Regulation of nuclear translocation of Forkhead transcription factor AFX by protein kinase B (original) (raw)
Abstract
The regulation of intracellular localization of AFX, a human Forkhead transcription factor, was studied. AFX was recovered as a phosphoprotein from transfected COS-7 cells growing in the presence of FBS, and the phosphorylation was eliminated by wortmannin, a potent inhibitor of phosphatidylinositol (PI) 3-kinase. AFX was phosphorylated in vitro by protein kinase B (PKB), a downstream target of PI 3-kinase, but a mutant protein in which three putative phosphorylation sites of PKB had been replaced by Ala was not recognized by PKB. In Chinese hamster ovary cells (CHO-K1) cultured with serum, the AFX protein fused with green fluorescence protein (AFX-GFP) is localized mainly in the cytoplasm, and wortmannin induced transient nuclear translocation of the fusion protein. The AFX-GFP mutant in which all three phosphorylation sites had been replaced by Ala was detected exclusively in the cell nucleus. AFX-GFP was in the nucleus when the cells were infected with an adenovirus vector encoding a dominant-negative form of either PI 3-kinase or PKB, whereas the fusion protein stayed in the cytoplasm when the cells expressed constitutively active PKB. In CHO-K1 cells expressing AFX-GFP, DNA fragmentation was induced by the stable PI 3-kinase inhibitor LY294002, and the expression of the active form of PKB suppressed this DNA fragmentation. The phosphorylation site mutant of AFX-GFP enhanced DNA fragmentation irrespective of the presence and absence of PI 3-kinase inhibitor. These results indicate that the nuclear translocation of AFX is negatively regulated through its phosphorylation by PKB.
Phosphatidylinositol (PI) 3-kinase mediates the signal from various growth factors to regulate cell proliferation and survival (1, 2). A Ser/Thr protein kinase, termed protein kinase B (PKB) or Akt, is identified as a downstream target of PI 3-kinase. This protein kinase is activated by interaction of its pleckstrin homology domain with PI 3-kinase products and/or by phosphorylation of its catalytic domain by some upstream protein kinases (3, 4). The potential role of PKB in insulin action has been explored extensively (2–4). In Caenorhabditis elegans, DAF-2, AGE-1, and Ce-Akt, which are homologues of the mammalian insulin receptor, p110 catalytic subunit of PI 3-kinase, and PKB, respectively, have been isolated (5–7). In this organism, DAF-16, a transcription factor containing the Forkhead motif, is a major downstream target of the AGE-1/Ce-AKT signaling cascade (7–9). This protein is shown to mediate insulin-like metabolic and longevity signals, and genetic analysis reveals that the AGE-1/Ce-AKT pathway suppresses the activity of DAF-16 for gene transcription. DAF-16 contains three repeats of the consensus sequence for phosphorylation by PKB (10), Arg-Xaa-Arg-Xaa-Xaa-Ser/Thr, where Xaa is any amino acid, and thus this protein is thought to be a direct target of Ce-Akt (7).
Some members of the Forkhead family of human transcription factors, FKHR (11), its related gene products (FKHRL1 and FKHR1) (12, 13), and AFX (14, 15), are structurally related to the Nematoda DAF-16 protein. All of these human proteins conserve the three potential sites for PKB phosphorylation. Recent studies strongly suggest that these proteins are downstream targets of the PI 3-kinase pathway, and their nuclear localization is negatively regulated by phosphorylation by PKB. Namely, FKHRL1 is phosphorylated by PKB to promote cell survival by suppressing its transcriptional activity, and site-directed mutagenesis of the three potential sites retains this protein within the nucleus, as judged by immunohistochemistry (16). Similarly, FKHR is shown to serve as a substrate of PKB, and its transcriptional activity is negatively regulated by this phosphorylation (17–19). More recently, FKHR1, another member of the FKHR subclass of the Forkhead family, was shown to be phosphorylated by PKB to negatively regulate the transcriptional activity, and its nuclear export is induced by its phosphorylation (13).
On the other hand, AFX, another entity of the mammalian Forkhead proteins, is recently shown to serve as a substrate of PKB both in vitro and in vivo, and its transcriptional activity is inhibited by phosphorylation (20). We describe here that the subcellular distribution of AFX is regulated by PKB in the PI 3-kinase signaling pathway by visualizing the intracellular localization of this protein fused with green fluorescence protein (GFP). The role of AFX was also studied by monitoring DNA fragmentation in cells expressing an AFX protein with mutated phosphorylation sites.
