Identification of Vascular Lineage-Specific Genes by Transcriptional Profiling of Isolated Blood Vascular and Lymphatic Endothelial Cells (original) (raw)
Abstract
In mammals, the lymphatic vascular system develops by budding of lymphatic progenitor endothelial cells from embryonic veins to form a distinct network of draining vessels with important functions in the immune response and in cancer metastasis. However, the lineage-specific molecular characteristics of blood vascular versus lymphatic endothelium have remained poorly defined. We isolated lymphatic endothelial cells (LECs) and blood vascular endothelial cells (BVECs) by immunomagnetic isolation directly from human skin. Cultured LECs but not BVECs expressed the lymphatic markers Prox1 and LYVE-1 and formed LYVE-1-positive vascular tubes after implantation in vivo. Transcriptional profiling studies revealed increased expression of several extracellular matrix and adhesion molecules in BVECs, including versican, collagens, laminin, and N-cadherin, and of the growth factor receptors endoglin and vascular endothelial growth factor receptor-1/Flt-1. Differential immunostains of human skin confirmed the blood vessel-specific expression of these genes. During embryonic development, endoglin expression was gradually down-regulated on lymphatic endothelium whereas vascular endothelial growth factor receptor-1 was absent from lymphatics. We also identified several genes with specific expression in LECs. These results demonstrate that some lineage-specific genes are only expressed during distinct developmental stages and they identify new molecular markers for blood vascular and lymphatic endothelium with important implications for future studies of vascular development and function.
The lymphatic system consists of a vascular network of thin-walled capillaries that drain protein-rich lymph from the extracellular spaces within most organs and that play major roles in the immune response and in tumor metastasis. 1,2 Lymphatic vessels provide the conduit for antigen-presenting cells from the organ exposed to antigens to the regional lymph nodes, involving active recruitment of antigen-presenting cells by chemokines and other mediators secreted by lymphatic endothelium. 3 Moreover, the early dissemination of malignant tumors frequently occurs via lymphatic vessels to regional lymph nodes, and the recent discovery of active tumor lymphangiogenesis and its role in cancer metastasis has drawn considerable attention to the molecular mechanisms that control activation and proliferation of lymphatic endothelium. 2,4 In particular, overexpression of the lymphangiogenesis factors vascular endothelial growth factor (VEGF)-C and VEGF-D by tumor cells has been shown to promote tumor lymphangiogenesis by activation of VEGF receptor-3 (VEGFR-3) on tumor-associated lymphatic endothelium, resulting in enhanced rates of lymph node metastasis. 5-8
In contrast to the rapid progress made in elucidating the formation and molecular control of the blood vascular system, 9,10 the mechanisms controlling the normal development of lymphatic vessels and the molecular regulation of their biological function have remained poorly understood, mainly because of the lack of molecular tools to specifically distinguish lymphatic vessels from blood vessels and to functionally characterize the lymphatic endothelium. 11 Consequently, our understanding of the function of the lymphatic system and its role in disease is still rudimentary. Recently, several novel markers have been reported to be predominantly expressed by lymphatic endothelium. VEGFR-3, a receptor for the lymphangiogenesis factors VEGF-C and VEGF-D, is expressed by both blood vascular and lymphatic endothelium during embryonic development, whereas its expression becomes restricted to lymphatic vessels in adult life. 12 However, VEGFR-3 has been detected on blood vascular endothelium associated with tumors and healing wounds, 13,14 and blockade of VEGFR-3 also resulted in inhibition of tumor angiogenesis. 15 Recently, podoplanin, a transmembrane mucoprotein, has been reported as a novel lymphatic-vessel marker, 16 and both podoplanin and VEGFR-3 have been used for the isolation of lymphatic endothelial cells (LECs). 17,18 The hyaluronan receptor LYVE-1 19 is specifically expressed by LECs, but not by blood vessels in most organs including the skin, 5,20-22 and recent studies have identified the transcription factor Prox1, 2 a homeobox gene required for the development of the lymphatic system, 23 as a master control gene in the program specifying LEC fate. 22,24 However, besides these few markers with specific expression in lymphatic endothelium, little is known about the distinct molecular characteristics of blood vascular endothelial cells (BVECs) versus LECs, and a comprehensive comparison of the lineage-specific differentiation of these cell types has been lacking.
To identify novel lineage-specific molecules involved in the biological function of these distinct vascular cell types, we developed a new protocol for the selective isolation of LECs and BVECs directly from human skin. Here, we report that immunomagnetic selection of CD34−/CD31+ cells yields pure cultures of LECs that maintain expression of the specific lymphatic markers Prox1 and LYVE-1, whereas BVECs remain negative for these markers. Importantly, gene array profiling, combined with quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and double-immunofluorescence stains of normal skin, identified several novel genes with specific expression in lymphatic or blood vascular endothelium. Together, these results provide important new tools for molecular and functional studies of lymphatic and blood vascular endothelium in vascular development, tumor progression, and the immune response.
Materials and Methods
Isolation of Human Dermal BVECs and LECs
Neonatal human foreskins were obtained after routine circumcisions. After enzymatic digestion, the epidermis was removed and dermal cells were mechanically released as previously described. 25 CD34-positive BVECs were isolated by immunomagnetic purification 25 with an anti-human CD34 antibody (BD Pharmingen, San Diego, CA) conjugated to immunomagnetic beads (Dynal, Lake Success, NY). Thereafter, the remaining CD34-negative cells were incubated with an immunomagnetic beads-conjugated anti-human CD31 antibody (Dynal) to isolate LECs. LECs were seeded onto fibronectin-coated (10 μg/ml; BD Biosciences, Bedford, MA) culture dishes and were propagated in endothelial cell basal medium (BioWhittaker, Walkersville, MD), supplemented with 10 μg/ml of hydrocortisone acetate, 2.5 × 10−2 mg/ml _N_-6,2′-_O_-dibutyryl-adenosine 3′,5′-cyclic monophosphate (Sigma, St. Louis, MO), 2 mmol/L l-glutamine, 20% fetal bovine serum (Life Technologies, Inc., Grand Island, NY), antibiotics, and 20 ng/ml of recombinant human VEGF165 (R&D Systems, Minneapolis, MN). VEGF was omitted after the first passage. LECs remained negative for CD34 expression for at least eight passages, as evaluated by immunocytochemistry. BVECs were cultured in the same medium without addition of VEGF. Confluent primary BVEC cultures were further purified by immunomagnetic E-selectin selection after 6 hours of stimulation with recombinant human tumor necrosis factor-α as described. 25
Immunostains
Immunofluorescence stainings were performed on 6-μm cryostat sections of neonatal human foreskin or on 10-μm sections of mouse embryos as previously described, 22,26 using antibodies to Prox1, 22 murine or human LYVE-1 (kindly provided by Dr. D. Jackson, John Radcliffe Hospital, Oxford, UK 20 ), human CD34 and CD31 (BD Pharmingen), mouse VEGFR-1 (MF1; kindly provided by Dr. DJ Hicklin, Imclone Systems, New York, NY) and human VEGFR-1/Flt-1 (Sigma), N-cadherin (Transduction Laboratories, San Diego, CA), versican (clone 12C5; Developmental Studies Hybridoma Bank, University of Iowa, Ames, IA), mouse endoglin (BD Pharmingen) and human endoglin (Neomarkers, Fremont, CA), macrophage mannose receptor (BD Biosciences), desmoplakin (Serotec, Raleigh, NC), MIP-3-α (Santa Cruz Biotechnology, Santa Cruz, CA), and corresponding secondary antibodies labeled with AlexaFluor488 or AlexaFluor594 (Molecular Probes, Eugene, Oregon). Nuclei were counterstained with 20 μg/ml of Hoechst bisbenzimide. 27 Sections were examined by using a Nikon E-600 microscope (Nikon, Melville, NY) and images were captured with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI). Antibody staining of cultured cells was observed using a Leica TCS NT4D confocal imaging system (Leica, Heidelberg, Germany).
