Traction Forces of Fibroblasts are Regulated by the Rho-Dependent Kinase but not by the Myosin Light Chain Kinase (original) (raw)
. Author manuscript; available in PMC: 2007 Jun 15.
Published in final edited form as: Arch Biochem Biophys. 2006 Oct 11;456(2):224–231. doi: 10.1016/j.abb.2006.09.025
Abstract
Adhesive cells show complex mechanical interactions with the substrate, however the exact mechanism of such interactions, termed traction forces, is still unclear. To address this question we have measured traction forces of fibroblasts treated with agents that affect the myosin II-dependent contractile mechanism. Using the potent myosin II inhibitor blebbistatin, we demonstrate that traction forces are strongly dependent on a functional myosin II heavy chain. Since myosin II is regulated by both the myosin light chain kinase (MLCK) and, directly or indirectly, the Rho-associated kinase (ROCK), we examined the effects of inhibitors against these kinases. Interestingly, inhibition of the myosin light chain kinase had no detectable effect, while inhibition of the Rho-dependent kinase caused strong inhibition of traction forces. Our results indicate that ROCK and MLCK play non-redundant roles in regulating myosin II functions, and that a subset of myosin II, regulated by the Rho small GTPase, may be responsible for the regulation of traction forces in migrating fibroblasts.
Introduction
Cultured cells are known to generate contractile forces, which may play a role in various events of cell migration including forward propulsion, tail retraction, and deadhesion [1]. Contractile forces may also be involved in maintaining the cell shape and in mediating extracellular and intracellular physical communications. At least a part of these contractile forces, referred to as traction forces, are transmitted to the substrate and detectable as wrinkling of silicon sheets in earlier studies [2, 3, 4]. Recent development of traction force microscopy allows quantitative measurements of traction forces through the deformation of flexible polyacrylamide substrates embedded with fluorescent particles [5, 6].
Earlier experiments with poorly defined inhibitors such as BDM have implicated myosin II in the generation of traction forces [7]. The involvement of myosin II also appeared to be supported by morphological/behavior responses of cells to the potent non-muscle myosin II inhibitor blebbistatin [8], including the inhibition of fibroblasts to remodel collagen fibers [9], invade the matrices [10] and contract floating matrices [11]. However these effects could also be associated with the disruption of cell shape and directional migration, in addition or instead of effects on traction forces.
Equally important is the mechanism for the regulation of myosin II, which is known to involve phosphorylation of the regulatory light chain (MRLC) and possibly the heavy chain [12, 13, 14]. In vitro phosphorylation of MRLC at Thr18 and Ser19 stimulates the actin-activated ATPase of myosin II and filament assembly [15]. However, while manipulating the phosphorylation state of MRLC by overexpression of Thr18/Ser19 mutants has some effects on cell migration [16, 17, 18], other studies with pharmacological agents suggest that phosphorylation of MRLC is not necessary for migration [19]. The analysis is complicated by the involvement of multiple Ca2+ dependent and Ca2+ independent pathways in regulating MRLC phosphorylation at Thr18/Ser19; the former is mediated by the myosin light chain kinase (MLCK) downstream of Ca2+-calmodulin, while the latter may involve the Rho-dependent kinase (ROCK), which may act directly on MRLC or through the myosin light chain phosphatase [20]. There are indications that these pathways may regulate distinct cellular functions. For example, MLCK has been implicated in the formation of actin bundles along the cell periphery while ROCK is required for maintaining stress fibers in the central region of the cell [21, 22].
In this study we have directly addressed the role of myosin contractility in the production of traction forces in migrating fibroblasts, by applying traction force microscopy to cells treated with various pharmaceutical agents that affect either myosin II directly or regulatory pathways for MRLC phosphorylation. We show that myosin II and ROCK are required for the production of traction forces, while MLCK surprisingly is not essential in this regard.
Materials and Methods
Cell Culture, Treatments, and Immunoblotting
NIH-3T3 mouse embryonic fibroblasts were purchased from ATTC. Cells were maintained in DMEM supplemented with 10% donor calf serum (Hyclone), 50 U/ml penicillin, 50 μg/ml streptomycin and 2 mM L-glutamine (GIBCO, Grand Island, NY). Pharmaceutical reagents purchased from commercial sources include ML-7 (an MLCK inhibitor [23]; Calbiochem, San Diego, CA), blebbistatin (a non-muscle myosin II inhibitor [24]; Toronto Research, Toronto, Canada), Y-27632 (a ROCK inhibitor [25]; Mitsubishi Pharma, Osaka, Japan), and wortmannin (an inhibitor of both MLCK and phosphatidylinositol 3-kinase [26]; MP Biochemicals, Irvine, CA). These reagents were stored as stock solutions in DMSO at −20°C (50 mM for ML-7, 100 mM for blebbistatin, 20 mM for Y-27632 and 1 mM for wortmannin). BATI peptide, a cell-permeable peptide inhibitor of MLCK, was synthesized according to Wu et al. [27] by Peptide Institute Inc., Osaka, Japan, and stored as a 20 mM stock solution in distilled deionized water at −20°C. All the reagents were diluted from the stock solution 1:1000 into the medium immediately before use. Immunoblotting samples were prepared by homogenizing treated or untreated NIH3T3 cells in a SDS sample buffer. SDS gel electrophoresis and blotting were performed following standard procedures and the blots were probed with anti-monophosphorylated MRLC antibodies (Cell Signaling Technology, Danvers, MA) at a dilution of 1:100 or anti-chicken gizzard actin (Chemicon, Temecula, CA) at a dilution of 1:1000, followed by alkaline phosphatase conjugated secondary antibodies (Promega, Madison, WI) at a dilution of 1:5000. The blots were then developed with Nitro Blue tetrazolium and BCIP (Nacalai Tesque, Japan).
