Perturbation of Hyaluronan Interactions by Soluble CD44 Inhibits Growth of Murine Mammary Carcinoma Cells in Ascites (original) (raw)
Abstract
Hyaluronan accumulates in ascites during intraperitoneal proliferation of TA3/St murine mammary carcinoma cells and at sites of their invasion of the peritoneal wall. To determine whether hyaluronan is functionally involved in these events, ascites tumor formation was compared in mice injected intraperitoneally with stable transfectants of TA3/St cells that overexpress soluble CD44, a hyaluronan-binding protein, versus in mice injected with transfectants expressing mutated soluble CD44 that does not bind hyaluronan. The soluble CD44 transfectants temporarily grew at a reduced rate within the peritoneal cavity, then went into G1 arrest and were subsequently cleared from the peritoneum. However, transfectants overexpressing mutant soluble CD44 that does not bind hyaluronan exhibited similar ascites accumulation, growth rates, and cell-cycle profiles in vivo to wild-type and vector-transfected TA3/St cells, all of which continued to grow until the tumors became fatal. The soluble CD44-transfected TA3/St cells also failed to attach to and form tumors in the peritoneal wall. When grown in vitro in soft agar, the soluble CD44 transfectants exhibited a dramatic reduction in colony formation compared to wild-type, vector-transfected, and mutant soluble CD44-transfected TA3/St cells. Thus, perturbation of hyaluronan interactions by soluble CD44 has a direct effect on the growth characteristics of these tumor cells, leading to inhibition of anchorage-independent growth in vitro and ascites growth in vivo.
Breast cancer cells metastasize directly through the vasculature to organs distant from the original tumor site, but they also invade and exfoliate into body cavities, especially the pleural space, where they grow in suspension within effusions. 1 The rapid accumulation of these effusions is believed to result from increased permeability of the vasculature lining such cavities under the influence of tumor cell products, eg, vascular endothelial growth factor. 2 The breast cancer cells eventually attach to and invade tissues lining the cavity wall. The tumor cells then gain access to the many blood vessels contained therein, leading to further dissemination of malignant cells to other organs. 3
In a past study, we showed that hyaluronan accumulates in the ascites, and at initial sites of attachment and invasion of tumor cells at the mesothelial surface of the peritoneal wall, after introduction of murine ovarian tumor cells or mammary carcinoma cells into the peritoneal cavity of syngeneic mice. 4 Several types of malignant solid tumors contain elevated levels of hyaluronan, a ubiquitous glycosaminoglycan that contributes both to the structure of extracellular matrix and to cell-matrix interactions that influence cell behavior. 5,6 The enrichment of hyaluronan in tumors can result from increased production by tumor cells themselves 7,8 or from interactions between tumor cells and surrounding stromal cells that induce increased production by the latter. 9-11 High levels of hyaluronan correlate with tumor spread and with poor survival rates in human patients with a variety of tumor types, 12-15 and experimental evidence in animal models directly implicates hyaluronan in solid tumor progression. 16-20 In the present study our objective was to determine whether hyaluronan also contributes to ascites growth and tumor cell invasion of the peritoneal wall.
We have shown that stable transfection of TA3/St murine mammary carcinoma cells with cDNA encoding soluble CD44 prevents formation of metastatic nodules in the lung after introduction of the TA3/St cells into the vasculature. 17 In that study, soluble CD44 presumably acted as a competitive inhibitor of crucial hyaluronan-protein interactions because transfection with mutant soluble CD44 that does not bind hyaluronan had no effect on invasion and metastasis. This supposition was confirmed by experiments showing that soluble CD44 transfection prevents hyaluronan-mediated clustering of endogenous membrane CD44 that is in turn required for binding of gelatinase B (MMP-9) to the tumor cell surface and for invasiveness. 21 In the present study, we show that stable transfection of TA3/St cells with soluble CD44 not only inhibits tumor invasion but also prevents tumor cell proliferation in ascites and that this inhibition is because of a direct effect on growth characteristics of the tumor cells rather than, or in addition to, an indirect effect on other events in vivo. These changes in tumor cell growth characteristics depend on hyaluronan interactions because mutated soluble CD44 that does not bind hyaluronan does not cause these changes.
Materials and Methods
Cell Lines and Culture Conditions
The TA3/St cell line was established from an ascites subline originally derived from a spontaneous mouse mammary adenocarcinoma. 2,22 TA3/St cells were maintained by weekly passages in the peritoneal cavities of syngeneic, 4- to 6-week-old female A/Jax mice or in culture in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Inc., Rockville, MD) supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories Inc., Logan, UT). Transfected TA3/St cells were cultured in DMEM supplemented with 10% FBS and 0.5 mg/ml geneticin (G418 sulfate, Life Technologies, Inc., Grand Island, NY).
Transfection of TA3/St Cells with Soluble CD44 Constructs
Soluble CD44 constructs were prepared and analyzed as described previously. 17 For transfection, TA3/St cells were treated with either pCR3-Uni eukaryotic expression vector alone (InVitrogen Corp., San Diego, CA) or pCR3-Uni vector containing cDNAs encoding soluble CD44 isoforms, in the presence of lipofectamine. These isoforms included either variant exons v8-v10 or v6-v10, where v10 is a new insert containing a stop codon, thus leading to truncation before the transmembrane domain; 17,23 v6-v10 was used with or without the R43A mutation that leads to loss of hyaluronan binding capacity. 17,24 G418-resistant colonies were selected and seven clones were chosen for further study: two transfectants containing v6-v10 (v6-v10a and v6-v10b), one containing v8-v10, two containing the v6-v10 mutant (v6-v10 R43A), and two mock transfectants containing vector only. The transfectants were analyzed by reverse transcriptase-polymerase chain reaction, fluorescence-activated cell sorting, and Western blotting as described previously 17 to confirm that each clone expresses the appropriate CD44 protein. All transfectants and wild-type cells produced similar amounts of surface-associated, standard, and variant CD44 isoforms. However the soluble CD44 transfectants, including the mutant soluble CD44 transfectants, also produced soluble, secreted CD44. 17
Tumorigenicity Assay
TA3/St cells in log phase growth were trypsinized, washed with DMEM containing 10% FBS, and resuspended in Hanks’ balanced salt solution (HBSS; Life Technologies, Inc.) for counting. Suspensions of TA3/St cell lines were injected, using a 25-gauge needle, into the peritoneal cavities of 4- to 6-week-old female A/Jax mice (The Jackson Laboratory, Bar Harbor, ME) at 1 × 10 6 cells/200 μl HBSS each, and allowed to grow in vivo for a period of 7 to 19 days. For each cell line and time point, six mice were given injections. Mice were observed daily for signs of ascites tumor development and monitored twice daily after the tumor symptoms appeared: abdominal bloating, decreased movement, loss of grooming behavior, and hunched posture. If mice were not expected to survive overnight they were sacrificed before conclusion of the experimental protocol. Mice that did not exhibit the above symptoms were sacrificed according to experimental parameters. The peritoneal walls from each of the mice were removed, cut into strips (∼4 mm × 8 mm), and fixed in 4% paraformaldehyde (Tousimis, Rockville, MD) in phosphate-buffered saline (PBS) for histological analysis.
