumor cell invasion, we transfected breast carcinoma MDA-MB-231 cells with MT1-MMP-GFP and embedded them in three-dimensional matrices (3D-Col I). Live cell confocal imaging showed, for the first time, activity and dynamics of MT1-MMP during cell invasion (Figure 1A and Supplementary Video 1). Fluorescence and reflection images, revealing MT1-MMP-GFP localization and collagen fiber organization respectively, showed MT1-MMP dynamic redistribution to collagen fiber adhesion sites at the PM, and subsequent collagen fiber degradation (Figure 1A and Supplementary Video 1). Cells overexpressing large amounts of MT1-MMP-GFP were used only to monitor MT1-MMP activity during invasion as they produced a high extent of matrix destruction, thus clearly showing that MT1-MMP-GFP retains protease activity. However, subsequent live cell invasion studies were carried out using cells expressing low amounts of MT1-MMP-GFP, which behaved in a more physiological manner. This was accomplished by choosing cells with dim GFP fluorescence producing punctual degradation of collagen fibers during the invasive process. A non-linear pattern of MT1-MMP localization at membrane protrusions in contact with the underlying 3D matrix was also shown for endogenous MT1-MMP in MDA-MB-231 and primary carcinoma cells as revealed by immunostaining with specific anti-MT1-MMP antibodies (Abs) (Figure 1B and C).
Figure 1.
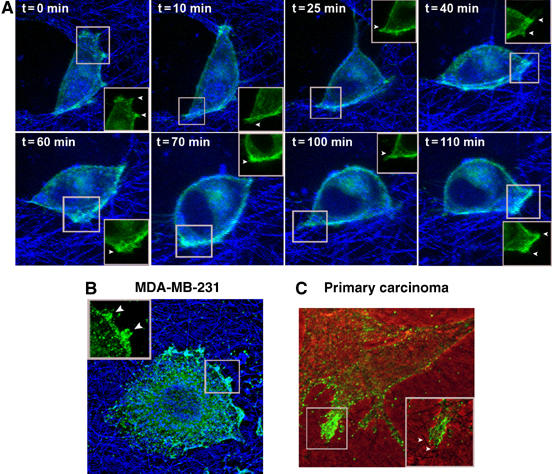
MT1-MMP accumulates at the sites of active collagen degradation during invasion of 3D-Col I matrices. (A) Live cell confocal imaging of MT1-MMP-GFP expressed in MDA-MB-231 cells embedded into 3D-Col I. Overlay of MT1-MMP-GFP fluorescence (green) and collagen fiber reflection (blue) images obtained at the indicated time points during the course of a time-lapse experiment is shown (see Supplementary Video 1). (B, C) Localization of endogenous MT1-MMP revealed by immunofluorescence staining with Lem-2/15 mAb of MDA-MB-231 cells and endometrial carcinoma primary cultured cells embedded into 3D-Col I. (B) Overlay of MT1-MMP staining (green) and collagen fiber reflection (blue) images is shown. (C) Overlay of phase contrast and fluorescence images. Insets show membrane sites engaging bundles of collagen fibers. GFP concentration at invasive structures is pointed by arrowheads.
Polarized vesicle traffic is responsible for the accumulation of MT1-MMP at collagen contact sites
Localization of MT1-MMP to invasive structures has been described previously (Nakahara et al, 1997; Lehti et al, 2000; Mori et al, 2002), although the underlying mechanism responsible for its redistribution on the cell surface has not been elucidated. MT1-MMP localization was analyzed in MDA-MB-231 cells embedded into 3D-Col I. Interestingly, a novel compartmentalization of MT1-MMP-positive vesicles at submembranous pools in invasive structures was observed (Figure 2A). Clear evidence of recruitment of MT1-MMP-carrying vesicles to invasive structures was obtained when monitoring the formation of a new membrane protrusion event (Figure 2A, pointed by arrowheads and Supplementary Video 2). Cell adhesion to collagen fibers leading to PM protrusion was accompanied by local recruitment of MT1-MMP-positive intracellular vesicles to a submembranous area, followed by the local accumulation of MT1-MMP at the protrusive membrane (Figure 2A and Supplementary Video 2). Visualization of vesicle trajectories by fast scanning confocal imaging revealed highly complex traffic going to and from the PM in different directions. Traffic from the cell center to the periphery is not obvious, although there is clear PM transport from submembranous pools localized at the polarized areas (see Supplementary Figure 3 and Supplementary Video 4, 5 and 6). Polarized MT1-MMP vesicle traffic was also induced by adhered Col I-coated beads but not control BSA-coated beads (Figure 2B). Dynamic live cell studies show very active vesicle recruitment to collagen-coated beads, where MT1-MMP is accumulated (Supplementary Figure 7 and Supplementary Video 8). To gain insight into the cues that induced MT1-MMP vesicle recruitment, we allowed cells to interact with beads coated with different ECM matrix proteins. Quantitative analysis showed MT1-MMP-specific mobilization induced by Col I, Fn or β1 integrin clustering Abs, but not by BSA. Col I-induced MT1-MMP recruitment could be specifically impaired by function blocking anti-β1 Abs (Figure 2C). Altogether, these results show that recruitment of MT1-MMP vesicles induced by collagen engagement in MDA-MB-231 cells is mediated by β1 integrin-dependent adhesive events.
Figure 2.
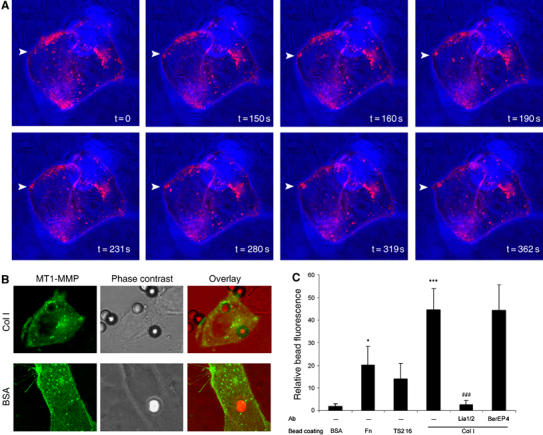
MT1-MMP intracellular vesicle recruitment toward collagen contact sites at the PM of MDA-MB-231 cells. (A) Live cell imaging of MDA-MB-231 cells transfected with MT1-MMP-GFP and embedded into 3D-Col I. Overlay of fluorescence and phase-contrast images showing MT1-MMP-GFP localization (pink) and cell morphology/collagen fiber distribution respectively, acquired at different time points is shown (see Supplementary Video 2). Arrowheads point to a new contact established between the cell membrane and a meshwork of collagen fibers, where active vesicle recruitment is observed. (B) MDA-MB-231 cells expressing MT1-MMP-GFP (green), cultured on glass coverslips, were incubated with Col I- or BSA-coated beads for 1 h, fixed and imaged. Fluorescence image (showing GFP), phase-contrast image (showing cell morphology and bead localization) and their overlay are presented. (C) Beads coated with BSA, Fn, anti-β1 Ab (TS2/16) or Col I were allowed to interact for 1 h with MDA-MB-231 cells that had been previously treated with or without not with a blocking anti-β1 (Lia1/2) or control (BerEP4) Abs. Cells were then fixed and imaged by confocal microscopy. Bars represent relative fluorescence intensity at the bead surrounding area normalized for background fluorescence calculated at 10–15 beads for each of the three independent experiments performed. The statistical significance of relative bead fluorescence comparing the different bead coatings and control (BSA) values (*) and antibody-treated compared to isotype control values (#) was evaluated using Student's _t_-test. (*P<0.05; ***/###P<0.001).
