IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization (original) (raw)
Abstract
Cytokines play an important role in modulating inflammatory responses and, as a result, airway tone. IL-10 is a regulatory cytokine that has been suggested for treatment of asthma because of its immunosuppressive and anti-inflammatory properties. In contrast to these suggestions, we demonstrate in a model of allergic sensitization that mice deficient in IL-10 (IL-10−/−) develop a pulmonary inflammatory response but fail to exhibit airway hyperresponsiveness in both in vitro and in vivo assessments of lung function. Reconstitution of these deficient mice with the IL-10 gene fully restores development of airway hyperresponsiveness comparable to control mice. These results identify an important role of IL-10, downstream of the inflammatory cascade, in regulating the tone of the airways after allergic sensitization and challenge.
One of the basic characteristics of asthma is airway hyperresponsiveness (AHR), which increases after exposure to allergen. The level of responsiveness is demonstrated by showing increased responses to bronchoconstrictors such as methacholine (MCh). This heightened responsiveness is thought to result from a complex inflammatory cascade involving several cell types, including T lymphocytes and eosinophils (1, 2). T lymphocytes exert many of their effects by secreting an array of cytokines. In allergic asthma, type 2 T helper (Th) cell (Th2) cytokines dominate over Th1 cytokines and several studies suggest a critical role for IL-4, IL-5, and IL-13 in the development of AHR (3). The mechanisms underlying cytokine-mediated influences on the tone of the airways are still largely unknown.
IL-10 originally was described in mice as a factor inhibiting cytokine production from murine Th1 clones (4). Subsequent studies showed that IL-10 also can down-regulate Th2 clones and their production of IL-4 and IL-5 (5). In addition, IL-10 expresses a wide variety of effects on other immune cells, including stimulation of B cell differentiation and Ig secretion (6). The true biological effects of IL-10 have been difficult to delineate because the activities of this molecule on immune responsiveness vary considerably (7). However, it is known that adult mice deficient in IL-10 (IL-10−/−) develop a CD4 T cell-dependent and IFN-γ-mediated enterocolitis (8).
The data concerning the role of IL-10 in allergic inflammation and AHR are contradictory. A few reports found reduced IL-10 mRNA expression both in peripheral blood mononuclear cells and bronchoalveolar lavage (BAL) lymphocytes of asthmatic patients (5) whereas others have demonstrated elevated levels in asthmatics (9–11). Because of its immunosupressive properties in vitro and in animal models, IL-10 has been suggested as a potential therapy of allergic inflammation and asthma (12).
To define the role of IL-10 in controlling the development of allergic inflammation and AHR, we used an established mouse model of eosinophilic airway inflammation and allergen-driven alterations in airway function. Here, we describe that IL-10-deficient mice, sensitized and challenged to ovalbumin (OVA), fail to develop AHR despite a significant eosinophilic airway inflammatory response. Only after reconstitution with IL-10 could changes in airway responsiveness be detected. These data imply a major role for IL-10 in the regulation of airway function downstream of the inflammatory cascade.
Methods
Animals.
Homozygous IL-10-deficient mice (IL-10−/−) on a C57BL/6 background [C57BL/6-IL-10(tm1Cgn)] (13) originally were obtained for use in our institute from Werner Müller, Institut für Genetik der Universität zu Köln, Cologne, Germany. These mice were housed in specific pathogen-free conditions and maintained on an OVA-free diet in the Biological Resources Center at the National Jewish Medical and Research Center. Control wild-type (WT) C57BL/6 mice were purchased from The Jackson Laboratory. Both female and male mice, 6–10 weeks of age, were used in the experiments. Controls were matched with the deficient mice with regard to both age and gender in each experimental group.
Sensitization and Airway Challenge.
Mice were sensitized by i.p. injection of 20 μg of OVA (grade V; Sigma) emulsified in 2.25 mg alum (AlumImuject; Pierce), or they received PBS alone in a total volume of 100 μl on days 0 and 14. Mice were challenged via the airways by OVA (1% in PBS) or PBS for 20 min on days 28, 29, and 30 by ultrasonic nebulization (De Vilbiss Health Care, Somerset, PA, particle size 1–5 μm). On day 32, airway function was measured as described below after which mice were killed and specimens were collected for further analysis (14).
For studies involving assessment of airway responses to electrical field stimulation (EFS), mice were sensitized by exposure to aerosolized OVA [1% (wt/vol) in PBS] 20–30 min per day on 10 consecutive days (15).
Determination of Airway Resistance and Dynamic Compliance (Cdyn).