Histology
Fixed strips of peritoneal wall were washed in PBS, dehydrated through 30%, 70%, 95%, 100% ethanol and xylene, and then embedded in paraffin wax (Fisher, Columbia, MD). Sections (5 μm) were cut, mounted on poly-l-lysine (Sigma)-coated slides, and stained with Mayers modified hematoxylin (Poly Scientific Research, Bay Shore, NY) or hematoxylin and eosin (Richard-Allen Medical, Richland, MI) after deparaffinization in xylene and rehydration through 100%, 95%, 70%, 35% ethanol, PBS, and water.
In Vivo Cell Proliferation Assay
Transfected TA3/St cells in log phase growth were trypsinized, washed with DMEM containing 10% FBS, and resuspended in HBSS for counting. Suspensions of the transfected TA3/St cells were seeded into the peritoneal cavities of female A/Jax mice at 2 × 10 6 cells/200 μl HBSS each and allowed to grow in vivo for a period of 2 to 15 days. At each of five different time points (2, 5, 7, 10, and 15 days), groups of six mice were sacrificed and cells were harvested from the peritoneal cavities with two 6-ml intraperitoneal lavages of calcium- and magnesium-free PBS (PBS−; Life Technologies, Inc.). Harvested cells were then washed three times with PBS−, using low-speed centrifugation with each wash to remove any red blood cells that were withdrawn along with tumor cells from the peritoneal cavity; the tumor cells formed a pellet while the red blood cells remained in the supernatant during these centrifugations. The washed cells were counted in a Coulter Counter (Coulter Electronics, Hialeah, FL) by diluting aliquots of cells resuspended in PBS− to concentrations between 200 to 20,000 cells/ml.
Cell-Cycle Analyses
Transfected and wild-type TA3/St cells were grown intraperitoneally for 7 days, harvested, and washed as described in the previous section. They were then resuspended in 70% EtOH and kept at −20°C until all samples had been collected for cell-cycle analysis. After removal from the freezer, the cells were washed twice with PBS−, resuspended in PBS− containing 0.1 mmol/L EDTA, pH 7.4, 50 mg/ml propidium iodide, 50 mg/ml RNase A (Boehringer Mannheim, Indianapolis, IN), and 1% Triton X-100, and incubated overnight at 4°C. Cell samples were then analyzed by fluorescence-activated cell sorting in a FACScan (Becton Dickinson, Mountain View, CA).
Transfected and wild-type cells were cultured in vitro in DMEM plus 10% FBS, then harvested during log phase of growth, and analyzed in the same way as above.
In Vitro Cell Proliferation Assay
Each cell line, in log growth phase, was trypsinized, washed with DMEM containing 10% FBS, and resuspended in the same media for culture. Cells were plated at 5 × 10 4 cells per well in 6-well plates (60-mm wells) and allowed to grow in 4 ml of medium at 37oC for 1 to 5 days. Every 24 hours, triplicate wells for each cell line were trypsinized, washed with DMEM, and resuspended in PBS−. The harvested cells were then counted in a Coulter Counter after dilution in PBS− to concentrations of 200 to 10,000 cells/ml.
Soft Agar Assay
Soft agar assays were performed in 60-mm dishes containing 2 ml of 1.2% agarose diluted with 2× DMEM containing 20% FBS to yield a final agarose concentration of 0.6%. Cells were harvested from monolayer culture in log growth phase by trypsinization, washing, and resuspension in DMEM containing 10% FBS for counting. The cells were then suspended in 0.33% agarose in DMEM containing 10% FBS and plated at 5000 cells/well on top of the 0.6% agarose base. After each agarose layer was allowed to solidify (10 minutes at 25oC), three additional 1-ml volumes of 0.33% agarose were layered on top of the cells. Each cell line was plated in triplicate and grown at 37oC for 28 days. Total numbers of colonies per well containing >30 cells or >200 cells per colony were counted separately using a microscope grid. The two classes of colony size were assessed by counting cells in numerous colonies under the microscope and correlating these numbers with colony size. The two classes could be distinguished readily because the great majority of colonies were found to contain between 30 and 100 cells; the large colonies (>200 cells) were very easily distinguished from the majority of colonies (30 to 100 cells) and there were virtually no colonies with <30 cells.