Materials and Methods
Cell Culture.
African green monkey COS-7 and Chinese hamster ovary cells (CHO-K1) were maintained in DMEM and Ham’s F-12 medium, respectively, supplemented with 10% FBS at 37°C in a 5% CO2/95% air incubator. Sf-9 cells were cultured in Grace’s insect medium containing yeastolate, lactalbumin hydrolysate, and glutamine (GIBCO/BRL), supplemented with 10% FBS.
Expression Vectors.
The cDNA of human AFX (14) was isolated from a human brain cDNA library (CLONTECH) by PCR. The full-length AFX was introduced into pTB701-FLAG (21) and BS340 (22) vectors. The resulting mammalian expression plasmids having the FLAG-epitope tag sequence at the NH2 terminus and the GFP sequence at the COOH terminus are designated FLAG-AFX and AFX-GFP, respectively. Three putative phosphorylation sites of AFX (10), Thr-28, Ser-193, and Ser-258 (14), were each replaced by Ala through site-directed mutagenesis, and the resulting point mutants were referred to as T28A, S193A, and S258A, respectively. The mutant molecule having Ala at all of these three sites, was designated triple mutant. These mutants were introduced into BS340 vector. The DNA sequences of these constructs were confirmed by the dideoxynucleotide chain-termination method by using a DNA Sequencing System model 373A (Applied Biosystems). COS-7 and CHO-K1 cells were transfected with each expression plasmid by electroporation by using Gene Pulser (Bio-Rad) and by a polyamine transfection reagent Trans IT (Panvera, Madison, WI), respectively. The expression construct of the glutathione _S_-transferase (GST)-fusion protein of rat PKBα, designated GST-PKBα (23), was cloned into pAcGHLT vector (PharMingen), and was recombined into the Autographa california strain of the nuclear polyhedrosis virus (AcNPV) by using a Baculogold transfection kit according to the manufacturer’s protocol (PharMingen). A baculovirus that encodes the catalytic subunit of PI 3-kinase (p110α) (24) was kindly provided by M. D. Waterfield (Ludwig Institute for Cancer Research, London). Adenovirus vectors encoding the dominant-negative PI 3-kinase (AxCAΔp85) (25), the dominant-negative PKB (AxCAAkt-AA) (25), and the active form of PKB (AxCAMyr-Akt) (26) were prepared as described, and the control virus encoding β-galactosidase (AxCALacZ) was kindly provided by I. Saito (University of Tokyo).
Immunoprecipitation.
The following procedures were carried out at 0–4°C. After washing with PBS, the cells were lysed in 20 mM Tris⋅HCl (pH 7.5) containing 1 mM EDTA, 1 mM EGTA, 10 mM 2-mercaptoethanol, 1% Triton X-100, 150 mM NaCl, 10 mM NaF, 1 mM Na3VO4, and 50 μg/ml PMSF. The lysate was centrifuged, and the supernatant was incubated for 1 h with either an anti-FLAG mAb (Sigma) or an anti-GFP polyclonal Ab (A-6455; Molecular Probes). Then, protein A-Sepharose (Pharmacia) was added to the mixture and incubated for 30 min. The immunoprecipitates were collected by centrifugation and washed four times each time with 20 mM Tris⋅HCl (pH 7.5) containing 150 mM NaCl and 1% Triton X-100.
Immunoblot Analysis.
Immunoblot analysis was carried out as described (27). Anti-GFP Ab, anti-FLAG Ab, anti-p85 subunit of PI 3-kinase Ab (28), or anti-PKB Ab (25) was employed as the primary Ab. The anti-p85 subunit of PI 3-kinase Ab was kindly provided by Y. Fukui (University of Tokyo). Alkaline phosphatase-conjugated anti-rabbit or anti-mouse Ab (Promega) was employed as the secondary Ab. The color reaction used 5-bromo-4-chloro-3-indolyl-phosphate and nitro blue tetrazolium as substrates.
Metabolic Labeling.
COS-7 cells transfected with FLAG-AFX were cultured for 1 h in phosphate-free DMEM supplemented with dialyzed FBS, and then metabolically labeled for 3 h with [32P]orthophosphate (18 MBq per 6-cm dish). After treatment with wortmannin for 30 min, the cells were lysed. FLAG-AFX was immunoprecipitated and the immunoblot analysis was carried out by using the anti-FLAG Ab as described above. The 32P radioactivity incorporated into FLAG-AFX was determined by a Bio-Imaging Analyzer (BAS-2000; FUJIX, Tokyo).