Northern and Western Blot Analyses
Total cellular RNA was isolated from confluent LEC and BVEC cultures at passages 4 and 5, using the TRIzol reagent (Invitrogen, Carlsbad, CA). Samples of RNA (10 μg each) were subjected to Northern blot analyses as described, 26 using a 2.0-kb human Prox1 cDNA probe and a 956-bp human type XVIII collagen probe (kindly provided by Dr. Y. Ninomiya). A 36B4 ribosomal-associated protein cDNA probe was used as a control for equal RNA loading. 28 For Western blot analyses, confluent LEC and BVEC cultures were homogenized in lysis buffer as described. 5 Ten μg of protein per sample were analyzed by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were immunoblotted with polyclonal antibodies against human VEGFR-2/KDR or VEGFR-3/Flt4 (Santa Cruz Biotechnology) as described. 5
Real-Time Quantitative RT-PCR
The ABI Prism 7000 Sequence Detection System was used to perform either SYBR-Green based or dual-labeled probe based real-time RT-PCR reactions as described. 29 Sequences of the primers used for this study are provided in Table 3 ▶ . For SYBR-Green based reactions, at least three sets of primers were used for each gene of interest. SYBR-Green PCR Master Mix was used for all SYBR-Green reactions with the addition of MultiScribe reverse transcriptase (Applied Biosystems, Foster City, CA). For dual-labeled probe-based real-time RT-PCR reactions, probes labeled with 6-FAM and TAMRA at their 5′ and 3′ end, respectively, were multiplexed with a GAPDH probe labeled with Joe and TAMRA at the 5′ and 3′ end as an internal control. TaqMan EZ RT-PCR Core Reagent was used for dual-labeled probe based reactions. Total RNAs were isolated as described above and were treated with RNase-free RQ-DNase (Promega, Madison, WI) before analyses. Twenty ng of total RNA were used for each reaction. The primers and probes were designed using Primer Express software (Perkin Elmer Life Sciences, Boston, MA) and were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). Expression data were normalized based on the expression levels of GAPDH mRNA.
Table 3.
Names of Genes and Primer Sequences for the SYBR-Green-Based Real-Time RT-PCR
| Gene names | Forward primers | Reverse primers |
|---|---|---|
| CD44 | AAAGGAGCAGCACTTCAGGA | CTGTCTGTGCTGTCGGTGAT |
| CEA-CAM | GTAGCAAAGCCCCAAATCAA | AACGGATGGAGATTCCAGTG |
| Chondroitin sulfate proteoglycan 2 (versican) | TTTGCCACCCAGTTACAACA | GGGCCACAAGGACAGTAGTC |
| Collagen type I, alpha 2 chain | AGGAGTTGTTGGACCACAGG | TCCCTTCAATCCATCCAGAC |
| G protein-coupled receptor 39 | AGCACAGAACAGAGGGGCTA | GAGCAGGAGGGAGAGACAGA |
| Integrin alpha4 | GAGGAATTCCCACCACTTCA | ATTTCATGGGCACAAAACC |
| Integrin beta3 (GPIIIa, CD61) | TGGTCCTGCTCTCAGTGATG | GAATTCTTTTVCGGTCGTGGA |
| Intestinal trefoil factor | CCAGGCACTGTTCATCTCAG | GAGCATGGGACCTTTATTCG |
| Macrophage mannose receptor (MRC1) | GTGGCCGGAGTAGTCATCAT | TCTTGAGGTAGGTGCACACG |
| Membrane glycoprotein gp130 | TGAACGAGGGGAAGAAAATG | TGTGTGTTGCCCATTCAGAT |
| N-cadherin | TGGAGAACCCCATTGACATT | TGATCCCTCAGGAACTGTCC |
| Reelin | AGGACCGTTATGCTGGACAC | ACATGTCAAAGGCGATCCTC |
| VEGF receptor-1/Flt1 | GGCCTCTGATGGTGATTGTT | GTGCTGCATCCTTGTTGAGA |
| Names of Genes and Primer/Probe Sequences for the Taqman-Based Real-Time RT-PCR | |||
|---|---|---|---|
| Gene names | Forward primers | Reverse primers | Taqman probes |
| CCL21/SLC | GGTTCTGGCCTTTGGCATC | AGGCAACAGTCCTGAGCCC | FAM-CCAGGACCCAAGGCAGTGATGGA-TAMRA |
| GAPDH | GATTCCACCCATGGCAAT | GAAGATGGTGATGGGATTTC | JOE-CAAGCTTCCCGTTCTCAGCC-TAMRA |
| LYVE-1 | AGCTATGGCTGGGTTGGAGA | CCCCATTTTTCCCACACTTG | FAM-TTCGTGGTCATCTCTAGGATTAGCCCAAACC-TAMRA |
| Podoplanin | AGGCGGCGTTGCCAT | GTCTTCGCTGGTTCCTGGAG | FAM-CCAGGTGCCGAAGATGATGTGGTC-TAMRA |
| Prox1 | ACAAAATGGTGGCACGGA | CCTGATGTACTTCGGAGCCTG | FAM-CCCAGTTTCCAAGCCAGCGGTCTCT-TAMRA |
| VEGF-C | CACCACCAAACATGCAGCTG | TGAAAATCCTGGCTCACAAGC | FAM-CGGCCATGTACGAACCGCCAG-TAMRA |
| VEGFR3 (Flt4) | TCTGCTACAGCTTCCAGGTGG | GCAGCCAGGTCTCTGTGGAT | FAM-ATGGAGTTCCTGGCTTCCCGAAAGTG-TAMRA |
In Vitro and in Vivo Tube Formation Assays
Confluent LEC and BVEC cultures at passage 5 were labeled with 5-chloromethylfluorescein diacetate (CMFDA, Cell Tracker Green; Molecular Probes) or with 5(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR; Cell Tracker Orange) according to the manufacturer’s instructions.LECs or BVECs (2.5 × 105) were seeded onto each well of Matrigel-coated (Becton Dickinson, Franklin Lakes, NJ) 24-well plates and were incubated for 24 hours at 37°C. Cells were analyzed by using a Nikon TE-300 microscope and images were captured with a SPOT digital camera. In vivo tube formation was investigated by mixing 1 × 106 LECs or BVECs with 500 μl of Matrigel, followed by subcutaneous injection into SCID CV17 mice (Charles River Laboratories, Wilmington, MA). All experiments were performed three times with comparable results. After 7 days, mice were sacrificed and tissue samples were fixed with 4% paraformaldehyde overnight and embedded in paraffin. Six-μm paraffin sections were either stained with hematoxylin and eosin or were double-stained with antibodies against human CD31 and LYVE-1 as described above.
Oligonucleotide Array Analyses
Total cellular RNA was extracted from confluent fifth passage LEC and BVEC cultures, maintained in complete endothelial growth medium without addition of VEGF. Oligonucleotide array analyses were performed using the human 95Av2 (12,625 genes) gene arrays (GeneChip; Affymetrix, Santa Clara, CA) according to the manufacturer’s instructions. Arrays were scanned using an Affymetrix confocal scanner and analyzed by the Microarray Suite 5.0 software (Affymetrix). Intensity values were scaled so that the overall fluorescence intensity of each chip of the same type was equivalent. Genes with a P value of ≤0.002 were considered as significantly increased or decreased.
