Gene–gene interaction associated with neural reward sensitivity (original) (raw)
Abstract
Reward processing depends on dopaminergic neurotransmission and is modulated by factors affecting dopamine (DA) reuptake and degradation. We used fMRI and a guessing task sensitive to reward-related activation in the prefrontal cortex and ventral striatum to study how individual variation in genes contributing to DA reuptake [DA transporter (DAT)] and degradation [catechol-o-methyltransferase (COMT)] influences reward processing. Prefrontal activity, evoked by anticipation of reward irrespective of reward probability and magnitude, was COMT genotype-dependent. Volunteers homozygous for the Met allele, associated with lower enzyme activity and presumably greater DA availability, showed larger responses compared with volunteers homozygous for the Val allele. A similar COMT effect was observed in the ventral striatum. As reported previously, the ventral striatum was also found to code gain-related expected value, i.e., the product of reward magnitude and gain probability. Individual differences in ventral striatal sensitivity for value were in part explained by an epistatic gene–gene interaction between COMT and DAT. Although most genotype combinations exhibited the expected activity increase with more likely and larger rewards, two genotype combinations (COMT Met/Met DAT 10R and COMT Val/Val 9R) were associated with blunted ventral striatal responses. In view of a consistent relationship between reduced reward sensitivity and addiction, our findings point to a potential genetic basis for vulnerability to addiction.
Keywords: COMT, dopamine, dopamine transporter, functional MRI, genetics
Dopamine (DA) is critical to motivational and reward-related functions of the brain, including adaptation through reinforcement learning (1, 2) and decision making (3). Considerable interindividual differences with respect to decision making have been observed (4), and it has been speculated that genetic variability in the dopaminergic system could be related to these differences (5, 6). In addition, interindividual variation in dopaminergic function has been hypothesized as a major factor contributing to inheritable personality traits (7) and addiction (8). However, little is known about how variation in DA-related genes modulates the described physiological properties of the dopaminergic reward system (1–3, 9, 10) and how such physiological variation affects reward processing. To bridge this gap between genetics and behavior, we combined genetics and personality assessment with fMRI measures of brain activation as an intermediate (endo)phenotype (11, 12), an approach based on the assumption that brain activation is causally more directly linked to genotype than is behavior (12).
In the study of individual differences in DA system physiology, a useful conceptual distinction is often made between tonic and phasic dopaminergic neurotransmission (13, 14). In the striatum, a basal level of extracellular DA results from tonic, slow, and irregular “background” firing of dopaminergic neurons originating in the ventral tegmental area. By contrast, burst firing of ventral tegmental area neurons induces phasic DA release, a mechanism involved in signaling behaviorally relevant stimulus attributes, such as the magnitude and probability of anticipated rewards (1, 2). Phasically released DA is normally eliminated by rapid reuptake through the DA transporter (DAT) (14), which is abundant in the striatum (15).
The precise effect of catechol-o-methyltransferase (COMT) on dopaminergic neurotransmission is less well established. COMT is known to regulate extrasynaptical DA breakdown, which is mainly true for the prefrontal cortex (PFC), where COMT is relatively more abundant than in the striatum (16). COMT might also have a small, local effect in DA metabolism in the striatum (17). More importantly, an interaction between prefrontal COMT and subcortical function has been suggested (12), probably mediated by excitatory glutamatergic efferents from the PFC to the striatum, which are thought to translate a high prefrontal into a high striatal dopaminergic tone (13).
Within the genes coding for DAT and COMT, common functional polymorphisms have been described (18, 19). A single nucleotide exchange in the COMT gene, causing a valine to methionine (Val/Met-158) substitution (18), entails a 4-fold reduction in COMT activity in Met relative to Val homozygotes, with heterozygotes demonstrating intermediate activity (20). As a consequence, it is expected that Met homozygotes have increased tonic DA levels in the PFC and possibly also in the striatum (13).
In samples of European ancestry, the DAT gene has two common alleles with either 9 or 10 repeats (9R or 10R) of a 40-base pair sequence in its 3′ region (19). Although contradictory results have been obtained in in vivo binding studies (21, 22), in vitro data (23–25) suggest that the 9R allele is associated with lower DAT expression than the more frequent 10R allele. Lower DAT expression associated with the 9R allele may reduce synaptic DA clearance and therefore augment phasic DA levels.