Immunofluorescence
NIH3T3 cells were treated with reagents or vehicle and fixed in 4% formaldehyde in phosphate buffered saline (PBS) for 10 min followed by permeabilization in 0.2% Triton X-100 in PBS for 2 min. They were blocked with 1% BSA/PBS for 1 min at room temperature then incubated for 1 hour at 37°C with 1:500 dilution of Alexa-488 phalloidin (Invitrogen, Eugene, OR) and anti-vinculin monoclonal antibodies (clone VIN11-5; Sigma, St. Louis, MO), followed by anti-mouse Alexa-546 secondary antibodies (Invitrogen). Cells were imaged on a Ziess Axiovert S100TV microscope illuminated with a 100W mercury arc lamp. Images were collected with a cooled charge-coupled device camera equipped with a back-illuminated frame-transfer chip (EEV Type57; Roper Scientific, Trenton, NJ) and processed for background subtraction with the use of custom programs.
Preparation of Polyacrylamide Substrates
Substrates composed of 5% acrylamine/0.08% bis-acrylamide were prepared as previously described [28, 29] using 40% w/v acrylamide (BioRad, Hercules, CA) and 2% w/v N,N-methylene-bis-acrylamide (BioRad), and 1:100 dilution of fluorescent latex beads (0.2-μm Fluospheres; Invitrogen). Type I collagen was covalently attached to the surface of the polacrylamide as previously described [29]. Young’s modulus of the substrate was determined as previously described based on the Hertz theory [30]. This method yielded a modulus of 2.4x104 N/m2.
Traction Force Microscopy and interference reflection microscopy (IRM)
Traction force microscopy has been described previously in detail [5]. NIH3T3 fibroblasts were plated on the substrates overnight before data collection. Cells and beads were imaged with a 40x N.A. 0.75 Plan Neofluar phase objective on a Ziess Axiovert S100TV microscope equipped with a custom stage incubator and a 100W quartz halogen lamp. Following time-lapse collection of the cell and bead images, a microneedle was used to remove the cell to obtain an image of the substrate without traction forces. A pattern recognition algorithm was used to determine the deformation of the substrate caused by traction forces relative to an unstressed substrate, based on changes in bead distribution. A Bayesian maximum likelihood method was then applied to determine traction stress [31]. IRM was performed by placing a half-reflecting mirror in the epi-illumination path and closing down the epi-illumination aperture diaphragm, using a Zeiss Axiovert-200 microscope equipped with a 100x N.A. 1.30 NeoFluar phase contrast objective lens.
Results
Myosin II Plays a Crucial Role in Generating Traction Forces
To assess the contribution of non-muscle myosin II motor activities to the production of traction forces, we applied traction force microscopy to NIH 3T3 fibroblasts treated with the inhibitor blebbistatin. Treatment with 10 μM blebbistatin for 30 minutes caused strong inhibition of traction forces from an average of 0.084±0.04 dynes/cm2 to the noise level of 0.0065±0.002 dynes/cm2 (Figure 1). These results support the notion that myosin II produces most if not all of traction forces. However, consistent with previous studies [32, 33], cells treated with blebbistatin maintained the ability to migrate for at least two hours, while the inhibition of traction forces took place within minutes. These results suggest that traction forces as detected by traction force microscopy are not directly required for migration, but may play a subtler role such as maintaining the persistence or guidance [34].
Figure 1. Inhibition of traction force production by a myosin II inhibitor.
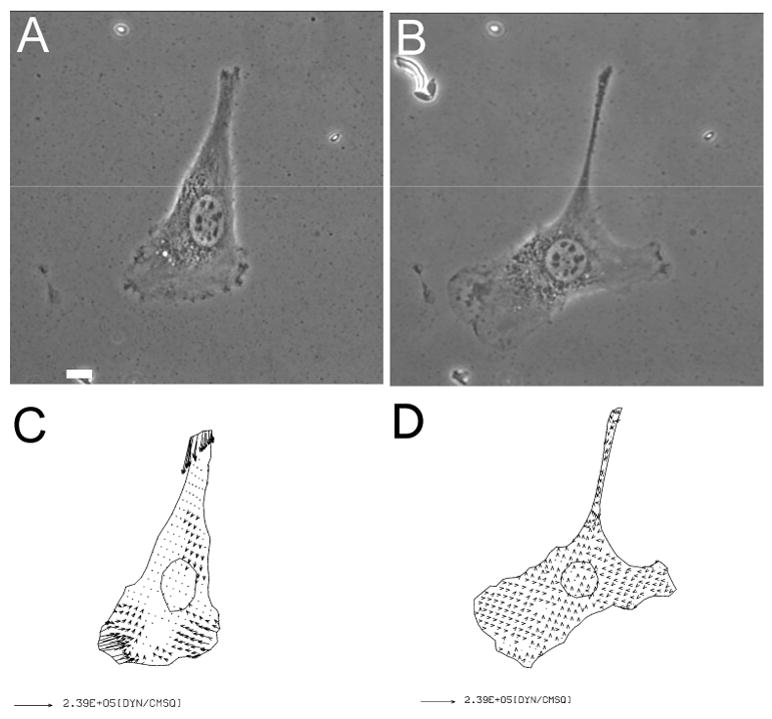
Phase images of an NIH3T3 fibroblast on collagen-coated polyacrylamide substrate are shown before (A), and after (B), treatment with 10 μM blebbistatin for 30 minutes. Vector plots show the corresponding traction stress before (C), and after (D), the treatment. Note the presence of lamellipodium despite the strong inhibition of traction forces. Bar, 10 μm.
Production of Traction Forces Is Independent of MLCK Activities
To determine how myosin II-dependent traction forces are regulated, we treated NIH 3T3 cells with a number of inhibitors against MLCK. Surprisingly, treatment for 30–60 minutes with conventional chemical inhibitors of MLCK, including ML-7 at 50 μM and wortmannin 1 μM, caused no significant inhibition of traction forces despite the partial retraction of some cells. Since these agents are known to inhibit other enzymes such as the PI-3 kinase and may induce complicated compensation, we applied a newly developed peptide, referred to as BATI, which specifically inhibits MLCK by combining the auto-inhibitory domain with a cell-permeable sequence [35]. This peptide has previously been shown to block MRLC phosphorylation as for ML-7 [18]. Immunoblotting showed inhibition of MRLC phosphorylation by both BATI peptide and ML-7 (Figure 2). However, as for chemical inhibitors, treatment with 20 μM BATI peptide caused no detectable effect on either the magnitude or the spatial pattern of traction forces (Figure 3). These results suggest that phosphorylation of the MRLC by MLCK does not play a major role in the production of traction forces.