Airway resistance and Cdyn were determined before and after inhalation of aerosolized MCh. Anesthetized, tracheostomized mice were mechanically ventilated, and lung function was assessed by a modification of previously described work (14). A four-way connector was attached to the tracheostomy tube (stainless-steel cannula, 18G), with two ports connected to the inspiratory and expiratory sides of two ventilators. Ventilation was achieved at a rate of 160 breaths/min, tidal volume of 150 μl with a positive end-expiratory pressure of 2–3 cm H2O (ventilator model 683; Harvard Apparatus). Aerosolized MCh was administered for 10 breaths at a rate of 60 breaths/min, tidal volume of 500 μl by the second ventilator (model SN-480–7-3–2T; Shinano Manufacturing, Tokyo) in increasing concentrations (6.25, 12.5, 25, 50, and 100 mg/ml). After each aerosol MCh challenge, the data were continuously collected for 1–5 min and maximum values of lung resistance (RL) and minimum values of Cdyn were taken to express changes in these functional parameters.
EFS of Trachea in Vitro.
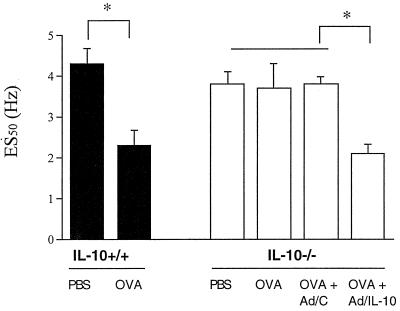
Airway responsiveness to EFS was measured 48 h after the last OVA aerosol challenge as described (15). Tracheas were removed, and 0.5-cm long preparations were placed in Krebs–Henseleit solution suspended by triangular supports transducing the force of contractions. EFS with an increasing frequency from 0.5 to 30 Hz was applied, and the contractions were measured. The duration of the stimulation was 1 ms. Frequencies resulting in 50% of the maximal contractions (ES50) were calculated from linear plots for each individual animal and were compared between the different groups.
mAb Treatments.
Anti-mouse IL-5 mAb, TRFK-5 (IgG2b), was used in this study for studying effects on AHR (16). One hundred micrograms of the stock mAb was diluted with PBS in a total volume of 100 μl, which then was given to i.p.-sensitized mice as a single i.v. injection 2 h before the first airway challenge. As a control, purified rat IgG2b at the same dose and volume was given.
Administration of Adenoviral Construct.
Replication-deficient human type 5 adenoviral constructs carrying the transgene for murine IL-10 in the E1 region of the viral genome (17) was delivered intranasally. As control, we included an E1-deleted replication-deficient human type 5 adenoviral construct carrying no transgene. Mice were anesthetized with an i.p. injection of tribromoethanol (Avertin, 250 mg/kg of 2.5% solution in PBS) after which 1 × 108 plaque-forming units of either construct was applied in the nostril with a micropipette in a total volume of 30 μl of PBS vehicle (two 15-μl administrations, 2 min apart).
In the in vivo airway resistance measurement experiments, the constructs were administered 24 h before the initial airway challenge. In the in vitro airway function experiments after 10 consecutive days of OVA nebulization, the constructs were administered 4 days before measurement of the response to EFS (day 12).
BAL.
After assessment of RL and Cdyn, lungs were lavaged via the tracheal tube with Hanks' balanced salt solution, (1 × 1 ml, room temperature). The volume of collected BAL fluid (BALF) was measured in each sample and the number of BALF cells was counted by cell counter (Coulter Counter). Cytospin slides were stained with Leukostat (Fisher Diagnostics, Pittsburgh, PA) and differentiated in a blinded fashion by counting at least 200 cells under light microscopy.
Measurement of Serum Igs.
Serum levels of total IgE, OVA-specific IgE, and IgG1 were measured by ELISA as described (18). The anti-OVA antibody titers of samples were related to internal pooled standards and expressed as ELISA units. Total IgE level was calculated by comparison with known mouse IgE standard (PharMingen). The limit of detection was 100 pg/ml for IgE.
Measurement of Cytokines in BALF.
IFN-γ, IL-4, and IL-5 in the BALF supernatants were detected by enzyme immunoassay (EIA) as described (19). For IL-10, the OptEIA set was used according to the manufacturer's directions (PharMingen). For IL-13, a commercial kit was used (R & D Systems). Cytokine levels were determined by comparison with the known standards. The limits of detection were 30 pg/ml for IL-10 and 10 pg/ml for the other cytokines.
Measurement of BALF Peptide Leukotrienes and Eosinophilic Peroxidase (EPO).