Proliferation Assays
BVECs or LECs (3.5 × 104) were seeded onto fibronectin-coated six-well plates. Triplicate dishes were treated without or with 100 ng/ml of recombinant human placental growth factor-1 (PlGF-1, R&D Systems), 100 ng/ml human VEGF-C (kindly provided by Dr. K. Alitalo), 100 ng/ml human VEGF-D (R&D Systems), or 10 ng/ml recombinant human VEGF-A (VEGF165, R&D Systems) in endothelial growth medium. After 48 hours, cells were trypsinized and cell numbers were determined using a hematocytometer. For thymidine incorporation assays, 2 × 104 cells were seeded into quadruplicate wells of 24-well plates in the presence or absence of the above growth factors. Thymidine incorporation was assessed after 36 hours as previously described. 30 For apoptosis assays, 1 × 104 cells were seeded into triplicate wells of 96-well plates in endothelial cell basal medium containing 2% fetal calf serum, and were treated with the above growth factors for 72 hours. Apoptosis rates were determined by the Cellular DNA Fragmentation ELISA kit (Roche, Germany) according to the manufacturer’s instructions. Statistical analyses were performed using the paired Student’s _t_-test.
Results
Selective Isolation of Lymphatic and Blood Vascular Endothelial Cells from Human Skin
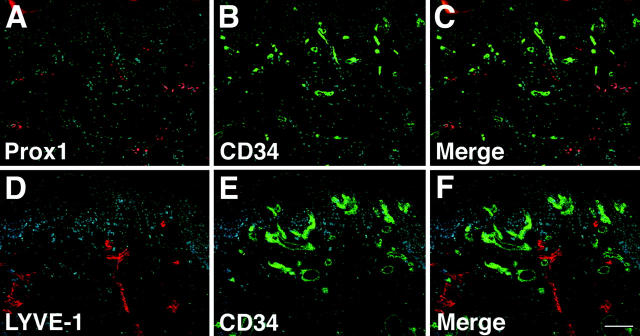
Using the lymphatic-specific transcription factor Prox1 22 as a specific marker for lymphatic endothelium, we found that CD34 was selectively expressed by Prox1-negative blood vessels in human skin, whereas Prox1-positive lymphatic vessels did not express CD34 (Figure 1; A to C) ▶ . These results were confirmed by immunofluorescence double stains with antibodies against the lymphatic-specific hyaluronan receptor LYVE-1 and against CD34, demonstrating mutually exclusive staining of lymphatic vessels with LYVE-1 and of blood vessels with CD34 (Figure 1; D to F) ▶ . All Prox1-, LYVE-1-, or CD34-positive vessels also expressed the pan-vascular marker CD31 (data not shown). These results revealed that CD34 was specifically expressed by blood vascular, but not by lymphatic, endothelium. We next isolated CD34-positive BVECs and CD34-negative/CD31-positive LECs by immunomagnetic purification directly from enzymatically digested neonatal human foreskins. After seeding on fibronectin-coated culture dishes, both LECs and BVECs maintained a typical cobblestone-like endothelial morphology for at least eight passages (Figure 2, A and B) ▶ .
Figure 1.
Specific detection of lymphatic and blood vessels in normal human skin. A–C: The homeobox gene Prox1 is specifically expressed by LECs (A, red), whereas blood vessels express CD34 (B, green). Double immunostains for Prox1 (red) and CD34 (green) reveal mutually exclusive vascular expression in lymphatic and blood vessels (C). Nuclei are labeled blue (Hoechst stain). D–F: Specific staining of lymphatic vessels with an antibody against the hyaluronan receptor LYVE-1 (D, red) and of blood vessels with an anti-CD34 antibody (E, green). F: Double immunostains for LYVE-1 (red) and CD34 (green) demonstrate mutually exclusive expression in lymphatics and blood vessels. Scale bar, 100 μm.
Figure 2.
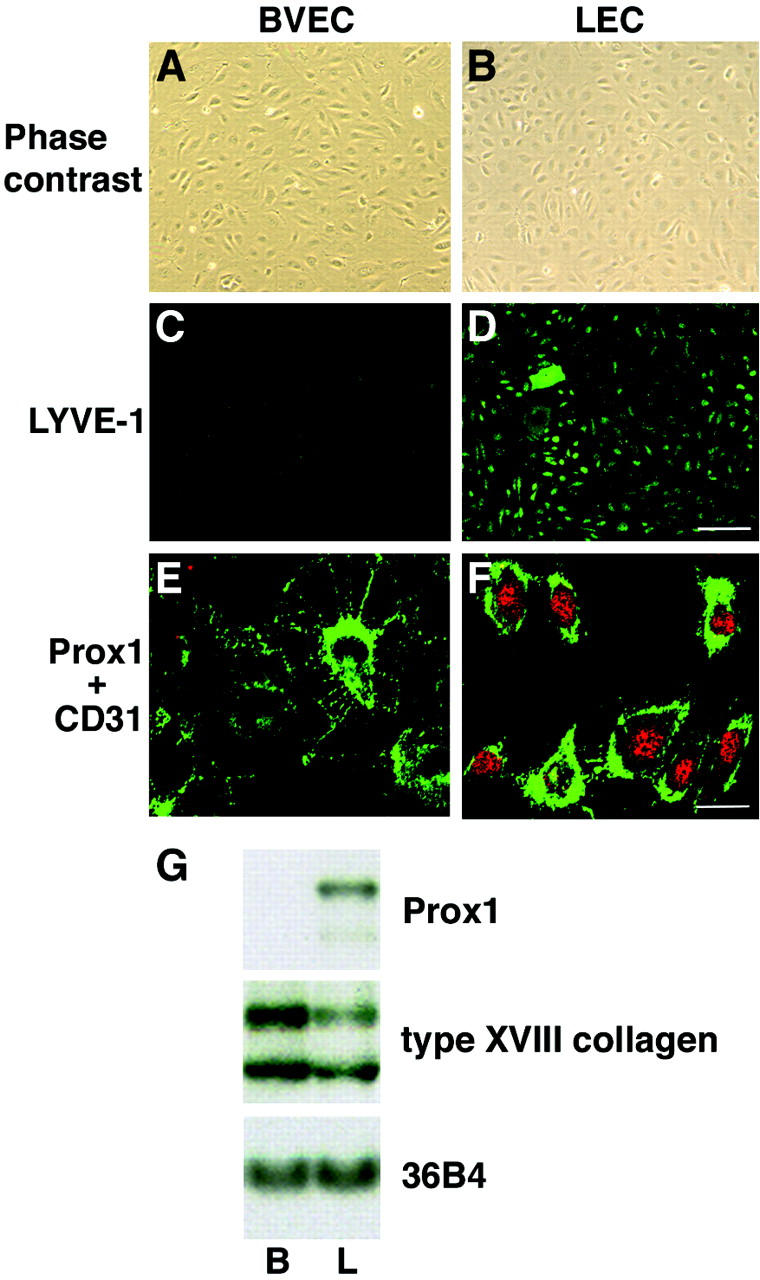
Cultured human dermal LECs and BVECs maintain their lineage-specific differentiation in vitro. A and B: Cultured BVECs and LECs (passage 4) show comparable endothelial morphology with characteristic cobblestone appearance. Phase-contrast micrograph. C and D: All LECs express the lymphatic marker LYVE-1 in vitro (D), whereas BVECs are not labeled (C). E and F: Double immunostains for CD31 (green) and Prox1 (red) demonstrate CD31 membrane labeling in both cell types whereas Prox1 (nuclear stain, red) is selectively expressed in LECs. G: Northern blot analysis confirms selective Prox1 mRNA expression in cultured LECs (L), whereas no expression is found in BVECs (B). In contrast, the basement membrane component type XVIII collagen is expressed at higher levels in BVECs. Hybridization with a probe to ribosomal protein-associated RNA 36B4 demonstrates equal loading. Scale bars: 100 μm (D); 25 μm (F).
