Vasculogenic Mimicry: How Convincing, How Novel, and How Significant? (original) (raw)
In a recent publication, Maniotis et al 1 report that blood vessels of malignant eye tumors known as uveal melanomas are formed by tumor cells instead of endothelial cells. The authors use the term vasculogenic mimicry to describe this phenomenon and consider it a novel concept in the biology of tumor vascularization. The paper has received widespread attention and apparent validation through two commentaries, one published along with the paper in The American Journal of Pathology 2 and another published concurrently in Science. 3
Despite the paper’s impact the evidence is, in our view, unconvincing. The problems are, however, not easily detected by readers unfamiliar with the background or pitfalls of this specialized topic. Although it is intriguing and worthy of further study, the evidence presented in Maniotis et al for a functionally significant contribution of tumor cell-lined blood vessels to vascularization and blood flow in uveal melanomas is neither persuasive nor novel. The purpose of this commentary is to examine the evidence for vasculogenic mimicry and the reasons for our assessment. (Note: This commentary does not address the in vitro or microarray data presented by Maniotis et al, because the interpretation of these results is dependent on the histological, immunohistochemical, and electron microscopic evidence that is the focus of our remarks. Also, this commentary does not question the validity of the relationship between the periodic acid-Schiff (PAS) staining pattern of uveal melanomas and clinical outcome, as reported by Folberg et al in several publications. 4,5 This correlation may be clinically useful even if the PAS staining pattern does not faithfully represent the microvascular architecture. Neither does our commentary question the usefulness of microvascular density as a prognostic factor for survival in uveal melanomas. 6,7 Indeed, PAS staining pattern and microvascular density may offer complementary indices of the lethality of these tumors. 6-9 )
How Convincing?
A definitive demonstration of tumor cell-lined blood vessels would address several key questions. 1) Are the structures under consideration actually blood vessels, defined as routes through which blood circulates; ie, do they contribute meaningfully to blood flow? 2) Can the presence or absence of endothelial cells and tumor cells in contact with the vascular lumen be established using unambiguous markers? 3) If erythrocytes are used as markers, are they located inside or outside blood vessels? 4) Where is the interface between endothelial cells and tumor cells in blood vessel walls? 5) How extensive is the presumptive contribution of tumor cells to the lining of blood vessels?
The first two of these questions are addressed in Maniotis et al, but the approach is not on target and the results are not straightforward or convincing. Consider the following five problems.
PAS-Stained Networks in Uveal Melanomas Do Not Reflect the Microvascular Architecture
In a search for tumor cell-lined blood vessels, a key step is the identification of the vessels in question as routes for circulating blood. Maniotis et al used periodic acid-Schiff to stain the “patterned vascular channels” in histological sections of uveal melanomas. The interpretation of PAS-stained networks in uveal melanomas as reflecting the microvascular architecture of the tumors stems from a report in 1992 by Folberg et al 4 that defines nine different vascular patterns in these tumors based on PAS staining of what was assumed to be periendothelial basement membrane. The term network was used for the most complex pattern consisting of three or more PAS-stained back-to-back loops. Folberg, who is one of the authors of the Maniotis et al paper, applied this approach in numerous subsequent papers. 5,10-23 The presence of one or more PAS-stained networks was reported to indicate an unfavorable clinical outcome. 4,5