Samples for leukotriene measurements were prepared by adding 50 μl of 100% methanol to 200 μl of the BALF supernatants. These samples were loaded onto C-18 Sep-Pak chromatography columns (Varian). Methanol-water (80% vol/vol) was used to rinse out the tubes and to elute the bound peptide-leukotrienes, which then were evaporated to dryness on a rotary evaporator at 30°C. The dry pellet was dissolved in 500 μl of EIA buffer, which then was used for ELISA analysis. Peptide-leukotrienes were assayed by using leukotriene EIA kits (Cayman Chemicals, Ann Arbor, MI). The range of the EIA standard curve was 7–1,000 pg/ml, with 50% binding at 53 pg/ml. The rabbit antiserum against leukotriene had the following cross reactivities: leukotriene C4 (LTC4) (100%), leukotriene D4 (100%), leukotriene E4 (LTE4) (67%), and _N-_acetyl-LTE4 (10.5%), but not 5,12,15-hydroxyeicosatetraenoic acid, leukotriene B4 (LTB4), 20-hydroxy-LTB4, or prostaglandins (<0.01%). The limit of detection was 12 pg/ml.
EPO was measured in BAL supernatants with _o_-phenylenediamine hydrochloride substrate as described (20). Horseradish peroxidase was used as a standard starting from 1,000 pg/ml with 3-fold dilutions to create a standard curve. EPO levels of the samples were calculated based on this standard curve.
Histologic and Immunohistochemistry Studies.
After obtaining the BALF, lungs were inflated through the tracheal tube with 2 ml air and fixed in 10% formalin. Blocks of lung tissue were cut around the main bronchus and embedded in paraffin blocks. Tissue sections, 5 μm thick, were affixed to microscope slides and deparaffinized. The slides were stained with hematoxylin and eosin and periodic acid Schiff (PAS) for identification of mucus-containing cells, and examined under light microscopy.
Cells containing major basic protein (MBP) in lung sections were identified by immunohistochemical staining as described by using rabbit anti-mouse MBP (provided by J. J. Lee, Mayo Clinic, Scottsdale, AZ) (16). The slides were examined in a blinded fashion with a Nikon microscope equipped with a fluorescein filter system. The number of eosinophils in the perivascular, peribronchial, and peripheral tissues were evaluated by using iplab2 software (Signal Analytics, Vienna, VA) for the Macintosh computer counting five sections per animal (three mice per group).
Statistical Analysis.
The data were analyzed with the jmp statistical software package (SAS Institute, Cary, NC). ANOVA was used to determine the levels of difference between all groups. Comparisons for all pairs were performed by Tukey–Kramer honest significant difference test. Significance levels were set at P value of 0.05. Values for all measurements are expressed as mean ± SEM.
Results
Allergic Sensitization Does Not Lead to AHR in IL-10−/− Mice.
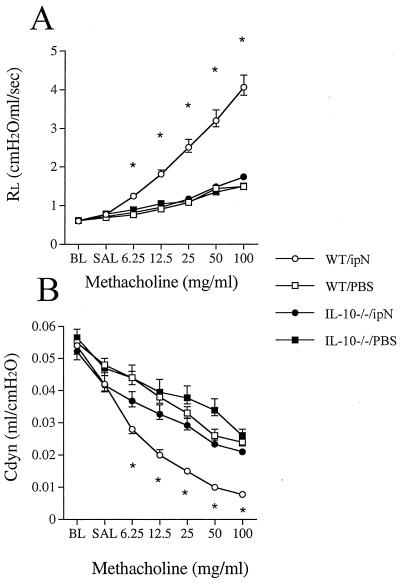
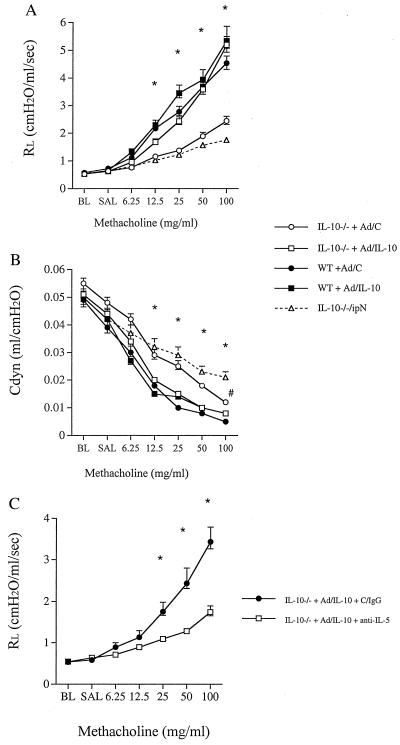
Intraperitoneal OVA sensitization and airway challenge of mice is an established model consistently leading to allergic sensitization and AHR in BALB/c and C57BL/6 mice (19). WT mice that were sensitized according to this protocol developed significant AHR to inhaled MCh. Fig. 1 illustrates RL and Cdyn in response to increasing concentrations of inhaled MCh. In contrast, OVA-sensitized and challenged IL-10−/− mice did not develop any increase in RL above nonsensitized and OVA-challenged control mice. Similarly, monitoring Cdyn, there were no significant differences between the sensitized and nonsensitized IL-10-deficient mice whereas there were major differences compared with normal WT mice at all doses of MCh.