Figure 2. Decrease of MRLC phosphorylation after the treatment with inhibitors.
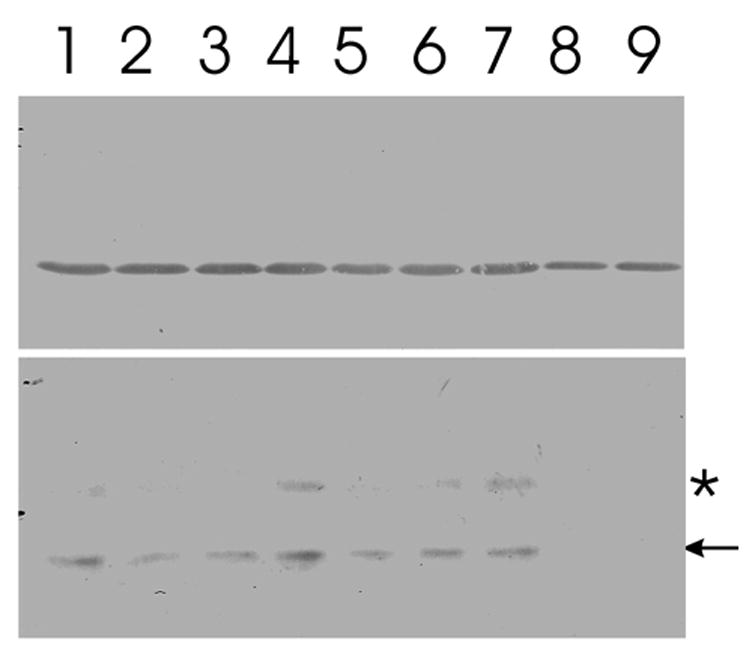
Lysates of NIH3T3 fibroblasts, treated with medium alone (lane 1), 10 μM Y-27632 for 30 (lane 2) or 60 (lane 3) minutes, 20 μM BATI inhibitory peptide for 30 (lane 4) or 60 (lane 5) minutes, 10 μM ML-7 for 30 (lane 6) or 60 (lane 7) minutes, 50 μM ML-7 for 30 (lane 8) or 60 (lane 9) minutes at 37°C are subjected to immnoblotting. Upper lanes are probed with anti-actin antibodies, as a loading control, lower lanes are probed with anti-monophospho-MRLC antibodies. Arrow in the lower panel indicates the position of MRLC. The anti-phospho-MLCK antibody also reacts with a band of slightly higher molecular weight (asterisk), which likely represents an MRLC isoform [44]. Y-27632 causes a rapid but partial inhibition of MRLC phosphorylation, which shows no apparent change beyond 30 minutes of incubation. In contrast, the effect of BATI does not become clear until 60 minutes in this assay. Treatment with 10 μM ML-7 causes little inhibition of MRLC phosphorylation even after prolonged incubation, while treatment with 50 μM ML-7 shows a rapid, strong inhibition.
Figure 3. Lack of effects of the inhibition of MLCK activity on traction forces.
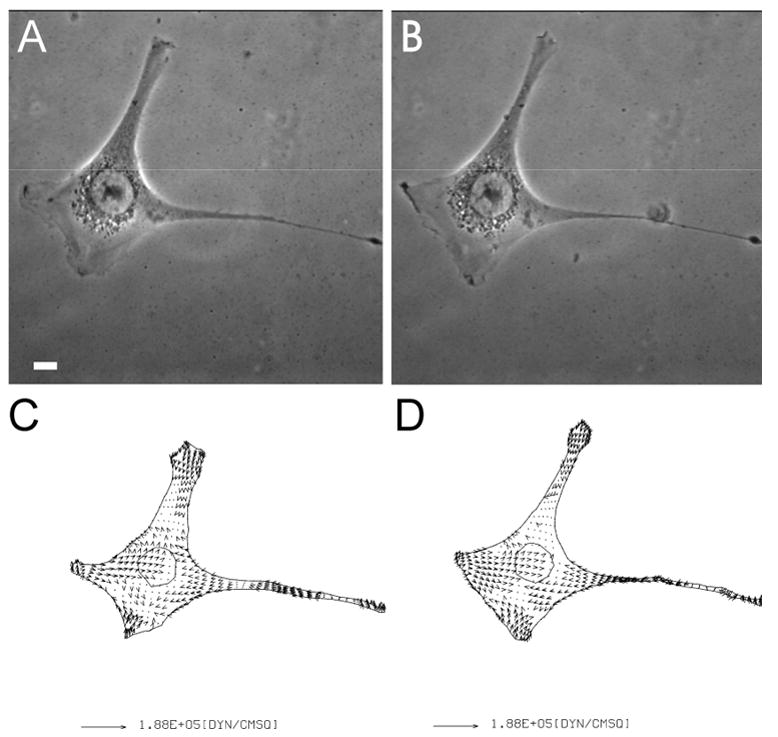
Phase images of an NIH3T3 fibroblast on collagen coated polyacrylamide substrate are shown before (A), and after (B), treatment with 20 μM BATI inhibitory peptide for 30 minutes. Vector plots show the corresponding traction stress before (C), and after (D), the treatment. Bar, 10 μm.
ROCK Activity Is Essential for the Production of Traction Forces
We turned to an alternative mechanism driven by the small GTPase Rho and ROCK, which promotes MRLC phosphorylation primarily through the inhibition of dephosphorylation by myosin phosphatase [35]. Treatment of NIH3T3 fibroblasts with 20 μM ROCK inhibitor Y-27632 resulted in partial inhibition of MRLC phosphorylation but striking inhibition of traction forces within minutes of application (Figures 2 and 4), while treatment with DMSO alone had no effect (Figure 4). Similar effects were observed upon the microinjection of the Rho inhibitor C3 (data not shown). As for cells in blebbistatin, cells maintained their ability to form lamellipodia and to migrate in the presence of Y-27632 despite the inhibition of traction forces [18] (Figure 4). These results suggest that traction forces of fibroblasts are regulated primarily by the Ca2+-independent Rho pathway, and that cell migration can take place without strong traction forces.
