A Nonhuman Primate Model for the Selective Elimination of CD8+ Lymphocytes Using a Mouse-Human Chimeric Monoclonal Antibody (original) (raw)
Abstract
Nonhuman primates provide valuable animal models for human diseases. However, studies assessing the role of cell-mediated immune responses have been difficult to perform in nonhuman primates. We have shown that CD8+ lymphocyte-mediated immunity in rhesus monkeys can be selectively eliminated using the mouse-human chimeric anti-CD8 monoclonal antibody cM-T807. In vitro, this antibody completely blocked antigen-specific expansion of cytotoxic T cells and decreased major histocompatibility complex class I-restricted, antigen-specific lysis of target cells but did not mediate complement-dependent cell lysis. In vivo administration of cM-T807 in rhesus monkeys resulted in near total depletion of CD8+ T cells from the blood and lymph nodes for up to 6 weeks. This depletion was not solely complement-dependent and persisted longer in adults than in juveniles. Preservation of B cell and CD4+ T cell function in monkeys depleted of CD8+ lymphocytes was demonstrated by their ability to develop humoral immune responses to the administered chimeric monoclonal antibody. Furthermore, during CD8+ lymphocyte depletion, monkeys developed delayed-type hypersensitivity reactions comprised only of CD4+ T cells but not CD8+ T cells. This CD8+ lymphocyte depletion model should prove useful in defining the role of cell-mediated immune responses in controlling infectious diseases in nonhuman primates.
Defining the role of the cellular and humoral components of the immune response to pathogens has furthered our understanding of the pathophysiology of various infectious diseases. Knowledge of these immune responses has also been valuable in designing immunization and other prophylactic strategies to prevent infection by these organisms. Animal models that permit passive administration of immunoglobulins or adoptive transfer of lymphocytes to naive hosts have been crucial for demonstrating the contribution of specific components of the immune response in controlling certain infections.
Numerous experimental approaches have been used to study the role of CD8+ cell-mediated immunity in the control of infections. Studies demonstrating the importance of cellular immunity in various viral infections have been performed by adoptive transfer of lymphocytes in syngeneic mice. 1,2 Genetic knockout mice in which the CD8 or β2 microglobulin genes have been disrupted have been useful for defining the immunopathogenic role of cytotoxic T lymphocytes (CTL) in specific infectious agents. 3,4 Finally, rodents depleted of CD8+ lymphocytes by administration of CD8-specific monoclonal antibodies have been useful in determining the role of CTL in controlling pathogens. 5 However, these approaches have been used only in small laboratory animals. The immune responses to many human pathogens cannot be studied in rodent models.
Nonhuman primates provide unique models for a number of important infectious diseases. These models have been instrumental in characterizing disease pathogenesis and in testing immunization approaches to prevent infection by hepatitis viruses, herpes viruses, and HIV. 6,7 However, the inbred or gene-disrupted nonhuman primates that would be needed for studies of cellular immunity do not exist. Previous attempts to deplete T cell subpopulations in nonhuman primates have had only limited success. Administration of monoclonal antibodies targeting the CD8 molecule have produced only transient and incomplete depletion of CD8-bearing lymphocytes from blood. 8,9 More importantly, these approaches failed to deplete this cell subset consistently from secondary lymphoid organs.
In this report, we describe a rhesus monkey model of CD8+ lymphocyte depletion using a mouse-human chimeric monoclonal antibody. Intravenous administration of this antibody resulted in nearly total depletion of CD8+ lymphocytes from the blood and lymph nodes for 2–6 weeks. However, CD4 cell-mediated immune responses remained intact and all monkeys were capable of mounting humoral immune responses.
Materials and Methods
Monoclonal Antibody Generation and Production
The cMT-807 mAb was prepared as described previously. 10 The heavy and light chain variable region genes were isolated from the murine M-T807 hybridoma 11 and ligated to the human γ1 heavy chain and κ light chain genes, respectively, in separate expression plasmids and transfected into SP2/0-AG14 cells. The secreted mouse-human chimeric mAb was purified using protein A affinity chromatography as previously described. 10
An isotype-matched mouse-human chimeric monoclonal antibody (chimeric 1129) directed against respiratory syncytial virus (MedImmune, Inc., Gaithersburg, MD) was used as a control monoclonal antibody. The CHO DG44 cell line, which was stably transfected with the plasmid that codes for this chimeric monoclonal antibody, was grown in Minimum Essential Medium Alpha without ribonucleosides or deoxyribonucleosides and supplemented with fetal bovine serum, glutamine, and methotrexate. Secreted chimeric antibody was routinely purified using a protein G column and concentrated in phosphate-buffered saline (PBS).
In Vitro Proliferation of Antigen-Specific CTL
To evaluate the effect of the anti-CD8 antibody cM-T807 on the proliferation of antigen-specific CD8+ T cells, we used the simian immunodeficiency virus of macaques (SIVmac) model of infection, where viral peptides and rhesus monkey major histocompatibility complex (MHC) class I alleles capable of presenting these peptides have been defined. 12 Peripheral blood lymphocytes (PBL) were obtained by density gradient centrifugation from 3 monkeys chronically infected with SIVmac expressing the MHC class I allele Mamu A*01. PBL were then cultured for 3 days at 3 × 10 6 cells/ml in the presence of 1 μg/ml of the 9-mer SIVmac Gag peptide p11C, C-M (CTPYDINQM), and either 20 μg/ml cM-T807 or control monoclonal antibody. Cells were then maintained an additional 7 days in medium supplemented with recombinant interleukin-2 (IL-2) (20 U/ml) and either cM-T807 or control antibody (20 μg/ml). To quantitate expansion of SIVmac peptide-specific CD8+ T cells, the cells were washed and stained with anti-rhesus monkey CD3 (FN-18; gift of David M. Neville, Jr., National Institutes of Health, Bethesda, MD) directly coupled to APC and PE-coupled tetrameric complexes of Mamu-A*01/p11C, C-M as described previously. 13,14 The percentage of T cells binding tetramer was then evaluated by flow cytometry.