Figure 1.
Airway responsiveness to MCh after sensitization with OVA and challenge with either OVA or PBS in IL-10-deficient (IL-10−/−) and WT mice. Airway responsiveness was monitored by measuring RL (A) and Cdyn (B) as described in Methods. The results for each group are expressed as means ± SEM. Data represent two comparable experiments with 10 mice per group. *, Significant differences between the groups (ANOVA and Tukey-Kramer, P < 0.05). BL, baseline; SAL, saline.
Lung Inflammation in IL-10−/− and WT Mice.
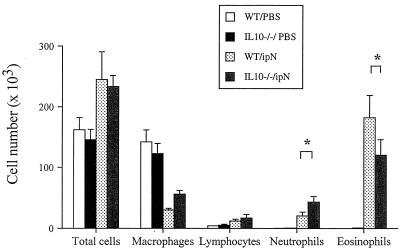
To account for these differences in lung function the inflammatory cell populations in the BALF were examined (Fig. 2). Eosinophils comprised up to 70% of the cells in WT mice and approximately 50% in the IL-10−/− mice (P < 0.01). Neutrophils, on the other hand, were 15% of the total cell population in the IL-10−/− mice and approximately 5% in the WT mice (P < 0.01). There were no significant differences in total cell numbers, macrophages, or numbers of lymphocytes.
Figure 2.
Cellular composition of BALF. IL-10-deficient (IL-10−/−) and WT mice were sensitized and challenged as described in Methods. BALF was obtained from the same groups described in the legend to Fig. 1. The results for each group are expressed as means ± SEM. *, Significant differences between the groups (ANOVA and Tukey-Kramer, P < 0.05).
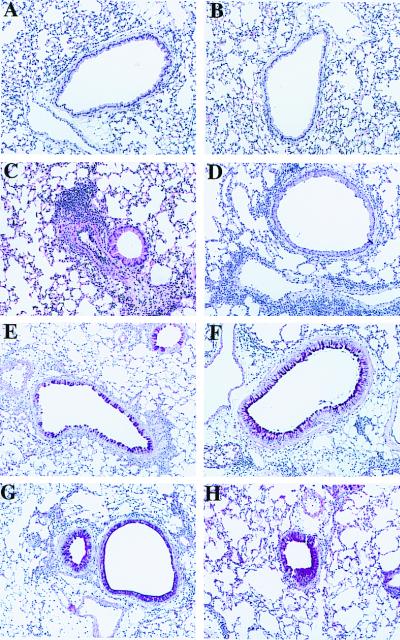
Lung histology showed a heavy infiltration of inflammatory cells in the perivascular and to a slightly lesser extent peribronchial spaces in the OVA-sensitized and OVA-challenged IL-10−/− and in the WT mice. Both strains of mice exposed to only 3 days of nebulization with OVA alone had no signs of inflammation (Fig. 3 A and B). There were no obvious differences between the two mouse strains when sections stained with hematoxylin and eosin were examined (Fig. 3 C and D). Staining of the mucus producing goblet cells with PAS-stain failed to reveal any differences between the strains of mice (Fig. 3 E and F). Numbers of eosinophils in the lung tissue were evaluated by immunohistochemistry staining for the major basic protein (MBP). Comparison of the numbers of MBP+ cells in peribronchial, perivascular, and parenchymal areas of the lung did not reveal significant differences between sensitized and challenged WT and IL-10−/− mice (the numbers ranged from 400 to 900 eosinophils/mm2 in these areas).
Figure 3.
Histologic sections of murine lungs. Normal airways and vessels after sensitization with OVA and exposure to nebulized PBS in WT mice (A) and IL-10−/− mice (B). Representative sections after sensitization and challenge with OVA from a WT mouse (C) and an IL-10−/− mouse (D). Cellular infiltration can be seen perivascularly and to some extent peribronchially. PAS-stained section from a WT mouse (E) and an IL-10−/− mouse (F) sensitized and challenged with OVA. Note the staining of single goblet cells within the respiratory epithelium. PAS-stained section from an IL-10−/− mouse sensitized and challenged with OVA and administered IL-10 by adenovirus-mediated gene transfer. Hyperplasia of goblet cells and excessive mucin production partly filling the airway lumen can be seen in a WT mouse (G) and an IL-10−/− mouse (H).
Cytokine Levels in BALF.