Human Dermal LECs and BVECs Maintain Their Lineage-Specific Differentiation in Vitro
All cultured LECs maintained expression of the hyaluronan receptor LYVE-1 during the first eight passages (Figure 2D) ▶ , whereas LYVE-1 expression was absent from BVEC cultures (Figure 2C) ▶ . Double-immunofluorescence stains with antibodies to the endothelial junction molecule CD31 (PECAM-1 31 ) and the lymphatic-specific transcription factor Prox1 revealed membrane labeling for CD31 in all cultured cells, whereas only LECs showed nuclear staining for Prox1 (Figure 2, E and F) ▶ . Northern blot analyses confirmed that Prox1 mRNA was exclusively expressed in LECs but not in BVECs (Figure 2G) ▶ . In contrast, the vascular basement membrane molecule type XVIII collagen was more strongly expressed in BVECs than in LECs (Figure 2G) ▶ , in accordance with the only rudimentary basement formation in lymphatic vessels in vivo. 1 To further investigate the distinct mRNA expression of marker genes that have been previously reported to be specific for lymphatic vessels in normal human skin, we performed TaqMan quantitative real-time RT-PCR analysis of cultured LECs versus BVECs. We found a 74.1-fold higher expression of Prox1 mRNA expression in LECs, as compared with BVECs (Table 1) ▶ . Moreover, LECs showed highly increased expression of the lymphatic markers podoplanin, LYVE-1, VEGFR-3, and CCL21/secondary lymphoid chemokine, whereas the levels of VEGF-C were higher in BVECs than in LECs (Table 1) ▶ . Western blot analyses confirmed increased VEGFR-3 protein expression in LECs, whereas comparable levels of VEGFR-2 were found in both cell types (data not shown).
Table 1.
TaqMan Real-Time RT-PCR Analysis of Lineage-Specific Vascular Markers in Cultured LEC and BVEC
| Fold increase in LEC over BVEC | Fold increase in BVEC over LEC |
|---|---|
| Prox1 | 74.1 |
| Podoplanin | 54.6 |
| LYVE-1 | 6.7 |
| CCL21 | 4.6 |
| VEGFR-3/Flt4 | 4.4 |
| VEGF-C | 3.7 |
Isolated LECs and BVECs Maintain Their Lineage-Specific Differentiation during Tube Formation in Vivo
Twenty-four hours after seeding onto Matrigel-coated culture tissues, both BVECs and LECs efficiently formed a network of tube-like structures in vitro (Figure 3, A and B) ▶ . We next mixed BVECs or LECs with Matrigel that was implanted subcutaneously into immunodeficient mice. Seven days after injection, both LECs and BVECs had formed tube-like structures in vivo, occasionally with a distinguishable lumen (Figure 3, C and D) ▶ . Whereas both types of endothelial cells expressed CD31 in vivo (Figure 3, E and G) ▶ , only LECs (Figure 3H) ▶ , but not BVECs (Figure 3F) ▶ expressed the hyaluronan receptor LYVE-1. Double-immunofluorescence stains confirmed that LYVE-1 expression was restricted to human CD31-positive LECs in vivo (Figure 3, K and L) ▶ , whereas human CD31-positive BVECs did not express LYVE-1 (Figure 3, I and J) ▶ .
Figure 3.
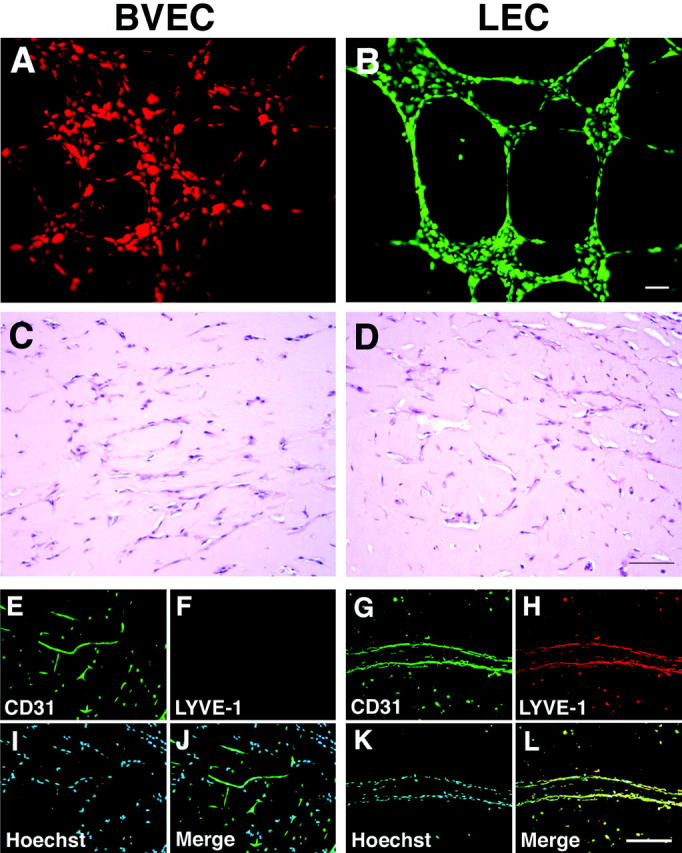
BVECs and LECs maintain lineage-specific differentiation after formation of tube-like structures in vivo. A and B: Cultured BVECs and LECs were fluorescently labeled with CMTMR (orange) or CMFDA (green), respectively, and were seeded onto Matrigel. Both BVECs (A) and LECs (B) demonstrate formation of tube-like structures in vitro, a characteristic feature of endothelial cells. C–L: Isolated BVECs or LECs were mixed with Matrigel and were implanted subcutaneously into SCID mice. Efficient formation of tube-like structures by BVECs (C) and LECs (D) after 7 days in vivo. H&E stains. Both BVECs (E) and LECs (G) express CD31 (green) in vivo, whereas only tubes formed by LECs (H), but not by BVECs (F) are also positive for LYVE-1 (red). I and K: Nuclear stain with Hoechst (blue). J and L: Double immunostains for CD31 (green) and LYVE-1 (red), combined with nuclear stains (blue), confirm lineage-specific tube formation in Matrigel. Scale bars: 50 μm (B); 100 μm (D, L).
Identification of Genes with Specific Expression in Blood Vascular Endothelium by Gene Array Analysis of Cultured BVECs versus LECs
Comparative gene array analyses of cultured BVECs and LECs revealed that only a small percentage of genes (1.2%) showed at least twofold increased expression in BVECs (1.6% in LECs) and that only 0.37% of all genes were increased by fourfold or more in BVECs (0.39% in LECs), in agreement with the close lineage connection between both types of vascular cells. We focused our analysis on growth factors, chemokines, their receptors, extracellular matrix and adhesion molecules that likely play essential roles in the development and function of the blood vascular system. BVECs showed highly increased gene expression of several extracellular matrix molecules, including the chondroitin sulfate proteoglycan versican; type I, III, and VI collagen; SPARC; and fibronectin (Table 2) ▶ . In accordance with the in vivo formation of a regular vascular basement membrane by BVECs, but not LECs, the expression of the basement membrane molecules laminin, type IV collagen, and perlecan was increased in cultured BVECs. BVECs also expressed significantly higher levels of several adhesion molecules and adhesion receptors, including N-cadherin; integrins α4, α5, β3, and β5; and neural cell adhesion molecule (Table 2) ▶ . In addition to VEGF-C, several other vascular growth factors showed increased expression by BVECs, most prominently PlGF and VEGF-B and their receptor VEGFR-1/Flt1. Moreover, endoglin, a low-affinity receptor for transforming growth factor-β, and the hyaluronan receptor CD44 were more strongly expressed by BVECs than by LECs (Table 2) ▶ .
Table 2.