In Vitro Lysis of Antigen-Expressing Target Cells
To evaluate the effect of cM-T807 on the lytic functional activity of CD8+ T cells, SIVmac-specific lysis of autologous target cells was performed as previously described 12 in the presence of either cM-T807 or control monoclonal antibody. Briefly, PBL were generated from SIVmac-infected rhesus monkeys by nonspecific stimulation with Con A (5 μg/ml) (Sigma Chemical Co., St. Louis, MO) or SIV Gag peptide p11C, C-M for 3 days and then maintained in IL-2-supplemented medium for an additional 8 days. Autologous B lymphoblastoid cell lines (B-LCL), which served as targets, were incubated with 50 μg/ml 12-mer peptide p11C (EGCTPYDINQML) or a negative control peptide p11B (ALSEGCTPYDIN) for 90 minutes during labeling with Na251CrO4 (ICN Pharmaceuticals, Inc., Costa Mesa, CA). Effector lymphocytes were cultured in duplicate using U-bottom microtiter plates with 10 4 target B-LCL at different effector-to-target cell ratios in the presence of either 20 μg/ml cM-T807 or control monoclonal antibody. Plates were incubated in a humidified incubator at 37°C for 4 hours. Specific release was calculated as [(experimental release-spontaneous release)/(maximum release-spontaneous release)] × 100. Spontaneous release was <20% of maximal release with detergent (1% Triton X-100, Sigma Chemical) in all assays.
Complement-Mediated Lysis
Because there was no control antibody available that would cross-react with the rhesus monkey CD8 molecule and show significant complement activation, we used human PBL as targets. PBL from three healthy human donors were isolated by density gradient centrifugation, stained for 20 minutes with anti-CD3-APC (UCHT1, Beckman Coulter, Inc., Miami, FL) and anti-CD4-FITC (OKT4, Ortho Diagnostics, Raritan, NJ), and washed twice. These two mAbs did not show complement activation. The cells were incubated with cM-T807, another monoclonal anti-CD8 antibody (ASH, Biodesign International, Kennebunk, ME), or control monoclonal antibody at 20 μg/ml for 30 minutes on ice. The cells were washed twice and incubated with normal human serum as source of complement at a final concentration of 1:2 for 2 hours at 37°C. The cells were washed once with ice-cold PBS and incubated for 5 minutes with propidium iodide (PI) (50 μg/ml) on ice. The percentage of CD3+4− T cells (equivalent to CD3+8+ T cells) that were lysed and, therefore, stained with PI was determined by flow cytometry.
Antibody Administration to Animals
Rhesus monkeys (Macaca mulatta) were administered cM-T807 or control monoclonal antibody by intravenous injection. For optimal depletion of the monkeys’ CD8+ lymphocytes, 3–5 mg/kg of cM-T807 or control antibody was administered three times (days 0, 4, and 7). For all monoclonal antibody administration and biopsy procedures, animals were anesthetized with ketamine HCl.
All animals were maintained in accordance with the guidelines of the Committee on Animals for the Harvard Medical School and the Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, DC, National Academic Press, 1996).
Purification of Cobra Venom Factor (CVF) and Administration to Animals
CVF was purified from the venom of the species Naja naja by sequential column chromatography 15 and was shown to be free of endotoxin using the E-TOXATE reagent (Sigma Chemical) (data not shown). Intravenous administration of CVF to rhesus monkeys to consume complement was performed as previously described. 16 The first dose of CVF (200 μg/kg body weight) was administered 1 day before administration of cM-T807. The second dose of CVF (100 μg/kg body weight) was administered immediately before cM-T807 administration (5 mg/kg body weight). Two additional administrations of CVF (100 μg/ml) were performed 2 and 4 days after administration of cM-T807. The serum complement hemolytic activity was evaluated by CH50 assay using antibody-sensitized sheep erythrocytes (Sigma Chemical) as targets as described. 17
Immunophenotyping of PBL and Lymph Node Cells
Lymphocytes in peripheral blood were immunophenotyped using EDTA-anticoagulated blood specimens in a whole blood lysis technique. Lymph node lymphocyte suspensions were obtained by gently teasing peripheral lymph node specimens that had been obtained by excisional biopsy. Cells in lymph node cell suspension were adjusted to 106/ml in RPMI 1640/10% fetal bovine serum. Fluorochrome-conjugated antibodies were incubated with 100 μl of whole blood or lymph node cell suspension for 20 minutes at room temperature. Antibodies used were anti-CD3(FN18)-APC, anti-CD4-FITC (OKT4, Ortho Diagnostic Systems), anti-CD8-PE (DK25, Dako, Inc., Carpenteria, CA), and anti-CD20-ECD (B1, Beckman Coulter). Erythrocytes were lysed using a ImmunoPrep Reagent System and a Q-Prep Workstation (Beckman Coulter). To reduce the background level of staining, the ImmunoPrep procedure was modified and lysed samples were washed with 1.0 ml PBS, centrifuged for 3 minutes at 300 × g, and fixed in PBS/1% formalin. Specimens were routinely analyzed for 3-color immunofluorescence using a manually determined light scattergate to gate lymphocytes. Absolute lymphocyte counts on blood specimens were obtained using a T540 Hematology Analyzer (Beckman Coulter).
To determine the extent to which cM-T807 would interfere with binding of the anti-CD8 monoclonal antibody used to detect CD8+ lymphocytes by flow cytometry, we incubated PBL after Ficoll gradient centrifugation with saturating amounts (20 μg/ml) of cM-T807 for 30 minutes on ice. PBL were then stained with anti-CD8-PE and analyzed as described above. The mean CD8-PE fluorescence on the cM-T807-pretreated T lymphocytes was compared to the mean fluorescence on T lymphocytes that were not pretreated.