Figure 4. Inhibition of traction force production by a ROCK inhibitor.
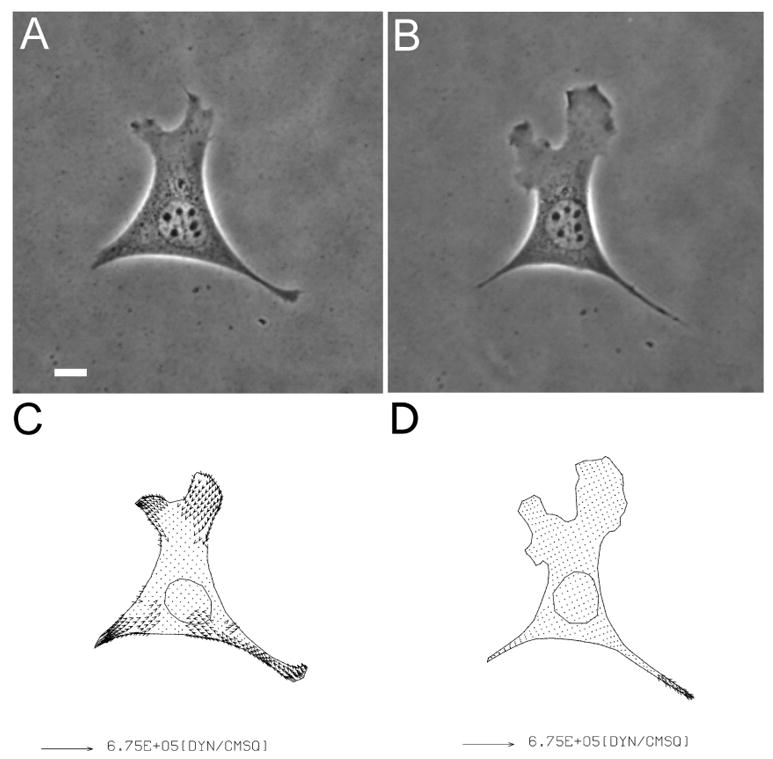
Phase images of an NIH3T3 fibroblast on collagen coated polyacrylamide substrate are shown before (A), and after (B), treatment with 20 μM Y-27632 for 30 minutes. Vector plots show the corresponding traction stress before (C), and after (D), the treatment. Note the presence of lamellipodium despite the strong inhibition of traction forces. Bar, 10 μm.
Differential Effects of MLCK and ROCK on Focal Adhesions
Since traction forces are likely generated and transmitted through stress fibers and focal adhesions, we performed immunofluoresence to examine these structures under conditions that did and did not affect traction forces. Treatment with MLCK inhibiting peptide BATI or ML-7 did not cause pronounced perturbations to stress fibers. Focal adhesions remained intact, although some of them appeared more punctate than in control cells (Figure 5). In contrast, treatment with blebbistatin or the ROCK inhibitor Y-27632 drastically changed the organization of both structures. Stress fibers were either absent or reduced to very thin fibrils in the central region of the cell (Figure 5). In addition, cells treated with blebbistatin showed a dramatic disappearance of vinculin structures, with some aggregates remaining near the central region, while cells in Y-27632 maintained a band of vinculin at the leading edge and small punctuate structures elsewhere after 30 minutes of treatment.
Figure 5. Disruption of the organization of actin and vinculin by agents that affect myosin II.
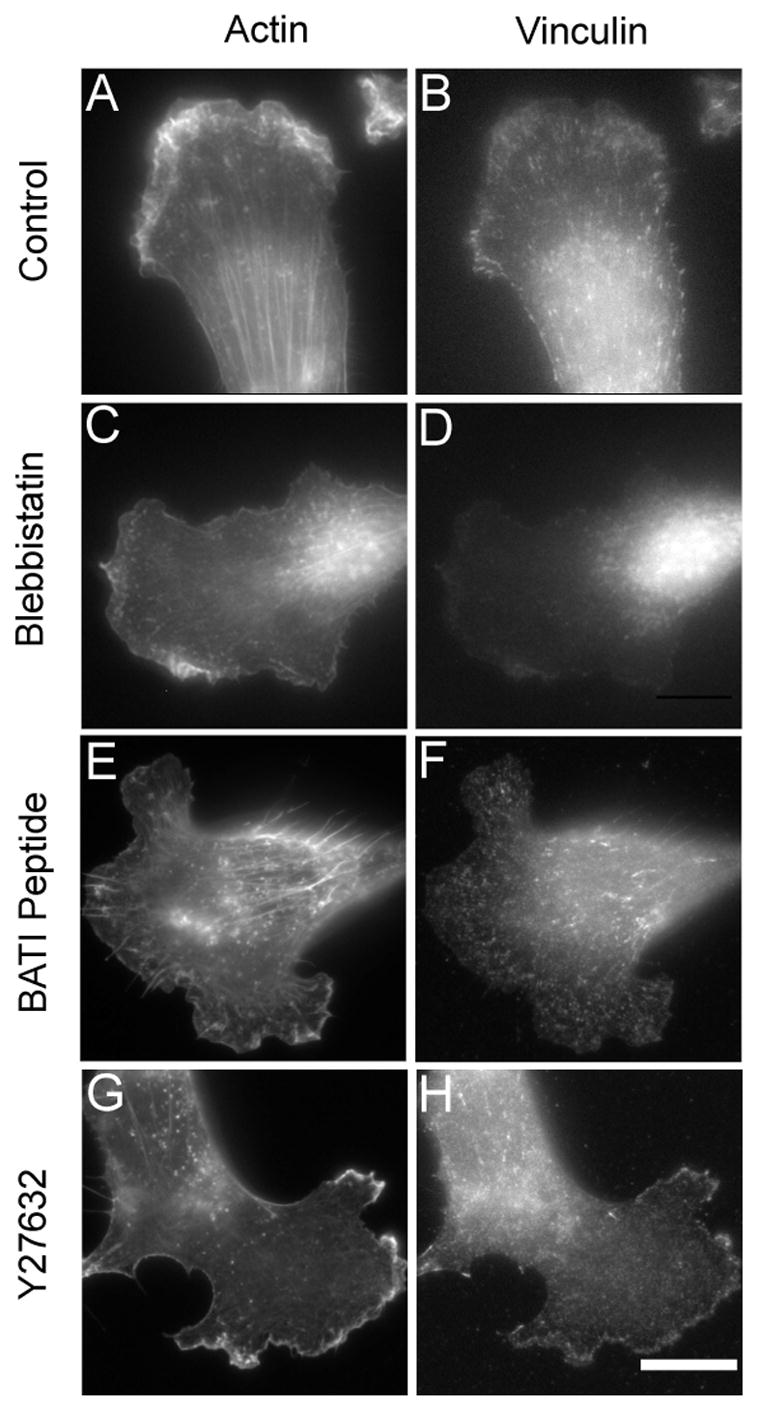