This approach is not in concert with multiple lines of evidence showing that PAS-stained networks do not represent blood vessels, do not pinpoint the location of blood vessels, and do not define the microvascular architecture of uveal melanomas. Immunohistochemistry for the endothelial cell marker Factor VIII-related antigen shows scattered discrete profiles of blood vessels instead of loops and networks around clusters of tumor cells. 6,8 In response to this immunohistochemical evidence, Folberg 24 has argued that PAS staining, though not specific for blood vessels, precisely matches the microvascular pattern by staining the perivascular connective tissue. This argument is inconsistent with other evidence. For example, “silent” regions with no PAS staining, which Folberg et al 4 interpreted as avascular regions of the tumors, contain abundant vessels that are immunoreactive for Factor VIII 8 and another endothelial cell marker, CD34. 7 Furthermore, the pattern of CD34 immunoreactivity matches the pattern of Factor VIII staining regardless of whether PAS staining is present. 7,9 The apparent similarity of PAS staining to the staining pattern of the endothelial cell marker Ulex europeus agglutinin I lectin, as reported by Folberg, 4 is likely to be an artifact of connective tissue autofluorescence. 8
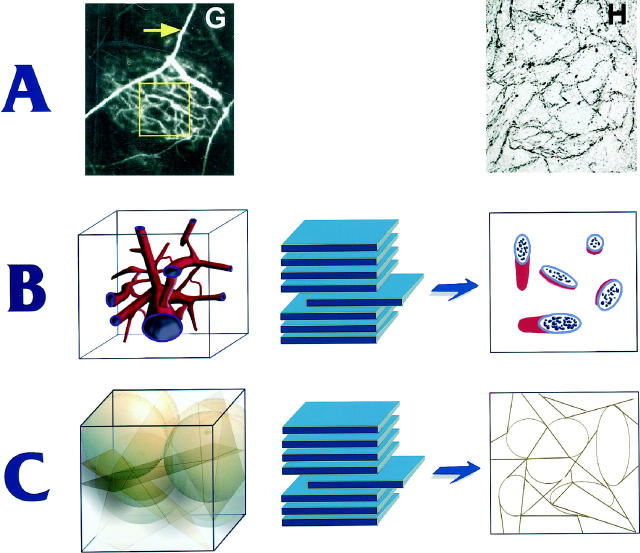
Maniotis et al report that the PAS-stained pattern in tissue sections precisely matches the microvascular architecture seen in angiograms. However, geometric considerations dictate that vascular patterns seen in angiograms, which are 2-dimensional projections of 3-dimensional networks, should not match the pattern of blood vessels visible in corresponding thin histological sections, which are essentially 2-dimensional. 8 The structure of the PAS-stained networks as viewed in 2-dimensional sections indicates that they are curved sheets, not tubes or sinusoids (Figure 1) ▶ . A 3-dimensional anastomosing network of tubes or sinusoids would appear in 2 dimensions as discontinuous segments of tubes, which when cut in various planes of section would range in appearance from circles or ellipses to longitudinal sections of cylinders (Figure 1) ▶ . A network of blood vessels would not appear in 2 dimensions as a continuous, interconnected network of lines. Also, it is extremely unlikely that an angiogram would have precisely the same appearance as a histological section because the network of blood vessels shown in the angiogram should appear as discrete vessel profiles in thin histological sections (Figure 1) ▶ . The same argument applies to ultrasound images of the tumors. 14
Figure 1.
Relationship between 3-dimensional structures and individual 2-dimensional sections from the same structures. Figures in A are adapted from Maniotis et al. The left-hand figure, showing an angiogram of a uveal melanoma, is an image of a 3-dimensional structure and must correspond to the example in B, which illustrates a 3-dimensional network of interconnecting tubes (left) and the resulting ellipses seen in a section (right). The right-hand figure of A, however, is a 5-μm section of the tumor shown in the angiogram and resembles more closely the structure in C, which shows a hypothetical clustering of planar and ellipsoidal objects (left) and the interconnected networks seen in a corresponding section (right).
Thus, the PAS-stained networks called “patterned vascular channels” are unlikely to represent networks of blood vessels. Instead, these networks appear to consist of septa of connective tissue and extracellular matrix around clusters of tumor cells. Vessels are located in some of the septa, but most of the continuous, interconnected dark lines around tumor cells shown in PAS-stained histological sections are not blood vessels.
If the PAS-stained pattern does not closely represent the arrangement of blood vessels, then indeed it would not be expected to match the distribution of endothelial cell markers. Therefore, the mismatch between the PAS pattern and endothelial cell distribution would be the expected finding rather than novel evidence of endothelial cell absence and blood vessel formation by tumor cells.