Results
Transfection of TA3/St Cells with Soluble CD44 cDNA
Stable transfectants overexpressing the naturally occurring soluble CD44 isoforms, v6-v10 and v8-v10, 23 the mutant isoform, v6-v10 R43A, 24 or vector alone were selected and analyzed for CD44 production and secretion as described in Materials and Methods. All cell lines produced similar amounts of membrane-bound CD44. Only the soluble CD44 transfectants, including the mutant soluble CD44 transfectants, produced secreted CD44; clones were selected that produced similar amounts of soluble CD44. 17 Binding of hyaluronan to the soluble CD44 transfectants and their adhesion to a hyaluronan substratum were shown previously to be reduced compared to wild-type TA3/St cells and vector controls. 17,21 Stable transfectants producing mutated soluble CD44 (v6-v10 R43A) exhibited high levels of hyaluronan binding and adhesion to hyaluronan, similar to wild-type and vector controls. 17,21
Overexpression of Soluble CD44 Inhibits Growth in Ascites and Peritoneal Wall Invasion by TA3/St Mammary Carcinoma Cells
Syngeneic A/Jax mice were injected intraperitoneally, at 1 × 10 6 cells per animal, with wild-type TA3/St cells or with TA3/St transfectants expressing soluble CD44 isoforms, mutant soluble CD44 (v6-v10 R43A), or vector alone. Tumor growth and invasion were assessed as described in Methods. Because results obtained in pilot experiments were virtually identical for the wild-type cells, both vector transfectants and both mutant soluble CD44 transfectants, only one of each control transfectant was examined in detail. Three soluble CD44 transfectant clones, two expressing v6-v10 and one expressing v8-v10, were examined in detail. Table 1 ▶ summarizes the results of one such experiment in which ascites accumulation, tumor growth, and tumor invasion were compared in the above manner in groups of six animals that were injected with either wild-type TA3/St cells, one of the vector transfectants, one of the mutant soluble CD44 transfectants, or the two v6-v10 soluble CD44 transfectants. Identical results to those shown in Table 1 ▶ for the two v6-v10 transfectants were obtained for the v8-v10 soluble CD44 transfectant in other similar experiments. The results of these experiments are discussed below.
Table 1.
Soluble CD44 Transfectants of TA3/St Mammary Carcinoma Cells Have Lost Their Tumorigenicity in Vivo
| Cell type | Numbers of animals | ||
|---|---|---|---|
| Attachment* | Growth/invasion† | Ascites‡ | |
| Controls | |||
| Wild-type TA3/St | 6 /6 | 6 /6 | 6 /6 |
| Vector transfectant | 6 /6 | 6 /6 | 6 /6 |
| Soluble CD44 v6–v10 R43A | 6 /6 | 6 /6 | 6 /6 |
| Soluble CD44 transfectants§ | |||
| Soluble CD44 v6–v10a | 0 /6 | 1¶ /6 | 0 /6 |
| Soluble CD44 v6–v10b | 0 /6 | 2¶ /6 | 0 /6 |
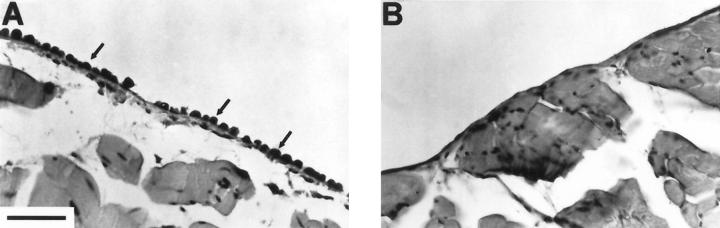
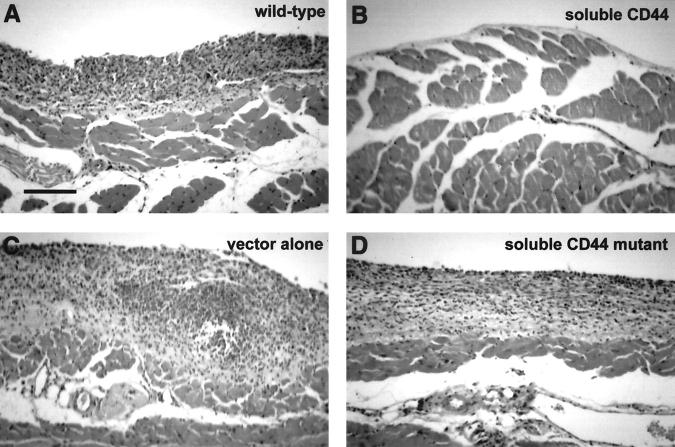
The mice injected with wild-type, vector-transfected, or mutant soluble CD44 (v6-v10 R43A)-transfected cells accumulated ascites fluid and exhibited abdominal bloating, symptomatic of ascites tumor development. Mice injected with either of the two v6-v10 soluble CD44 transfectants or with the v8-v10 transfectant did not accumulate ascites fluid or exhibit abdominal bloating. At 7 days or at 14 to 19 days after injection of cells, the mice were sacrificed and their peritoneal walls were fixed and stained. At seven days, tumor cells had attached to regions of the peritoneal wall in wild-type, vector-transfected, or mutant soluble CD44 (v6-v10 R43A)-transfected controls (Figure 1A) ▶ . However, no tumor cells were observed attached to the peritoneal walls of mice injected with the v6-v10 or the v8-v10 soluble CD44 transfectants (Figure 1B) ▶ . Mice injected with wild-type, vector-transfected, or mutant soluble CD44-transfected cells exhibited widespread tumorigenesis and invasion of the mesothelium and muscle layers of the peritoneal walls by 14 to 19 days (Figure 2 ▶ ; A, C, and D). In contrast, mice injected with v6-v10 or v8-v10 soluble CD44-transfected cells showed little or no tumor cell attachment to the mesothelium and no invasion of the peritoneal wall (Figure 2B) ▶ .
Figure 1.
Soluble CD44 transfectants do not attach to the peritoneal wall in vivo. TA3/St transfectants were injected into the peritoneal cavity of syngeneic A/Jax mice (1 × 10 6 cells each), then the animals were sacrificed after 7 days, and their peritoneal walls were fixed and stained. A: Control TA3/St cells transfected with vector alone; B: TA3/St cells transfected with soluble CD44. The control cells attached to the peritoneal wall (arrows) whereas the soluble CD44 transfectants did not. Similar attachment to that shown in A for vector transfectants was also obtained with wild-type and mutant soluble CD44 (R43A)-transfected cells; no attachment was observed with soluble CD44 transfectants v6-v10a, v6-v10b (see Table 1 ▶ ), or v8-v10. Scale bar, 50 μm.