Delayed-Type Hypersensitivity (DTH) in CD8+ Lymphocyte-Depleted Monkeys
To sensitize rhesus monkeys for later assessment of cutaneous DTH responses, 2 monkeys were immunized intramuscularly with 0.5 ml of tetanus toxoid (Tetanus Toxoid, USP, adsorbed, Connaught Labs, Seattle, WA). Two weeks after immunization, 0.1 ml of tetanus toxoid (Tetanus Toxoid, USP, plain, Connaught Labs) was injected intradermally. At 3 days after intradermal injection of tetanus toxoid, a 6-mm full-thickness punch biopsy of skin was taken at the injection site. For CD8+ lymphocyte depletion, the monkeys received 3 administrations of cM-T807 as described above. Eleven days after initiation of CD8+ lymphocyte depletion the monkeys received another intradermal injection of tetanus toxoid. Three days after injection a skin biopsy was taken as described above. Two weeks after reappearance of CD8+ lymphocytes the monkeys received as a control an intradermal injection of saline and skin biopsies were taken 3 days later as described above. Another 2 weeks later, the monkeys received a third intradermal injection of tetanus toxoid and the final skin biopsy was taken.
Anti-Chimeric Monoclonal Antibody Responses
Antibody responses in monkeys directed against cM-T807 or the control monoclonal antibody were measured by enzyme-linked immunosorbent assay. Chimeric monoclonal antibodies were diluted in PBS (10 μg/ml) and plated in 96-well microtiter plates at 100 μl/well overnight at 4°C. Wells were washed and blocked with PBS/1% nonfat dry milk/2% fetal bovine serum. Test plasma specimens were serially diluted in PBS/1% nonfat dry milk. Aliquots (50 μl) were added to blocked wells, incubated for 2 hours at 37°C, and then washed. Binding of monkey Ig to chimeric monoclonal antibodies was detected using a rhesus monkey IgG-specific mouse monoclonal antibody (B3, kindly provided by Ronald C. Kennedy), followed by horseradish peroxidase-conjugated anti-mouse IgG. Binding of conjugated antibody was detected using tetramethylbenzidine substrate. Endpoint dilutions were determined as optical density reading >2 SD over background.
Histology and Immunohistochemistry
Skin biopsy specimens were bisected. One half of each specimen was fixed in 10% neutral buffered formalin and routinely processed for histological examination and the other half embedded in OCT (Miles Scientific, Naperville, IL), snap-frozen, and kept at −70°C until immunohistochemistry was performed. Routine histological examination of skin was performed on formalin-fixed tissues embedded in paraffin and stained with hematoxylin and eosin.
Immunohistochemical examination of T cell subsets in skin was performed as previously described. 18 Briefly, skin biopsies were snap-frozen, sectioned on a cryostat, and then fixed in ice-cold acetone for 10 minutes. To visualize CD8+ lymphocytes, sections were incubated with the first antibody (anti-CD8, DK25, Dako), the secondary antibody conjugated to biotin, and then an avidin-biotin alkaline phosphatase complex (Vector Labs, Burlingame, CA) with Vector Red as the chromogen. To visualize CD4+ lymphocytes, the staining procedure was repeated using an anti-CD4 primary antibody (NuTh/1, provided by Yokoyama and Matsuo, Nichirei Research Institute, Tokyo) and Vector Blue as the chromogen. Control procedures included omission of the primary antibody and substitution of an isotype-matched irrelevant antibody.
Lymph node biopsy specimens were cut into three portions. One portion was fixed in 10% neutral buffered formalin and routinely processed for histological examination and a second portion was embedded in OCT, snap-frozen, and kept at −70°C until immunohistochemistry was performed. The third portion was used for preparation of single cells for flow cytometric evaluation of lymphocyte subsets. Routine histological examination of lymph nodes was performed on formalin-fixed tissues embedded in paraffin and stained with hematoxylin and eosin or Giemsa stain.
Immunohistochemical identification of CD8+ lymphocytes in lymph nodes was performed on snap-frozen lymph node biopsy specimens that were sectioned (6 μm) on a cryostat and fixed in 2% paraformaldehyde at room temperature for 15 minutes. Sections were rinsed in PBS and incubated with a cocktail of anti-CD8 antibodies (Leu-2a, Becton Dickinson, Heidelberg, Germany and C8/144B, Dakopatts, Hamburg, Germany) or with a cocktail of anti-CD4 antibodies (Leu-3a, Becton Dickinson, and NCL CD4-IF6, Novocastra Laboratories Ltd., Newcastle-on-Tyne, UK) or anti-CD20 antibody (Dako Diagnostika GmbH, Hamburg, Germany). Binding of the primary antibodies was visualized by the alkaline phosphatase anti-alkaline phosphatase technique using New Fuchsin as the chromogen. Sections were counterstained with hematoxylin and mounted.
Results
In Vitro Effect of cM-T807
Because the CD8 molecule is crucial to CTL function, 19 we determined the in vitro effect of cM-T807 on the effector function of CD8+ T cells. To accomplish this, we made use of the simian immunodeficiency virus of macaques (SIVmac) infection model in which SIVmac CTL epitope peptides and restricting rhesus monkey MHC class I molecules have been defined. 7 However, it was first necessary to determine whether pretreatment of PBL with cM-T807 would interfere with the binding of the anti-CD8 antibody used for flow cytometric detection of CD8+ lymphocytes. As shown in Figure 1A ▶ , pretreatment of PBL with saturating concentrations with cM-T807 resulted in a one-half log decrease in the intensity of CD8-PE fluorescence as compared to untreated PBL. However, T cells pretreated with cM-T807 stained positively with anti-CD8-PE with fluorescence intensity >1 log brighter than background staining, assuring that CD8+ lymphocyte could be detected readily in the presence of the chimeric anti-CD8 antibody.
Figure 1.