On the basis of these physiological and genetic findings, we conjectured that hemodynamic responses to reward anticipation in the ventral striatum, an indirect index of phasic DA release (3, 9, 10, 26), are modulated by DAT and COMT genotypes.
Results
Genotyping.
The allelic distribution of both genotypes of interest (COMT and DAT) was in Hardy–Weinberg equilibrium [COMT Val/Met, χ2 (1) = 0.17, P = 0.7; DAT VNTR, χ2 (1) = 0.39, P = 0.52]. For additional demographics, see supporting information (SI) Table 2.
To further control for the effects of stratification, genotyping was also performed for unrelated genes (see Methods). Using a contingency table approach (27), we found that the allelic distributions between COMT Val/Val and Met/Met subjects, as well as between DAT VNTR 10R and 9R subjects, did not differ (SI Table 3). This finding makes genetic inhomogeneity of the tested population unlikely.
fMRI.
To reliably activate the mesolimbic DA system, we used a previously established guessing paradigm, which allows the independent manipulation of reward probability and reward magnitude (26), in 105 healthy male volunteers. Each trial began with the presentation of the backside of eight playing cards. Volunteers had to place a given amount of money (1€ or 5€) on certain playing cards, allowing for the control of expected reward magnitude. In some trials, the bet had to be placed on a single card and in others on the corners of four adjacent cards, which allowed for the control of expected reward probability (low for a single card and high for four cards).
Effect of Genotype on Activation During Anticipation of Reward.
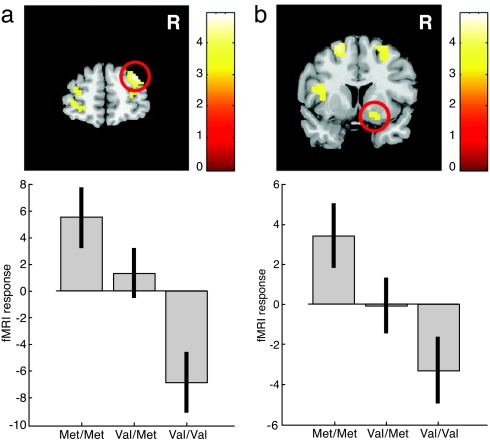
Examination of the individual influence of COMT and DAT genotypes on fMRI signal changes during reward anticipation, averaged across all reward probabilities and magnitudes against baseline, showed the right PFC signal to be strongly dependent on the COMT genotype (Fig. 1a). Specifically, there was a relative deactivation in volunteers homozygous for the Val allele, compared with Met homozygotes (peak x, y, z: 33, 54, 30 mm; Z = 4.6, P < 0.05, corrected), supporting previous findings (28). An activation in the left PFC was also COMT-dependent, but did not survive our statistical threshold (peak x, y, z: −48, 36, 24 mm; Z = 4.0, P < 0.001 uncorrected; P = 0.19, corrected; Fig. 1a). No effect of the DAT genotype on prefrontal activation was observed.
Fig. 1.
Reward anticipation-related activation across all reward probabilities and magnitudes in the PFC and the ventral putamen is affected by the COMT genotype only. (a) Right PFC activation as a function of the COMT genotype. Volunteers with the Met/Met genotype (less enzyme activity) show highest activation levels, whereas Val/Val volunteers show the lowest. Activation is superimposed on a coronal section. Genotype effects in the left hemisphere did not reach corrected significance. (b) A similar pattern was observed in the right ventral striatum.
In addition, we observed a significant main effect of COMT on signal changes in the right (peak x, y, z: 21, 9, −15 mm; Z = 3.6, P < 0.05; Fig. 1b) and left (peak x, y, z: −15, 12, −6 mm; Z = 2.9, P < 0.05) ventral putamen. In contrast to the effect in the PFC, a relative deactivation in volunteers homozygous for the Val allele compared with Met homozygotes was observed.
Effect of Genotype on Neural Encoding of Expected Value During Anticipation of Reward.