Figure 2.
Soluble CD44 transfectants do not form tumors in the intraperitoneal wall. TA3/St transfectants expressing soluble CD44 or soluble CD44 with a point mutation (R43A) in the hyaluronan-binding domain (mutant soluble CD44), vector-transfectants, or wild-type TA3/St murine mammary carcinoma cells were injected into the peritoneal cavity of syngeneic A/Jax mice. At 14- to 17-days postinjection, the mice were sacrificed, and their peritoneal walls were fixed and stained. Mice injected with wild-type (A), vector-transfected (C), or mutant soluble CD44-transfected cells (D) exhibited widespread tumor growth and invasion of the mesothelium and muscle layers of their peritoneal walls. Mice injected with soluble CD44-transfected cells (B) were shown to have normal peritoneal walls without tumor cell growth and invasion or, in a few cases, a small number of tumor cells attached to the mesothelium; similar results were obtained with v6-v10a, v6-v10b (see Table 1 ▶ ), and v8-v10 transfectants. Scale bar, 100 μm.
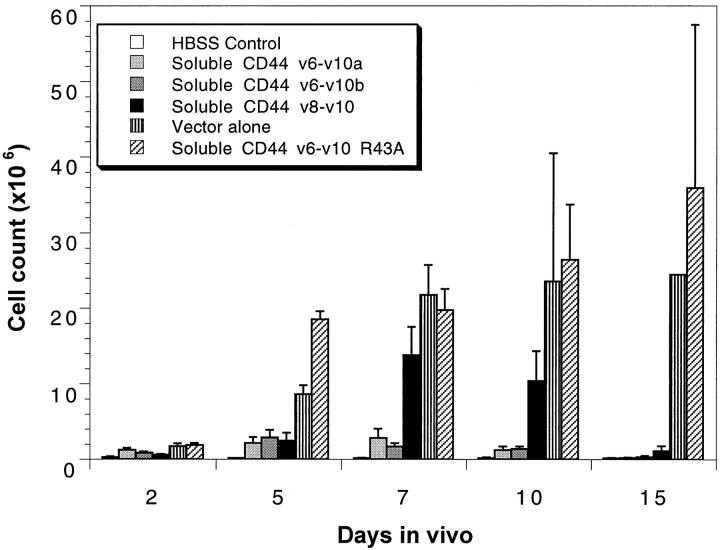
Because transfection with soluble CD44 cDNA eliminated ascites accumulation and invasion of the peritoneal wall, we directly analyzed growth of the injected cells to determine their fate in vivo. To facilitate high cell recovery from the peritoneal cavity, a larger number of cells, ie, 2 × 106, was injected into the abdomen of each host mouse than in the experiments above. The cells were recovered by intraperitoneal saline lavage at 2, 5, 7, 10, and 15 days postinjection and counted (Figure 3) ▶ . Vector-transfected and soluble CD44 R43A-transfected cells grew rapidly for the first 10 days after injection; however, most of these animals then became terminally ill and were sacrificed before day 15. Consequently only one to two animals remained for analysis at the 15-day point in these cases (in all other situations at least six animals were analyzed at each of the five time points). For the first 5 to 7 days, the soluble CD44 transfectants grew significantly, but at a diminished rate compared to the controls. Subsequently, between 10 and 15 days postinjection, their numbers became reduced to baseline (Figure 3) ▶ . This rise and fall in number of cells is particularly evident for the transfectant overexpressing the v8-v10 soluble CD44 isoform, where the ascites cell number rose to ∼14 × 10 6 at day 7 after injection but fell back to baseline by 15 days (Figure 3) ▶ . None of the animals carrying the soluble CD44 transfectants accumulated ascites and most survived indefinitely without any signs of tumor formation in the peritoneal wall. A small number of these animals slowly developed solid tumors outside the peritoneum near the site of tumor cell injection, presumably arising from cells that leaked from the peritoneum during injection or healing.
Figure 3.
Overexpression of soluble CD44 alters tumor cell proliferation in ascites. Cell numbers were counted as described in Methods for soluble CD44-transfected, vector-transfected, and soluble CD44 R43A-transfected TA3/St cells throughout 15 days in vivo. The soluble CD44 transfectants (v6-v10a, v6-v10b, v8-v10) grew at a diminished rate and dropped back to baseline (HBSS control) between 10- to 15-days postinjection with 2 × 10 6 cells, whereas the vector-transfected and the soluble CD44 R43A-transfected cells continued to grow. Growth rates of cells within the peritoneal cavity of six mice per condition and time point are represented, except in the case of the vector-transfected and soluble CD44 R43A-transfected cells at 15-days postinjection, where many of the animals became ill and had to be sacrificed between days 10 and 15.