It has been proposed that MT1-MMP delivery to invasive structures is mediated by CD44-dependent membrane transport, although our live cell studies prompted us to hypothesize that intracellular vesicle traffic was responsible for the accumulation of MT1-MMP at invasive structures. We addressed this issue by combining fluorescence recovery after photobleaching (FRAP) at the PM with fluorescence loss in photobleaching (FLIP) at the underlying submembranous compartment. Recovery of fluorescence monitored at the FRAP region quantitatively estimates the extent to which MT1-MMP membrane localization is dependent on membrane transport, independently of the contribution of vesicle income from the intracellular compartment. In contrast to the lateral PM (Figure 3A–F), no relocalization of fluorescent MT1-MMP at the invasive PM was observed (Figure 3G–L) when the intracellular pool of vesicles was continuously bleached. Additional example is shown in Supplementary Figure 9. Hence, at the invasive lamella, MT1-MMP membrane diffusion is compromised and intracellular traffic is most likely the source of MT1-MMP accumulation at invading structures.
Figure 3.
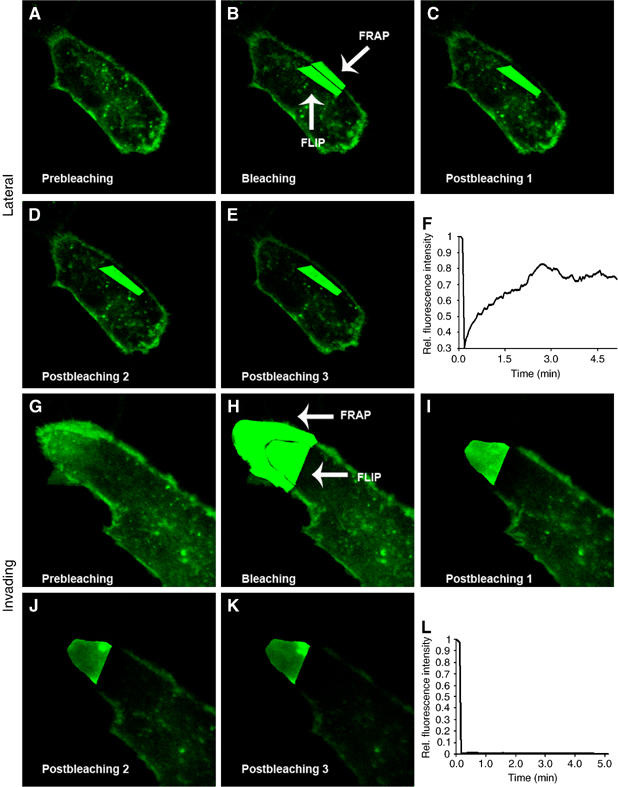
MT1-MMP FRAP/FLIP experiments reveal that intracellular vesicle traffic is responsible for the accumulation of MT1-MMP at the invasive PM. MT1-MMP-GFP expressing MDA-MB-231 cells embedded into 3D-Col I were subjected to FRAP-FLIP photobleaching experiments. Images showing prebleaching, bleaching and post-bleaching at the PM (FRAP region) during continuous photobleaching of the submembranous compartment (FLIP region) at the lateral (A–E) and invading (G–K) PM. Fluorescence recovery quantification at the FRAP region is calculated at the lateral (F) and invading (L) PM and represented in the graph.
MT1-MMP is found in the biosynthetic, not the recycling compartment in invasive MDA-MB-231 cells
To explore the involvement of the biosynthetic pathway in MT1-MMP-polarized exocytosis, we performed colocalization studies using a classical marker of this route, VSV-G, as a reporter. MDA-MB-231 cells coexpressing VSV-G-YFP and MT1-MMP-mRFP were embedded into 3D-Col I; cells were then incubated at 20°C to allow accumulation at the TGN (Ang et al, 2003), where both proteins were found colocalizing (not shown). When shifting to 32°C to allow rapid exit of VSV-G from the TGN, a number of vesicles displayed strong colocalization of MT1-MMP and VSV-G, and were found to translocate to the PM at invasive sites (Figure 4A). These results suggest that biosynthetic exocytic traffic is involved in the recruitment of MT1-MMP to invasive structures at the PM.
Figure 4.
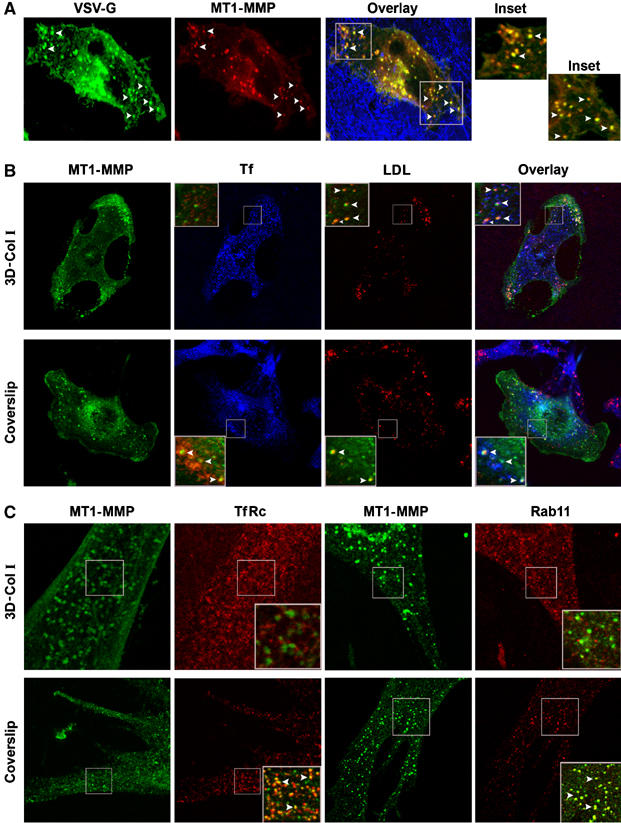
MT1-MMP colocalization with markers of the biosynthetic/recycling and degradative routes. (A) MDA-MB-231 cells cotransfected with MT1-MMP-mRFP and VSV-G-YFP were embedded into 3D-Col I. Cells were incubated overnight at 40°C, then transferred to 20°C for 2 h and finally shifted to 32°C for 1 h. Cells were then fixed and imaged. Arrowheads point to vesicles positive for both VSV-G (green) and MT1-MMP (red). Overlay image shows colocalization (yellow) and fiber reflection (blue). (B) MDA-MB-231 cells transfected with MT1-MMP-GFP were either embedded into 3D-Col I (upper panel) or plated on coverslips (lower panel) and incubated with labelled Tf and LDL for 1 h at 37°C to allow their internalization. Images show localization of MT1-MMP-GFP (green), Tf (blue), LDL (red), and their overlay. (C) Primary lung adenocarcinoma cells were either embedded into 3D-Col I (upper panel) or plated on coverslips (lower panel) and immunostained with specific Abs for TfRc or Rab11 (red), and MT1-MMP (green), as indicated. Insets show superimposed fluorescence images pseudocolored in green/red; arrowheads point colocalization vesicles (shown in yellow).