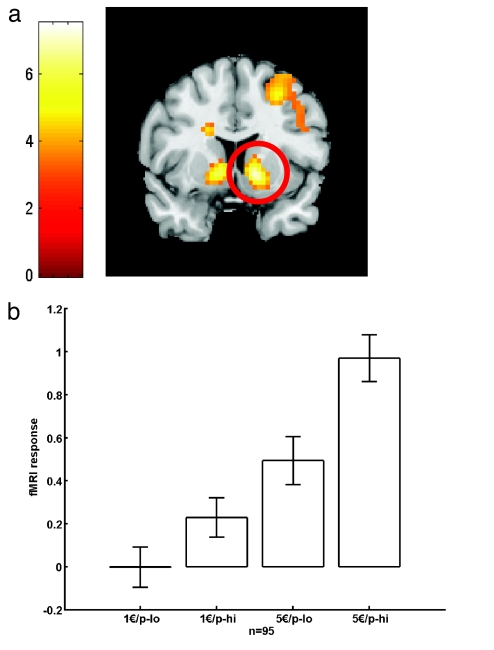
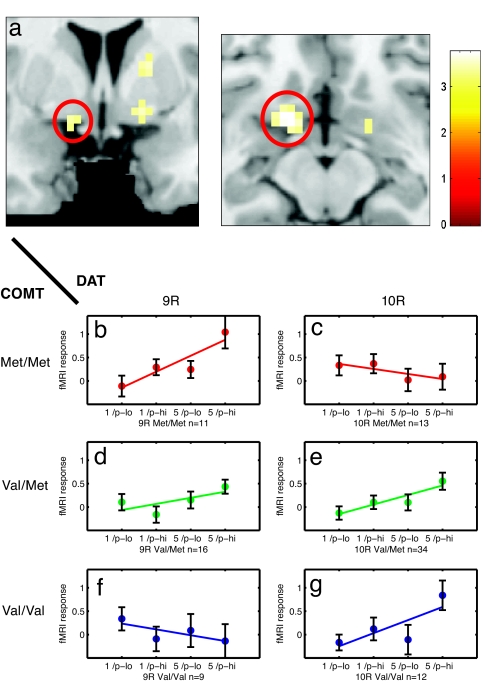
In contrast to the PFC, the ventral striatum also showed activation that scaled as a function of both reward probability and magnitude (10, 26) (peak x, y, z: −12, 6, −3 mm; Z = 5.6; and x, y, z: 12, 9, −3 mm; Z = 6.3, both P < 0.05, corrected; Table 1 and Fig. 2), consistent with the described role of this area in quantitative encoding of reward (1, 2, 10, 26). The slope of the striatal activation increase with more probable and greater rewards was not affected by the COMT and DAT genotypes when examining both genes in isolation. However, when considering a possible combined effect, we found that an epistatic gene–gene interaction between COMT and DAT explained a significant amount of the interindividual variance in ventral striatal responses (peak x, y, z: −15, 9, −9 mm; Z = 3.6; and x, y, z: 15, 3, −9 mm; Z = 3.4, both P < 0.05, corrected; Fig. 3a; see also SI Fig. 4). No other brain area showed a significant COMT–DAT interaction when correcting for multiple comparisons (SI Table 4 and SI Fig. 4_h_).
Table 1.
Frontal and ventral striatal activations during reward anticipation
| Effect | Region | x, y, z (mm) | Z |
|---|---|---|---|
| Activation pooled across reward probabilities and magnitudes | Ventral striatum R | 9, 6, −3 | 5.9 |
| Ventral striatum L | −9, 3, −3 | 6.4 | |
| Ventrolateral prefrontal cortex R | 33, 21, −15 | 10.3 | |
| Ventrolateral prefrontal cortex L | −30, 12, −18 | 9.4 | |
| Lateral prefrontal cortex R | 54, 27, 9 | 5.3 | |
| Lateral prefrontal cortex L | −57, 24, 9 | 6.0 | |
| Medial prefrontal cortex | −3, 51, 30 | 7.4 | |
| fMRI signal increase with increasing magnitude and probability (gain-related expected value) | Ventral striatum R | 12, 9, −3 | 6.3 |
| Ventral striatum L | −12, 6, −3 | 5.6 | |
| Ventrolateral prefrontal cortex R | 36, 24, −6 | 5.0 | |
| 33, 57, −3 | 4.8 | ||
| Ventrolateral prefrontal cortex L | −30, 27, −3 | 5.6 | |
| Superior frontal gyrus R | 27, 9, 51 | 4.6 |
Fig. 2.
Reward anticipation-related activation in the ventral striatum as a function of expected reward magnitude and probability for 95 volunteers (see Methods). (a) Activation in bilateral ventral striatum superimposed on a coronal section. (b) The strongest activation is observed for highly likely (p-hi) and large (5€) rewards (peak x, y, z: 12, 9, −3 mm; Z = 6.3, P < 0.05, corrected). The smallest activation is observed for unlikely (p-lo) and small (1€) rewards. The slope of this activation increase is used as a measure of neural reward sensitivity.