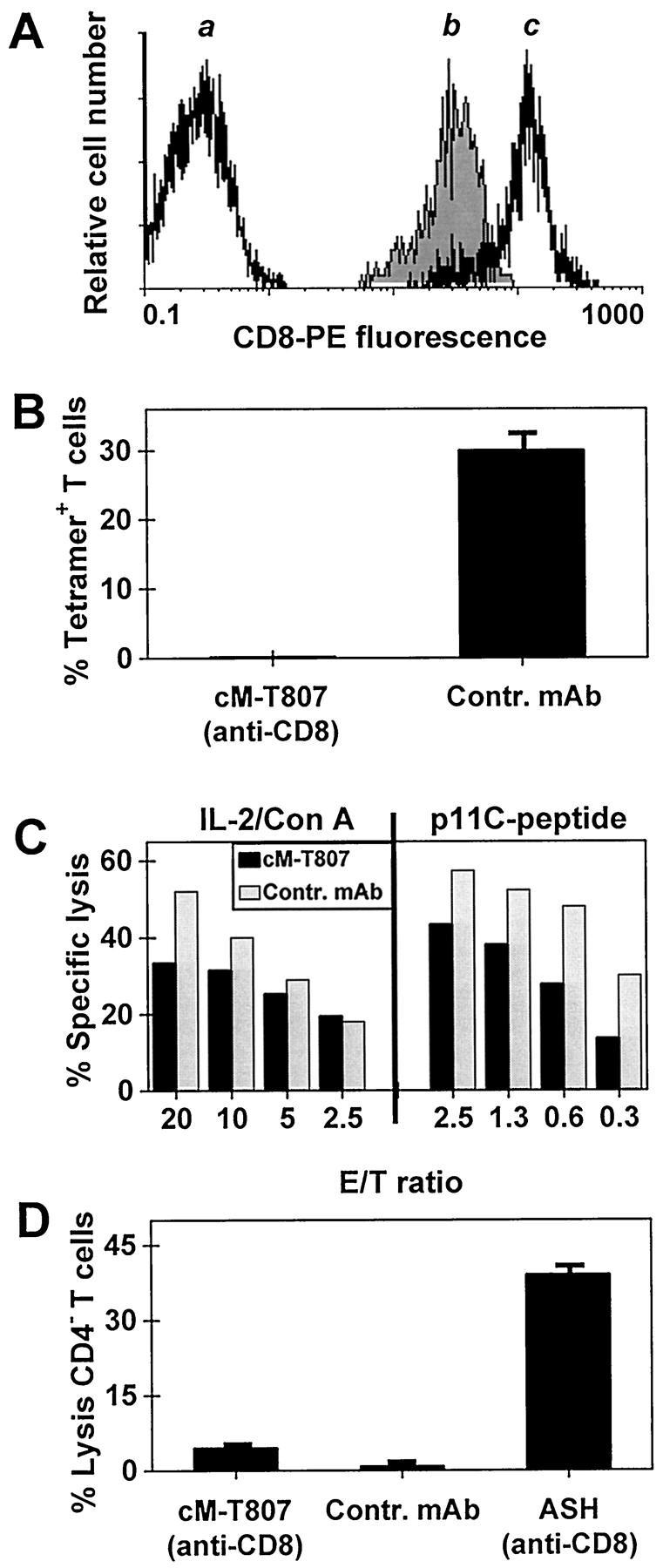
Activity of cM-T807 monoclonal antibody on CD8+ lymphocytes in vitro. A: Pretreatment of PBL with saturating concentrations with cM-T807 did not block the detection of CD8+ T cells using anti-CD8-PE. a, CD8- T cells (background staining); b, CD8+ T cells pretreated with cM-T807, then stained with anti-CD8-PE; c, CD8+ T cells stained with anti-CD8-PE without pretreatment. B: cM-T807 blocked the in vitro expansion of viral antigen-specific CD8+ T cells. PBL from a monkey infected with SIVmac were incubated with IL-2 and a MHC class I-restricted SIVmac peptide. Expansion of antigen-specific CD8+ T cells after 7 days of incubation in the presence of cM-T807 or a control monoclonal antibody was determined by the percentage of T cells that bound MHC-I/peptide tetrameric complexes (mean ± SD, n = 3). C: cM-T807 decreased antigen-specific, MHC class I-restricted cell lysis. PBL from 2 SIVmac-infected monkeys were expanded by nonspecific stimulation (Con A/IL-2) or by specific stimulation with a MHC class I-restricted SIVmac peptide (p11C-peptide) and then incubated with SIVmac peptide-pulsed, autologous target cells. Lysis of target cells in the presence of cM-807 (black bars) or control antibody (shaded bars) was determined by chromium release assay. D: cM-T807 caused marginal complement-dependent cell lysis. Human PBL were incubated with complement and cM-T807, control antibody or an anti-CD8 antibody (ASH) known to be lytic. Lysis of CD8+ T cells was determined flow cytometrically by quantitating CD3+4− cells that showed uptake of PI (mean ± SD, n = 3). E/T ratio, effector cell-to-target cell ratio.
To determine the functional effects of cM-T807, PBL from chronically SIVmac-infected monkeys were expanded in vitro by specific stimulation using the SIV Gag peptide p11C. The p11C-specific CTL were evaluated using the flow cytometry-based tetramer technology as described previously. 13,14 Whereas the addition of a control monoclonal antibody had no effect on the expansion of virus-specific CTL, addition of cM-T807 caused nearly complete inhibition of SIV Gag peptide p11C-specific CTL expansion (Figure 1B) ▶ .
We next wished to determine whether cM-T807 would interfere with the ability of CD8+ T cells to lyse target cells following antigen-specific MHC class I-restricted recognition. Effector cells expanded in vitro by either antigen-specific or nonspecific stimulation were incubated with autologous B-LCL target cells expressing the SIVmac Gag CTL epitope peptide. The addition of cM-T807 resulted in a decrease, but not a total inhibition, of target cell lysis using effector cells that had been expanded by either nonspecific or antigen-specific stimulation using the SIV Gag peptide p11C (Figure 1C) ▶ .