Levels of IL-4, IL-5, IL-13, and IFN-γ were assayed in IL-10+/+ and IL-10−/− mice after sensitization and challenge. Essentially, no significant differences were detected. In both strains of mice sensitization/challenge resulted in increases in IL-4 and IL-5 levels whereas IFN-γ levels remained unchanged (data not shown). After reconstitution of the IL-10 gene in IL-10−/− mice, we failed to detect any changes in cytokine levels when compared with mice receiving the control vector.
Leukotriene and EPO Levels.
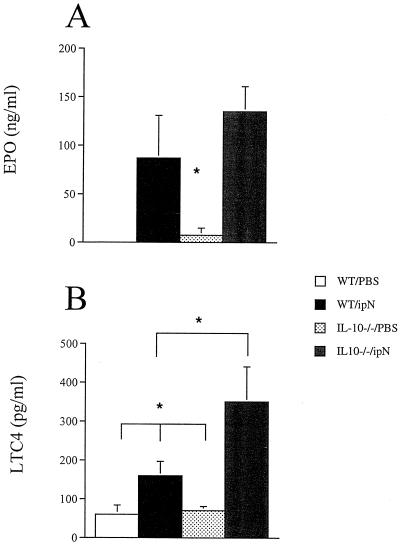
To account for the failure to develop AHR despite the strong eosinophil inflammatory response, we determined whether IL-10−/− mice failed to activate eosinophils accounting for the absence of AHR. We measured EPO and LTC4 levels in the BALF. OVA-sensitized IL-10-deficient mice actually had higher EPO and LTC4 levels than the WT mice (Fig. 4). This difference was statistically significant for LTC4 and when adjusted to the number of eosinophils present in the BALF, EPO levels were also significantly higher in the IL-10−/− mice. The concentrations of both mediators were low in naive mice.
Figure 4.
(A) EPO in mice sensitized and challenged with OVA or PBS. EPO was measured in BALF collected 48 h after the last airway challenge and measured as described in Methods. (B) LTC4 levels in the same mice measured as described in Methods. The results for each group are expressed as means ± SEM (n = 8 per group). *, Significant difference between IL-10−/− and all other groups (P < 0.05).
Serum Igs.
The total IgE level was more than 7-fold higher and serum levels of OVA-specific IgE more than 2-fold higher in the IL-10−/− mice than the WT mice (Table 1). OVA-specific IgG1 and IgG2a levels were also significantly higher in the IL-10−/− mice.
Table 1.
Concentration of total IgE and OVA-specific IgE and IgG1 in the sera of IL-10−/− and WT mice
| Group | Total IgE, ng/ml | OVA-specific, ELISA units/ml | ||
|---|---|---|---|---|
| IgE | IgG1 | IgG2a | ||
| WT/PBS | 5.75 ± 4.0 | 0 | 0 | 0 |
| IL10−/−/PBS | 4.5 ± 3.0 | 0 | 0 | 0 |
| WT/ipN* | 37.6 ± 13.0 | 12.3 ± 5.3 | 20.7 ± 20.7 | 5.1 ± 3.8 |
| IL10−/−/ipN | 273.5 ± 59.0† | 31.6 ± 4.1† | 255 ± 60.2† | 53.1 ± 15.0 |
| WT/ovaneb‡ | 1.5 ± 1.5 | 0 | 0 | 0 |
| IL10−/−/ovaneb | 99.0 ± 52.6† | 0 | 0.12 ± 0.12 | 0 |
IL-10−/− Mice Are Hyporesponsive After EFS of Trachea Smooth Muscle.
To assess whether there was a difference in the smooth muscle reactivity of these mice in vitro, we subjected isolated tracheal smooth muscle preparations to EFS (15). In these experiments, mice were sensitized by exposure to aerosolized OVA or PBS for 10 consecutive days, and 2 days after the last challenge, tracheas were isolated and exposed to EFS. In contrast to the sensitization and challenge approach described above, this approach to sensitization and development of increased sensitivity to EFS was shown to be IgE/IgG1 dependent (18). The electrical frequency required to induce 50% of the maximum contractile response (ES50) was significantly lower in the OVA-exposed WT mice than in their PBS-exposed controls (2.3 ± 0.37 Hz vs. 4.3 ± 0.36 Hz, P = 0.0003) (Fig. 5). In the IL-10−/− mice, no significant differences could be found between the sensitized and nonsensitized mice (ES50 3.7 ± 0.6 Hz vs. 3.8 ± 0.3 Hz). The maximum tension/contractility curves in response to MCh were similar in both strains of mice. These experiments suggest that the sensitized IL-10-deficient mice fail to develop altered airway reactivity to EFS as well as to inhaled MCh.