Genes with Significantly (P ≤ 0.002) Increased Expression in LEC or BVEC
| Accession no. | Fold increase in LEC over BVEC | Fold increase in BVEC over LEC |
|---|---|---|
| Growth factors and chemokines | ||
| Vascular endothelial growth factor-C* | X94216 | 42.2 |
| Placenta growth factor | X54936 | 3.0 |
| Vascular endothelial growth factor-B | U43368 | 1.7 |
| Transforming growth factor-beta | M60315 | 1.4 |
| Platelet-derived growth factor-B | M12783 | 1.4 |
| Platelet-derived growth factor-A | X06374 | 1.2 |
| Melanoma growth stimulatory activity (Gro1alpha) | X54489 | 1.2 |
| MCP-1 | M26683 | 1.2 |
| Exodus-1 (CCL20, MIP-3alpha) | U64197 | 39.4 |
| Fibroblast growth factor-12 | AL119322 | 14.9 |
| Transforming growth factor-alpha | X70340 | 4.0 |
| Thrombospondin-1 | U12471 | 3.2 |
| Follistatin | M194812.6 | |
| Stromal-cell derived factor-1 (SDF-1) | L36033 | 2.0 |
| RANTES | M21121 | 1.6 |
| Angiopoietin-2 | AF004327 | 1.5 |
| Receptors | ||
| G protein-coupled receptor 39* | AI936826 | 11.3 |
| Chemokine receptor X (CKRX) | AF014958, AF410948 | 9.2, 9.2 |
| VEGF receptor-1/Flt1* | U01134 | 6.5 |
| Interleukin-13 receptor | U70981 | 6.1 |
| CD44* | AF098641, M59040 | 3.5, 2.8 |
| Latent TGF-beta binding protein | Z37976 | 3.2 |
| CXCR4 | L06797 | 3.0 |
| Colony stimulating factor 2 receptor, beta | H04668 | 2.5 |
| Thrombin receptor | M62424 | 2.3 |
| Complement Iq receptor | U94333 | 2.1 |
| Endoglin | X72012 | 1.7 |
| Insuline-like growth factor II receptor | Y00285 | 1.6 |
| Interleukin 4 receptor | X52425 | 1.6 |
| TRAIL receptor 2 | AF016266 | 1.4 |
| Jagged2 | AF029778 | 1.2 |
| Macrophage mannose receptor | M93221 | 22.6 |
| Proteinase-activated receptor-2 | U34038 | 4.6 |
| Membrane glycoprotein gp 130* | M57230 | 3.7 |
| 46 kDa Coxsackievirus and adenovirus receptor | Y07538 | 3.5 |
| Orphan G protein-coupled receptor | AI674208 | 3.5 |
| Glycine receptor beta subunit | U33267 | 2.5 |
| Intracellular hyaluronic acid binding protein | AF032862 | 1.7 |
| Jagged1 | U77914, AF003837 | 1.6, 1.3 |
| CGRP type 1 receptor | L76380 | 1.5 |
| Extracellular matrix molecules | ||
| Chondroitin sulfate proteoglycan 2 (versican)* | D32039, X15998 | 59.7, 13.9 |
| Collagen type III, alpha 1 chain | AB007902 | 45.2 |
| Collagen type VI, alpha3 chain | X52022 | 8.0 |
| Collagen type I, alpha 2 chain* | J03464, V00503 | 4.9, 4.3 |
| Collagen type VI, alpha 1 chain | X15580 | 3.0 |
| Bone morphogenetic protein 1 | M22488 | 2.6 |
| Fibulin 5 | AF093118 | 2.6 |
| Fibulin-like extracellular matrix protein 2 | AF093119 | 2.1 |
| SPARC/osteonectin | J03040 | 2.0 |
| Laminin, beta 2 chain | M55210, X79683 | 1.9, 1.7 |
| Laminin, beta 1 chain | M61916 | 1.7 |
| Fibronectin | M10905, X02761 | 1.7, 1.5 |
| Procollagen type V, alpha 2 chain | Y14690 | 1.7 |
| Perlecan | M85289 | 1.3 |
| Proteoglycan 1 | X17042 | 1.3 |
| Collagen type IV, alpha 2 chain | X05610 | 1.2 |
| Reelin* | U79716 | 26.0 |
| Extracellular matrix protein (MFAP3) | L35251 | 3.0 |
| Adhesion Molecules | ||
| Integrin alpha4* | X16983 | 13.0 |
| Desmoglein 1 | AF097935 | 13.0 |
| N-cadherin* | M34064 | 4.0 |
| Integrin beta3 (GPIIIa, CD61)* | M35999 | 2.5 |
| Integrin alpha 5 | X06256 | 2.1 |
| Platelet/endothelial cell adhesion molecule (CD31) | AA100961, L34657 | 1.7, 1.5 |
| Neural cell adhesion molecule (NrCAM) | AB002341 | 1.7 |
| H-cadherin | U59289 | 1.7 |
| Protocadherin 42 | L11370 | 1.7 |
| Integrin beta5 | X53002, M35011 | 1.6, 1.5 |
| CD151 | D29963 | 1.6 |
| Intercellular adhesion molecule-2 (ICAM-2) | X15606 | 1.6 |
| VE-cadherin | X79981 | 1.3 |
| CEA-CAM* | S71326, X16354 | 59.7, 3.5 |
| Desmoplakin-1 | AL031058 | 4.6 |
| Galectin 8 | L78132 | 2.6 |
| Integrin alpha 6B | S66213 | 1.9 |
| Plakoglobin | M23410 | 1.3 |
| Miscellaneous | ||
| Von Willebrand factor | M10321 | 2.3 |
| Endothelial cell-specific molecule 1 | X89426 | 2.3 |
| Podoplanin | A1660929, AF030428 | 16.0, 113 |
| Proxl | U44060 | 4.6 |
| Intestinal trefoil factor* | AI985964 | 3.2 |
| Down syndrome critical region gene 2 | AJ006291 | 1.7 |
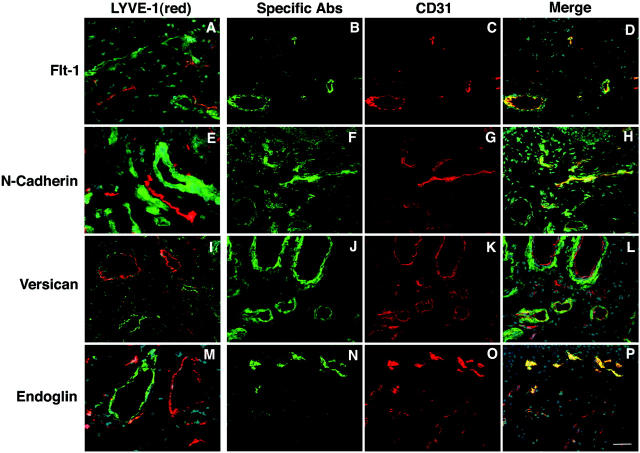
We next used double-immunofluorescence stains for the pan-vascular marker CD31 or the lymphatic marker LYVE-1 to investigate the expression of several of the BVEC-expressed genes in normal skin in situ. These studies revealed that VEGFR-1/Flt1, N-cadherin, and versican were exclusively expressed by LYVE-1-negative, CD31-positive blood vessels, but not by lymphatic endothelium (Figure 4; A to L) ▶ . Strong endoglin expression was detected on all blood vessels, whereas its expression was low or absent in LYVE-1-positive lymphatic vessels (Figure 4; M to P) ▶ .
Figure 4.
Blood vessel-specific expression of VEGFR-1/Flt1, N-cadherin, versican, and endoglin in normal human skin. Differential immunostains reveal mutually exclusive expression of LYVE-1 (red) on lymphatic vessels and of VEGFR-1/Flt-1 (A), N-cadherin (E), versican (I), and endoglin (M) on cutaneous blood vessels (green). Nuclear Hoechst counterstain (blue) was used to depict skin morphology. Double immunostains for the pan-endothelial marker CD31 (red) and selected blood vascular markers (green) demonstrate that only a fraction of all vessels also express VEGFR-1/Flt1 (B–D), N-cadherin (F–H), versican (J–L), or endoglin (N–P). Scale bar, 50 μm.