The biosynthetic transport of proteins to the cell surface occurs via the recycling endosomes (Futter et al, 1995; Leitinger et al, 1995; Ang et al, 2004; Lock and Stow, 2005). Moreover, recycling has been proposed as a mechanism of MT1-MMP recruitment to the leading edge during cell migration (Remacle et al, 2003; Wang et al, 2004). We therefore sought to determine the involvement of recycling in MT1-MMP-polarized exocytosis. We allowed MT1-MMP-GFP-transfected cells to uptake transferrin (Tf) and low-density lipoprotein (LDL) to label recycling and lysosomal compartments, and analyzed their colocalization with MT1-MMP-GFP (Figure 4B and Supplementary Figure 10). Surprisingly, MT1-MMP showed almost negligible colocalization with Tf and a strong colocalization with LDL in 3D-Col I-embedded cells, suggesting that MT1-MMP is absent from recycling compartment, and instead is being sorted to lysosome degradation in invasive cells. However, when we used the same experimental conditions as previous studies that demonstrated MT1-MMP recycling (Remacle et al, 2003; Wang et al, 2004), that is, plating cells in coverslips, we confirmed MT1-MMP localization at recycling compartments (Figure 4B). In addition, primary tumor cells also showed overlap of the TfRc/Rab11-positive recycling compartment with endogenous MT1-MMP in cells grown on coverslips but not in 3D-Col I-embedded cells (Figure 4C). Therefore, MT1-MMP is confined within the biosynthetic, although it is absent from the recycling compartment in invasive cells.
Rab8 but not Rab11 codistributes with MT1-MMP at exocytic vesicles, and is specifically mobilized by Col I
Because Rab8 GTPase has been involved in polarized membrane transport of PM proteins during the formation of membrane protrusions, we sought to determine its involvement in MT1-MMP exocytic delivery to invasive structures. We found a strong colocalization of MT1-MMP and Rab-8 in intracellular vesicles (Figure 5A). Time-lapse confocal imaging revealed the presence of MT1-MMP in Rab8-positive vesicles being transported to the invasive PM (Figure 5B and Supplementary Video 11), whereas colocalization of MT1-MMP with Rab11 was negligible (Figure 5C). Vesicles recruited to Col I-coated beads also showed a strong colocalization of Rab8 and MT1-MMP (Figure 5D). Interestingly, we observed a striking colocalization of MT1-MMP and Rab8 within membranes deposited at degraded matrix (Figure 5E). Deposition of cell fragments within the extracellular matrix caused by exocytic release of vesicles has been related to rear retraction during tumor cell invasion (Friedl and Wolf, 2003; Mayer et al, 2004), and MT1-MMP has been previously shown to be released within these fragments in endothelial cells (Taraboletti et al, 2002). These results strongly suggest that traffic and fusion of exocytic vesicles carrying MT1-MMP to matrix degradation sites is regulated by Rab8. Moreover, collagen-coated beads specifically induced recruitment of Rab8- but not Rab11-positive vesicles (Figure 5F), indicating that Rab8-mediated traffic is induced by collagen interaction.
Figure 5.
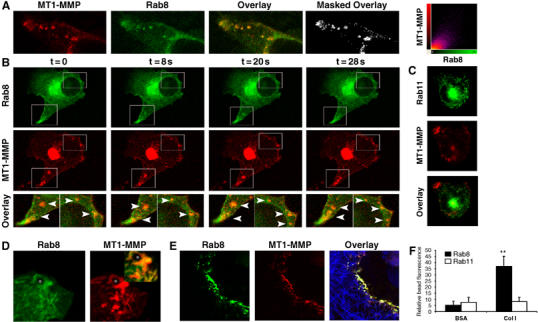
Rab8 but not Rab11 codistributes with MT1-MMP during vesicle transport to the PM. MDA-MB-231 transfected with MT1-MMP-mRFP and Rab8-GFP were embedded into 3D-Col I and analyzed by confocal imaging. (A) MT1-MMP-mRFP fluorescence (red), Rab8-GFP (green) and the superimposed images where colocalization can be seen in yellow, as well as the image showing exclusively colocalizing pixels (white) are shown. 2D colocalization histogram corresponding to these images obtained using Imaris software (Bitplane AG, Zurich, Switzerland) is also shown. Live cell imaging of 3D-Col I invading MDA-MB-231 cells transfected with MT1-MMP-mRFP and either Rab8-GFP (B) or Rab-11-GFP (C). Images acquired at the indicated time points show Rab8 or Rab11 (green) and MT1-MMP (red) localization during the course of the experiment (see Supplementary Video 11). (D) MDA-MB-231 cells expressing MT1-MMP-mRFP (red) and Rab8-GFP (green), cultured on glass coverslips, were incubated with Col I-coated beads for 1 h, then fixed and imaged. Overlay of fluorescence images is presented in inset. Asterisk indicates bead localization. (E) Confocal images of MT1-MMP-mRFP (red) and Rab8-GFP (green) fluorescence and collagen fiber reflection (blue) shows colocalization of MT1-MMP and Rab8 attached to degraded collagen fibers. (F) Beads coated with BSA or Col I were allowed to interact with Rab8-GFP- or Rab11-GFP-expressing MDA-MB-231 cells. Bars represent relative fluorescence intensity at the bead surrounding area normalized to background fluorescence calculated at 10–15 beads for each of the three independent experiments performed.
Rab8 regulates traffic of MT1-MMP to invasive structures, and MT1-MMP-dependent collagen degradative activity and invasion
The involvement of Rab8 in the regulation of MT1-MMP activity was first evaluated by quantitative experiments of MT1-MMP vesicle recruitment in cells expressing wtRab8, Rab8-activated mutant (Rab8Q67L) or Rab8ΔC (inactive mutant with impaired membrane localization owing to loss of the prenylation site). MT1-MMP-mRFP vesicle recruitment to Col I-coated beads was significantly induced by overexpression of Rab8-activated mutant, but not by Rab8ΔC control (Figure 6A). Specificity of Rab8 effect was demonstrated by examining CD44 recruitment to hyaluronic acid (HA)-coated beads, which was unaffected by the expression of Rab8 constructs (Supplementary Figure 12). Furthermore, transwell collagen invasion assays showed that similar to MT1-MMP overexpression, Rab8Q67L and wtRab8 induced an increase in cell invasion, whereas ΔC control was shown to be ineffective (Figure 6B). Pericellular collagenolysis evaluated in cells expressing the different constructs revealed that Rab8 overexpression and activation induced collagen degradative activity (Figure 6C). Function blocking anti-MT1-MMP Ab (Lem-2/15) significantly abrogated Rab8-induced invasion and collagen degradation (Figure 6B, C), thus indicating its endogenous MT1-MMP dependence. These studies demonstrate the involvement of Rab8 in regulating MT1-MMP delivery to the PM and its collagenolytic and proinvasive activities.