Figure 5.
Airway responsiveness measured by EFS. Airway responsiveness was studied by EFS of tracheal smooth muscle preparations. Results are expressed as the electrical frequency (Hz) required to induce a 50% of the maximum contractile response (ES50). The results for each group are expressed as means ± SEM (n = 5 per group). *, Significant difference between groups (P < 0.05).
Adenovirus-Mediated Transfer of the IL-10 Gene Reconstitutes AHR in IL-10−/− Mice.
To address whether the absence of IL-10 was solely responsible for the failure to respond to inhaled MCh or EFS after allergen sensitization and challenge, we reconstituted the genetically deficient animals with IL-10 by using adenovirus-mediated gene transfer. A replication-deficient adenovirus/IL-10 (Ad/IL-10) or a corresponding control construct carrying no transgene (Ad/C) was administered intranasally at a dose of 1 × 108 plaque-forming units, 24 h before the first aerosolized challenge (5 days before measurement of airway function). The expression of IL-10 was transient in the airways but there were still detectable concentrations of the cytokine in the BALF 5 days after the administration of this concentration of the viral construct (33 ± 25 pg/ml). Active gene transfer reconstituted both RL and Cdyn to the levels observed in WT mice in response to inhaled MCh (Fig. 6 A and B). Ad/IL-10 alone did not cause AHR either in the naive IL-10−/− or the WT mice (RL at 100 mg/ml MCh 1.8 and 1.7, respectively). Ad/C induced a low-level, but not significant, increase in airway resistance in the sensitized and challenged IL-10−/− mice compared with those mice receiving no construct (Fig. 6 A and B). Significant differences between Ad/IL-10- and Ad/C-treated (−/−) mice were observed for both airway resistance and Cdyn in response to all MCh doses. WT mice administered the Ad/IL-10 showed a minor additional increase in airway resistance compared with the WT mice administered Ad/C (Fig. 6A).
Figure 6.
Airway responsiveness to MCh after sensitization and challenge with OVA in IL-10-deficient (open symbols in A and B) and WT mice (filled symbols in A and B). Mice were administered either empty replication-deficient Ad/C (circles) or Ad/IL-10 (squares) 24 h before the first airway challenge with aerosolized OVA. IL-10−/− mice with no treatment (triangle) also are shown. Airway responsiveness was monitored by measuring RL (A) and Cdyn (B) as described in Methods. Mice administered with Ad-IL-10 were treated with control IgG (circles) or a mAb against IL-5 (squares) and airway responsiveness was monitored by measuring RL (C). The results for each group are expressed as means ± SEM (n = 8 per group). Significant differences (P < 0.05) are indicated by * (IL-10−/− + Ad/C and IL-10−/−/ipN vs. other groups in A and B) and # (IL-10−/− + Ad/C vs. IL-10−/−/ipN). BL, baseline; SAL, saline.
Reconstitution of the mice with the Ad/IL-10 construct 24 h before the initial airway challenge did not affect levels of OVA-specific antibodies (data not shown) but also increased mucus production both in the IL-10−/− and WT mice as seen in PAS-stained sections (Fig. 3 G and H). As shown above, allergen-sensitized IL-10−/− mice had fewer eosinophils but more neutrophils in the BALF than the WT mice. After reconstitution, the neutrophils decreased from 16% in mice administered the empty control vector to 8% in mice receiving Ad/IL-10 vector (P < 0.05). Correspondingly, the percentage of eosinophils increased from 52% to 65% (P < 0.05).
To define whether IL-10-mediated reconstitution of AHR was IL-5/eosinophil-dependent as shown in other models using a similar sensitization and challenge protocol (16, 21), mice sensitized to OVA and administered Ad/IL-10 were treated with anti-IL-5 antibody 2 h before the first airway challenge with OVA. This process resulted in a dramatic decrease in airway eosinophil numbers (from 55% to 6%) and concomitant normalization of lung function (Fig. 6C). Thus, the effects of IL-10 on airway function depend, at least in part, on allergen-induced eosinophilic inflammation.
Adenovirus-mediated IL-10 reconstitution also was assessed in the in vitro measurements of airway function after 10 consecutive days of OVA exposure. As in the in vivo system, Ad/IL-10, but not Ad/C, reconstituted the response to EFS [ES50 (50% of the maximal contractions) 2.1 ± 0.2 Hz and 3.8 ± 0.2 Hz, respectively] (Fig. 5).