In Vitro Phosphorylation of AFX-GFP.
Sf-9 cells were coinfected with the baculovirus vectors of GST-PKBα and the p110α subunit of PI 3-kinase. GST-PKBα showing a high protein kinase activity was purified from the cell lysate by affinity chromatography on a glutathione-Sepharose (Pharmacia) column (29). AFX-GFP and the mutant proteins were immunoprecipitated from the transfected CHO-K1 cells, which were pretreated with wortmannin (Sigma) at 1 μM for 30 min. The reaction mixture (25 μl) containing 20 mM Tris⋅HCl (pH 7.5), 10 mM MgCl2, 20 μM ATP, 15–50 kBq of [γ-32P]ATP, 100 ng of GST-PKBα, and AFX-GFP or each mutant protein was incubated for 30 min at 30°C. The phosphorylation reaction was stopped by the addition of SDS sample buffer. The phosphorylated proteins were separated by SDS/PAGE and visualized by using the Bio-Imaging Analyzer.
Observation of AFX-GFP Translocation.
CHO-K1 cells transfected with AFX-GFP or its mutants were spread onto glass-bottom culture dishes (MatTek, Ashland, MA) and maintained for at least 16 h before observation. The culture medium was replaced by a buffer composed of 5 mM Hepes (pH 7.3), 135 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 10 mM glucose. The cells were then treated with either wortmannin or LY294002 (Sigma). These PI 3-kinase inhibitors were dissolved in DMSO, and the final concentrations of DMSO in the culture buffer were 0.01% and 0.1% for wortmannin and LY294002, respectively. The fluorescence of the proteins was monitored with a confocal laser scanning fluorescent microscope (model LSM 410 invert; Zeiss) at 488-nm argon excitation by using a 515-nm long-pass barrier filter as described (22, 30). Where indicated, transfected CHO-K1 cells were infected with the adenovirus vector and cultured for 24 h and then spread onto glass-bottom culture dishes for microscopic observation.
Flow Cytometry.
The cells were fixed with 70% ethanol on ice for 20 min, treated with RNase A (0.5 μg/ml) for 20 min, and stained with propidium iodide (50 μg/ml). DNA distribution in the cells was analyzed by flow cytometry (Cyto ACE-300; Jasco, Tokyo) by using a 640-nm long-pass filter for propidium iodide (31).
Results
Phosphorylation of AFX in Vivo and in Vitro.
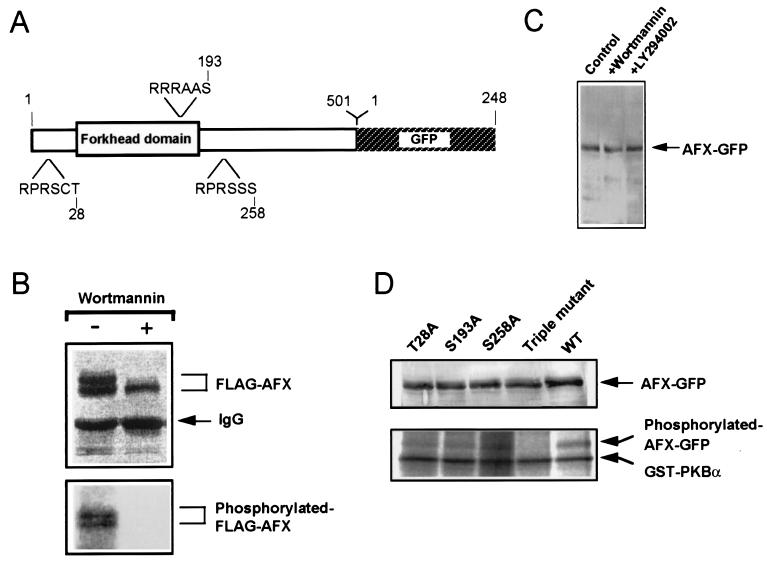
AFX has three putative phosphorylation sites for PKB, Thr-28, Ser-193, and Ser-258 (10, 14, 15) (Fig. 1A). In the presence of FBS, FLAG-AFX was recovered as a phosphoprotein from the transfected COS-7 cells metabolically labeled with [32P]orthophosphate, and the treatment of the cells with wortmannin, a potent inhibitor of PI 3-kinase, eliminated the phosphorylation (Fig. 1B). FLAG-AFX was detected as a doublet by immunoblot analysis, and both of these two bands were clearly phosphorylated. When treated with wortmannin, the upper band disappeared and only the lower band of FLAG-AFX was recovered. The upper band presumably represents a multiply phosphorylated form of AFX (20). To study in vitro phosphorylation of AFX and its intracellular localization as described below, AFX-GFP was constructed and expressed in CHO-K1 cells (Fig. 1C). To confirm whether AFX is directly phosphorylated by PKB, AFX-GFP and its mutant proteins in which the putative phosphorylation sites are replaced by Ala were expressed and immunoprecipitated from the lysates of the transfected cells (Fig. 1D). Prior to preparation of the lysates, the cells were treated with wortmannin to prevent PI 3-kinase-dependent phosphorylation of the fusion proteins. AFX-GFP and its mutant proteins thus isolated were separately incubated with PKB in vitro in the presence of [γ-32P]ATP. The results showed that AFX-GFP was efficiently phosphorylated by PKB, whereas the triple mutant of AFX-GFP did not serve as a substrate of PKB (Fig. 1D). The mutant proteins with one of the three phosphorylation sites replaced were still phosphorylated by PKB, but to lesser extents. It may be concluded that PKB phosphorylates all of the three sites, but not other amino acid residues in AFX.