Endothelial Cell-Lined Blood Vessels Are Present in Uveal Melanomas
Maniotis et al conclude that blood vessels in aggressive uveal melanomas are formed by cancer cells, based in part on their failure to find any endothelial cells in these tumors. In their hands, immunoreactivity for the endothelial cell markers Factor VIII-related antigen, CD31, CD34, and KDR (flk-1) was weak, focal, and discontinuous, and most of the PAS-stained networks were unlabeled by these markers. CD34 immunoreactivity and KDR immunoreactivity were found in the lumen of blood vessels, not in the wall, and CD31 immunoreactivity appeared to be located in the nuclei of perivascular tumor cells.
In sharp contrast to these findings, several studies have documented the presence of Factor VIII-related antigen, CD31, and CD34 immunoreactivity of blood vessels in aggressive uveal melanomas. 6-9,16,18 Some of the authors of the Maniotis et al paper contributed to this evidence. 16,18 All of these papers show distributions of immunoreactivity that would be expected for tumor microvasculature, not PAS-stained networks. Because the PAS-stained septa are themselves not blood vessels, they would not be expected to have immunoreactivity for endothelial cell markers. Only the blood vessels within them and elsewhere would have these features. Although Maniotis et al acknowledge the presence of Factor VIII-related antigen, CD31, CD34, and KDR, they argue that these molecules are expressed by tumor cells, not endothelial cells. Yet, to fit the distribution of immunoreactivity, they argue that the expression is restricted to those tumor cells that line blood vessels. In our view, this argument is circular.
Immunoreactivity for Factor VIII-related antigen, CD31, CD34, and KDR and Ulex lectin histochemistry would be expected to show the location of vascular endothelial cells. Indeed, the focal, discontinuous regions of immunoreactivity illustrated by Maniotis et al fit the expected distribution of endothelial cells in tumor vessels. Surprisingly, however, this finding is interpreted as showing that the immunoreactivity was associated with the contents of the tumor vessels, not the vessels themselves. A more straightforward interpretation would be that the antibodies identified endothelial cells in tumor vessels that were collapsed or poorly preserved. Fixation by immersion in formalin combined with the high tissue pressure in tumors would predispose to vessel collapse. 25 Also, because the 234 tumors described in this study were removed before 1993 and the tumors were fixed in formalin and embedded in paraffin, 5 the preservation of the specimens was unlikely to be optimal for immunohistochemistry at sufficient resolution. Bleaching of melanin to improve visibility in the histological sections would further degrade immunoreactivity. 8
Extravasated Erythrocytes in Extracellular Matrix Can Be Misinterpreted as Vascular Channels
A convincing argument for the presence of blood vessels lined by tumor cells depends on the unequivocal identification of the structures in question as vessels connected to the bloodstream. In the Maniotis et al paper, key evidence for this identification came from transmission electron microscopic studies that produced the illustrated example of an alleged tumor cell-lined blood vessel. In this illustration, the “vessel” was identified by the presence of erythrocytes, and the “lumen” is lined by basement membrane. The possibility of extravascular erythrocytes was not considered.
There are three problems with this part of the argument. First, two earlier reports by Folberg and colleagues, both of which are cited in Maniotis et al, document the presence of endothelial cells in highly invasive uveal melanomas examined by transmission electron microscopy. 4,10 A recent report (Foss AJE, Munro P, Cree I, submitted for publication) confirms these earlier observations. Electron micrographs in the earlier papers by Folberg et al 4,10 clearly show endothelial cells that are in contact with the vessel lumen and are surrounded by basement membrane. The point is made that the endothelial cells do not have intact intercellular junctions and, therefore, do not have a normal barrier function. 4,10 By contrast, after examining the same cases, Maniotis et al now claim that endothelial cells are not present at all and that the blood is in direct contact with basement membrane and tumor cells.
Second, the ultrastructural example in Maniotis et al is not convincing because the erythrocytes shown are likely to be extravascular. Extravasated erythrocytes and hemorrhage are such common features of tumors, including uveal melanomas, that the location of erythrocytes cannot be assumed to define the interior of blood vessels. 26-31 Hemorrhage in uveal melanomas can occur during surgical removal of the eye as well as spontaneously. 26,31 Recent studies have begun to elucidate the mechanism of the propensity for hemorrhage in tumors. 32 Therefore, the presence of erythrocytes next to tumor cells does not justify an inference of tumor cell-lined vascular channels.