Discussion
Important roles for a number of cytokines, including IL-4, IL-5, and IL-13, have been shown in the development of allergic asthma in humans and increased airway responsiveness in experimental models (2, 3). Similarly, a group of negative regulators of allergic inflammation also have been implicated in asthma pathogenesis, among them IL-10. In the present study, we arrived at somewhat different conclusions. Here, the role of IL-10 in the development of AHR and pulmonary inflammation in an experimental model of allergic sensitization was identified by using genetically deficient mice. The major finding in this study was that IL-10−/− mice, sensitized and challenged to OVA, failed to develop AHR in response to inhaled MCh (altered RL and Cdyn) whereas, under the same conditions, WT mice developed AHR. This failure to respond to inhaled MCh monitoring airway responsiveness in vivo was paralleled in in vitro studies of tracheal smooth muscle responsiveness to EFS. This latter system detects increased acetylcholine release from nerves and muscarinic (M2) receptor dysfunction after allergen exposure (15, 22). Thus, IL-10 appears to play a major role in the development of altered airway function. Evidence that this was not simply the consequence of a developmental defect was provided by IL-10 gene reconstitution experiments, which showed that both in vivo or in vitro altered airway function could be fully restored.
Inflammation, particularly eosinophilic inflammation, is a hallmark of asthma. In many, but not all, animal models, development of altered airway function, in vivo or in vitro, has been linked to eosinophil accumulation in the lungs (14, 19, 21, 23). In these studies, prevention of eosinophilic accumulation in the lungs was associated with attenuation of AHR. In the absence of AHR, IL-10-deficient mice were shown to exhibit a robust airway eosinophil response. In addition, indirect evidence for eosinophil activation in the deficient mice was provided by the elevated levels of EPO and LTC4 after sensitization and challenge. Further, after reconstitution of the deficient mice with IL-10, which reconstituted AHR, anti-IL-5 administration markedly reduced eosinophil inflammation and normalized lung function, suggesting that the development of altered airway function in these mice did not follow an aberrant pathway but was associated with eosinophil accumulation as in WT mice. Cumulatively, these data imply that IL-10 modulates airway function in allergic mice, but downstream of the eosinophil inflammatory cascade.
Somewhat in contrast to our findings, Grünig et al. (3) found that IL-10−/− mice developed comparable AHR as controls after bronchopulmonary aspergillosis. In addition, they demonstrated exaggerated airway inflammation in the IL-10-deficient mice. Bronchopulmonary aspergillosis is a complex combination of both infection and allergic sensitization involving the activation of several different types of inflammatory reactions, including both Th1 and Th2 responses. In this model, the effects of IL-10 deficiency on airway function appear to have been overcome, confirming that airway responsiveness in IL-10−/− mice is not intrinsically abnormal.
Reconstitution with the IL-10 gene before allergen challenge in the present study did not result in diminished pulmonary inflammation as reported by Zuany-Amorim et al. (24) nor did it alter levels of IL-4, IL-5, IL-13, or IFN-γ in the BALF. They administered IL-10 (protein) to normal mice at the time of allergen challenge and showed a significant reduction in eosinophil inflammation; airway function was not measured. In contrast, genetic reconstitution of IL-10-deficient mice resulted in an increase in eosinophil numbers and mucus production in our study; this increase in mucus production was observed in both deficient and WT mice. Cumulatively, the data indicate that IL-10 is not required for eosinophilic inflammation and activation, cytokine release, or IgE production. These findings suggest the possibility that, in the presence of eosinophilic inflammation, IL-10 acts on smooth muscle directly or via an intermediate that is not eosinophil-derived. Direct effects of cytokines on airway smooth muscle constrictor responses recently have been identified (25).
In summary, these studies reveal a critical role for IL-10 in the development of AHR after allergic sensitization. This role appears to be downstream but nevertheless dependent on the airway inflammatory cascade, including eosinophil accumulation and activation. Whether this is a direct effect on smooth muscle function or on the regulation of a mediator(s) of smooth muscle function remains to be determined.
Acknowledgments
This work was funded in part by National Institutes of Health Grants HL-36577 and HL-61005 and Environmental Protection Agency Grant R825702 (to E.W.G.). M.J.M. was partially supported by the Academy of Finland and the Pediatric Research Foundation of Finland.