Soluble CD44-Transfected TA3/St Mammary Carcinoma Cells Enter G1 Arrest in Ascites
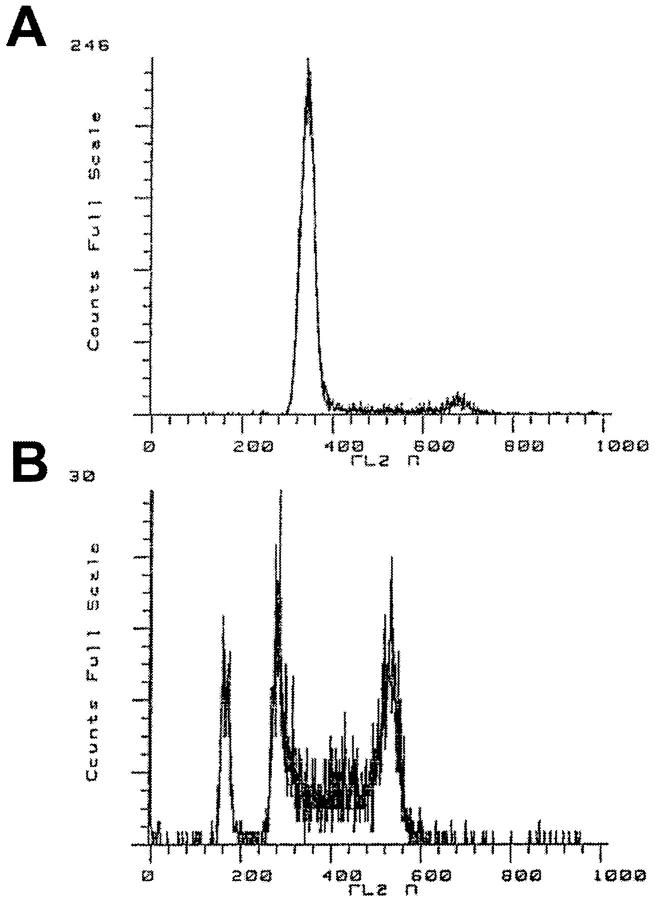
Wild-type and transfected TA3/St cells were harvested at 7-days postinjection from the peritoneal cavity of mice injected with 2 × 10 6 cells. These cells were then analyzed by fluorescence-activated cell sorting to establish a cell-cycle profile for each cell line in vivo. Cells transfected with soluble CD44 isoforms exhibited G1 arrest, whereas wild-type, vector-transfectant, and soluble CD44 R43A-transfected cells demonstrated a cell-cycle profile typical of an asynchronously cycling cell population (Figure 4 ▶ ; Table 2 ▶ ). The proportion of cells in G0/G1 for each population was calculated to be approximately 30 to 40% for the various control populations compared to 75 to 85% for the soluble CD44 transfectants (Table 2) ▶ .
Figure 4.
Soluble CD44 transfectants grown in ascites enter G1 arrest. A: Cell-cycle analysis of soluble CD44-transfected cells grown in ascites; B: vector-transfected control. Quantitative data are given in Table 2 ▶ . A similar cell-cycle pattern to that of the vector-transfected cells (B) was obtained with wild-type and mutant soluble CD44-transfected cells; the cell-cycle patterns seen with all three soluble CD44 transfectants, v6-v10a, v6-v10b, and v8-v10, were similar to that shown in A (see Table 2 ▶ ).
Table 2.
Soluble CD44 Transfectants Exhibit G1 Arrest in Vivo
| Cell type | % Cells in G0/G1 | |
|---|---|---|
| In vitro | In vivo | |
| Controls | ||
| Wild-type TA3/St | 44.1± 0.5 | 30.0± 2.1 |
| Vector transfectant | 28.3± 1.5 | 38.2± 5.4 |
| Soluble CD44 v6–v10 R43A | 33.3± 0.9 | 39.1± 4.8 |
| Soluble CD44 transfectants | ||
| Soluble CD44 v6–v10a | 44.7± 1.6 | 86.2± 1.0 |
| Soluble CD44 v6–v10b | 37.0± 2.1 | 76.4± 4.1 |
| Soluble CD44 v8–v10 | 53.0± 1.2 | 74.3± 2.8 |
For comparison, the various cell lines were grown in monolayer tissue culture instead of in ascites. In this case, both the soluble CD44 transfectants and the control cell types exhibited similar cell-cycle profiles, ranging from ∼30 to 50% cells in G0/G1 (Table 2) ▶ .
In a further attempt to understand the fate of the soluble CD44-transfected TA3/St cells after inoculation into the peritoneum, we allowed the transfectants to grow in vivo for 5 days, checked that they had gone into G1 arrest, as found above, then placed the cells in culture to see whether they would recover. The soluble CD44-transfected cells failed to attach and grow, whereas vector controls grew in similar fashion to that before inoculation in vivo (data not shown). Thus we conclude that, in ascites, the soluble CD44 transfectants irreversibly entered G1 arrest, subsequently died, and were cleared from the peritoneum.
Soluble CD44-Transfected TA3/St Mammary Carcinoma Cells Have Lost the Capacity for Anchorage-Independent Growth in Vitro
It is not clear from the results obtained above whether overexpression of soluble CD44 has a direct effect on tumor cell growth or whether its effect was an indirect consequence of another event in vivo. Thus we sought additional evidence to discriminate between these two possible explanations.
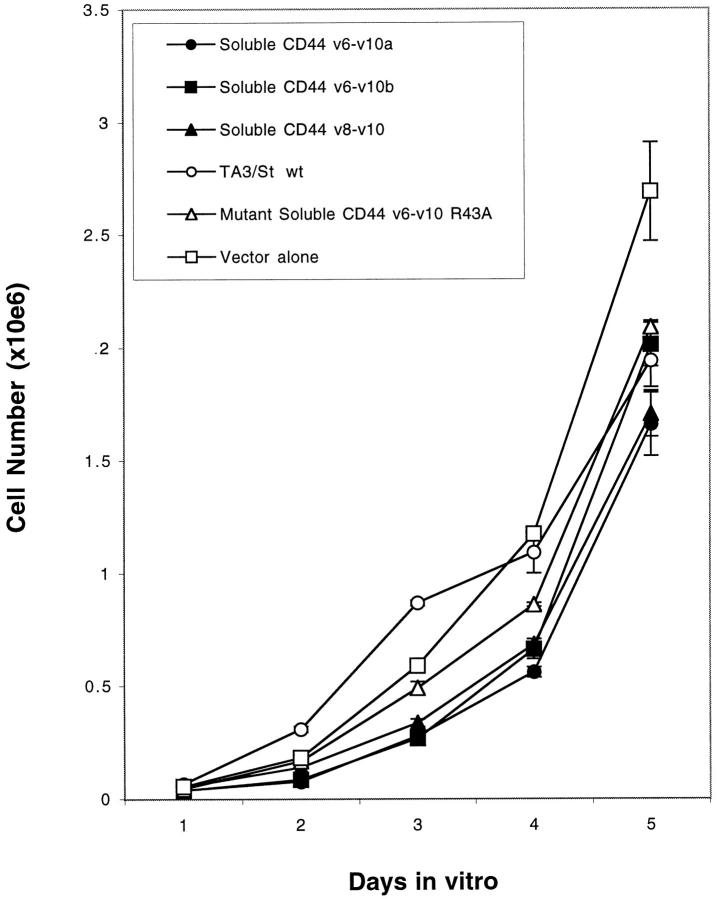
First, proliferation of the soluble CD44 transfectants and control cells was compared in monolayer culture in tissue culture wells. Each cell line grew at approximately the same rate during a 5-day period (Figure 5) ▶ and exhibited similar cell-cycle profiles (Table 2) ▶ .