Third, because cellular membranes are not preserved in the electron micrographs shown in Maniotis et al, cellular boundaries cannot be seen, and it is unclear how many and what types of cells are present in addition to the tumor cells.
Tumor Cells Next to the Vessel Lumen Must Contact Endothelial Cells Somewhere
If blood vessels in uveal melanomas are lined by tumor cells, somewhere there must be a junction between tumor cells in contact with the vessel lumen and endothelial cells. The identification of the junction between the two systems would provide the “smoking gun” in the list of evidence for tumor cell-lined vessels. No such junction was identified, described, or discussed.
Tumor Cell-Lined Vessels in Uveal Melanomas, if Present, Are Likely to Be Infrequent
Maniotis et al do not report the number or proportion of presumptive tumor cell-lined blood vessels in aggressive uveal melanomas. Did these structures constitute 1%, 10%, or 100% of the vessels? Only one example is used to illustrate the electron microscopic observations, and the reader is not told the number of presumptive vessels or the number of tumors that were examined in this way. Because no data were presented that would limit the interpretation to a particular subset of blood vessels, the reader is led to believe that all of the vessels in these tumors are lined by tumor cells. Yet this inference is inconsistent with the results of numerous earlier studies, including some from the same group, that have identified endothelial cells in vessels of aggressive uveal melanomas. 4,6-10,16,18 As mentioned above, three different methods (lectin staining, immunohistochemistry, and transmission electron microscopy) have given consistent and complementary results: most blood vessels in aggressive uveal melanomas are lined by endothelial cells and have the same general features as vessels in other tumors. If tumor cell-lined vessels are present in these tumors, they must be infrequent.
How Novel?
The possibility that cancer cells participate in the formation of blood vessels in tumors has been recognized for many years. It is not surprising that cancer cells with features of endothelial cells line blood vessels of tumors of vascular origin such as angiosarcomas. 33 However, cancer cells have been reported to line vessels in other types of tumors as well. In his book on the pathology of tumors published in 1948, Willis states that “in rapidly growing tumors, [vessels] consist of little more than irregular channels lined by endothelium only or by naked tumor cells.” 34 Although Willis does not use the terminology of vasculogenic mimicry, he clearly sets out the concept that tumor cells can acquire a new phenotype and participate in the formation of blood vessels. In the 1960s and 1970s, François, 35,36 Jensen, 37,38 and Duke-Elder and Perkins 26 reported that tumor cells in some uveal melanomas line cavernous spaces or cyst-like blood lakes that may communicate with the microvasculature. Warren, 39 Prause and Jensen, 40 and Hammersen 41 subsequently added ultrastructural evidence of the contribution of cancer cells to the walls of tumor vessels. Warren 39 included “blood vessels without endothelial lining” among his nine categories of tumor vessels. In 1989, Konerding et al 42 used the term endothelial imitation to describe the role of tumor cells in the formation of vascular channels. This concept has been addressed in reviews on tumor blood flow 43,44 and in a textbook of general pathology. 45 Another example of cells other than endothelial cells that form blood vessels can be found in placental cytotrophoblasts creating hybrid fetal/material vessels of the endometrium through a process referred to as pseudo-vasculogenesis. 46 Neither the paper by Maniotis et al nor the two commentaries acknowledges any of these precedents.
How Significant?
The contribution of cancer cells to the formation and lining of blood vessels in tumors has broad biological and medical significance, with pathophysiological and therapeutic implications ranging from predisposition to blood-borne spread of tumor cells, to facilitated entry of drugs into tumors, to the efficacy of conventional anti-cancer drugs as anti-angiogenic agents, and to potential ineffectiveness of endothelial cell-targeted angiogenesis inhibitors.
Because of the potential significance, the issue of endothelial cells versus cancer cells lining blood vessels of tumors needs careful, systematic investigation. Earlier studies have all faced the problems of distinguishing between tumor cells and endothelial cells and between intravascular and extravascular erythrocytes. The issue has only begun to be studied with the broad range of contemporary methods such as reporter genes that uniquely identify tumor cells and endothelial cells, and markers that unambiguously label the vessels through which blood circulates. There is also a need to determine the magnitude of the contribution of tumor cells to blood vessels and of such vessels to tumor blood flow, using quantitative methods with appropriate sampling. By growing human uveal melanomas in immunodeficient mice, it would be possible to label the tumor cells and vasculature with distinctive markers, visualize them by intravital microscopy, and preserve the tissues under optimal conditions for morphological and morphometric examination.