Figure 1.
Expression and phosphorylation of AFX. (A) Schematic structure of the AFX-GFP fusion protein. The numbers on the top show the amino acid residues of AFX and GFP. Three putative phosphorylation sites by PKB containing the motif sequence Arg-Xaa-Arg-Xaa-Xaa-Ser/Thr are indicated. (B) Phosphorylation of AFX in vivo. COS-7 cells transfected with FLAG-AFX were metabolically labeled with [32P]orthophosphate in the presence of FBS. After incubation for 30 min with or without wortmannin at 200 nM, FLAG-AFX was immunoprecipitated, and the immunoblot analysis was carried out by using the anti-FLAG Ab (Upper), and the 32P radioactivity incorporated into FLAG-AFX was visualized (Lower). (C) Expression of AFX-GFP. The lysates of CHO-K1 cells transfected with AFX-GFP were subjected to SDS/PAGE, and immunoblot analysis was carried out by using the anti-GFP Ab. Cells treated with either wortmannin at 1 μM or LY294002 at 10 μM for 60 min and control cells without treatment were employed. (D) Phosphorylation of AFX-GFP and its mutants by PKB in vitro. CHO-K1 cells transfected with either the wild-type AFX-GFP (WT) or each AFX-GFP mutant were pretreated with wortmannin, the lysates were subjected to SDS/PAGE, and immunoblot analysis using the anti-GFP Ab was carried out (Upper). The immunoprecipitates from the lysates by the anti-GFP Ab were incubated with GST-PKBα in the presence of [γ-32P]ATP, and the phosphorylated proteins were separated and visualized (Lower).
Nuclear Translocation of AFX Induced by PI 3-Kinase Inhibitors.
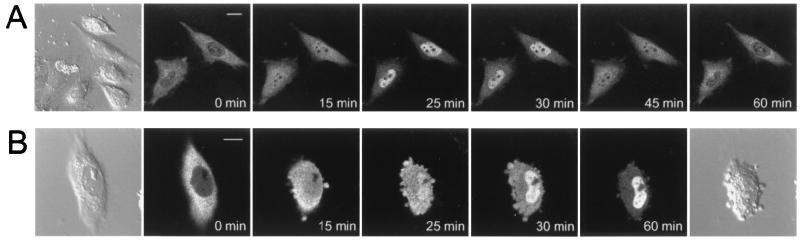
In the next set of experiments, the intracellular localization of AFX was investigated by using its fusion protein with GFP. Transfection of the DNA construct in CHO-K1 cells resulted in the expression of AFX-GFP with an approximate molecular mass of 83 kDa, corresponding to the calculated molecular mass of 80,442 Da (Fig. 1C). Analysis of the cells cultured with 10% FBS under the confocal fluorescence microscope revealed that AFX-GFP is located in the cytoplasm but not in the nucleus (Fig. 2). If, however, the cells were treated with wortmannin at 200 nM, the fusion protein was found in the nucleus. The nuclear localization was maximal at 25–30 min, and the protein was returned to the cytoplasm 60 min after the treatment (Fig. 2A). The transient nuclear translocation of AFX-GFP is probably due to the fact that wortmannin is metabolically unstable and rapidly disappears from the culture medium within 1 h (32, 33). A similar nuclear translocation of AFX-GFP was observed after treatment of the cells with LY294002, another PI 3-kinase inhibitor, at 10 μM (Fig. 2B). This inhibitor is more stable than wortmannin (32, 33) and caused sustained nuclear localization of AFX-GFP. LY294002 was added to the culture medium in a DMSO solution, and the high concentration of DMSO made the cell shape round. However, the AFX-GFP protein remained intact during the entire period of experiments, as judged by the immunoblot using the anti-GFP Ab, indicating that the fluorescence observed under the microscope reflects the localization of AFX-GFP.