Figure 5.
Overexpression of soluble CD44 does not affect tumor cell growth in monolayer culture. Cells were grown in standard monolayer conditions. Cell numbers were counted in triplicate cultures at each time point as described in Methods. No significant differences were observed among the various cell lines.
We then examined anchorage-independent growth of the various cell lines in soft agar. Dramatic differences in size and number of colonies formed between the soluble CD44 transfectants and control cells were observed (Table 3) ▶ . The wild-type, vector-transfected, and mutant soluble CD44-transfected cells formed many times more colonies than the soluble CD44 transfectants, and the colonies formed by the control cells were larger than those few colonies formed by the soluble CD44 transfectants (Table 3) ▶ .
Table 3.
Soluble CD44 Transfectants Fail to Form Colonies in Soft Agar
| Cell type | Number of colonies* | |
|---|---|---|
| >30 cells | >200 cells | |
| Controls | ||
| Wild-type TA3/St | 205 ± 28 | 30 ± 2 |
| Vector transfectant | 83 ± 21 | 25 ± 9 |
| Soluble CD44 v6–v10 R43A | 409 ± 11 | 39 ± 5 |
| Soluble CD44 transfectants | ||
| Soluble CD44 v6–v10a | 1 ± 0 | 0 ± 0 |
| Soluble CD44 v6–v10b | 16 ± 4 | 5 ± 1 |
| Soluble CD44 v8–v10 | 8 ± 1 | 0 ± 0 |
Discussion
In the work presented here we have demonstrated that stable transfection of malignant TA3/St mammary carcinoma cells with cDNAs encoding soluble CD44 isoforms directly alters their growth and adhesion characteristics such that they are unable to form ascites tumors or to invade the tissues of the host animal after intraperitoneal injection. Transfection with mutated cDNA encoding soluble CD44 that does not bind hyaluronan, and thus does not interfere with hyaluronan binding or cellular adhesion to hyaluronan, 17,21 failed to inhibit the tumorigenicity of TA3/St cells. Thus soluble CD44 most likely acts by competitively disrupting an interaction involving hyaluronan.
A particularly striking finding of this study was the failure of TA3/St transfectants overexpressing soluble CD44 to form ascites tumors. For each of the three soluble CD44 transfectants tested, growth took place for several days in the peritoneum subsequent to inoculation. However, the rate of growth of the soluble CD44 transfectants was slower than for controls and the former cells went into G1 arrest; the control cells, however, continued to increase in number to a point that became fatal for the host animals. Growth of the soluble CD44-transfected cells not only ceased but the numbers of cells in the ascites decreased back to an insignificant level. Depending on the particular transfectant, 3 to 14 million cells per mouse were lost from the peritoneum between 5 and 15 days postinoculation, implying that the soluble CD44 transfectants not only went into G1 arrest but also subsequently died and were cleared from the peritoneal cavity. In a parallel study, we have compared the ability of the soluble CD44-transfected and vector-transfected TA3/St cells studied herein to form metastases in the lung after intravenous injection. 17 In that study, overexpression of soluble CD44 was shown to induce apoptosis subsequent to entry of the cells into lung tissue, and consequently formation of metastatic nodules was dramatically inhibited. In the current study it is also probable that the soluble CD44 transfectants became apoptotic, although we were unable to capture the cells for analysis during the window of time between appearance of apoptotic characteristics and clearance of the cells from the ascites. TA3/St cells transfected with mutated soluble CD44 (R43A) behaved like vector-transfected controls (Figure 3) ▶ , indicating that an hyaluronan-mediated interaction is involved in these effects of soluble CD44 on growth.
Interestingly, the cell number reached in the peritoneum for the soluble CD44 transfectants, 5 to 7 days after inoculation, was sufficient for widespread attachment to the peritoneal wall to occur in the case of the controls. However, no attachment of soluble CD44 transfectants was detected. This observation suggests that perturbed hyaluronan-CD44 interactions lead both to altered growth characteristics within the ascites and to inhibition of peritoneal wall implantation. Previous studies have also implicated interactions between tumor cell surface CD44 and mesothelial cell-derived hyaluronan in tumor cell attachment to the peritoneal wall. 4,25,26
Although decreased attachment of the soluble CD44 transfectants to the peritoneal wall is consistent with past findings, the altered growth characteristics of soluble CD44 transfectants within the ascites were not predicted. Overproduction of soluble CD44 could influence any one of several events necessary for ascites tumor growth. For example, hyaluronan binds to fibrinogen; 27 thus, excess soluble CD44 may disrupt formation in the ascites of a provisional matrix rich in fibrin and hyaluronan that is important for tumor progression. 2,3 Hyaluronan-CD44 interactions may also be involved in angiogenesis, 28,29 in which case soluble CD44 could again be potentially disruptive. Consequently we attempted to determine whether or not perturbation of endogenous tumor cell surface hyaluronan interactions by soluble CD44 gives rise to direct inhibitory effects on tumor cell growth. We have shown that the soluble CD44 transfectants, but not the mutant soluble CD44 transfectant, have lost their ability to exhibit anchorage-independent growth in soft agar, a commonly used indicator of the transformed state of cells. 30,31 Thus it would seem that endogenous hyaluronan produced by the tumor cells themselves serves an important function in anchorage-independent growth. This conclusion is supported by recent experiments showing that increased expression of hyaluronan, driven by transfection with cDNA for hyaluronan synthase, leads to acquisition of the ability to grow in soft agar. 19 However, it is unlikely that the effect of overexpression of soluble CD44 is because of changes in hyaluronan synthesis because none of the parent or transfected cell lines produce large amounts of hyaluronan. Rather, it is more likely that soluble CD44 disrupts the organization of endogenous pericellular hyaluronan with respect to its interactions with CD44 or other hyaluronan-binding proteins that are important for the transformed behavior of the parent and control cells, eg, CD44-mediated docking of MMP-9 (see below). Also, in vivo, both parent and soluble CD44 transfectants induce high hyaluronan levels in surrounding stromal tissue, 17 indicating that this is not the underlying difference in their behavior in vivo.