Finally, we wished to determine whether cM-T807 could affect CD8+ lymphocyte function and viability through complement-mediated lysis. To accomplish this, we incubated human PBL with cM-T807, a control monoclonal antibody, or another anti-CD8 antibody (ASH; IgM-isotype) with known lytic activity. Lysis of CD8+ T cells was determined by PI uptake in the CD3+4− T cell subset. In contrast to the anti-CD8 antibody ASH, cM-T807 showed only a low complement-mediated lysis of CD4− T cells, similar to that seen in control monoclonal antibody-treated cells (Figure 1D) ▶ .
In Vivo Administration of cM-T807 to Rhesus Monkeys
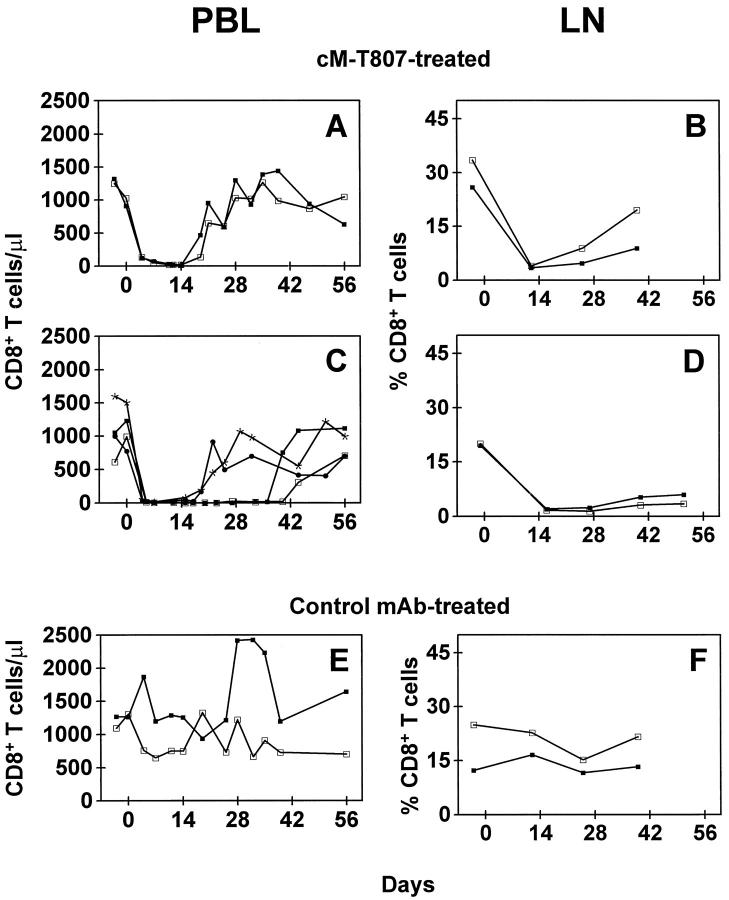
To determine the effect of cM-T807 in vivo, we administered 3–5 mg/kg body weight of the anti-CD8 antibody or a control monoclonal antibody intravenously to 2-year-old juvenile rhesus monkeys 3 times over a 7-day period. cM-T807 induced an immediate depletion of CD8+ lymphocytes in peripheral blood (up to 99%) in the 2 cM-T807-treated monkeys. This treatment resulted in elimination of both CD8+ T cells (CD3+8+) and CD8+ NK cells (CD3−8+). CD8+ T cells (Figure 2A) ▶ and CD8+ NK cells (data not shown) first reappeared in these monkeys 14 days after the first injection. A significant (80–100%) reduction in percentage of CD8+ T cells in lymph nodes was also observed (Figures 2B, 3A, and 3B) ▶ ▶ .
Figure 2.
Intravenous administration of cM-T807 depleted blood and lymph node of CD8+ T cells. Rhesus monkeys received three injections of cM-T807 or control monoclonal antibody (3–5 mg/kg) on days 0, 3, and 7. CD8+ T cells were depleted from the blood (A, C) and lymph nodes (B, D). In monkeys 2 years of age (A, B), duration of depletion was shorter than in monkeys >5 years of age (C, D). The identical treatment protocol with a control monoclonal antibody resulted in no change in CD8+ T cells (E, F). LN, lymph node. Each line illustrates the results from one monkey.
Figure 3.