Figure 2.
Translocation of AFX-GFP in CHO-K1 cells. (A) Wortmannin-induced nuclear localization. (B) LY294002-induced nuclear localization. CHO-K1 cells expressing AFX-GFP were treated with either 200 nM wortmannin or 10 μM LY294002. The fluorescence of the fusion protein was monitored with a confocal laser scanning fluorescent microscope. Morphology of the cells observed under a Nomarski interfering microscope at 0 min is shown at the left end in A and B, and morphology observed 60 min after the LY294002 treatment is shown at the right end in B. (Bars, 10 μm.)
Phosphorylation and Nuclear Translocation of AFX.
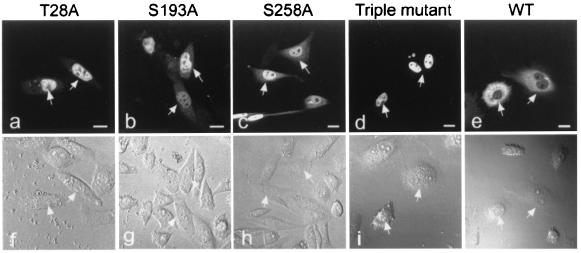
Further evidence for the role of phosphorylation of AFX in its intracellular localization was provided further by the fact that the triple mutant of AFX-GFP was detected exclusively in the nucleus (Fig. 3). Wortmannin did not affect the subcellular localization of the triple mutant. The point mutant proteins, T28A, S193A, or S258A, remained largely in the nucleus, but some fluorescence was detected in the cytoplasm, supporting the notion that all of the three potential phosphorylation sites equally take part in regulating the intracellular localization of AFX.
Figure 3.
Expression of AFX-GFP mutants in CHO-K1 cells. CHO-K1 cells were transfected with either the wild-type AFX-GFP (WT) or each mutant, as indicated at the top, and the fluorescence of the proteins (a–e) and morphology of the cells observed under a Nomarski interfering microscope (f–j) are shown. The positions of the nuclei of the cells expressing AFX-GFP are indicated by arrows. (Bars, 10 μm.)
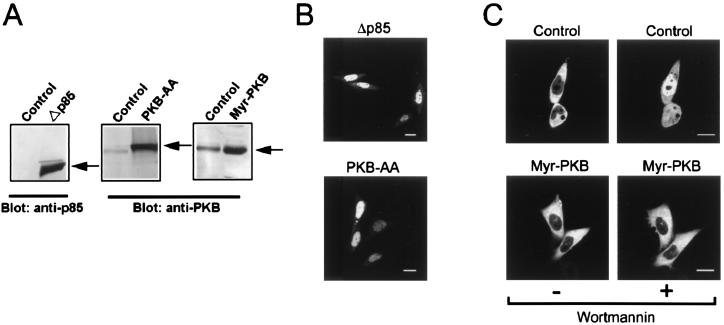
Localization of AFX in Cells Expressing PI 3-Kinase and PKB Mutants.