Abbreviations
Ad/C
adenovirus control
Ad/IL-10
adenovirus/IL-10
AHR
airway hyperresponsiveness
BAL
bronchoalveolar lavage
BALF
BAL fluid
Cdyn
dynamic compliance
EFS
electrical field stimulation
EIA
enzyme immunoassay
EPO
eosinophil peroxidase
LTC4
leukotriene C4
MCh
methacholine
OVA
ovalbumin
PAS
periodic acid Schiff
RL
lung resistance
Th
T helper
WT
wild type
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100118997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100118997
References
- 1.Gelfand E W, Irvin C G. Nat Med. 1997;3:382–384. doi: 10.1038/nm0497-382. [DOI] [PubMed] [Google Scholar]
- 2.Wills-Karp M. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 3.Grünig G, Warnock M, Wakil A E, Venkayya R, Brombacher F, Rennick D M, Sheppard D, Mohrs M, Donaldson D D, Locksley R M, Corry D B. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiorentino D F, Bond M W, Mosmann T R. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borish L. J Allergy Clin Immunol. 1998;101:293–297. doi: 10.1016/S0091-6749(98)70238-6. [DOI] [PubMed] [Google Scholar]
- 6.Moore K W, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 7.Lelievre E, Sarrouilhe D, Morel F, Preud'Homme J L, Wijdenes J, Lecron J C. Cytokine. 1998;10:831–840. doi: 10.1006/cyto.1998.0371. [DOI] [PubMed] [Google Scholar]
- 8.Rennick D M, Fort M M, Davidson N J. J Leukocyte Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 9.Robinson D S, Tsicopoulos A, Meng Q, Durham S, Kay A B, Hamid Q. Am J Respir Cell Mol Biol. 1996;14:113–117. doi: 10.1165/ajrcmb.14.2.8630259. [DOI] [PubMed] [Google Scholar]
- 10.Tillie-Leblond I, Pugin J, Marquette C H, Lamblin C, Saulnier F, Brichet A, Wallaert B, Tonnel A B, Gosset P. Am J Respir Crit Care Med. 1999;159:487–494. doi: 10.1164/ajrccm.159.2.9805115. [DOI] [PubMed] [Google Scholar]
- 11.Magnan A, van Pee D, Bongrand P, Vervloet D. Allergy. 1998;53:1092–1095. doi: 10.1111/j.1398-9995.1998.tb03821.x. [DOI] [PubMed] [Google Scholar]
- 12.Pretolani M, Goldman M. Immunol Today. 1997;18:277–280. doi: 10.1016/s0167-5699(97)80023-0. [DOI] [PubMed] [Google Scholar]
- 13.Kühn R, Lohler J, Rennick D, Rajewsky K, Müller W. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Hamelmann E, Joetham A, Shultz L D, Larsen G L, Irvin C G, Gelfand E W. J Exp Med. 1997;186:449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen G L, Renz H, Loader J E, Bradley K L, Gelfand E W. J Clin Invest. 1992;89:747–752. doi: 10.1172/JCI115651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamelmann E, Oshiba A, Loader J, Larsen G L, Gleich G, Lee J, Gelfand E W. Am J Respir Crit Care Med. 1997;155:819–825. doi: 10.1164/ajrccm.155.3.9117011. [DOI] [PubMed] [Google Scholar]
- 17.Xing Z, Ohkawara Y, Jordana M, Graham F L, Gauldie J. Gene Ther. 1997;4:140–149. doi: 10.1038/sj.gt.3300371. [DOI] [PubMed] [Google Scholar]
- 18.Oshiba A, Hamelmann E, Takeda K, Bradley K L, Loader J E, Larsen G L, Gelfand E W. J Clin Invest. 1996;97:1398–1408. doi: 10.1172/JCI118560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haczku A, Takeda K, Redai I, Hamelmann E, Cielewicz G, Joetham A, Loader J, Lee J J, Irvin C, Gelfand E W. Am J Respir Crit Care Med. 1999;159:1638–1643. doi: 10.1164/ajrccm.159.5.9711040. [DOI] [PubMed] [Google Scholar]
- 20.Hamelmann E, Oshiba A, Schwarze J, Bradley K, Loader J, Larsen G L, Gelfand E W. Am J Respir Cell Mol Biol. 1997;16:674–682. doi: 10.1165/ajrcmb.16.6.9191469. [DOI] [PubMed] [Google Scholar]
- 21.Foster P S, Hogan S P, Ramsay A J, Matthaei K I, Young I G. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen G L, Fame T M, Renz H, Loader J E, Graves J P, Gelfand E W. Am J Physiol. 1994;266:L263–L270. doi: 10.1152/ajplung.1994.266.3.L263. [DOI] [PubMed] [Google Scholar]
- 23.Evans C M, Fryer A D, Jacoby D B, Gleich G J, Costello R W. J Clin Invest. 1997;100:2254–2262. doi: 10.1172/JCI119763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuany-Amorim C, Haile S, Leduc D, Dumarey C, Huerre M, Vargajtig B B, Pretolani M. J Clin Invest. 1995;95:2644–2651. doi: 10.1172/JCI117966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakonarson H, Maskeri N, Carter C, Chuang S, Grunstein M M. J Clin Invest. 1999;104:657–667. doi: 10.1172/JCI7137. [DOI] [PMC free article] [PubMed] [Google Scholar]