Expression of VEGFR-1/Flt1 and Endoglin during Embryonic Development of the Lymphatic Vascular System
Throughout the murine embryonic development of the lymphatic vascular system, VEGFR-1/Flt-1 was expressed selectively on BVECs with a particularly high expression on endothelial cells of the dorsal aorta (Figure 5A) ▶ . In contrast, VEGFR-1 was not detected on Prox1-expressing lymphatic progenitor endothelial cells located in the cardinal vein or on Prox1-positive cells that had already budded from the vein. In contrast, endoglin was expressed on both blood vascular and LECs at E11.5 (Figure 5B) ▶ , with low or absent expression in single Prox1-positive LECs after budding. At E14.5, endoglin was strongly expressed on blood vessels, whereas lymphatic vessels show reduced expression (Figure 5C) ▶ .
Figure 5.
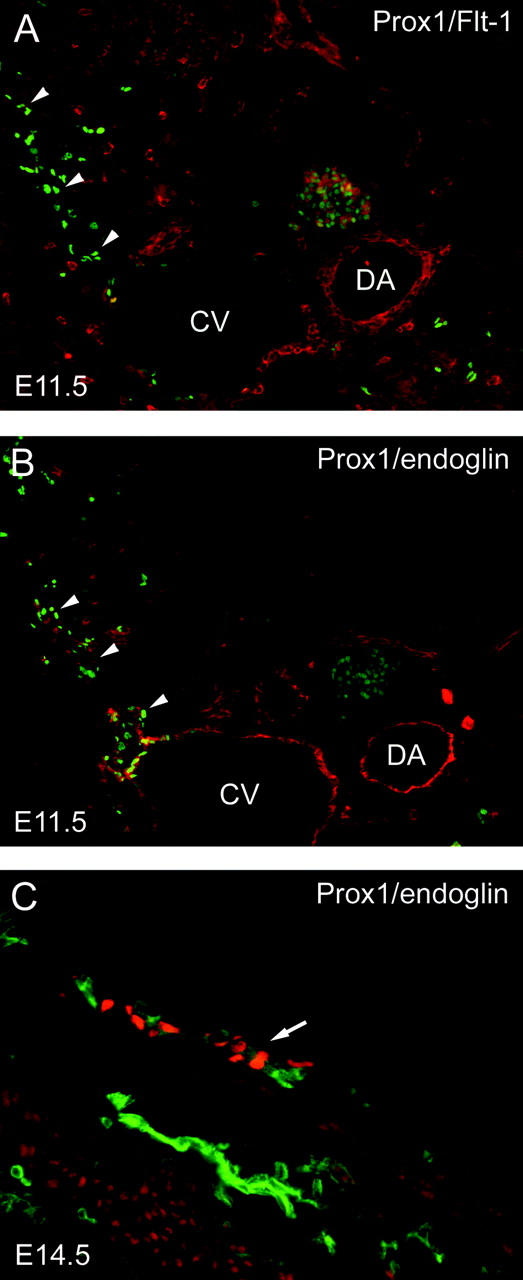
Expression of endoglin and VEGFR-1/Flt-1 during embryonic development of the lymphatic vascular system. Throughout the embryonic development of the lymphatic vascular system, Flt-1 is expressed selectively on BVECs, whereas endoglin is expressed on both blood vascular and LECs. A: Flt-1 expression (red) is limited to the vascular endothelium at E11.5 and is particularly high on endothelial cells of the dorsal aorta (DA). In contrast, Flt-1 is not detected on LECs located in or budding from (arrowheads) the cardinal vein (CV). Lymphatic endothelial cells are identified by the expression of Prox1 (green). B: In an adjacent section, endoglin expression (red) is detected on both blood vascular and LECs. C: At E14.5, endoglin is strongly expressed on blood vessels (green), whereas lymphatic vessels show reduced expression (arrow, detected by expression of Prox1, red).
VEGF-A Stimulates Proliferation of Both BVECs and LECs
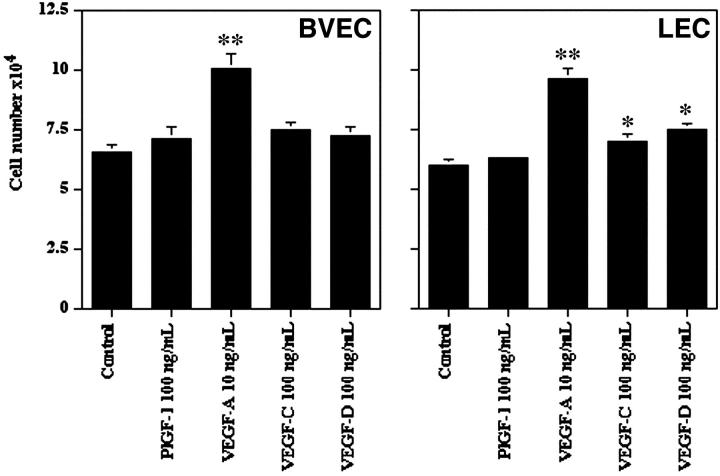
Both LECs and BVECs responded equally well to the mitogenic stimulation by VEGF-A (Figure 6) ▶ , a ligand for both VEGFR-1 and for VEGFR-2 that was equally expressed on both cell types. Moreover, VEGF-A treatment potently stimulated DNA synthesis, as measured by 3H-thymidine incorporation, of both BVECs (+147.4%, P < 0.05) and LECs (+281.9%, P < 0.01), and also reduced cellular apoptosis rates of both BVECs (−15.0%, P < 0.05) and LECs (−23.2%, P < 0.01). In contrast, the lymphangiogenesis factors VEGF-C and VEGF-D, ligands for VEGFR-2 and VEGFR-3, promoted LEC growth more potently than BVEC proliferation (Figure 6) ▶ , in accordance with the higher levels of VEGFR-3 expression in LECs.
Figure 6.
LEC and BVEC proliferation was equally stimulated by VEGF-A. Both VEGF-C and VEGF-D induced LEC proliferation with only minor effects on BVECs. Mean values ± SD. *, P < 0.05; **, P < 0.01.
Identification of Genes with Predominant Expression in Lymphatic Endothelium by Gene Array Analysis
LECs expressed increased levels of proxl, podoplanin and several chemokines including stromal cell-derived factor-1 (SDF-1), RANTES, and exodus-1/CCL20/MIP3-α, a molecule involved in dendritic cell attraction to lymphatic endothelium (Table 2) ▶ . Moreover, we detected increased expression of the extracellular matrix protein reelin and of several molecules involved in adhesion, including CEA-CAM, galectin 8, integrin α 6B, desmoplakin, and plakoglobin. LECs also expressed higher levels of the macrophage mannose receptor, the membrane glycoprotein gp130 and intestinal trefoil factor (Table 2) ▶ . Immunofluorescence double stains of normal human skin confirmed that desmoplakin, CCL20/MIP-3α, and macrophage mannose receptor were selectively expressed by LYVE-1-positive lymphatic vessels but not by CD31-positive/LYVE-1-negative blood vessels (Figure 7) ▶ .
Figure 7.