Even though evidence that cancer cells can become lining cells and participate in the formation of blood vessels in tumors has been discussed for many years, the extent and pathophysiological significance of this phenomenon are still unclear and can only be determined by rigorous examination of the issue. Compelling evidence that supports or refutes the concept would be timely and welcome. Unfortunately, the paper by Maniotis et al promised a new level of understanding but did not solve this vexing problem.
Acknowledgments
We thank Drs. Peter Baluk, Robert Kerbel, Guido Majno, and Gavin Thurston for valuable discussions, insights, and suggestions during the preparation of this commentary. Research in the authors’ laboratories relating to some issues discussed in this commentary is supported in part by National Institutes of Health grants HL-24136 and HL-59157 from the National Heart, Lung and Blood Institute (to D. M.) and R35-CA-56591 from the National Cancer Institute (to R. J.).
Footnotes
Address reprint requests to Dr. Donald M. McDonald, Cardiovascular Research Institute, Room S-1363, University of California, San Francisco, CA 94143-0130, E-mail: dmcd@itsa.ucsf.edu.
References
- 1.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ: Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999, 155:739-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissell MJ: Tumor plasticity allows vasculogenic mimicry, a novel form of angiogenic switch: a rose by any other name? Am J Pathol 1999, 155:675-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barinaga M: New type of blood vessel found in tumors. Science 1999, 285:1475. [DOI] [PubMed] [Google Scholar]
- 4.Folberg R, Pe’er J, Gruman LM, Woolson RF, Jeng G, Montague PR, Moninger TO, Yi H, Moore KC: The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: a matched case-control study. Hum Pathol 1992, 23:1298-1305 [DOI] [PubMed] [Google Scholar]
- 5.Folberg R, Rummelt V, Parys-Van Ginderdeuren R, Hwang T, Woolson RF, Pe’er J, Gruman LM: The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology 1993, 100:1389–1398 [DOI] [PubMed]
- 6.Foss AJ, Alexander RA, Jefferies LW, Hungerford JL, Harris AL, Lightman S: Microvessel count predicts survival in uveal melanoma. Cancer Res 1996, 56:2900-2903 [PubMed] [Google Scholar]
- 7.Makitie T, Summanen P, Tarkkanen A, Kivela T: Microvascular density in predicting survival of patients with choroidal and ciliary body melanoma. Invest Ophthalmol Vis Sci 1999, 40:2471-2480 [PubMed] [Google Scholar]
- 8.Foss AJ, Alexander RA, Hungerford JL, Harris AL, Cree IA, Lightman S: Reassessment of the PAS patterns in uveal melanoma. Br J Ophthalmol 1997, 81:240-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makitie T, Summanen P, Tarkkanen A, Kivela T: Microvascular loops and networks as prognostic indicators in choroidal and ciliary body melanomas. J Natl Cancer Inst 1999, 91:359-367 [DOI] [PubMed] [Google Scholar]
- 10.Rummelt V, Folberg R, Rummelt C, Gruman LM, Hwang T, Woolson RF, Yi H, Naumann GO: Microcirculation architecture of melanocytic nevi and malignant melanomas of the ciliary body and choroid: a comparative histopathologic and ultrastructural study. Ophthalmology 1994, 101:718-727 [DOI] [PubMed] [Google Scholar]
- 11.Pe’er J, Rummelt V, Mawn L, Hwang T, Woolson RF, Folberg R: Mean of the ten largest nucleoli, microcirculation architecture, and prognosis of ciliochoroidal melanomas. Ophthalmology 1994, 101:1227-1235 [DOI] [PubMed] [Google Scholar]