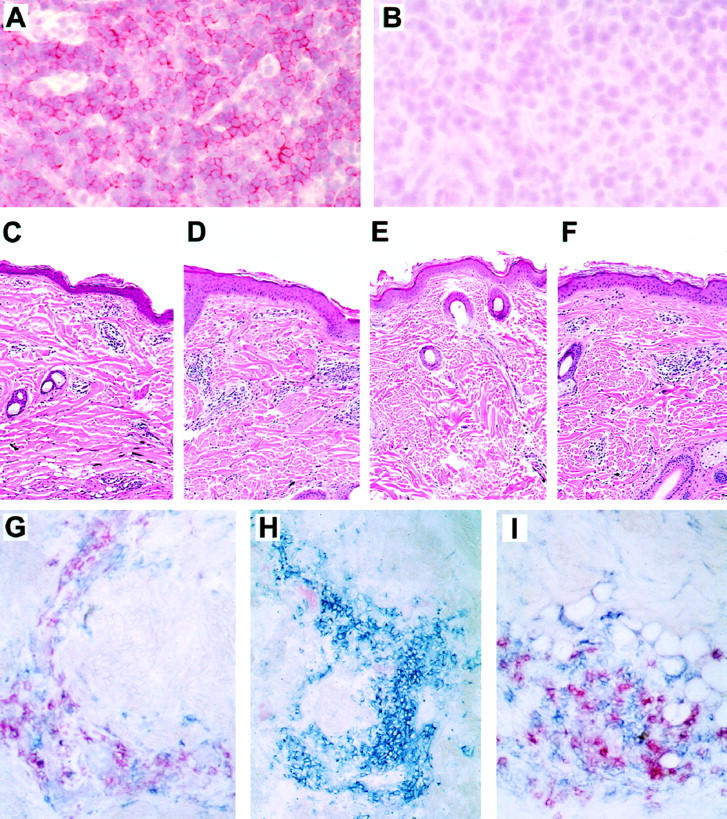
Histology and immunohistochemistry of lymph nodes and skin. A-B: cM-T807-treatment resulted in nearly total depletion of CD8+ lymphocytes from lymph nodes as shown in the T-cell zone of lymph nodes. Sections of lymph nodes taken before (A) or 14 days after (B) treatment with cM-T807 were stained for CD8. Original magnification, ×100. C-F: Cellular infiltration in cutaneous DTH reactions in tetanus toxoid-sensitized monkeys. Mononuclear cells infiltrated dermal and subdermal regions of skin at the site of tetanus toxoid injection before treatment (C) and at 2 weeks after cM-T807 treatment when CD8+ lymphocytes were depleted (D). Two to four weeks after the reappearance of CD8+ lymphocytes, saline injection resulted in no cellular infiltrates (E), but tetanus toxoid caused the typical DTH reaction (F). G-I: Immunophenotype of cells infiltrating DTH reaction. Before cM-T807 treatment, both CD4+ (blue) and CD8+ (red) cells appear at the site of the DTH reaction (G). After CD8+ lymphocyte depletion, only CD4+ cells are recruited into the DTH reaction (H), whereas both CD4+ (blue) and CD8+ (red) cells infiltrate skin after reappearance of CD8+ lymphocytes (I). (A, B, immunohistochemistry using New Fuchsin as chromogen; C-E, hematoxylin and eosin staining; G-F, Vector Red and Vector Blue chromogens.)
Although we have shown above that cM-T807 does not inhibit the detection of CD8+ T cells by the anti-CD8-PE reagent, we could not rule out that CD3+CD8− lymphocytes may arise after prolonged exposure to cM-T807. However, during cM-T807-mediated CD8+ lymphocyte depletion >95% of all remaining lymphocytes were either CD20+ B cells or CD4+ T lymphocytes, suggesting that any CD3+CD8− cells represented only a very small subset.
The absolute numbers of circulating CD4+ T cells and B cells did not change during this treatment. Similarly, in the lymph nodes of cM-T807-treated monkeys, the number and distribution of CD4+ lymphocytes or CD20+ B cells did not differ from that seen in control antibody-treated monkeys (data not shown). Studies in humans 20 and monkeys 21 have suggested that the duration of lymphocyte depletion may be related to the age-dependent regenerative function of the thymus. Therefore, we evaluated the CD8 lymphocyte-depleting efficacy of cM-T807 in mature animals (>5 years of age). Indeed, we noted a longer duration of depletion of CD8+ lymphocytes from blood and lymph nodes in more mature animals, persisting 21–40 days after the first injection of cM-T807 (Figure 2C and D) ▶ . Control monoclonal antibody-treatment resulted in no change of CD8+ T cells in peripheral blood or lymph nodes (Figure 2, E and F) ▶ .
Mechanism of Depletion Is Not Solely Complement-Dependent
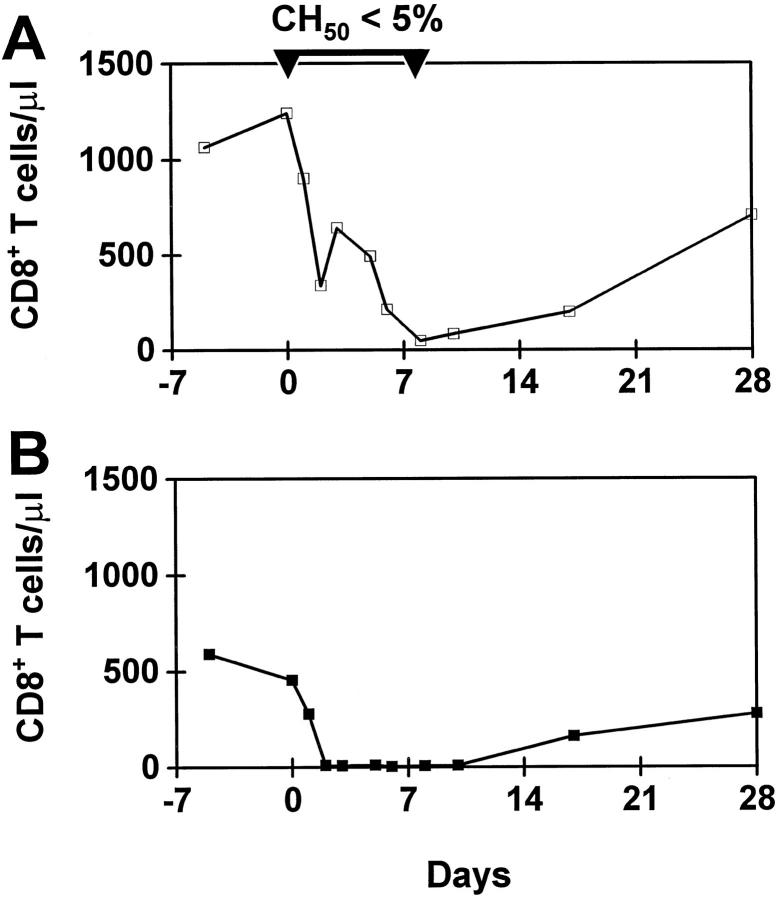
Several mechanisms may contribute to the lymphocyte-depleting effect of a mAb in vivo. Complement proteins and their activation products may play a major role in this process. Although our in vitro results suggested that cM-T807 was a poor activator of complement, we directly assessed the role of complement proteins in the cM-T807-mediated depletion of CD8+ lymphocytes in vivo. Administration of CVF leads to a significant reduction of the hemolytic activity of complement by activation of the alternative pathway. 22 CVF treatment can be used to deplete complement in vivo in a variety of animal species including rhesus monkeys. 16 To determine whether cM-T807 could deplete CD8+ lymphocytes in monkeys with significantly reduced hemolytic activity of complement, CVF was administered 1 day before and on days 0, 2, and 4 after cM-T807-administration (Figure 4A) ▶ . This treatment reduced the hemolytic activity to <5% of pretreatment levels for 8 days. Despite reduction in the complement activity, we still observed a 50–80% reduction of CD8+ T cells during the first 7 days following cM-T807 administration. It appeared, however, that the CD8+ lymphocyte depletion was less efficient in the complement-depleted monkey as compared to the control monkey that received cM-T807 but not CVF (Figure 4B) ▶ . These results suggest that complement-independent mechanisms are primarily responsible for the cM-T807-induced CD8+ lymphocyte depletion in vivo.