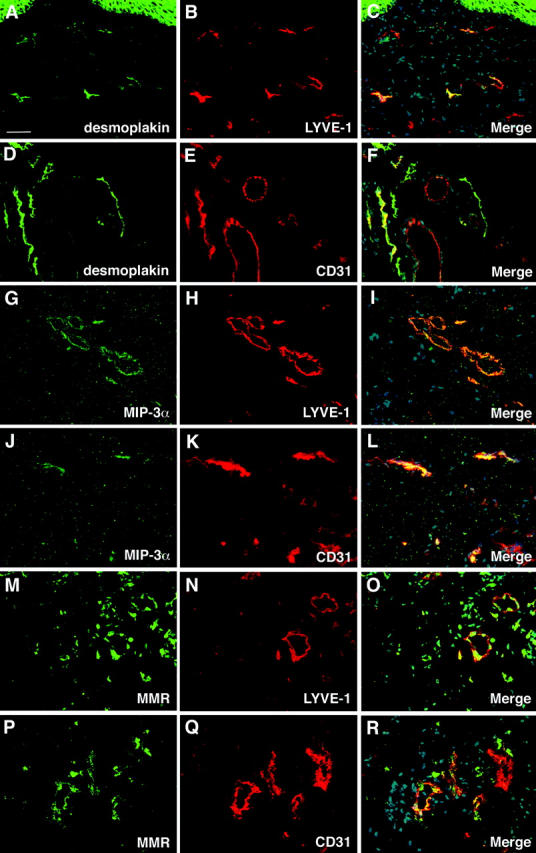
Lymphatic endothelium-specific expression of desmoplakin, CCL20/MIP-3α and macrophage mannose receptor in normal human skin. A–C: All desmoplakin-positive lymphatic vessels also express LYVE-1, whereas only a fraction of CD31-positive vessels also expresses desmoplakin (D–F). G–I: Co-localization of MIP-3α and LYVE-1 in cutaneous lymphatic vessels. J–L: Blood vessels (red in L) do not express MIP-3α. M–O: Expression of macrophage mannose receptor (MMR) is detected on all LYVE-1-positive small lymphatic vessels (orange in O), whereas MMR expression is absent from blood vessels (P–R); blood vessels are depicted in red in R. Scale bar, 50 μm.
Discussion
We developed a novel immunomagnetic isolation technique for the direct purification of LECs and BVECs from human skin, based on the recent findings that the expression of the lymphatic hyaluronan receptor LYVE-1 and of CD34 on blood vessels was mutually exclusive in postnatal murine skin. 5,20,22 Whereas these findings suggested that CD34 is specifically expressed by blood vascular but not by lymphatic endothelium, co-expression of the proposed lymphatic marker podoplanin and of CD34 was recently reported in a subpopulation of endothelial cells in human skin. 17 However, we found that in normal human skin, CD34 expression was absent from lymphatic vessels that all expressed the specific markers Prox1 22,32 and LYVE-1 19 and, conversely, that all CD34-positive vessels were negative for these lymphatic markers. Although the function of CD34 expressed by mature blood vascular endothelium has remained poorly defined, recent studies suggest that CD34 may recognize L-selectin if decorated by 6-sulfosialyl Lewis X saccharides and the MECA-79 epitope. 33
Based on these findings, we isolated BVECs directly from human foreskins by positive selection for CD34 and LECs by consecutive positive selection of the remaining CD34-negative cells for expression of the endothelial junction molecule CD31 31 that is expressed by both blood vascular and lymphatic endothelium. 2 Immunostains revealed that expression of Prox1 and of LYVE-1 was completely absent from cultured BVECs, whereas all LECs expressed both lymphatic-specific markers, confirming that the isolation procedure yielded pure cell populations and that cultured LECs and BVECs maintain cell lineage-specific differentiation even after multiple passages in vitro. These findings were confirmed by the highly increased expression levels of the lymphatic markers Prox1, LYVE-1, VEGFR-3/Flt4, 34 podoplanin, 16 and CCL21 (secondary lymphoid chemokine) 35 in LECs. Our finding that expression of the lymphangiogenesis factor VEGF-C was markedly higher in BVECs than in LECs is in accordance with previously reported results. 17,18
Isolated LECs efficiently formed tube-like structures after seeding onto Matrigel in vitro and after subcutaneous transplantation into immunosuppressed mice in vivo, which maintained the expression of the lymphatic-specific hyaluronan receptor LYVE-1 for at least 1 week after transplantation. Whereas future studies are needed to investigate whether LEC tubes will also function in lymphatic drainage of tissue fluid, these results demonstrate, for the first time, that transplanted LECs can reassemble to form lymphatic vascular structures in vivo, with possible implications for future strategies to treat lymphedemas in humans.
Our findings that only a small percentage of all genes investigated by gene profiling studies showed differential expression in LECs versus BVECs further corroborates the close relationship of the blood vascular and the lymphatic vascular system. These results are in accordance with the proposed development of lymphatic progenitor endothelial cells from embryonic veins 2,22 and with our recent findings that ectopic expression of the transcription factor Prox1, a master control gene in the program specifying LEC fate, in blood vascular endothelium was sufficient to program these cells to adopt a LEC phenotype. 24 Importantly, we found that several genes involved in the formation of the vascular basement membrane showed increased expression in cultured BVECs, as compared with LECs. Because both types of cells were cultured under identical in vitro conditions, these results suggest that the absent or only rudimentary basement membrane formation of lymphatic vessels in vivo is not merely because of the lack of the appropriate environmental stimuli, but represents an intrinsic, lineage-specific feature of lymphatic endothelium. Indeed, recent studies in mouse embryos have revealed that budding lymphatic endothelial progenitor cells gradually down-regulate the expression of laminin, whereas blood vascular endothelium maintained strong expression of this basement membrane component. 22 In contrast, _Prox1_-deficient endothelial cells failed to down-regulate laminin expression after initial budding from embryonic veins. 22
Mutations in the gene for the low-affinity transforming growth factor-β receptor endoglin have been found to be associated with the autosomal-dominant vascular malformations of hereditary hemorrhagic teleangiectasia type I. 36 We found that endoglin expression was increased in cultured BVECs and that endoglin was strongly expressed on blood vessels, but was absent from or only sparsely expressed in lymphatic endothelium in vivo, in accordance with the observed vascular, but not lymphatic, abnormalities in hereditary hemorrhagic teleangiectasia. VEGFR-1, a receptor for VEGF-A, VEGF-B, and PlGF, was more strongly expressed by BVECs than LECs in vitro, and was specifically expressed by blood vascular endothelium, but not by lymphatic endothelium, during embryonic development and postnatally in vivo. These results provide a potential explanation for the recent findings that intradermal injection of a VEGF-A-encoding adenovirus into mouse ears potently induced the formation of both lymphatic and blood vessels, whereas injection of an adenovirus encoding PlGF, a specific ligand for VEGFR-1, 37 selectively induced blood vascular angiogenesis but not lymphangiogenesis. 38
Lymphatic endothelium secretes the chemokine CCL21 (secondary lymphoid chemokine) that binds to CC chemokine receptor 7 (CCR7), 3,17,35 leading to chemoattraction and migration of mature dendritic cells from the skin to regional lymph nodes 3 and to enhanced lymph node metastasis of CCR7-expressing malignant melanoma xenotransplants. 39 Using gene array profiling studies, we found additional chemokines with enhanced expression in LECs, including stromal cell-derived factor-1, RANTES, and CCL20 (MIP-3α, exodus-1). Recently, low-level expression of CCL20 was detected in podoplanin-positive cultured LECs but not in podoplanin-negative cells, whereas both types of cells potently up-regulated CCL20 expression on stimulation with inflammatory cytokines. 17 We confirmed our in vitro findings by differential immunostains of normal human skin, demonstrating that CCL20 was specifically expressed on lymphatic vessels in noninflamed skin. Our results suggest that additional chemokines, secreted by lymphatic endothelium, might participate in the recruitment of antigen-presenting cells and, possibly, tumor cells to regional lymph nodes.
It is of interest that angiopoietin-2 showed increased expression in cultured LECs since recent studies in angiopoietin-2-deficient mice suggest an important role of angiopoietin-2 for the final developmental steps of lymphatic network patterning and lymphatic vessel maturation. 40 Reelin was one of the genes with the highest increase of expression in LECs, as compared with BVECs. During the review process of this manuscript, another article also described results of gene array studies of LECs and BVECs, including several genes of the present study such as angiopoietin-2 and reelin. 41 Recently, it has been shown that autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human reelin mutations. 42 Although we were unable to detect specific reelin protein expression in cutaneous lymphatic vessels with the available antibodies, some humans with reelin mutations also show congenital lymphedema, 42 suggesting a potential function of this gene in lymphatic development.