Recent work from one of our laboratories 21 has demonstrated binding of MMP-9 to CD44 at the surface of TA3/St murine mammary carcinoma and MC human melanoma cells. This binding of MMP-9 to CD44 is dependent on hyaluronan-induced clustering of CD44 in the plasma membrane. Overexpression of soluble CD44 disrupts clustering of endogenous membrane CD44 and thus inhibits complex formation with MMP-9. Complex formation between CD44 and MMP-9 has also been observed in other mammary carcinoma cell lines. 32 Docking of MMP-9 at the surface of TA3/St cells promotes its activity, possibly via protection from tissue inhibitors of MMPs, which in turn leads to enhanced tumor invasion and angiogenesis. 21,33 Cell surface-bound MMP-9 acts, at least in part, by activating latent transforming growth factor-β1 (TGF-β) which then stimulates new blood vessel formation in vitro and in vivo. 33 In similar fashion to solid tumors, ascites tumor growth is accompanied by extensive angiogenesis within the peritoneal wall. 3 Thus, it is possible that TGF-β, activated in the above manner by MMP-9, stimulates peritoneal angiogenesis and thus ascites tumor growth. However, promotion of angiogenesis would not explain the involvement of hyaluronan in anchorage-independent growth in vitro, as discussed above. The effects of TGF-β on growth characteristics are complex but, in many cases, loss of responsiveness to inhibitory effects of TGF-β is associated with malignancy. 34,35 Although TGF-β is usually thought of as a tumor suppressor, it promotes late stages of carcinoma progression. 36,37 TGF-β also induces anchorage-independent growth in fibroblasts 38 and in immortalized, nontumorigenic epithelial cells. 39 Thus it is conceivable that TGF-β is at least partially responsible for the effects seen herein. Alternatively MMP-9, or another metalloproteinase bound to CD44 in an analogous manner, might cause release of a factor from the tumor cell surface that stimulates transformation and/or tumor growth directly. 40 Thus, hyaluronan- and CD44-dependent presentation of a metalloproteinase at the cell surface could explain the role of hyaluronan in tumor cell growth characteristics and the effects of overexpression of soluble CD44 demonstrated in the present study. Irrespective of the underlying mechanism, our findings lead to the conclusion that hyaluronan interactions at the cell surface are, at least under some circumstances, crucial to tumor cell growth characteristics in vitro and in vivo.
Acknowledgments
We thank Ms. Danielle Garneau for her exceptional technical assistance.
Footnotes
Address reprint requests to Bryan P. Toole, Tufts University School of Medicine, Department of Anatomy and Cellular Biology, 136 Harrison Avenue, Boston, MA 02111. E-mail: btoole@infonet.tufts.edu.
Supported by United States Army Medical Research and Materiel Command fellowship DAMD17–96-1–6060 (to R. M. P.), by National Institutes of Health grants CA55735 and GM48614 (to I. S.), and by National Institutes of Health grant CA73839 and a grant from Mizutani Foundation for Glycoscience (to B. P. T.).
References
- 1.Shulman LN, Sugarbaker DJ: Malignant effusions. Harris JR Lippman ME Morrow M Hellman S eds. Diseases of the Breast. 1996, :pp 833-840 Lippincott-Raven, Philadelphia [Google Scholar]
- 2.Nagy JA, Masse EM, Herzberg KT, Meyers MS, Yeo KT, Yeo TK, Sioussat TM, Dvorak HF: Pathogenesis of ascites tumor growth: vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res 1995, 55:360-368 [PubMed] [Google Scholar]
- 3.Nagy JA, Morgan ES, Herzberg KT, Manseau EJ, Dvorak AM, Dvorak HF: Pathogenesis of ascites tumor growth: angiogenesis, vascular remodeling, and stroma formation in the peritoneal lining. Cancer Res 1995, 55:376-385 [PubMed] [Google Scholar]
- 4.Yeo TK, Nagy JA, Yeo KT, Dvorak HF, Toole BP: Increased hyaluronan at sites of attachment to mesentery by CD44-positive mouse ovarian and breast tumor cells. Am J Pathol 1996, 148:1733-1740 [PMC free article] [PubMed] [Google Scholar]
- 5.Knudson W, Biswas C, Li XQ, Nemec RE, Toole BP: The role and regulation of tumour-associated hyaluronan. Ciba Found Symp 1989, 143:150-159 [DOI] [PubMed] [Google Scholar]
- 6.Knudson, W: Tumor-associated hyaluronan. Providing an extracellular matrix that facilitates invasion. Am J Pathol 1996, 148:1721–1726 [PMC free article] [PubMed]
- 7.Kimata K, Honma Y, Okayama M, Oguri K, Hozumi M, Suzuki S: Increased synthesis of hyaluronic acid by mouse mammary carcinoma cell variants with high metastatic potential. Cancer Res 1983, 43:1347-1354 [PubMed] [Google Scholar]
- 8.Zhang L, Underhill CB, Chen L: Hyaluronan on the surface of tumor cells is correlated with metastatic behavior. Cancer Res 1995, 55:428-433 [PubMed] [Google Scholar]
- 9.Toole BP, Biswas C, Gross J: Hyaluronate and invasiveness of the rabbit V2 carcinoma. Proc Natl Acad Sci USA 1979, 76:6299-6303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knudson W, Biswas C, Toole BP: Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci USA 1984, 81:6767-6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asplund T, Versnel MA, Laurent TC, Heldin P: Human mesothelioma cells produce factors that stimulate the production of hyaluronan by mesothelial cells and fibroblasts. Cancer Res 1993, 53:388-392 [PubMed] [Google Scholar]
- 12.Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, Agren U, Alhava E, Kosma VM: Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res 1998, 58:342-347 [PubMed] [Google Scholar]