Figure 6.
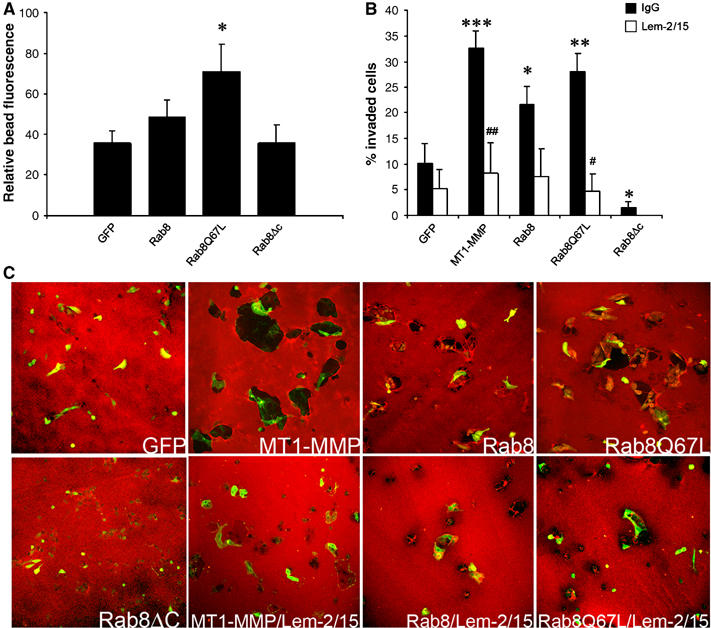
Rab8 activation induces recruitment of MT1-MMP vesicles, MT1-MMP-dependent collagen degradation and invasion. (A) MDA-MB-231 cells were cotransfected with MT1-MMP-mRFP and either GFP, wtRab8-GFP, Rab8Q67L-GFP or Rab8ΔC-GFP, and, allowed to interact with Col I-coated beads for 1 h, then fixed and analyzed by confocal microscopy. Bars represent the percentage of MT1-MMP-mRFP fluorescence intensity around the bead calculated in 10–18 cells expressing the different GFP constructs from three independent experiments. (B) MDA-MB-231 cells were transfected with GFP, wtRab8-GFP, Rab8Q67L-GFP, Rab8ΔC-GFP or MT1-MMP-GFP. Cells were then allowed to migrate for 48 h on transwell filters coated with 3D-Col I to FCS containing media in the presence of isotype control IgG (solid bars), or function blocking anti-MT1-MMP Ab (Lem-2/15) (open bars). Bars represent the percentage of invaded GFP-expressing cells quantified in seven independent experiments by counting four different fields for each experiment. (C) MDA-MB-231 cells transfected with GFP, wtRab8-GFP, Rab8Q67L-GFP, Rab8ΔC-GFP or MT1-MMP-GFP were cultured on 2D-Col I layers for 48 h in the presence or absence of function blocking anti-MT1-MMP Ab (Lem-2/15), then fixed and labelled with anti-Col I antibody to evaluate degradation. Representative overlay images of Col I staining (red) and expression of the different constructs (green) is shown. The statistical significance comparing expression of different constructs to control (GFP) values (*) and antibody-treated compared to isotype control values (#) was evaluated using Student's _t_-test (*/#P<0.05; **/##P<0.01; ***/###P<0.001).
To demonstrate further the role of Rab8 in MT1-MMP exocytic traffic to the PM, we performed gene silencing studies. Stable cell lines carrying short-hairpin RNA (shRNA) targeted Rab8a and Rab11a showed protein depletions of approximately 80 and 60%, respectively, as assessed by Western blotting analysis (Figure 7A and B). The possibility of having off-target effects was ruled out by analyzing the levels of Rab11 protein in Rab8 knocked down cells and vice versa control and Rab8-silenced cells displayed similar levels of surface MT1-MMP expression (10 and 9.3 mean fluorescence intensity) as revealed by flow cytometry analysis. Thus, steady-state expression of MT1-MMP at the cell surface was unaffected by Rab8 knockdown. However, endogenous MT1-MMP vesicle recruitment to Col I-coated beads (Figure 7C), MT1-MMP-induced tumor cell invasion (Figure 7D) and collagen degradation (Figure 7E) were impaired in cells expressing shRNA for Rab8 but not Rab11. Moreover, ectopic expression of Rab8 coding sequence tagged with mRFP carrying four silent mutations in Rab8shRNA1 targeting sequence reconstituted these functions (Supplementary Figure 13). Transiently transfected Rab8shRNA in mammalian expression vectors rendered similar effects (Supplementary Figure 14). CD44 recruitment to HA-coated beads was unaffected by Rab8shRNA, further demonstrating that polarized distribution of other surface proteins is independent of Rab8 (Supplementary Figure 12). These results clearly demonstrate that Rab8 GTPase specifically mediates regulated, not constitutive, transport of MT1-MMP to the PM, MT1-MMP-dependent collagen degradation and invasion.
Figure 7.
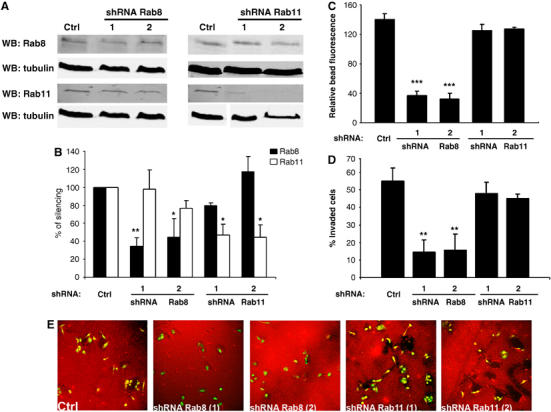
Rab8 but not Rab11 knockdown with shRNA decreases MT1-MMP vesicle recruitment, collagen degradation and invasion. MDA-MB-231 cells stably expressing PMCSV Pig (control), or PMCSV carrying Rab8shRNA sequences 1 and 2 or Rab11shRNA sequences 1 and 2. (A) The levels of Rab8 protein was assessed by Western blot analysis. Control tubulin blotting is also shown. (B) Quantification of Rab8 protein levels normalized using tubulin as a loading control is represented in the bar diagram. Endogenous vesicle recruitment (C), transwell invasion (D) and collagen degradation (E) were evaluated as described in Figure 6. (E) Representative images show Col I staining (red) and GFP expression from PMCSV vector (green). Asterisks indicate statistical significance comparing the expression of the different shRNAs to control (PMCSV Pig) values.