Fig. 3.
Functional gene–gene interaction between the COMT and DAT genotypes in the left ventral striatum. (a) Coronal and axial sections show the average ventral striatal fMRI response to reward anticipation across all subjects overlaid on a template high-resolution MR image (for confidence intervals of each slope, see SI Table 5). (b–g) Individual fMRI responses from the left ventral striatum (peak x, y, z: −15, 9, −9 mm) as a function of reward probability, magnitude, and genotype. The activation increase related to more probable and greater rewards depends on a gene–gene interaction between the COMT and DAT genotypes. A positive slope (b, d, e, and g) indicates more activation for 5€ high-probability (p-hi) trials as compared with 1€ low-probability (p-low) trials. In some combinations of the COMT and DAT genotypes (c and f), the slope is blunted, presumably reflecting suboptimal neural encoding of reward.
Although most COMT–DAT genotype combinations (Fig. 3 b, d, e, and g) showed an activity increase with more probable and greater anticipated rewards, the genotypes COMT Met/Met DAT 10R and COMT Val/Val DAT 9R showed a blunted striatal response to increasing expected value, suggesting that these individuals possibly failed to encode anticipated reward in proportion to its magnitude and probability (Fig. 3 c and f). The 95% confidence intervals (as estimated by bootstrap resampling) for the slightly negative regression slope in the two genotype combinations (see Fig. 3 c and f; left ventral striatum, peak x, y, z: −15, 9, −9 mm) contained zero. We therefore refrain from interpreting those responses as decreases (see SI Table 5).
Cross-validation by dividing the whole sample into odd and even samples (i.e., volunteers) revealed significant effects (P < 0.05) for the reported COMT–DAT interaction in bilateral ventral striatum in both independent samples at the coordinates (x, y, z: −15, 9, −9 and 15, 3, −9 mm, respectively) reported for the whole group.
Discussion
Our data show that neuronal activity during reward anticipation is modulated by the COMT genotype in the PFC and ventral striatum. In addition to this additive genotype effect, we observed a nonlinear multiplicative effect of the COMT and DAT genotypes on ventral striatal responses. This gene–gene interaction affected the ability of the ventral striatum to encode increasing and more likely rewards, i.e., gain-related expected value.
Modulation of prefrontal responses to emotional stimuli by the COMT genotype has been reported previously (28). As in the previous study, we observed the largest responses associated with the Met COMT allele, which encodes a less efficient enzyme isoform. As a consequence, prefrontal DA levels may be elevated in Met allele carriers, a potential explanation for the augmented neuronal response in these volunteers.
A similar main effect of the COMT genotype was also observed in the ventral striatum. Although local effects of COMT in the ventral striatum have been hypothesized (13), current evidence (17) favors an explanation based on prefrontal regulation of striatal DA metabolism via top–down projections (16).
Considerable interindividual differences exist with respect to reward processing and decision making (5). In the context of prospect theory (29), currently the most influential model of decision under risk, the impact of probabilities has been shown to differ remarkably between individuals (4), and it has been speculated that interindividual differences, e.g., genetic variability in the dopaminergic system, could be related to this effect (5). Although the framework of expected value assumes a simpler (i.e., linear) influence of probability, our data show that expected value, which is partly determined by reward probability, is affected by genetic variability in genes related to dopamine metabolism.
Recently, it has been shown that response properties in the ventral striatum were related to individual preferences for immediate over delayed rewards in a guessing task (6). The authors speculated that these interindividual differences might be related to dopamine-regulating polymorphisms. Although we did not assess individual reward discounting functions, our study supports this notion because we can directly show that the response pattern of the ventral striatum is related to variation in dopamine-regulating genes.
Importantly, on its own, the COMT genotype did not influence ventral striatal sensitivity to reward magnitude and probability, although overall it did affect anticipatory activity in the ventral striatum. The latter stimulus properties are known to be encoded by the activity of burst-firing neurons originating in the midbrain and terminating in the ventral striatum (1, 2). Apart from diffusion (30), this phasic dopaminergic signal is regulated by reuptake of DA by the DA transporter. DAT genotype on its own also did not affect ventral striatal reward sensitivity. Only when considering the COMT and DAT genotypes together we observed a multiplicative, interactive genotype effect on hemodynamic responses. This observation is in line with a general notion (13) that basal dopaminergic tone, regulated by COMT, interacts with phasic DA release regulated by DAT.