Figure 4.
Effect of consumption of complement in vivo through administration of CVF on depletion of CD8+ lymphocytes. A rhesus monkey received 4 injections of CVF (500 μg/kg total dose) to consume complement in vivo during treatment with a single administration of cM-T807 (5 mg/kg, day 0). Although hemolytic complement was reduced to <5% by this treatment, cM-T807 caused substantial depletion of CD8+ T cells (A) as compared to the identical treatment of a control monkey with cM-T807 that did not receive CVF (B).
DTH Reactions in CD8+ Lymphocyte-Depleted Monkeys
To determine how administration of cM-T807 and subsequent CD8+ lymphocyte depletion affects other immune responses, we assessed cutaneous DTH reactions in monkeys that were CD8+ lymphocyte-depleted. Monkeys previously sensitized to tetanus toxoid received an intradermal injection of tetanus toxoid after the final administration of cM-T807. Cellular infiltration into the skin at the site of tetanus toxoid challenge was determined by histological examination of skin biopsies taken 3 days after antigen was injected. Superficial dermal perivascular mononuclear cell infiltration was accompanied by variable numbers of eosinophils. 23 As illustrated in Figure 3 ▶ , cellular infiltrates typical of a DTH reaction were evident before CD8+ lymphocyte depletion (Figure 3C) ▶ , during CD8+ lymphocyte depletion (Figure 3D) ▶ , and after reappearance of CD8+ lymphocytes (Figure 3F) ▶ , but not following saline injections (Figure 3E) ▶ . Immunophenotyping of the infiltrating mononuclear cells showed a mixture of CD4+ and CD8+ cells before CD8+ lymphocyte depletion (Figure 3G) ▶ and after reappearance of CD8+ lymphocytes (Figure 3I) ▶ . However, during CD8+ lymphocyte depletion, only CD4+ lymphocytes were present at the site of the DTH reaction (Figure 3H) ▶ . These results confirmed that no CD8+ lymphocytes capable of contributing to the DTH reaction remained in the cM-T807-treated monkeys. Furthermore, inflammatory responses mediated by CD4+ lymphocytes remained intact during the period of CD8+ lymphocyte depletion.
Humoral Immune Responses in CD8+ Lymphocyte-Depleted Monkeys
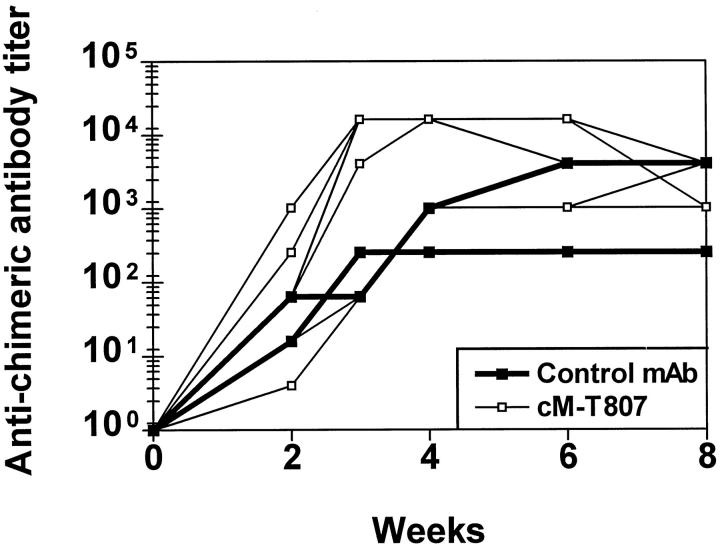
To determine the ability of CD8+ lymphocyte-depleted monkeys to mount humoral immune responses and to assess the antigenicity of mouse-human chimeric monoclonal antibodies in the treated monkeys, we measured the generation of IgG responses specific for the administered chimeric monoclonal antibodies. As shown in Figure 5 ▶ , monkeys that received either control or cM-T807 monoclonal antibodies developed IgG antibody responses that recognized the chimeric monoclonal antibody by 3 weeks after the first treatment. Titers of anti-chimeric IgG in control monoclonal antibody-treated monkeys were similar to those measured in monkeys that received cM-T807.
Figure 5.
Development of humoral immune responses to cM-T807 or control chimeric antibody in monkeys. Detection of monkey antibodies to mouse-human chimeric antibodies was performed by enzyme-linked immunosorbent assay. All monkeys that received either cM-T807 (open symbols) or control chimeric monoclonal antibody (closed symbols) developed humoral immune responses to the administered antibodies, peaking within 3–6 weeks.