Discussion
Focal degradation of the ECM barrier at the invading cell front is a key process in tumor invasion, and this is achieved by localization of proteases at the leading edge of migrating cells. There are clear evidences that MT1-MMP localizes at invasive structures (Nakahara et al, 1997; Lehti et al, 2000; Mori et al, 2002). However, how precisely the enzyme is targeted to the the invasion sites remains to be determined.
Three dimensional collagen matrices mimic the ‘in vivo' environment encountered by tumor cells, and so provide a surrogate of the tissue microenvironment, allowing us to perform live cell studies of tumor cell invasion. We herein show for the first time dynamic redistribution and activity of MT1-MMP at invasive structures, as visualized by live confocal imaging of MDA-MB-231 adenocarcinoma cell invasion of 3D-Col I. β1 Integrin-dependent adhesion was found to be the spatial cue leading to MT1-MMP recruitment in response to collagen engagement. Accordingly, integrin clustering stimulates cell-surface expression of MT1-MMP (Ellerbroek et al, 2001), and coclustering of β1 integrins and MT1-MMP has been shown in tumor cells invading 3D collagen matrices (Wolf et al, 2003), and endothelial cells adhered to collagen-coated surfaces, where the biochemical association of both MT1-MMP and β1 integrin was demonstrated (Galvez et al, 2002).
MT1-MMP proinvasive activity requires its redistribution to motility-related structures (Nakahara et al, 1997; Lehti et al, 2000; Mori et al, 2002). Seiki and co-workers have suggested the interaction of MT1-MMP with CD44, and the linkage of the latter to the actin cytoskeleton, as the mechanism driving the proteinase to the leading edge of migrating cells (Mori et al, 2002; Suenaga et al, 2005). Our data on 3D invasion models point out a completely novel mechanism, regulated exocytosis of MT1-MMP vesicles, mediating MT1-MMP recruitment to invasive structures. This hypothesis is based in several pieces of evidence: (1) dynamic visualization of MT1-MMP vesicles being recruited to the cell surface from intracellular locations at collagen fiber attachment sites preceding membrane protrusion; (2) FRAP/FLIP experiments showing that submembranous vesicle pool rather than membrane diffusion is required for the accumulation of MT1-MMP at the invasive PM; and (3) the requirement of active Rab8, a GTPase involved in exocytic traffic, for collagen-induced MT1-MMP recruitment to the membrane, MT1-MMP-dependent collagen degradation and invasion. In agreement with this hypothesis, an intracellular functional pool of MT1-MMP available for trafficking to the cell surface upon stimulation of HT1080 cells with ConA has been reported (Zucker et al, 2002).
The confinement of MT1-MMP within the biosynthetic and its absence from recycling compartments seems contradictory as there is increasing evidence showing that biosynthetic transport to the cell surface occurs via recycling endosomes (Futter et al, 1995; Leitinger et al, 1995; Ang et al, 2004; Lock and Stow, 2005). However, a number of live imaging studies (Lippincott-Schwartz et al, 2000; Lock and Stow, 2005) support the existence of a direct delivery pathway from the Golgi complex to the PM that bypasses recycling endosomes, which could be involved in the traffic of MT1-MMP to invasive structures. Our results showing that MT1-MMP intracellular compartmentalization depends on the extracellular context may provide a rationale for internalized MT1-MMP. MT1-MMP will recycle when cells are not involved in ECM degradation thus maintaining a controlled surface activity, while allowing intracellular pools to be stored for rapid trafficking if necessary. In contrast, MT1-MMP will be mobilized to a degradative compartment when cells are actively involved in ECM proteolytic processing to prevent accumulation of inactivated MT1-MMP (TIMP-2-inhibited or partially degraded molecules). We can, therefore, establish a strong parallelism between the homeostasis of MT1-MMP and the so-called constitutive cycling traffic reported for a number of membrane proteins (reviewed by Royle and Murrell-Lagnado, 2003). A good example is the glucose transporter GLUT4, which undergoes rapid constitutive internalization and subsequent slow recycling back to the surface, and therefore under basal conditions, exists predominantly within intracellular compartments (Dugani and Klip, 2005). Despite being engaged in a recycling loop, there is a more static secretory pool of GLUT4 storage vesicles ready to move directly to the cell surface in response to insulin stimulation (Dugani and Klip, 2005). Accordingly, both MT1-MMP and GLUT4 have been localized at Rab8-positive vesicles (our data and Miinea et al, 2005), and their transport to the membrane is dependent on syntaxin 4 (Widberg et al, 2003; Miyata et al, 2004).
Our studies reveal a novel pathway in the regulation of MT1-MMP and allow us to propose a model for MT1-MMP homeostasis (Figure 8). In this model, different traffic pathways of MT1-MMP are highlighted: (i) Rab8-regulated exocytic mobilization from an intracellular storage compartment different from recycling endosomes would account for polarized recruitment of MT1-MMP to the invasive PM engaged in matrix degradation (i.e. this report); (ii) constitutive cycling will be predominant in a stationary cell, where MT1-MMP is not involved in ECM proteolytic processing, being found in the recycling compartment instead; (iii) the possibility that, as reported for GLUT4 and Rab8, there is a transport loop between the storage compartment and recycling endosomes is not excluded; and (iv) endocytosis targeted to lysosome degradation will most likely be the fate of surface MT1-MMP, inactivated during the process of matrix degradation. This model would keep a potentially harmful enzyme away from the PM, where it could exert unwanted side-effects, despite being an extremely sensitive system for rapid and localized enzyme mobilization, avoiding the slow process of protein synthesis.
Figure 8.
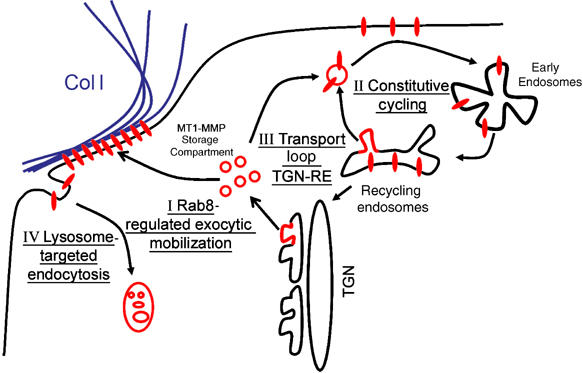
Model for MT1-MMP intracellular trafficking. The model depicts two main intracellular pathways (I) Rab8-regulated exocytic mobilization of MT1-MMP from a biosynthetic storage compartment induced by collagen engagement in invading cells (II) Constitutive cycling from recycling endosomes in a stationary cell involves MT1-MMP un-engaged in matrix degradation. Additional pathways could involve (III) transport loop between the biosynthetic storage and recycling compartments and (IV) endocytosis targeted to lysosome degradation of surface MT1-MMP involved in collagen degradative activity.