There are two possible mechanisms through which COMT activity could modulate ventral striatal tonic and phasic DA. First, extrasynaptic COMT in the ventral striatum may directly affect local DA levels (13). Second, glutamatergic projections from the PFC whose activity is regulated by prefrontal DA levels may impact on striatal DA (31, 32). In either case, lower COMT activity is thought to be associated with higher tonic DA in the ventral striatum. Supposedly the relationship between tonic and phasic DA levels is characterized by an inhibitory interaction in a way that high tonic DA levels (low COMT activity) inhibit phasic DA release through stimulation of presynaptic autoreceptors (13).
An interaction between COMT and DAT indicates a multiplicative effect of both genotypes on the observed BOLD response in the ventral striatum. More importantly, we observed a crossed interaction (Fig. 3), which by definition renders the ensuing function u-shaped or invertedly u-shaped. Plotting the slope of ventral striatal activity changes with increasing reward, our measure of neural reward sensitivity (as in Fig. 3 and SI Fig. 4) against genotype indeed showed such an inverted u-shaped pattern (SI Fig. 5). Importantly, this pattern emerged from the data irrespective of whether COMT (SI Fig. 5) or DAT (SI Fig. 6) was used as the major grouping factor. Based on the proposed effects of COMT and DAT on DA neurotransmission (13), it is possible that increasing fMRI signal with increasing reward (see Fig. 3 b, d, e, and g) is related to intermediate levels of phasic DA release. By contrast, in individuals in which phasic DA availability is presumably either lower or higher (Fig. 3 c and f), the ventral striatum does not appear to encode reward optimally.
This nonlinear, inverted u-shaped pattern of ventral striatal responses bears similarities with the dependence of working memory performance (and the associated PFC activation) on cortical DA (33). In working memory tasks, typically, suboptimal performance is associated with both very low and very high prefrontal DA levels (34). Because DAT expression in the PFC is low (35), interindividual variations in prefrontal function and activity are best explained on the basis of the COMT genotype only, in combination with nongenetic factors such as medication, stress, or disease state (34). Our own prefrontal data, albeit assessing an affective function, accord with this viewpoint. Our data from the ventral striatum, however, suggest that, at a genetic level, variation in subcortical neural function may rather result from an epistatic gene–gene interaction between two DA-related genes. Specifically, an inverted u-shaped model as proposed here is a physiologically motivated explanation of neural variation on a genetic basis. The model will have to be tested in future studies, ideally employing a combined genetic-pharmacological approach.
Given that no a priori stratification by genotypes was used, some genotype combinations were less frequently observed than others. However, even the smallest group (COMT Val/Val DAT 9R) contained nine volunteers, and the overall validity of the results was confirmed by using an odd–even sample cross-validation.
Finally, to ask whether the observed gene–brain relationship has behavioral relevance, we plotted the average individual sensation-seeking scores as a function of genotype (SI Figs. 5_c_ and 6_c_). Using COMT as the major grouping factor revealed a significant u-shaped pattern, with the highest sensation-seeking scores observed in those genotypes with suboptimal striatal reward encoding (SI Fig. 5_c_). When using DAT (SI Fig. 6_c_) as the major grouping factor, a similar picture emerged, but the fit of the u-shaped function only revealed a trend. The observed correlation between neural reward sensitivity and sensation-seeking scores is particularly interesting in the light of consistent findings of decreased excitability of the mesolimbic reward system and elevated sensation-seeking scores in addiction (8, 36). This correlation underlines the behavioral relevance of our genetic and neural analysis and suggests that a genetically modulated dysfunction in neural reward processing, in addition to a possible genetic effect on drug neurotoxicity, is related to motivational behavior that predisposes to addiction (37). However, given the marginal significance of the effect, future studies are necessary to answer the question of whether the observed interaction between dopamine-regulating genes is a crucial vulnerability factor for addiction.
In summary, our data show that the interaction of dopamine-regulating genes can modulate ventral striatal reward sensitivity and thus explain commonly observed interindividual differences in reward-related behavior.
Methods
Participants.