Infection of mammalian cells with the adenovirus vector of the dominant-negative form of PI 3-kinase (Δp85) and PKB (PKB-AA) has been shown to suppress the activity of endogenous PI 3-kinase and PKB, respectively (25). On the other hand, a constitutively active form of PKB was established by adding a myristoyl signal to the NH2 terminus of PKB (Myr-PKB) as described (26, 34). These recombinant proteins were coexpressed in CHO-K1 cells by using adenovirus vectors as shown by immunoblot analysis (Fig. 4A), and thus, the intracellular localization of AFX-GFP was explored in cells infected with these adenovirus vectors. AFX-GFP was located in the nucleus in the cells expressing either the dominant-negative form of PI 3-kinase (Δp85) or PKB (PKB-AA) (Fig. 4B), whereas the fluorescent protein was found in the cytoplasm in the cells infected with the control virus expressing β-galactosidase (AxCALacZ) (Fig. 4C). In contrast, AFX-GFP was always found in the cytoplasm, when the cells were infected with adenovirus vector of the constitutively active form of PKB (Myr-PKB), and wortmannin did not induce the nuclear translocation (Fig. 4C). The results indicate that AFX is phosphorylated by PKB kept in the cytoplasm and that dephosphorylation of this transcription factor triggers its nuclear translocation.
Figure 4.
Localization of AFX-GFP in CHO-K1 cells infected with the dominant-negative PI 3-kinase, the dominant-negative PKB, and the active form of PKB. (A) Immunoblot analysis of PI 3-kinase and PKB. The lysates prepared from the cells infected with the adenovirus vectors encoding the dominant-negative PI 3-kinase (Δp85), the dominant-negative PKB (PKB-AA), the active form of PKB (Myr-PKB), or β-galactosidase (Control) were subjected to immunoblot analysis using the anti-p85 subunit of PI 3-kinase Ab or the anti-PKB Ab. The positions of the immunoreactive bands are indicated by arrows. (B and C) Localization of AFX-GFP in infected cells. CHO-K1 cells expressing AFX-GFP were infected with each adenovirus vector as indicated at the top, and the fluorescence of AFX-GFP was observed. (C) The localization of AFX-GFP was observed before and after treatment with wortmannin at 1 μM for 30 min. (Bars, 10 μm.)
DNA Fragmentation in Cells Expressing AFX or Triple Mutant.
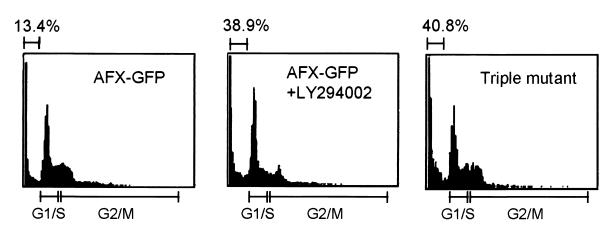
AFX and FKHRL1 bind to the insulin-responsive element (16, 20). This element has been identified in the promoter of several genes that are repressed by insulin and sensitive to PI 3-kinase. FKHRL1 also binds to the promoter of Fas ligand gene, and its expression by FKHRL1 binding promotes cell apoptosis (16). It is possible that one of the anti-apoptotic signals in the PI 3-kinase/PKB pathway may be mediated by phosphorylation, resulting in the inhibition of the Forkhead family transcription factors. The target genes of AFX in the survival signaling pathway have not been identified, but it is plausible that AFX has a role in the regulation of apoptosis analogously to FKHRL1. In fact, DNA fragmentation was induced when CHO-K1 cells expressing AFX-GFP were treated with LY294002 for 24 h (Fig. 5). The active form of PKB suppressed this DNA fragmentation (data not shown). Triple mutant AFX-GFP, which persistently stays in the nucleus, enhanced DNA fragmentation without addition of PI 3-kinase inhibitor. The results strongly suggest that AFX is involved in cell survival and that the nuclear translocation of AFX is sufficient to induce apoptosis.
Figure 5.
Flow cytometry of CHO-K1 cells. CHO-K1 cells expressing AFX-GFP or triple mutant were used. Where indicated, the cells were treated with LY294002 at 20 μM for 24 h. DNA distribution was analyzed by flow cytometry, and the cell fractions in G1/S and G2/M phases are indicated at the bottom. The numbers on top indicate the percentages of the cells containing sub-G1 DNA. Results shown are representative of three independent experiments.