- 13.Auvinen PK, Parkkinen JJ, Johansson RT, Agren UM, Tammi RH, Eskelinen MJ, Kosma VM: Expression of hyaluronan in benign and malignant breast lesions. Int J Cancer 1997, 74:477-481 [DOI] [PubMed] [Google Scholar]
- 14.Setala LP, Tammi MI, Tammi RH, Eskelinen MJ, Lipponen PK, Agren UM, Parkkinen J, Alhava EM, Kosma VM: Hyaluronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br J Cancer 1999, 79:1133-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anttila MA, Tammi RH, Tammi MI, Syrjanen KJ, Saarikoski SV, Kosma VM: High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res 2000, 60:150-155 [PubMed] [Google Scholar]
- 16.Bartolazzi A, Peach R, Aruffo A, Stamenkovic I: Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med 1994, 180:53-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Q, Toole BP, Stamenkovic I: Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med 1997, 186:1985-1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng C, Toole BP, Kinney SD, Kuo JW, Stamenkovic I: Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer 1998, 77:396-401 [DOI] [PubMed] [Google Scholar]
- 19.Kosaki R, Watanabe K, Yamaguchi Y: Overproduction of hyaluronan by expression of the hyaluronan synthase has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res 1999, 59:1141-1145 [PubMed] [Google Scholar]
- 20.Itano N, Sawai T, Miyaishi O, Kimata K: Relationship between hyaluronan production and metastatic potential of mouse mammary carcinoma cells. Cancer Res 1999, 59:2499-2504 [PubMed] [Google Scholar]
- 21.Yu Q, Stamenkovic I: Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev 1999, 13:35-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein G, Klein E: The transformation of a solid transplantable mouse carcinoma into an “ascites tumor.” Cancer Res 1951, 11:466–469 [PubMed]
- 23.Yu Q, Toole BP: A new alternatively spliced exon between v9 and v10 provides a molecular basis for synthesis of soluble CD44. J Biol Chem 1996, 271:20603-20607 [DOI] [PubMed] [Google Scholar]
- 24.Peach RJ, Hollenbaugh D, Stamenkovic I, Aruffo A: Identification of hyaluronic acid binding sites in the extracellular domain of CD44. J Cell Biol 1993, 122:257-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannistra SA, Kansas GS, Niloff J, DeFranzo B, Kim Y, Ottensmeier C: Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res 1993, 53:3830-3838 [PubMed] [Google Scholar]
- 26.Strobel T, Swanson L, Cannistra SA: In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: a novel role for CD44 in the process of peritoneal implantation. Cancer Res 1997, 57:1228-1232 [PubMed] [Google Scholar]
- 27.LeBoeuf RD, Raja RH, Fuller GM, Weigel PH: Human fibrinogen specifically binds hyaluronic acid. J Biol Chem 1986, 261:12586-12592 [PubMed] [Google Scholar]
- 28.Banerjee SD, Toole BP: Hyaluronan-binding protein in endothelial cell morphogenesis. J Cell Biol 1992, 119:643-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffioen AW, Coenen MJ, Damen CA, Hellwig SM, van Weering DH, Vooys W, Blijham GH, Groenewegen G: CD44 is involved in tumor angiogenesis; an activation antigen on human endothelial cells. Blood 1997, 90:1150-1159 [PubMed] [Google Scholar]
- 30.Macpherson I, Montagnier L: Agar suspension culture for the selective assay of cells transformed by polyoma virus. Virology 1964, 23:291-294 [DOI] [PubMed] [Google Scholar]
- 31.Freedman VH, Shin S: Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell 1974, 3:355-359 [DOI] [PubMed] [Google Scholar]
- 32.Bourguignon LY, Gunja-Smith Z, Iida N, Zhu HB, Young LJ, Muller WJ, Cardiff RD: CD44v(3,8–10) is involved in cytoskeleton-mediated tumor cell migration and matrix metalloproteinase (MMP-9) association in metastatic breast cancer cells. J Cell Physiol 1998, 176:206-215 [DOI] [PubMed] [Google Scholar]
- 33.Yu Q, Stamenkovic I: Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev 2000, 14:163-176 [PMC free article] [PubMed] [Google Scholar]
- 34.Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM: Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell 1998, 92:645-656 [DOI] [PubMed] [Google Scholar]
- 35.Tang B, deCastro K, Barnes HE, Parks WT, Stewart L, Bottinger EP, Danielpour D, Wakefield LM: Loss of responsiveness to transforming growth factor beta induces malignant transformation of nontumorigenic rat prostate epithelial cells. Cancer Res 1999, 59:4834-4842 [PubMed] [Google Scholar]
- 36.Oft M, Heider KH, Beug H: TGF-beta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol 1998, 8:1243-1252 [DOI] [PubMed] [Google Scholar]
- 37.Akhurst RJ, Balmain A: Genetic events and the role of TGF beta in epithelial tumour progression. J Pathol 1999, 187:82-90 [DOI] [PubMed] [Google Scholar]
- 38.Dalton SL, Scharf E, Davey G, Assoian RK: Transforming growth factor-β overrides the adhesion requirement for surface expression of α5β1 integrin in normal rat kidney fibroblasts. A necessary effect for induction of anchorage-independent growth. J Biol Chem 1999, 274:30139-30145 [DOI] [PubMed] [Google Scholar]
- 39.Wilder PJ, Rizzino A: Effects of transforming growth factor β on the anchorage-independent growth of murine epithelial JB6 cells. Cancer Res 1991, 51:5898-5902 [PubMed] [Google Scholar]
- 40.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Balck RA: An essential role for ectodomain shedding in mammalian development. Science 1998, 282:1281-1284 [DOI] [PubMed] [Google Scholar]