One hundred five healthy male volunteers from the greater Hamburg, Germany, area were enrolled in the study. We restricted our sample to male volunteers to exclude gender effects because it has been suggested that women have an increased endogenous striatal dopamine concentration (38). All participants underwent a structured psychiatric interview (39) performed by an experienced psychiatrist, as well as urine drug screening to exclude cocaine, amphetamine, cannabis, and opiate use. Seven volunteers were excluded from the sample due to a positive urine drug screen. Additionally, all subjects were asked to not smoke or drink alcoholic beverages at least 24 h before evaluation.
Subjects were of European ancestry, apart from one volunteer of Asian/German ancestry. The age range of the sample was 18–46 years (mean, 26.2 ± SD 5.4), and the years of education was 8–20 (mean, 14.9 ± SD 1.7). Sensation seeking was assessed by using Zuckerman's Sensation-Seeking Scale (Form V) (40). In addition, personality traits were assessed by using the revised NEO Personality Inventory (NEO-PI) self-report questionnaire (41), which covered each of the five-factor personality dimensions (neuroticism, extraversion, openness, agreeableness, and conscientiousness). To exclude pathological gambling, participants also completed a questionnaire of gambling behavior (Kurzfragebogen zum Glücksspielverhalten) (42). The study was approved by the Ethics Committee of the Medical Board in Hamburg, Germany, and all subjects gave written informed consent.
Blood Sampling and Genotyping.
Peripheral venous blood was drawn from all volunteers, and genomic DNA was extracted from the WBCs according to standard procedures by using the Qiagen FlexiGene DNA kit (Qiagen, Valencia, CA).
For fragment analysis of the DAT, 44-bp VNTR polymorphism (rs28363170) samples were amplified by PCR by using fluorescent-labeled forward primer 6FAM-5′-TCCTTGTGGTGTAGGGAACGG and reverse primer 5′-CTGGAGGTCACGGTCAAGG (Metabion International, Martinsried, Germany) with 1× Qiagen's Hotstar Buffer (Qiagen); 2.5 mM MgCl2; 200 μM each of dATP, dCTP, dGTP, and dTTP (Life Science Products, Frederick, CO); 2 units of Qiagen's Hotstar DNA polymerase; and 0.4 μM of each primer (Metabion International). PCR comprised 35 cycles (96°C for 30 sec, 64°C for 30 sec, and 72°C for 60 sec) with 25 ng of genomic DNA, and final extension was at 72°C for 25 min. PCR products were diluted with MegaBACE ET550-R size standard (GE Healthcare, Chalfont St. Giles, U.K.) following the manufacturer's recommendations, and samples were denatured at 98°C for 3 min, chilled on ice, and subjected to capillary electrophoresis on a MegaBACE 1000 DNA analyzer (GE Healthcare). Electrokinetic injection was at 3 kV for 55 sec, and electrophoresis was continued at 10 kV for 80 min. The data were analyzed with GE Healthcare's MegaBACE Fragment Profiler 1.2 software.
For cycle sequencing analysis of the COMT polymorphism (rs4680), samples were amplified by PCR by using forward primer 5′ACCCAGCGGATGG TGGATTTC and reverse primer 5′-GCCCTTTTTCCAGGTCTGAC (Metabion International) with 1× Qiagen's Hotstar Buffer (Qiagen); 1.5 mM MgCl2; 200 μM each of dATP, dCTP, dGTP, and dTTP (Life Sciences); 2 units of Qiagen's Hotstar DNA polymerase; and 0.4 μM of each primer. PCR comprised 35 cycles (94°C for 60 sec, 63°C for 60 sec, and 72°C for 60 sec) with 25 ng of genomic DNA, and final extension was at 72°C for 10 min. Before cycle sequencing, PCR products were purified by using Qiagen's QiaQuick 96 kit. For cycle sequencing of COMT PCR products, BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) was used. Each 10-μl reaction contained 2 μl of BigDye reaction mix and 10 pmol of forward (5′ACCCAGCGGATGG TGGATTTC) or reverse (5′-GCCCTTTTTCCAGGTCTGAC) primer, respectively. Cycle sequencing comprised 35 cycles (96°C for 10 sec, 55°C for 5 sec, and 60°C for 4 min). Sequence reactions were purified by using Sephadex G-50 columns. Samples were denatured at 95°C for 2 min, chilled on ice, and subsequently subjected to capillary electrophoresis on a MegaBACE 1000 DNA analyzer. Electrokinetic injection was at 2 kV for 75 sec, and electrophoresis was continued at 8 kV for 110 min. The data were analyzed with GE Healthcare's MegaBACE Sequence Analyzer 4.0 and Genecode's (Ann Arbor, MI) Sequencher 4.5 software.