NIH 3T3 fibroblasts treated with 10 μM blebbistatin (C, D), 20 μM BATI peptide (E, F), 20 μM Y-27632 (G, H), or carrier solution alone (A, B), for 30 minutes are double stained with fluorescent phalloidin (A, C, E, G) and antibodies against vinculin (B, D, F, H). Both blebbistatin and Y-27632 cause disappearance of stress fibers and focal adhesions, although cells in Y-27632 show a concentrated band of vinculin along the cell periphery. BATI induces no apparent effect on stress fibers, and a subtle change in the appearance of focal adhesions. Bar, 20μm.
The dynamic effects of Y-27632 and BATI were further investigated with time-lapse interference reflection microscopy (IRM; Figure 6 and 7). In control cells, IRM showed continuous assembly of new focal adhesions at the leading edge, while existing focal adhesions often showed forward or backward movements along their long axis as described previously [36]. Treatment with BATI peptide caused the transient formation of large patches of “close contacts”, without affecting the formation of new focal adhesions (Figure 6). However existing focal adhesions appeared less elongated than in control cells consistent with immunofluorescence. In contrast, Y-27632 inhibited both the formation of new focal adhesions and movements of pre-existing focal adhesions, without causing an immediate disassembly of pre-existing, mature focal adhesions (Figure 7). The continuous forward migration of the cell eventually caused these residual focal adhesions to accumulate in the central region of the cell.
Figure 6. Effects of MLCK inhibition on cell-substrate adhesions.
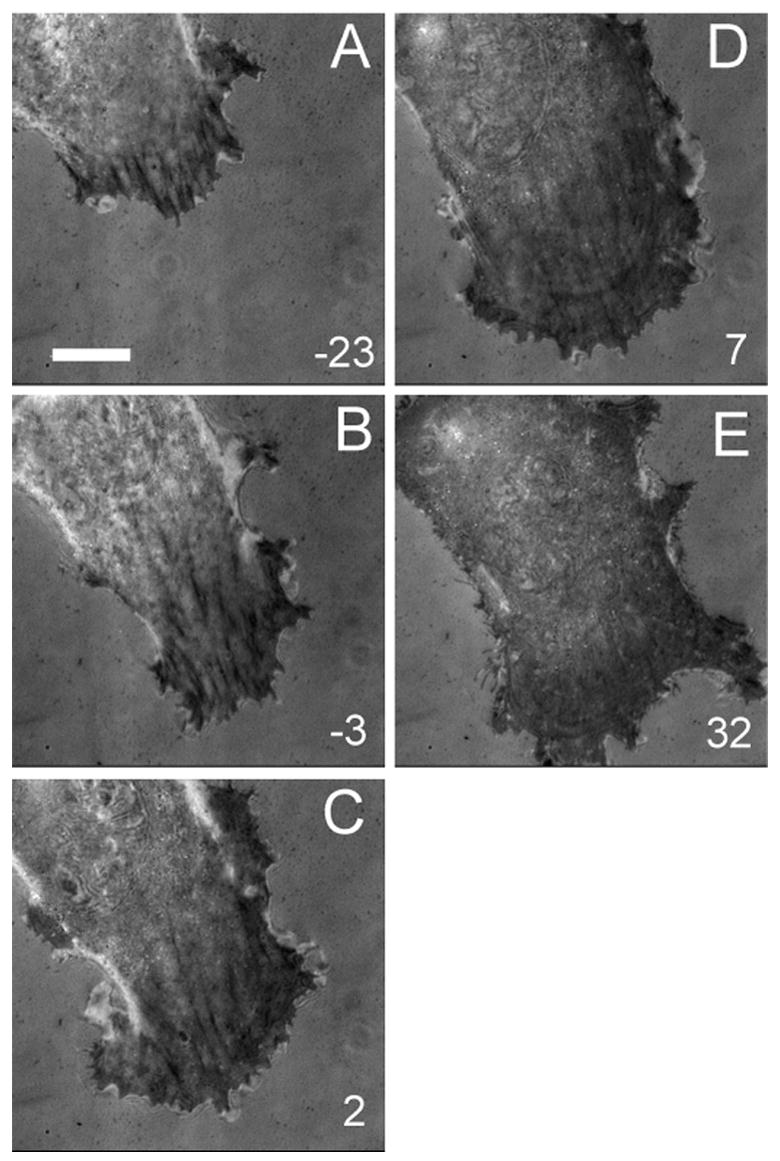
IRM images are shown before (A, B), and after (C, D, E), treatment with 20 μM BATI. Note the persistence of very dark adhesion plaques, the formation of dark patches of close contact near the leading edge, and the continuous forward migration of the cell. Numbers indicate minutes after treatment. Bar, 20 μm.