- 12.Rummelt V, Gardner LM, Folberg R, Beck S, Knosp B, Moninger TO, Moore KC: Three-dimensional relationships between tumor cells and microcirculation with double cyanine immunolabeling, laser scanning confocal microscopy, and computer-assisted reconstruction: an alternative to cast corrosion preparations. J Histochem Cytochem 1994, 42:681-686 [DOI] [PubMed] [Google Scholar]
- 13.Rummelt V, Folberg R, Woolson RF, Hwang T, Pe’er J: Relation between the microcirculation architecture and the aggressive behavior of ciliary body melanomas. Ophthalmology 1995, 102:844-851 [DOI] [PubMed] [Google Scholar]
- 14.Coleman DJ, Rondeau MJ, Silverman RH, Folberg R, Rummelt V, Woods SM, Lizzi FL: Correlation of microcirculation architecture with ultrasound backscatter parameters of uveal melanoma. Eur J Ophthalmol 1995, 5:96-106 [DOI] [PubMed] [Google Scholar]
- 15.Daniels KJ, Boldt HC, Martin JA, Gardner LM, Meyer M, Folberg R: Expression of type VI collagen in uveal melanoma: its role in pattern formation and tumor progression. Lab Invest 1996, 75:55-66 [PubMed] [Google Scholar]
- 16.Folberg R, Mehaffey M, Gardner LM, Meyer M, Rummelt V, Pe’er J: The microcirculation of choroidal and ciliary body melanomas. Eye 1997, 11:227-238 [DOI] [PubMed] [Google Scholar]
- 17.Mehaffey MG, Folberg R, Meyer M, Bentler SE, Hwang T, Woolson R, Moore KC: Relative importance of quantifying area and vascular patterns in uveal melanomas. Am J Ophthalmol 1997, 123:798-809 [DOI] [PubMed] [Google Scholar]
- 18.Silverman RH, Folberg R, Boldt HC, Lloyd HO, Rondeau MJ, Mehaffey MG, Lizzi FL, Coleman DJ: Correlation of ultrasound parameter imaging with microcirculatory patterns in uveal melanomas. Ultrasound Med Biol 1997, 23:573-581 [DOI] [PubMed] [Google Scholar]
- 19.Mehaffey MG, Gardner LM, Folberg R: Distribution of prognostically important vascular patterns across multiple levels in ciliary body and choroidal melanomas. Am J Ophthalmol 1998, 126:373-378 [DOI] [PubMed] [Google Scholar]
- 20.Rummelt V, Mehaffey MG, Campbell RJ, Pe’er J, Bentler SE, Woolson RF, Naumann GO, Folberg R: Microcirculation architecture of metastases from primary ciliary body and choroidal melanomas. Am J Ophthalmol 1998, 126:303-305 [DOI] [PubMed] [Google Scholar]
- 21.Mueller AJ, Bartsch DU, Folberg R, Mehaffey MG, Boldt HC, Meyer M, Gardner LM, Goldbaum MH, Pe’er J, Freeman WR: Imaging the microvasculature of choroidal melanomas with confocal indocyanine green scanning laser ophthalmoscopy. Arch Ophthalmol 1998, 116:31-39 [DOI] [PubMed] [Google Scholar]
- 22.Mueller AJ, Freeman WR, Folberg R, Bartsch DU, Scheider A, Schaller U, Kampik A: Evaluation of microvascularization pattern visibility in human choroidal melanomas: comparison of confocal fluorescein with indocyanine green angiography. Graefe’s Arch Clin Exp Ophthalmol 1999, 237:448-456 [DOI] [PubMed] [Google Scholar]
- 23.Mueller AJ, Folberg R, Freeman WR, Bartsch DU, Bergeron-Lynn G, Mehaffey MG, Kan-Mitchell J, Huang X, Jian G, Avila C, Taskintuna I, Cheng L, Wang J: Evaluation of the human choroidal melanoma rabbit model for studying microcirculation patterns with confocal ICG and histology. Exp Eye Res 1999, 68:671-678 [DOI] [PubMed] [Google Scholar]
- 24.Folberg R: Discussion of paper by Foss et al. Br J Ophthalmol 1997, 81:247-248 [Google Scholar]
- 25.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK: Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res 1999, 59:3776-3782 [PubMed] [Google Scholar]
- 26.Duke-Elder S, Perkins ES: Cysts and tumours of the uveal tract. Duke-Elder S eds. In System of Ophthalmology. 1966, :pp 754-937 C.V. Mosby Company, St. Louis [Google Scholar]