Discussion
With NIH 3T3 cells overproducing insulin receptor, Kops et al. (20) have recently shown that AFX is phosphorylated by PKB in vitro and in vivo, and that Ser-193 and Ser-258, but not Thr-28, are major sites of phosphorylation of this transcription factor. The authors also propose that an additional site of AFX is phosphorylated in a Ras-dependent but PI 3-kinase-independent mechanism, and predict that Ral-GEF, a GDP/GTP exchange factor of Ral, may take part in this insulin signaling pathway. The present study showed that, in CHO-K1 cells, the phosphorylation of AFX was totally blocked by PI 3-kinase inhibitor, and that Thr-28 was most likely an additional target site of PKB, suggesting that the PI 3-kinase/PKB pathway seems to be a major, if not the only, route of AFX phosphorylation in this cell line. This discrepancy is presumably because of differences of the cell lines employed by these two research groups, reflecting distinct links of the two signaling molecules, namely PI 3-kinase and Ras (20, 35). In fact, in our separate experiment with a truncated AFX protein having amino acid residues 1–192 fused with GFP, this mutated protein was mainly present in the cytoplasm. If, however, Thr-28 was replaced by Ala, then the resulting truncated protein was detected in the nucleus. Thus, Thr-28 may also take part as well during the nuclear import and export of the transcription factor AFX. This is consistent with the recent findings that the Thr residues located in the NH2 terminal portion of the FKHR-related transcription factors, Thr-32 in FKHRL1 (16), Thr-24 of FKHR (17, 18), and Thr-24 of FKHR1 (13), are deeply involved in their insulin-dependent translocation processes.
For FKHR1, the regions responsible for its nuclear translocation and return to the cytoplasm have been investigated (13). The regions of amino acid residues 147–251 and 347–380 of FKHR1 are required for the nuclear import and export of the protein, respectively. The former includes the Forkhead domain, and the latter contains a Leu-rich sequence at amino acid residues 368–377 (MENLLDNLNL). Perhaps export of AFX from the nucleus may be regulated in a manner analogous to FKHR1, because AFX also has a Leu-rich sequence in amino acid residues 300–309 (GLELLDGLNL) (14). It is important to investigate the role of these regions in the nuclear import and export processes of AFX regulated by its reversible phosphorylation. The phosphorylated FKHRL1 has been shown to bind to 14-3-3 proteins, which recognize phosphoserine and phosphothreonine residues and the protein complex stays in the cytoplasm (16). Nuclear factor of activated T cells (NFAT), another transcription factor family, translocates to the nucleus after dephosphorylation (36), and the dephosphorylated NFAT associates with an exportin protein Crm1 in the nucleus (37). It is attractive to surmise, then, that some binding proteins specific to the phosphorylated and dephosphorylated forms of AFX may be involved in the regulation of its intracellular movement. However, the transcriptional activity of AFX may be independent of its phosphorylation reaction per se, because the triple mutant of AFX, which stays persistently in the nucleus, induces DNA fragmentation as the triple mutant of FKHR does (18). Further studies are needed to clarify the mechanism of the nuclear transport and export as well as the transcriptional regulation of these Forkhead transcription factors.
Acknowledgments
We thank Drs. Michael D. Waterfield, Izumu Saito, and Yasuhisa Fukui for providing the baculovirus encoding the p110α subunit of PI 3-kinase, the adenovirus vectors of pAxCAwt and AxCALacZ, and the anti-p85 subunit of PI 3-kinase, respectively. We thank Drs. Tatsuo Furuyama and Nozomu Mori for discussions, and Ms. Yukiko Kimura for secretarial assistance. This study was supported in part by research grants from the Scientific Research Funds of the Ministry of Education, Science, Sports and Culture of Japan, the Suntory Institute for Bioorganic Research, and the Charitable Trust Osaka Cancer Researcher Fund.