Figure 7. Effects of ROCK inhibition on cell-substrate adhesions.
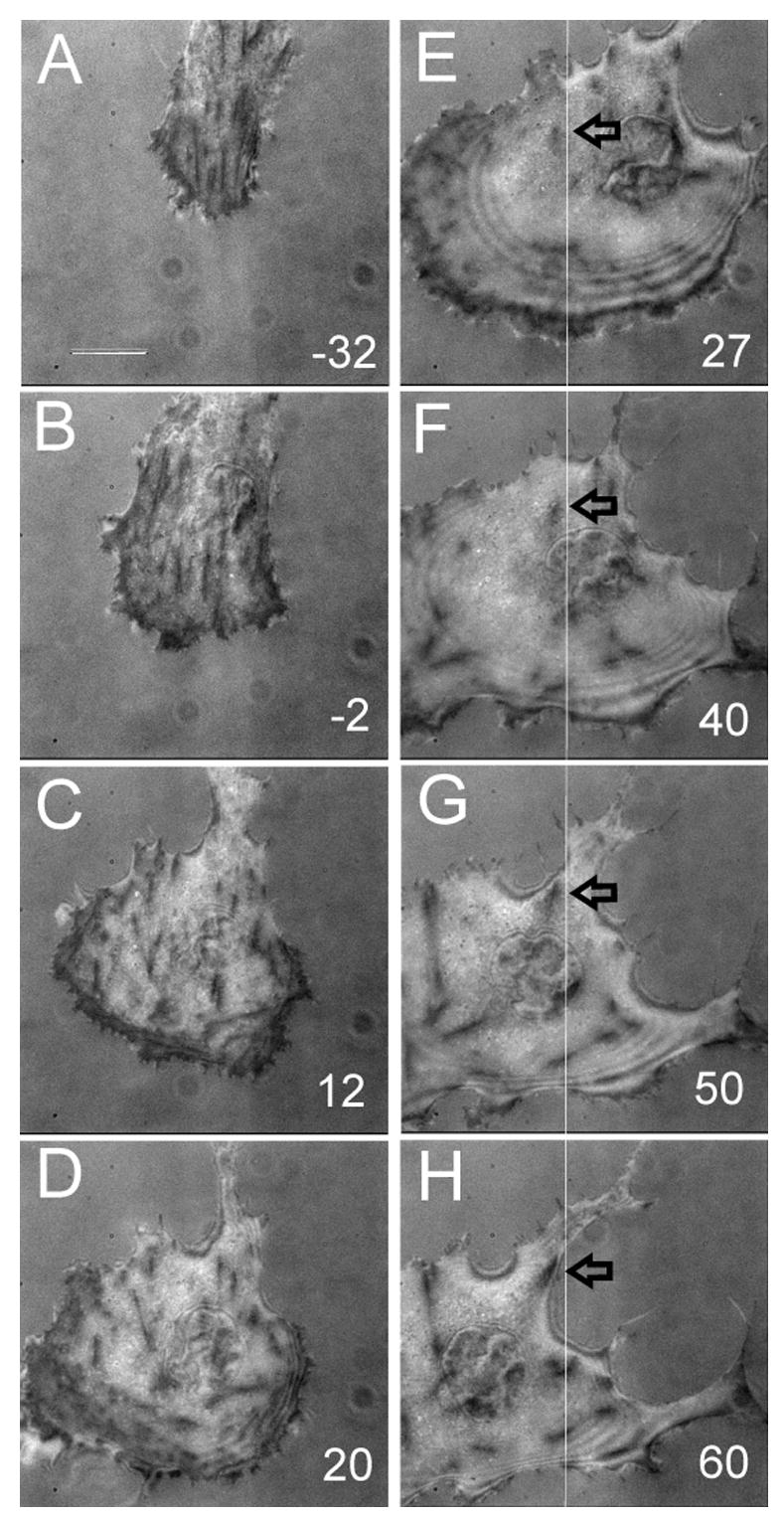
IRM images are shown before (A, B), and after (C-H), treatment with 20 μM Y-27632. Note the dramatic disappearance of most adhesion plaques near the leading edge upon prolonged treatment, while the cell expands and takes an abnormal shape. Numbers indicate minutes after treatment. The line in E-H serves as the reference for the visualization of shape change and the identification of residual adhesion plaques. Bar, 20 μm.
Discussion
Generation of strong traction forces represents a common function of adhesive cells including fibroblasts, epithelial cells, endothelial cells, and macrophages [37]. However, despite the advances in detection, the mechanism for the production and regulation of these forces has remained elusive. In this study we have examined the involvement of myosin II in force generation in cultured fibroblasts, using traction force microscopy in conjunction with a cache of small molecule and peptide inhibitors.
We have first confirmed myosin II as the primary contributor of traction forces. Previous studies with BDM and KT5926, which have ill-defined actions against myosin and possibly other proteins, have implicated myosin II in the generation of traction forces [38]. In addition, activation of protein kinase C, which phosphorylates myosin light chain at inhibitor sites, also inhibits traction forces [39]. Blebbistatin has been shown to be a potent inhibitor of non-muscle myosin II but not some muscle myosin II isoforms or unconventional myosins of class I, V and X [40]. It acts through binding to the large cleft of the motor domain and interfering with the opening and closing of the myosin during the contractile state [8, 40]. Since blebbistatin caused essentially complete removal of traction forces, the forces must be generated by a blebbistatin-sensitive motor, most likely non-muscle myosin II. In addition, the action likely involves both myosin IIA and IIB isoforms, which are enriched in different regions of the cell, as cells ablated of myosin IIB show only a partial inhibition of forces [34].