Rab8 was first described as an oncogene isolated as a transforming gene from a melanoma cell line (Nimmo et al, 1991), although its relevance in cancer has not been established yet. Notably, Rab8 search in Oncomine cancer profiling database (www.oncomine.org) showed its overexpression in tumoral versus normal tissues in different microarray data sets. Rab8 belongs to the family of Ras-like small GTPases that are major regulators of membrane trafficking in eukaryotic cells (Zerial and McBride, 2001). Although the traffic route regulated by Rab8 is still not clarified, there is however evidence that it is involved in the transport of PM proteins at membrane protrusions (Peranen et al, 1996; Hattula et al, 2002; Ang et al, 2003). Our results clearly show the involvement of Rab8 in the traffic of MT1-MMP to the PM and in MT1-MMP-dependent collagen degradation and invasion, which may help to explain Rab8-transforming activity. Considering the importance of MT1-MMP in tumor cell invasion and angiogenesis, there is a great interest in targeting this enzyme with new inhibitors. Cancer therapeutics designed to target protease activity by synthetic MMP inhibitors have proven ineffective. According to our results, an alternative strategy based on blocking MT1-MMP delivery to invasive structures by means of Rab8 targeting will be a more rational means of preventing invasion and metastasis mediated by MT1-MMP, without affecting the enzyme basal homeostasis. Important future work involving animal models should first be undertaken to validate Rab8 as a therapeutic cancer target.
Materials and methods
Cell culture, transfection and collagen inclusion
Breast adenocarcinoma MDA-MB-231 cells were maintained in DMEM supplemented with 10% FBS. Primary carcinoma cells were purified from fresh human endometrial and lung carcinoma tumor samples by enzymatic digestion as described elsewhere (Allinen et al, 2004) and cultured in HAMF10 medium supplemented with 10% FBS. Cell transfection was performed using lipofectamine 2000 (Invitrogen, Carlsbag, CA, USA) according to the manufacturer's instructions. At 24 h post-transfection, cells were trypsinized and mixed with readily prepared Col I solution (2,4 mg/ml bovine Col I (Vitrogen, Palo Alto, CA, USA), 1 × RPMI, 19 mM HEPES (Gibco), 0.19% sodium bicarbonate (Sigma) and 5% FBS) that was then allowed to polymerize for 2 h at 37°C (3D-Col I) or plated on rat tail Col I protein (Roche Diagnostics, Panzberg, Germany)-coated surfaces (2D-Col I layers).
Constructs and antibodies
EGFP-tagged MT1-MMP, Rab8wt, Rab8Q67L and Rab8ΔC constructs have been described previously (Galvez et al, 2002; Ang et al, 2003). MT1-MMP-mRFP was obtained by subcloning mRFP into EGFP restriction sites. Ts045 VSV-G-YFP and Rab11-GFP were kindly provided by Dr R Peppercok and Dr D Sheff, respectively. Abs used include LEM-2/15 anti-MT1-MMP (Galvez et al, 2002) and TS2/16 and Lia1/2 anti-human β1 integrin, kindly provided by Dr Sánchez-Madrid. Mouse mAb anti-TfRc and rabbit polyclonal Ab to Rab11 were from Zymed Laboratories (South San Francisco, CA, USA). Goat polyclonal anti-Rab8 Ab was from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and mAb anti-Col I and anti-tubulin DM1a Ab were from Sigma (St Louis, MO, USA). Anti-human epithelial antigen BerEP4 was from DakoCytomation (Glostrup, Denmark).
Immunofluorescence, Tf/LDL uptake and confocal microscopy
Cells were either plated onto coverslips or embedded into 3D-Col I, fixed at 4°C for 5 min with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100 and stained with the appropriate Abs. Tf/LDL uptake was monitored by incubating 1 h serum-starved cells with Tf-Alexa 647 (20 μg/ml) and dil-LDL (low-density lipoprotein conjugated to 3,3′-dioctadecylindocarbocyanine) (10 μg/ml) for 1 h at 37°C. Cell imaging was performed using a Leica TSC SP2 AOBS and SP5-RS AOBS with a 63 × Plan Apo 1.32 NA oil-immersion objective (Leica, Mannheim, Germany). Leica Confocal Software (LCS) was used for acquisition of images, which were later adjusted for contrast using Adobe Photoshop Software. Colocalization analysis was performed with Imaris software (Bitplane AG, Zurich, Switzerland).
Polystyrene bead assays
Polystyrene divinyl-benzene beads (5 μm) (Duke Scientific Corporation, Palo Alto, CA, USA) were incubated with 0.5% BSA, 100 μg/ml Col I (Vitrogen Palo Alto, CA, USA), 20 μg/ml Fn, 1 mg/ml HA (Sigma, St Louis, MO, USA) or TS2/16 Ab anti-β1 integrin culture supernatant. Cells expressing the different constructs were incubated for 1 h with coated beads at a cell to bead ratio of 1:40. For inhibition studies, cells were previously incubated with or without 10 μg/ml of blocking anti-β1 integrin Ab Lia1/2 or control BerEP4 Ab (anti-human epithelial antigen). Confocal images were analyzed for MT1-MMP fluorescence in a region around the bead and normalized to the overall background MT1-MMP fluorescence determined in three regions at irrelevant membrane areas of the cell. Relative bead fluorescence represents quantified bead fluorescencebackground fluorescence × 100 scored in at least 10 beads for each experimental condition.
Confocal photobleaching experiments
A combination of both FRAP and FLIP techniques was developed on a Leica TSC SP2 AOBS microscope using Leica Confocal Software (Leica, Mannheim, Germany). Live MT1-MMP-GFP expressing cells embedded into 3D-Col I gels were exposed to a bleaching regime consisting of (1) prebleach recording (scanning three images with laser AOTF 20%), (2) bleaching and scanning at two different regions of interest (membrane and submembranous compartments) using bleaching laser excitation settings (100% AOTF) in both regions and regular imaging scanning settings (20% AOTF) for the rest of the field and (3) post-bleaching recording. During the post-bleaching phase, the PM (FRAP region) was excited with regular imaging settings (20% AOTF,) whereas continuous bleaching settings (100% AOTF) were used at the FLIP region. The relative loss of intensity and recovery of fluorescence was calculated at the FRAP region after background subtraction using Siggia normalization (Siggia et al, 2000).
Collagen degradation and cell invasion assays
MDA-MB231 cells were transfected with the different constructs. At 24 h after transfection, cells were plated onto 2D-Col I layers and incubated for additional 48 h, fixed and immunostained for Col I. MDA-MB-231 cell invasion assays were performed in 8-μm pore 3D-Col I gel-coated transwell chambers (Costar). Cells were transfected with the different constructs and after 24 h, resuspended in serum-free medium and seeded at 5 × 104 cells/well. Cells were allowed to transmigrate to 10% FBS media for 48 h and then counted at the top and bottom of the chamber using Image J software (NIH, Bethesda, USA). Bars represent the percentage of invasive cells referred to the total number of cells considering only GFP or mRFP/GFP expressing cells.