In summary, our study reveals that LECs and BVECs maintain the distinct expression of a large number of lineage-specific genes in vitro and after transplantation in vivo, and it corroborates the close relationship between the blood vascular and lymphatic system. Moreover, these results demonstrate that some lineage-specific genes are only expressed during distinct developmental stages of vascular development, and they identify new molecular markers for blood vascular and lymphatic endothelium with important implications for vascular development, the regulation of the immune response, and the molecular control of tumor progression.
Acknowledgments
We thank J. Bertoncini, L. Janes, D. Lipoff, and L. Nguyen for expert technical assistance; H. Su for assistance with confocal microscopy; G. Oliver for helpful discussions and for providing Prox1 antibody; D. Jackson for the gift of LYVE-1 antibodies; K. Alitalo for the gift of human VEGF-C; C. A. Walsh for providing anti-reelin antibodies; and Y. Ninomiya for providing type XVIII collagen cDNA.
Footnotes
Address reprint requests to Michael Detmar, M.D., CBRC/Department of Dermatology, Massachusetts General Hospital, Building 149, 13th St., Charlestown, MA 02129. E-mail: michael.detmar@cbrc2.mgh.harvard.edu.
Supported by the National Institutes of Health/National Cancer Institute (grants CA69184, CA86410, and CA91861 to M. D.), the Susan G. Komen Breast Cancer Foundation (to M. D.), the American Cancer Society (program project grant 99-23901 to M. D.), the Sturge-Weber Foundation (to S. H.), and by the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd. Agreement (to M. D.).
References
- 1.Witte MH, Bernas MJ, Martin CP, Witte CL: Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech 2001, 55:122-145 [DOI] [PubMed] [Google Scholar]
- 2.Oliver G, Detmar M: The rediscovery of the lymphatic system. Old and new insights into the development and biological function of the lymphatic vascular system. Genes Dev 2002, 16:773-783 [DOI] [PubMed] [Google Scholar]
- 3.Saeki H, Moore AM, Brown MJ, Hwang ST: Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol 1999, 162:2472-2475 [PubMed] [Google Scholar]
- 4.Alitalo K, Carmeliet P: Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 2002, 1:219-227 [DOI] [PubMed] [Google Scholar]
- 5.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M: Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001, 7:192-198 [DOI] [PubMed] [Google Scholar]
- 6.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG: VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001, 7:186-191 [DOI] [PubMed] [Google Scholar]
- 7.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS: Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 2001, 20:672-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K: Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 2001, 61:1786-1790 [PubMed] [Google Scholar]
- 9.Gale NW, Yancopoulos GD: Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev 1999, 13:1055-1066 [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P: Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000, 6:389-395 [DOI] [PubMed] [Google Scholar]
- 11.Detmar M, Hirakawa S: The formation of lymphatic vessels and its importance in the setting of malignancy. J Exp Med 2002, 196:713-718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lymboussaki A, Partanen TA, Olofsson B, Thomas CJ, Fletcher CD, de Waal RM, Kaipainen A, Alitalo K: Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am J Pathol 1998, 153:395-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R, Alitalo K: VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol 1999, 154:1381-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K: Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol 2000, 156:1499-1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubo H, Fujiwara T, Jussila L, Hashi H, Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K, Yamaoka Y, Nishikawa SI: Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood 2000, 96:546-553 [PubMed] [Google Scholar]
- 16.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D: Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999, 154:385-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kriehuber E, Breiteneder GS, Groeger M, Soleiman A, Schoppmann SF, Stingl G, Kerjaschki D, Maurer D: Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med 2001, 194:797-808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maekinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K: Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J 2001, 20:4762-4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG: LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 1999, 144:789-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG: Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem 2001, 276:19420-19430 [DOI] [PubMed] [Google Scholar]
- 21.Skobe M, Detmar M: Structure, function and molecular control of the skin lymphatic system. J Invest Dermatol Symp Proc 2000, 5:14-19 [DOI] [PubMed] [Google Scholar]
- 22.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G: An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 2002, 21:1505-1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wigle JT, Oliver G: Prox1 function is required for the development of the murine lymphatic system. Cell 1999, 98:769-778 [DOI] [PubMed] [Google Scholar]
- 24.Hong Y-K, Harvey N, Noh Y-H, Schacht V, Hirakawa S, Detmar M, Oliver G: Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 2002, 225:351-357 [DOI] [PubMed] [Google Scholar]
- 25.Richard L, Velasco P, Detmar M: A simple immunomagnetic protocol for the selective isolation and long-term culture of human dermal microvascular endothelial cells. Exp Cell Res 1998, 240:1-6 [DOI] [PubMed] [Google Scholar]
- 26.Detmar M, Brown LF, Schön MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK: Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol 1998, 111:1-6 [DOI] [PubMed] [Google Scholar]
- 27.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M: Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol 2001, 159:893-903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Detmar M, Brown LF, Berse B, Jackman RW, Elicker BM, Dvorak HF, Claffey KP: Hypoxia regulates the expression of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptors in human skin. J Invest Dermatol 1997, 108:263-268 [DOI] [PubMed] [Google Scholar]
- 29.Hawighorst T, Skobe M, Streit M, Hong YK, Velasco P, Brown LF, Riccardi L, Lange-Asschenfeldt B, Detmar M: Activation of the tie2 receptor by angiopoietin-1 enhances tumor vessel maturation and impairs squamous cell carcinoma growth. Am J Pathol 2002, 160:1381-1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Detmar M, Mayer-da-Silva A, Stadler R, Orfanos CE: Effects of azelaic acid on proliferation and ultrastructure of mouse keratinocytes in vitro. J Invest Dermatol 1989, 93:70-74 [DOI] [PubMed] [Google Scholar]
- 31.Dejana E, Corada M, Lampugnani MG: Endothelial cell-to-cell junctions. EMBO J 1995, 9:910-918 [PubMed] [Google Scholar]
- 32.Wilting J, Papoutsi M, Christ B, Nicolaides KH, Von Kaisenberg CS, Borges J, Stark GB, Alitalo K, Tomarev SI, Niemeyer C, Rossler J: The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. FASEB J 2002, 16:1271-1273 [DOI] [PubMed] [Google Scholar]
- 33.Satomaa T, Renkonen O, Helin J, Kirveskari J, Makitie A, Renkonen R: O-glycans on human high endothelial CD34 putatively participating in L-selectin recognition. Blood 2002, 99:2609-2611 [DOI] [PubMed] [Google Scholar]
- 34.Kaipainen A, Korhonen J, Mustonen T, van HV, Fang GH, Dumont D, Breitman M, Alitalo K: Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 1995, 92:3566-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT: A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA 1998, 95:258-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shovlin CL, Scott J: Inherited diseases of the vasculature. Annu Rev Physiol 1996, 58:483-507 [DOI] [PubMed] [Google Scholar]
- 37.DiPalma T, Tucci M, Russo G, Maglione D, Lago CT, Romano A, Saccone S, Della VG, De GL, Dragani TA, Viglietto G, Persico MG: The placenta growth factor gene of the mouse. Mamm Genome 1996, 7:6-12 [DOI] [PubMed] [Google Scholar]
- 38.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar M, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF: Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF, VEGF-A) induces lymphangiogenesis as well as angiogenesis. J Exp Med 2002, 196:1497-1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiley HE, Gonzalez EB, Maki W, Wu MM, Hwang ST: Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst 2001, 93:1638-1643 [DOI] [PubMed] [Google Scholar]
- 40.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain C, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD: Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell 2002, 3:411-423 [DOI] [PubMed] [Google Scholar]
- 41.Petrova TV, Maekinen T, Maekelae TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Ylae-Herttuala S, Alitalo K: Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 2002, 21:4593-4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, Walsh CA: Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet 2000, 26:93-96 [DOI] [PubMed] [Google Scholar]