A number of regulatory mechanisms are known to affect myosin II activities. The best characterized involves phosphorylation of MRLC at Thr18 and Ser19, which induces filament assembly and activates the actin-activated Mg2+ATPase [15]. MRLC may be phosphorylated at these sites by both Ca2+-dependent and independent mechanisms [20]. The former is mediated by the MLCK and represents the primary mechanism of activation in smooth muscles. The latter involves a number of kinases including ROCK [41], DAPK [42], PAK [43], and the ZIP kinase [44]. In addition, ROCK is known to enhance MRLC phosphorylation by inhibiting a myosin light chain phosphatase [35]. Although the relationship among these mechanisms is largely unclear, previous studies have revealed differential sensitivities of actin-myosin structures to drugs inhibiting MLCK versus those inhibiting ROCK [18]. In cultured fibroblasts, inhibition of MLCK causes disassembly of peripheral structures, while inhibition of ROCK causes preferential dephosphorylation and disassembly of central stress fibers [18, 22]. These observations suggest that acto-myosin bundle structures in different regions of the cell may perform different functions and respond to different signals, a notion supported by the differential distributions of myosin [45], and tropomyosin [46], isoforms in different regions of cultured fibroblasts.
The present results suggest that ROCK and MLCK play non-redundant roles in regulating myosin II functions and cellular traction forces, even though both of them increase the phosphorylation level of MRLC at the same site(s). The inhibition of ROCK caused a strong inhibition of traction forces. Similarities between the effects of ROCK inhibitors and blebbistatin, including the strong inhibition of traction forces, focal adhesions, and stress fibers, support the notion that the effects of Y-27632 on traction forces are caused by the inhibition of MRLC phosphorylation, through a decrease in direct phosphorylation by ROCK and/or increase in phosphatase activities. However, ROCK does have additional targets besides MRLC and myosin phosphatase, including ERM, LIM-kinase, and adducin [47], which may explain the slightly different effects between Y-27632 and blebbistatin on vinculin-containing structures as shown in Figure 5.
Our results appear to raise questions on the functional role of MLCK in cultured fibroblasts, where MLCK has been shown to be active in the lamella region and to be involved in maintaining cell polarity and peripheral adhesion structures [18, 48]. . An explanation to the paradoxical lack of inhibition of traction forces is that traction forces may represent only a fraction of total force output by myosin II, and forces produced by MLCK-regulated myosin II may be counter-balanced by intracellular structures and never transmitted to the substrate as traction forces. Consistent with this explanation, active traction forces are highly concentrated near the leading edge and are associated with nascent focal adhesions [49], whereas contractile acto-myosin bundles and mature focal adhesions are distributed throughout much of spread fibroblasts.
The lack of inhibition of cell migration by blebbistatin and Y-27632 [18, 32, 33; unpublished observations], despite the nearly total inhibition of traction forces, raises serious questions about the biological function of traction forces, which were previously believed to be involved in overcoming adhesive resistance and propelling forward migration. However, as shown in the previous and present studies, inhibition of contractility also disrupts focal adhesions and weakens adhesions to the substrate [33], thus decreasing the demand of contractile forces to detach cells from the substrate to allow migration. In addition, non-adhesive cells such as neutrophils generate very weak traction forces (unpublished observations). Therefore the ability of cells to migrate in the absence of detectable traction forces does not necessarily contradict their role in cell migration. Traction forces may also play a role in maintaining cell shape and responses to adhesion signals. Treatment with Y-27632 and blebbistatin [18], and ablation of myosin IIB [34], disrupts the cell shape and causes cells to elongate and/or fragment, suggesting that traction forces may provide a surface tension-like mechanism to maintain the cell shape. In addition, although cells treated with Y-27632 show directional migration over a limited period of time [18], treatment with blebbistatin, Y-27632, and ablation of myosin IIB were found to inhibit the cell’s response to substrate rigidity [33, 34], indicating that traction forces are used for probing mechanical properties of the environment. However, the maintenance of cell polarity appears to involve both ROCK and MLCK, as inhibition of MLCK has also been reported to cause a random appearance of protrusions [18].
Finally, it is interesting to note that inhibition of ROCK causes striking disassembly of acto-myosin bundles near the cell center, while active traction forces are exerted exclusively near the leading edge. One possibility is that traction forces are generated not by the prominent stress fibers in the central region, but by fine actin fibers and myosin II minifilaments near the front [50]. Alternatively, different regions of the cell may be linked mechanically by structural components, such that forces generated by central stress fibers may be transmitted over a long distance to the leading edge. In summary, the present results demonstrate the complexity of myosin II regulation in cultured fibroblasts. Unlike contractions in skeletal or smooth muscle cells where a Ca2+-dependent regulatory mechanism dominates, the Ca2+-independent, Rho-dependent pathway plays a major role in regulating the traction force output. In addition, these different mechanisms do not function in a redundant manner, but appear to have distinct roles in cell migration, shape control, and mechanosensing.