Statistics.
For additional morphometric studies and as an additional control to rule out gross stratification effects, genotyping was also performed for the dopamine receptor D2 TaqIA restriction fragment length polymorphism on human chromosome 11q23 (rs1800497; DRD2A1), a 120-bp tandem duplication polymorphism (120-bp repeat) 1.2 kb upstream from the initiation codon in the promoter region of the dopamine D4 receptor (rs4646984; DRD4) on human chromosome 11p15, brain-derived neurotrophic factor (rs6265; BDNF) Val66Met polymorphism (human chromosome 11p13), the serotonin transporter (rs2066713; SLC6A4) fragment length polymorphism (human chromosome 17q11), and tryptophan hydroxylase TPHA218C (rs1800532) polymorphism (human chromosome 11p15). A contingency table approach (27) was used to test for differences in the allelic distributions of these additional markers for COMT Val/Val and Met/Met subjects or for either DAT VNTR 10R or 9R subjects. This analysis revealed no significant differences at P < 0.05 in allele frequencies for each locus (SI Table 3).
Imaging.
MR scanning was performed on a 3T MR Scanner (Siemens Trio, Erlangen, Germany) with a standard head coil. Thirty-eight continuous axial slices (2 mm thick) were acquired by using a gradient echo-planar T2*-sensitive sequence (TR = 2.22 sec, TE = 25 msec, flip angle 80°, matrix 64*64, field of view 192*192 mm). Subjects viewed the back-projected stimuli via a 45° mirror placed on top of the head coil. The task presentation and the recording of behavioral responses were performed with Cogent 2000v1.24 (www.vislab.ucl.ac.uk/cogent/index.html).
Image processing and statistical analyses were carried out by using SPM2 (www.fil.ion.ucl.ac.uk/spm). All volumes were realigned to the first volume, spatially normalized (43) to an echo planar imaging template in a standard coordinate system, resampled to a voxel size of 3 × 3 × 3 mm, and finally smoothed by using a 10-mm full-width at half-maximum isotropic Gaussian kernel.
All eight conditions of the paradigm were modeled separately in the context of the general linear model as implemented in SPM2. The anticipation and outcome phases were modeled as individual hemodynamic responses (3,034 msec and 7,241 msec after trial onset), leading to 16 regressors (2 × 2 × 2 conditions × 2 regressors). An additional covariate was incorporated into the model, representing the early response (3,034 after trial onset) modulated by the total amount of mouse movements in the choice period of this trial. This covariate ensured that movement-related activation during the early trial period was modeled independently from the regressors of interest (10).
Data were analyzed for each subject individually applying a high-pass filter with a cutoff of 120 sec to remove baseline drifts. Based on the ensuing parameter estimates, contrasts of interest were generated (i.e., main effect of anticipation-related responses against baseline and parametric increase for higher and more likely rewards).
The ensuing contrast images were then entered into the second-level analysis with subject entering as a random effect.
For the simple effect of COMT genotype (Fig. 1), an ANOVA with three groups (Met/Met, Val/Met, and Val/Val) was used. For the combined COMT–DAT analysis (Fig. 3), the general linear model comprised the following regressors: (1) the DAT genotype with levels 9R (including DAT 9/9R and 9/10R) and 10R (including DAT 10/10R), and (2) COMT with levels Met/Met, Val/Met, and Val/Val.
In the analysis of the COMT main effect, all 98 volunteers were tested. Three volunteers with shorter or longer DAT repeat variants were excluded from the COMT–DAT analysis, yielding 95 volunteers for the combined COMT–DAT analysis. Age-related effects were eliminated by modeling age as an additional covariate in both analyses.
For all of the above analyses, the threshold was set to P < 0.05 corrected for multiple comparisons. Based on previous data, correction for hypothesized regions was based on volumes of interest. In particular, correction for the ventral striatum was based on an 18-mm diameter sphere centered on x, y, z: ±15, 9, −9 mm, as identified by an independent study from a different laboratory (9). In other regions, the correction was based on the whole brain.
To test for the reliability of the reported fMRI signal changes in the ventral striatum (interaction between COMT and DAT), we performed additional data analyses. For each group of volunteers, we estimated the 95% confidence intervals of the regression slope by using bootstrap resampling (44) and the percentile t method (10,000 iterations for interval estimation; 200 iterations for variance estimation).