Rab8 gene silencing with shRNAs
Three different siRNA sequences were designed for silencing Rab8a and Rab11a with the help of web-based algorithms (http://side.bioinfo.ochoa.fib.es/) and (www.Invitrogen.com) (Rab8 (1): 5′-GAGAATTAAACTGCAGATA, Rab8 (2): 5′-GGAACTGGATTCGCAACATTG-3′ and Rab8 (3): 5′-GCTCGATGGCAAGAGAATTAA-3′), (Rab11 (1): 5′-AAGAGCACCATTGGAGTAGAGTT-3′, Rab11 (2): 5′-GTACGACTACCTCTTTAAA-3′ and Rab11 (3): 5′-GCAACAATGTGGTTCCTATTC-3′). shRNAs were cloned into the retroviral vector MSCV Pig, a modified version of MSCV-puro (Clontech), which contains GFP to report shRNA expression. HEK-293T cells were cotransfected with 10 μg of the plasmid containing the different shRNA and 10 μg of the amphotropic vector pCL-Ampho, retrovirus packaging vector. After 48 h, transfection retrovital supernatants were used as retroviral stock for transduction of MDA-MB 231 cells. Cells expressing the different shRNA constructs were selected with puromycin (0.5 μg/ml) for 5 days and GFP-expressing cells were sorted by flow cytometry to obtain stable shRNA-expressing cell lines. Only shRNA Rab8 (1), shRNA Rab8 (2), shRNA Rab11 (1) and shRNA Rab11 (2) showed significant depletion of Rab8 and Rab11 and were used for subsequent analysis. For shRNA rescue assays, four silent mutations were introduced to the shRNA Rab8 1 targeting sequence (nucleotides 165–183). The final mutated Rab8 sequence (aggattaagttgcaaata) was obtained by PCR and subcloned into mRFP vector. Rab8mut-mRFP was transiently transfected into shRNA Rab8 (1) -expressing stable cell line. Rab8 and Rab11 protein levels were analyzed by Western blotting. Alexa Fluor 680-conjugated secondary Abs were used to visualize and quantify the blots using the Odyssey Infrared Imaging System (Li-COr, Biosciences).
Statistical analysis
All numerical values reported represent mean±s.e. The statistical significance comparing differences between the experimental and control (GFP/BSA) values (*) and Ab-treated compared with isotype control values (#) was evaluated using Student's _t_-test. P<0.05 was taken as the limits of statistical significance (*/#P<0.05; **/##P<0.01; ***/###P<0.001).
Supplementary Material
Supplementary Figure 3
Supplementary Figure 7
Supplementary Figure 9
Supplementary Figure 10
Supplementary Figure 12
Supplementary Figure 13
Supplementary Figure 14
Supplementary Movie 1
Supplementary Movie 2
Supplementary Movie 4
Supplementary Movie 5
Supplementary Movie 6
Supplementary Movie 8
Supplementary Movie 11
Acknowledgments
We thank Drs MA del Pozo and MA Alonso for helpful advice and critical reading of the manuscript, Dr Rivera for help with biochemical studies, Dr M Malumbres for help with shRNA design and the Genomics Unit for help with shRNA cloning. Drs Mellman, Sánchez-Madrid, Pepperkok, Sheff and Tsien are acknowledged for providing us with reagents. Tumour cell samples were provided by the CNIO Tumour Bank Unit. This work was supported by a grant from Fondo de Investigaciones Sanitarias (FIS PI031324) to MCM. JJ B-C and R M-D are funded by the Ministry of Science and Technology of Spain (MCYT) and Fondo de Investigaciones Sanitarias (FIS), respectively.
References
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K (2004) Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 6: 17–32 [DOI] [PubMed] [Google Scholar]
- Ang AL, Folsch H, Koivisto UM, Pypaert M, Mellman I (2003) The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin–Darby canine kidney cells. J Cell Biol 163: 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I (2004) Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol 167: 531–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS, Aguado-Velasco C (1998) Membrane traffic during cell locomotion. Curr Opin Cell Biol 10: 537–541 [DOI] [PubMed] [Google Scholar]
- Chieregatti E, Meldolesi J (2005) Regulated exocytosis: new organelles for non-secretory purposes. Nat Rev Mol Cell Biol 6: 181–187 [DOI] [PubMed] [Google Scholar]
- Dugani CB, Klip A (2005) Glucose transporter 4: cycling, compartments and controversies. EMBO Rep 6: 1137–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2: 161–174 [DOI] [PubMed] [Google Scholar]
- Ellerbroek SM, Wu YI, Overall CM, Stack MS (2001) Functional interplay between type I collagen and cell surface matrix metalloproteinase activity. J Biol Chem 276: 24833–24842 [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3: 362–374 [DOI] [PubMed] [Google Scholar]
- Futter CE, Connolly CN, Cutler DF, Hopkins CR (1995) Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem 270: 10999–11003 [DOI] [PubMed] [Google Scholar]
- Galvez BG, Genis L, Matias-Roman S, Oblander SA, Tryggvason K, Apte SS, Arroyo AG (2005) Membrane type 1-matrix metalloproteinase is regulated by chemokines monocyte-chemoattractant protein-1/ccl2 and interleukin-8/CXCL8 in endothelial cells during angiogenesis. J Biol Chem 280: 1292–1298 [DOI] [PubMed] [Google Scholar]
- Galvez BG, Matias-Roman S, Yanez-Mo M, Sanchez-Madrid F, Arroyo AG (2002) ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol 159: 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattula K, Furuhjelm J, Arffman A, Peranen J (2002) A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell 13: 3268–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LA, Pimplikar S, Parton RG, Virta H, Zerial M, Simons K (1993) Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol 123: 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Seiki M (2006) MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol 206: 1–8 [DOI] [PubMed] [Google Scholar]
- Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, Aoki T, Seiki M (2001) Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J 20: 4782–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Lehti K, Wang X, Weiss SJ, Keski-Oja J, Pei D (2001) Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc Natl Acad Sci USA 98: 13693–13698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Maxfield FR (1995) Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature 377: 75–79 [DOI] [PubMed] [Google Scholar]
- Lehti K, Lohi J, Juntunen MM, Pei D, Keski-Oja J (2002) Oligomerization through hemopexin and cytoplasmic domains regulates the activity and turnover of membrane-type 1 matrix metalloproteinase. J Biol Chem 277: 8440–8448 [DOI] [PubMed] [Google Scholar]
- Lehti K, Valtanen H, Wickstrom SA, Lohi J, Keski-Oja J (2000) Regulation of membrane-type-1 matrix metalloproteinase activity by its cytoplasmic domain. J Biol Chem 275: 15006–15013 [DOI] [PubMed] [Google Scholar]
- Leitinger B, Hille-Rehfeld A, Spiess M (1995) Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc Natl Acad Sci USA 92: 10109–10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Roberts TH, Hirschberg K (2000) Secretory protein trafficking and organelle dynamics in living cells. Annu Rev Cell Dev Biol 16: 557–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, Stow JL (2005) Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell 16: 1744–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Maaser K, Daryab N, Zanker KS, Brocker EB, Friedl P (2004) Release of cell fragments by invading melanoma cells. Eur J Cell Biol 83: 709–715 [DOI] [PubMed] [Google Scholar]