Acknowledgments
Except for immunoblotting, experimental results were collected in the laboratory of Y.-L.W. through the support of NIH grant GM-32476. K.A.B. was responsible for experiments on the effects of ROCK on traction forces and for the preparation of the manuscript. K.H. performed the immunoblotting experiment. M.D. was responsible for computational analysis of traction stress, supported by NIH grant R01 GM-61806. Y.L.W. was responsible for the general conceptualization and supervision of the project. H.H. as a visiting scholar was responsible for collecting the majority of the experimental data. We are grateful to Mitsubishi Pharma of Osaka, Japan for providing Y-27632 before it was commercially available.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy GG, Parsons JT, Horwitz AR. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Harris AK, Wild P, Stopak D. Nature. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 3.Oliver T, Jacobson KA, Dembo M. Methods Enzymol. 1998;298:497–521. doi: 10.1016/s0076-6879(98)98042-9. [DOI] [PubMed] [Google Scholar]
- 4.Reinhart-King CA, Hammer DA, King M, editors. Principles of Cellular Engineering. Academic Press; 2006. p. 3.p. 24. [Google Scholar]
- 5.Munevar S, Wang YL, Dembo M. Biophy J. 2001;80:1744–1757. doi: 10.1016/s0006-3495(01)76145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beningo KA, Wang Y-L. Trends Cell Biol. 2002;12:79–84. doi: 10.1016/s0962-8924(01)02205-x. [DOI] [PubMed] [Google Scholar]
- 7.Pelham RJ, Wang Y-L. Mol Biol Cell. 1999;10:935–945. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Seller JR, Mitchison TJ. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 9.Sawhney RK, Howard J. Cell Motil Cytoskel. 2004;58:175–185. doi: 10.1002/cm.20004. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson S, Paterson HF, Marshall CJ. Nature Cell Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 11.Abe M, Ho CH, Kamm KE, Grinnell F. J Biol Chem. 2003;278:47707–47712. doi: 10.1074/jbc.M306228200. [DOI] [PubMed] [Google Scholar]
- 12.Moussavi RS, Kelley CA, Adelstein RS. Mol Cell Biochem. 1993;127–128:219–227. doi: 10.1007/BF01076773. [DOI] [PubMed] [Google Scholar]
- 13.Dulyaninova NG, Malashkevich VN, Almo SC, Bresnick AR. Biochemistry. 2005;44:6867–6876. doi: 10.1021/bi0500776. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg M, Ravid S. Mol Biol Cell. 2006;17:1364–1374. doi: 10.1091/mbc.E05-07-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan JL, Ravid S, Spudich JA. Ann Rev Biochem. 1992;61:721–759. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- 16.Uchimura T, Fumuto K, Yamamoto Y, Ueda K, Hosoya H. Cell Struct Func. 2002;27:479–486. doi: 10.1247/csf.27.479. [DOI] [PubMed] [Google Scholar]
- 17.Fumoto K, Uchimura T, Iwasaki T, Ueda K, Hosoya H. Biochem J. 2003;370:551–556. doi: 10.1042/BJ20021559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Totsukawa G, Wu Y, Sasaki Y, Hartshorne DJ, Yamakita Y, Yamashiro S, Matsumura F. J Cell Biol. 2004;164:427–439. doi: 10.1083/jcb.200306172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obara K, Nikcevic G, Pestic L, Nowak G, Lorimer DD, Guerriero V, Elson EL, Paul RJ, deLanerolle P. J Biol Chem. 1995;270:18734–18737. doi: 10.1074/jbc.270.32.18734. [DOI] [PubMed] [Google Scholar]
- 20.Somlyo AP, Somlyo AV. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 21.Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katoh K, Kano Y, Amano M, Kaibuchi K, Fujiwara K. Am J Physiol Cell Physiol. 2001;280:1669–1679. doi: 10.1152/ajpcell.2001.280.6.C1669. [DOI] [PubMed] [Google Scholar]
- 23.Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. J Biol Chem. 1987;262:7796–7801. [PubMed] [Google Scholar]
- 24.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 25.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi S, Kakita S, Takahashi I, Kawahara K, Tsukuda E, Sano T, Yamada K, Yoshida M, Yoshida M, Kase H, Matsuda Y. J Biol Chem. 1992;267:2157–2163. [PubMed] [Google Scholar]
- 27.Wu Y, Erdodi F, Muranyi A, Nullmeyer KD, Lynch RM, Hartshorne DJ. J Muscle Res Cell Motil. 2003;24:499–511. doi: 10.1023/b:jure.0000009810.36038.53. [DOI] [PubMed] [Google Scholar]
- 28.Pelham RJ, Wang YL. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beningo KA, Lo CM, Wang YL. Methods Cell Biol. 2001;69:325–339. doi: 10.1016/s0091-679x(02)69021-1. [DOI] [PubMed] [Google Scholar]
- 30.Lo CM, Wang HB, Dembo M, Wang YL. Biophy J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dembo M, Wang YL. Biophy J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Rooij J, Kerstens A, Danuser G, Shwartz MA, Waterman-Storer CM. J Cell Biol. 2005;171:153–164. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo WH, Frey MT, Burnham NA, Wang YL. Biophy J. 2005;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo C-M, Buxton DB, Chua GC, Dembo M, Adelstein RS, Wang YL. Mol Biol Cell. 2004;15:982–989. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Science. 1996;272:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 36.Smilenov LB, Mikhailov A, Pelham RJ, Marcantonio EE, Gunderson GG. Science. 1999;286:1172–1174. doi: 10.1126/science.286.5442.1172. [DOI] [PubMed] [Google Scholar]
- 37.Bershadsky AD, Balaban NQ, Geiger B. Ann Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 38.Pelham RJ, Wang YL. Mol Biol Cell. 1999;10:935–945. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danowski BA, Harris AK. Exp Cell Res. 1988;177:47–59. doi: 10.1016/0014-4827(88)90024-9. [DOI] [PubMed] [Google Scholar]
- 40.Allingham JS, Smith R, Rayment I. Nat Struct Mol Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 41.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 42.Jin Y, Blue EK, Dixon S, Hou L, Wysolmerski RB, Gallagher PJ. J Biol Chem. 2001;276:39667–39678. doi: 10.1074/jbc.M101886200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chew TL, Masaracchia RA, Goeckler ZM, Wysolmerski RB. J Muscle Res Cell Motil. 1998;19:839–854. doi: 10.1023/a:1005417926585. [DOI] [PubMed] [Google Scholar]
- 44.Murata-Hori M, Fukuta Y, Ueda K, Iwasaki T, Hosoya H. Oncogene. 2001;20:8175–8183. doi: 10.1038/sj.onc.1205055. [DOI] [PubMed] [Google Scholar]
- 45.Saitoh T, Takemura S, Ueda K, Hosoya H, Nagayama M, Haga H, Kawabata K, Yamaguchi A, Takahashi M. FEBS Lett. 2001;509:365–369. doi: 10.1016/s0014-5793(01)03186-6. [DOI] [PubMed] [Google Scholar]
- 46.Lin JJC, Hegman TE, Lin JL. J Cell Biol. 1988;107:563–572. doi: 10.1083/jcb.107.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amano M, Fukata Y, Kaibuchi K. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- 48.Chew TL, Wolf WA, Gallagher PJ, Matsumura F, Chisholm RL. J Cell Biol. 2002;56:543–553. doi: 10.1083/jcb.200110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. J Cell Biol. 2001;153:881–887. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svitkina TM, Verkhovsky AB, McQuade KM, Borisy G. JCell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]