Abbreviations
PI
phosphatidylinositol
PKB
protein kinase B
GFP
green fluorescence protein
GST
glutathione _S_-transferase
CHO
Chinese hamster ovary
References
- 1.Carpenter C L, Cantley L C. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd P R, Withers D J, Siddle K. Biochem J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemmings B A. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 4.Coffer P J, Jin J, Woodgett J R. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura K D, Tissenbaum H A, Ruvkun G. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 6.Morris J Z, Tissenbaum H A, Ruvkun G. Nature (London) 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 7.Paradis S, Ruvkun G. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin K, Dorman J B, Rodan A, Kenyon C. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 9.Ogg S, Paradis S, Gottlieb S, Patterson G I, Lee L, Tissenbaum H A, Ruvkun G. Nature (London) 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 10.Alessi D R, Caudwell F B, Andjelkovic M, Hemmings B A, Cohen P. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 11.Galili N, Davis R J, Fredericks W J, Mukhopadhyay S, Rauscher F J D, Emanuel B S, Rovera G, Barr F G. Nat Genet. 1993;5:230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 12.Anderson M J, Viars C S, Czekay S, Cavenee W K, Arden K C. Genomics. 1998;47:187–199. doi: 10.1006/geno.1997.5122. [DOI] [PubMed] [Google Scholar]
- 13.Biggs W H, III, Meisenhelder J, Hunter T, Cavenee W K, Arden K C. Proc Natl Acad Sci USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borkhardt A, Repp R, Haas O A, Leis T, Harbott J, Kreuder J, Hammermann J, Henn T, Lampert F. Oncogene. 1997;14:195–202. doi: 10.1038/sj.onc.1200814. [DOI] [PubMed] [Google Scholar]
- 15.Hillion J, Le Coniat M, Jonveaux P, Berger R, Bernard O A. Blood. 1997;90:3714–3719. [PubMed] [Google Scholar]
- 16.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 17.Nakae J, Byung-Chul P, Accili D. J Biol Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 18.Tang E D, Nuñez G, Barr F G, Guan K-L. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 19.Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. J Biol Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 20.Kops G J P L, de Ruiter N D, De Vries-Smits A M M, Powell D R, Bos J L, Burgering B M T. Nature (London) 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 21.Kuroda S, Tokunaga C, Kiyohara Y, Higuchi O, Konishi H, Mizuno K, Gill G N, Kikkawa U. J Biol Chem. 1996;271:31029–31032. doi: 10.1074/jbc.271.49.31029. [DOI] [PubMed] [Google Scholar]
- 22.Ohmori S, Shirai Y, Sakai N, Fujii M, Konishi H, Kikkawa U, Saito N. Mol Cell Biol. 1998;18:5263–5271. doi: 10.1128/mcb.18.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konishi H, Shinomura T, Kuroda S, Ono Y, Kikkawa U. Biochem Biophys Res Commun. 1994;205:817–825. doi: 10.1006/bbrc.1994.2738. [DOI] [PubMed] [Google Scholar]
- 24.Hiles I D, Otsu M, Volinia S, Fry M J, Gout I, Dhand R, Panayotou G, Ruiz-Larrea F, Thompson A, Totty N F, et al. Cell. 1992;70:419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotani K, Ogawa W, Hino Y, Kitamura T, Ueno H, Sano W, Sutherland C, Granner D K, Kasuga M. J Biol Chem. 1999;274:21305–21312. doi: 10.1074/jbc.274.30.21305. [DOI] [PubMed] [Google Scholar]
- 27.Konishi H, Matsuzaki H, Tanaka M, Ono Y, Tokunaga C, Kuroda S, Kikkawa U. Proc Natl Acad Sci USA. 1996;93:7639–7643. doi: 10.1073/pnas.93.15.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka S, Matsuda M, Nagata S, Kurata T, Nagashima K, Shizawa Y, Fukui Y. Jpn J Cancer Res. 1993;84:279–289. doi: 10.1111/j.1349-7006.1993.tb02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konishi H, Kuroda S, Kikkawa U. Biochem Biophys Res Commun. 1994;205:1770–1775. doi: 10.1006/bbrc.1994.2874. [DOI] [PubMed] [Google Scholar]
- 30.Sakai N, Sasaki K, Ikegaki N, Shirai Y, Ono Y, Saito N. J Cell Biol. 1997;139:1465–1476. doi: 10.1083/jcb.139.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe T, Ono Y, Taniyama Y, Hazama K, Igarashi K, Ogita K, Kikkawa U, Nishizuka Y. Proc Natl Acad Sci USA. 1992;89:10159–10163. doi: 10.1073/pnas.89.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones S M, Klinghoffer R, Prestwich G D, Toker A, Kazlauskas A. Curr Biol. 1999;9:512–521. doi: 10.1016/s0960-9822(99)80235-8. [DOI] [PubMed] [Google Scholar]
- 33.Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, Toki S, Matsuda Y, Onodera K, Fukui Y. J Biol Chem. 1994;269:18961–18967. [PubMed] [Google Scholar]
- 34.Kohn A D, Summers S A, Birnbaum M J, Roth R A. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 35.Burgering B M T, Coffer P J. Nature (London) 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 36.Shibasaki F, Price E R, Milan D, McKeon F. Nature (London) 1996;382:370–373. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, McKeon F. Nature (London) 1999;398:256–260. doi: 10.1038/18473. [DOI] [PubMed] [Google Scholar]