- Mazzone M, Baldassarre M, Beznoussenko G, Giacchetti G, Cao J, Zucker S, Luini A, Buccione R (2004) Intracellular processing and activation of membrane type 1 matrix metalloprotease depends on its partitioning into lipid domains. J Cell Sci 117: 6275–6287 [DOI] [PubMed] [Google Scholar]
- Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, Lane WS, Lienhard GE (2005) AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J 391: 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Ohnishi H, Suzuki J, Yoshikumi Y, Ohno H, Mashima H, Yasuda H, Ishijima T, Osawa H, Satoh K, Sunada K, Kita H, Yamamoto H, Sugano K (2004) Involvement of syntaxin 4 in the transport of membrane-type 1 matrix metalloproteinase to the plasma membrane in human gastric epithelial cells. Biochem Biophys Res Commun 323: 118–124 [DOI] [PubMed] [Google Scholar]
- Mori H, Tomari T, Koshikawa N, Kajita M, Itoh Y, Sato H, Tojo H, Yana I, Seiki M (2002) CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J 21: 3949–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada M, Yamada A, Takino T, Miyamori H, Takahashi T, Yamashita J, Sato H (2001) Suppression of membrane-type 1 matrix metalloproteinase (MMP)-mediated MMP-2 activation and tumor invasion by testican 3 and its splicing variant gene product, N-Tes. Cancer Res 61: 8896–8902 [PubMed] [Google Scholar]
- Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, Chen WT (1997) Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci USA 94: 7959–7964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo ER, Sanders PG, Padua RA, Hughes D, Williamson R, Johnson KJ (1991) The MEL gene: a new member of the RAB/YPT class of RAS-related genes. Oncogene 6: 1347–1351 [PubMed] [Google Scholar]
- Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M (2001) The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 107: 789–800 [DOI] [PubMed] [Google Scholar]
- Osenkowski P, Toth M, Fridman R (2004) Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP). J Cell Physiol 200: 2–10 [DOI] [PubMed] [Google Scholar]
- Peranen J, Auvinen P, Virta H, Wepf R, Simons K (1996) Rab8 promotes polarized membrane transport through reorganization of actin and microtubules in fibroblasts. J Cell Biol 135: 153–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle A, Murphy G, Roghi C (2003) Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J Cell Sci 116: 3905–3916 [DOI] [PubMed] [Google Scholar]
- Royle SJ, Murrell-Lagnado RD (2003) Constitutive cycling: a general mechanism to regulate cell surface proteins. Bioessays 25: 39–46 [DOI] [PubMed] [Google Scholar]
- Rozanov DV, Deryugina EI, Ratnikov BI, Monosov EZ, Marchenko GN, Quigley JP, Strongin AY (2001) Mutation analysis of membrane type-1 matrix metalloproteinase (MT1-MMP). The role of the cytoplasmic tail Cys(574), the active site Glu(240), and furin cleavage motifs in oligomerization, processing, and self-proteolysis of MT1-MMP expressed in breast carcinoma cells. J Biol Chem 276: 25705–25714 [DOI] [PubMed] [Google Scholar]
- Sato H, Takino T, Miyamori H (2005) Roles of membrane-type matrix metalloproteinase-1 in tumor invasion and metastasis. Cancer Sci 96: 212–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M (1994) A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370: 61–65 [DOI] [PubMed] [Google Scholar]
- Sesaki H, Ogihara S (1997) Protrusion of cell surface coupled with single exocytotic events of secretion of the slime in Physarum plasmodia. J Cell Sci 110: 809–818 [DOI] [PubMed] [Google Scholar]
- Siggia ED, Lippincott-Schwartz J, Bekiranov S (2000) Diffusion in inhomogeneous media: theory and simulations applied to whole cell photobleach recovery. Biophys J 79: 1761–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton H, Gavrilovic J, Atkinson SJ, d'Ortho MP, Yamada KM, Zardi L, Murphy G (1998) The activation of ProMMP-2 (gelatinase A) by HT1080 fibrosarcoma cells is promoted by culture on a fibronectin substrate and is concomitant with an increase in processing of MT1-MMP (MMP-14) to a 45 kDa form. J Cell Sci 111: 2789–2798 [DOI] [PubMed] [Google Scholar]
- Suenaga N, Mori H, Itoh Y, Seiki M (2005) CD44 binding through the hemopexin-like domain is critical for its shedding by membrane-type 1 matrix metalloproteinase. Oncogene 24: 859–868 [DOI] [PubMed] [Google Scholar]
- Tam EM, Wu YI, Butler GS, Stack MS, Overall CM (2002) Collagen binding properties of the membrane type-1 matrix metalloproteinase (MT1-MMP) hemopexin C domain. The ectodomain of the 44-kDa autocatalytic product of MT1-MMP inhibits cell invasion by disrupting native type I collagen cleavage. J Biol Chem 277: 39005–39014 [DOI] [PubMed] [Google Scholar]
- Taraboletti G, D'Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V (2002) Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol 160: 673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M, Hernandez-Barrantes S, Osenkowski P, Bernardo MM, Gervasi DC, Shimura Y, Meroueh O, Kotra LP, Galvez BG, Arroyo AG, Mobashery S, Fridman R (2002) Complex pattern of membrane type 1 matrix metalloproteinase shedding. Regulation by autocatalytic cells surface inactivation of active enzyme. J Biol Chem 277: 26340–26350 [DOI] [PubMed] [Google Scholar]
- Uekita T, Itoh Y, Yana I, Ohno H, Seiki M (2001) Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J Cell Biol 155: 1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ma D, Keski-Oja J, Pei D (2004) Co-recycling of MT1-MMP and MT3-MMP through the trans-Golgi network. Identification of DKV582 as a recycling signal. J Biol Chem 279: 9331–9336 [DOI] [PubMed] [Google Scholar]
- Widberg CH, Bryant NJ, Girotti M, Rea S, James DE (2003) Tomosyn interacts with the t-SNAREs syntaxin4 and SNAP23 and plays a role in insulin-stimulated GLUT4 translocation. J Biol Chem 278: 35093–35101 [DOI] [PubMed] [Google Scholar]
- Will H, Atkinson SJ, Butler GS, Smith B, Murphy G (1996) The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem 271: 17119–17123 [DOI] [PubMed] [Google Scholar]
- Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P (2003) Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102: 3262–3269 [DOI] [PubMed] [Google Scholar]
- Wu YI, Munshi HG, Sen R, Snipas SJ, Salvesen GS, Fridman R, Stack MS (2004) Glycosylation broadens the substrate profile of membrane type 1 matrix metalloproteinase. J Biol Chem 279: 8278–8289 [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117 [DOI] [PubMed] [Google Scholar]
- Zucker S, Hymowitz M, Conner CE, DiYanni EA, Cao J (2002) Rapid trafficking of membrane type 1-matrix metalloproteinase to the cell surface regulates progelatinase a activation. Lab Invest 82: 1673–1684 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 3
Supplementary Figure 7
Supplementary Figure 9
Supplementary Figure 10
Supplementary Figure 12
Supplementary Figure 13
Supplementary Figure 14
Supplementary Movie 1
Supplementary Movie 2
Supplementary Movie 4
Supplementary Movie 5
Supplementary Movie 6
Supplementary Movie 8
Supplementary Movie 11