To test for a within-sample replication of the COMT–DAT interaction, we performed a cross-validation dividing the whole sample into odd and even samples (i.e., volunteers) and testing these samples individually.
Acknowledgments
We thank the radiographer and information technology team of NeuroImage Nord for help with scanning, N. Dahmen and A. Gal for help with genetic analyses, B. Schott for supplying bootstrap routines, and M. Smolka for fruitful discussions. This work was supported by Volkswagenstiftung (J.Y., T.S., and C.B.), Studienstiftung des Deutschen Volkes (J.G.), Deutsche Forschungsgemeinschaft (C.B.), Bundesministerium Bildung und Forschung (C.B.), and the National Council of Technological and Scientific Development–CNPq, Brazil (J.Y.).
Abbreviations
DA
dopamine
DAT
DA transporter
PFC
prefrontal cortex.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tobler PN, Fiorillo CD, Schultz W. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 2.Fiorillo CD, Tobler PN, Schultz W. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 3.Montague PR, Hyman SE, Cohen JD. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez R, Wu G. Cogn Psychol. 1999;38:129–166. doi: 10.1006/cogp.1998.0710. [DOI] [PubMed] [Google Scholar]
- 5.Trepel C, Fox CR, Poldrack RA. Cognitive Brain Res. 2005;23:34–50. doi: 10.1016/j.cogbrainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin J, Osher Y, Kotler M, Gritsenko I, Nemanov L, Belmaker RH, Ebstein RP. Mol Psychiatry. 2000;5:96–100. doi: 10.1038/sj.mp.4000640. [DOI] [PubMed] [Google Scholar]
- 8.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 9.O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 10.Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 12.Meyer-Lindenberg A, Weinberger DR. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 13.Bilder RM, Volavka J, Lachman HM, Grace AA. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 14.Floresco SB, West AR, Ash B, Moore H, Grace AA. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 15.Jaber M, Jones S, Giros B, Caron MG. Mov Disord. 1997;12:629–633. doi: 10.1002/mds.870120502. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR. Neuroscience. 2003;116:127–137. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- 17.Huotari M, Santha M, Lucas LR, Karayiorgou M, Gogos JA, Mannisto PT. J Pharmacol Exp Ther. 2002;303:1309–1316. doi: 10.1124/jpet.102.043042. [DOI] [PubMed] [Google Scholar]
- 18.Lachman HM, Papoulos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- 20.Weinshilboum RM, Otterness DM, Szumlanski CL. Annu Rev Pharmacol Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- 21.van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, Baldwin RM, Innis RB, Gelernter J. J Nucl Med. 2005;46:745–751. [PubMed] [Google Scholar]
- 22.Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 23.Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. Pharmacogenomics J. 2001;1:152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- 24.VanNess SH, Owens MJ, Kilts CD. BMC Genet. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mill J, Asherson P, Browes C, D'Souza U, Craig I. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- 26.Yacubian J, Glascher J, Schroeder K, Sommer T, Braus DF, Büchel C. J Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond M, Rousset F. Evolution (Lawrence, Kans) 1995;49:1280–1283. doi: 10.1111/j.1558-5646.1995.tb04456.x. [DOI] [PubMed] [Google Scholar]
- 28.Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahneman D, Tversky A. Econometrica. 1979;4:263–291. [Google Scholar]
- 30.Cragg SJ, Rice ME. Trends Neurosci. 2004;27:270–277. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Grace AA. Brain Res Brain Res Rev. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 32.Sesack SR, Carr DB, Omelchenko N, Pinto A. Ann NY Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- 33.Williams GV, Goldman-Rakic PS. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 34.Tunbridge EM, Harrison PJ, Weinberger DR. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 37.Comings DE, Blum K. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- 38.Pohjalainen T, Rinne JO, Nagren K, Syvalahti E, Hietala J. Am J Psychiatry. 1998;155:768–773. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- 39.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 40.Zuckerman M. Behavioral Expressions and Biosocial Bases of Sensation Seeking. Cambridge: Cambridge Univ Press; 1994. [Google Scholar]
- 41.Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI): Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 42.Petry J. Psychotherapie der Glücksspielsucht. Weinheim: Beltz/Psychologie Verlags Union; 1996. [Google Scholar]
- 43.Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowiak RSJ. Human Brain Mapping. 1995;2:1–25. [Google Scholar]
- 44.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 45.Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, et al. J Neurosci. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]