- 27.Van den Brenk HA, Crowe M, Kelly H, Stone MG: The significance of free blood in liquid and solid tumours. Br J Exp Pathol 1977, 58:147-159 [PMC free article] [PubMed] [Google Scholar]
- 28.Schechter J, Ahmad N, Elias K, Weiner R: Estrogen-induced tumors: changes in the vasculature in two strains of rat. Am J Anat 1987, 179:315-323 [DOI] [PubMed] [Google Scholar]
- 29.Liwnicz BH, Wu SZ, Tew JM, Jr.: The relationship between the capillary structure and hemorrhage in gliomas. J Neurosurg 1987, 66:536–541 [DOI] [PubMed]
- 30.Specht CS, McLean IW, Biscoe BW: Traumatic enucleation for posterior uveal melanoma. Am J Ophthalmol 1990, 110:518-521 [DOI] [PubMed] [Google Scholar]
- 31.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM: Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol (in press) [DOI] [PMC free article] [PubMed]
- 32.Cheng SY, Nagane M, Huang HS, Cavenee WK: Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Sci USA 1997, 94:12081-12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meis-Kindblom JM, Kindblom LG: Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol 1998, 22:683-697 [DOI] [PubMed] [Google Scholar]
- 34.Willis RA: Pathology of Tumours. 1948:p 136 Butterworth & Co., Ltd., London
- 35.François J: Malignant melanomata of the choroid (Montgomery Memorial Lecture, 1961). Br J Ophthalmol 1963, 47:736-743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.François J, Neetens A: Physico-anatomical studies of spontaneous and experimental intraocular new growths: vascular supply. Bibl Anat 1967, 9:403-411 [PubMed] [Google Scholar]
- 37.Jensen OA: Malignant melanoma of the choroid of a peculiar cavernous type. Arch Ophthalmol 1964, 72:337-340 [DOI] [PubMed] [Google Scholar]
- 38.Jensen OA: The “Knapp-Rønne” type of malignant melanoma of the choroid: a haemangioma-like melanoma with a typical clinical picture. So-called “preretinal malignant choroidal melanoma.” Acta Ophthalmol (Copenh) 1976, 54:41-54 [DOI] [PubMed] [Google Scholar]
- 39.Warren BA: The vascular morphology of tumors. Peterson H-I eds. In Tumor Blood Circulation: Angiogenesis, Vascular Morphology and Blood Flow of Experimental and Human Tumors. 1979, :pp 1-48 CRC Press, Inc., Boca Raton [Google Scholar]
- 40.Prause JU, Jensen OA: Scanning electron microscopy of frozen-cracked, dry-cracked and enzyme-digested tissue of human malignant choroidal melanomas. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1980, 212:217-225 [DOI] [PubMed] [Google Scholar]
- 41.Hammersen F, Endrich B, Messmer K: The fine structure of tumor blood vessels. I. Participation of non-endothelial cells in tumor angiogenesis. Int J Microcirc Clin Exp 1985, 4:31-43 [PubMed] [Google Scholar]
- 42.Konerding MA, Steinberg F, Streffer C: The vasculature of xenotransplanted human melanomas and sarcomas on nude mice. II. Scanning and transmission electron microscopic studies. Acta Anat (Basel) 1989, 136:27-33 [DOI] [PubMed] [Google Scholar]
- 43.Jain RK: Determinants of tumor blood flow: a review. Cancer Res 1988, 48:2641-2658 [PubMed] [Google Scholar]
- 44.Vaupel P, Kallinowski F, Okunieff P: Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 1989, 49:6449-6465 [PubMed] [Google Scholar]
- 45.Majno G, Joris I: Cells, Tissue, and Disease: Principles of General Pathology, Cambridge, MA, Blackwell Science, 1996, p 783
- 46.Damsky CH, Fisher SJ: Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol 1998, 10:660-666 [DOI] [PubMed] [Google Scholar]