Discussion
These studies demonstrate the in vitro and in vivo functional activity of a unique CD8-specific monoclonal antibody in rhesus monkeys. The antibody completely blocked the in vitro expansion of antigen-specific CD8+ effector cells and partially inhibited antigen-specific, MHC class I-restricted lytic function of this cell population. Most interestingly, this monoclonal antibody caused an efficient in vivo depletion of CD8+ lymphocytes from the circulation and secondary lymphoid organs of rhesus monkeys. This extent and duration of depletion substantially exceeded that observed in previous attempts to eliminate CD8+ lymphocytes in nonhuman primates. 8,9 The loss of this subpopulation of lymphocytes appeared to be remarkably complete but did not affect immune responses mediated by CD4+ T cells and B cells.
Both CD4+ and CD8+ lymphocytes are observed in the DTH reaction in other animal models but the CD4+ lymphocytes appear to be the predominant effector cells. 24-26 Before treatment with the anti-CD8 monoclonal antibody, both CD4+ and CD8+ lymphocytes were present in the DTH infiltrate of the monkeys. During CD8+ lymphocyte depletion, however, the cellular infiltrate was comprised entirely of CD4+ lymphocytes. These findings confirmed the competence of CD4+ T cells. Furthermore, all monkeys treated with the anti-CD8 monoclonal antibody developed antibody responses against the administered chimeric immunoglobulin, indicating that humoral immune responsiveness was preserved.
The mechanism by which cM-T807 mediates depletion of CD8+ lymphocytes is unclear. Results from in vitro studies and observations in the complement-depleted monkey suggested that mechanisms independent of the lytic activity of complement played a substantial role in this process. However, complement components of the classical pathway, such as C1 and C4, not consumed during CVF treatment could potentially contribute to the cell depletion in vivo. Alternatively, Fc receptor-mediated interactions between cM-T807-coated CD8+ lymphocytes and granulocytes or macrophages may also be involved in the cell depletion.
The duration of CD8+ lymphocyte depletion varied among monkeys. Thymectomy has been shown to slow the rate of lymphocyte repopulation following mAb-mediated depletion in mice. 27 Similarly, a delay in lymphocyte repopulation has been seen after bone marrow transplantation in adults as compared to children. 28 Monkeys experimentally depleted of T cells showed age-related differences in the rates of lymphocyte repopulation. 21 Therefore, we reasoned that CD8+ lymphocyte depletion may persist for a longer period of time in older monkeys. In fact, lymphocyte depletion persisted in monkeys >5 years old for a longer period than in 2-year-old monkeys. Only 2 weeks of complete CD8+ lymphocyte depletion was achieved in juvenile monkeys. However, in 2 of 4 older monkeys, CD8+ lymphocytes reappeared only after 6 weeks. This duration of cell depletion should be sufficient to assess the role of CD8+ lymphocytes in an experimental setting.
This nonhuman primate model of CD8+ lymphocyte depletion will be extremely useful in clarifying the immune control mechanisms in diseases that cannot be studied in other animal models. The method described here will also have particular relevance in defining the immune correlates of vaccine protection. For example, the use of the live, attenuated nonhuman primate lentiviruses have proven to be one of the most effective vaccine approaches against challenge infection with a pathogenic SIV. 29 This nonhuman primate model of CD8+ lymphocyte depletion will allow the determination of the correlates of protective immunity in the live, attenuated vaccines as well as in other immunization strategies.
Acknowledgments
We thank Ronald C. Kennedy, Scott M. Koenig, Meryl A. Forman, and David M. Neville, Jr. for providing reagents and David E. Lee-Paritz, Kelledy H. Manson, Prahbat K. Sehgal, Birgit Raschdorff, and Gudrun Grosschupff for assistance in performing these studies.
Footnotes
Address reprint requests to Dr. Jörn E. Schmitz, Division of Viral Pathogenesis, Beth Israel Deaconess Medical Center, RE-113, P.O. Box 15732, Boston, MA 02215. E-mail: jschmitz@caregroup.harvard.edu.
Supported by DHHS Public Health Service grants RR-13150 and RR-00168 and by the German Ministry of Education and Research (BMBF 01 K1–9714 6) (to P. R. and K. T.-R.) and German Ministry of Education and Research AIDS Program (to J. E. S.).
References
- 1.Yap KL, Ada GL, McKenzie IFC: Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature 1978, 273:238-239 [DOI] [PubMed] [Google Scholar]
- 2.Riddell SR, Greenberg PD: Principles for adoptive T cell therapy of human viral diseases. Annu Rev Immunol 1995, 13:545-586 [DOI] [PubMed] [Google Scholar]
- 3.Fung-Leung W-P, Schilham MW, Rahemtulla A, Kundig TM, Vollenweider M, Potter J, van Ewijk W, Mak TW: CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell 1991, 65:443-449 [DOI] [PubMed] [Google Scholar]
- 4.Nesic D, Vukmanovic S: MHC class I is required for peripheral accumulation of CD8+ thymic emigrants. J Immunol 1998, 160:3705-3712 [PubMed] [Google Scholar]
- 5.Mielke ME, Niedobitek G, Stein H, Hahn H: Acquired resistance to Listeria monocytogenes in mediated by Lyt-2+ T cells independently of the influx of monocytes into granulomatous lesions. J Exp Med 1989, 170:589-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClure HM: Nonhuman primate models for human disease. Adv Vet Sci Comp Med 1984, 28:267-304 [DOI] [PubMed] [Google Scholar]
- 7.Lackner AA: Pathology of simian immunodeficiency virus induced disease. Current Topics in Microbiology and Immunology, Vol. 188, Simian Immunodeficiency Virus. Edited by NL Letvin, RC Desrosiers. Berlin, Springer Verlag, 1994, pp 35–64 [DOI] [PubMed]
- 8.Castro BA, Walker CM, Eichberg JW, Levy JA: Suppression of human immunodeficiency virus replication by CD8+ cells from infected and uninfected chimpanzees. Cell Immunol 1991, 132:246-255 [DOI] [PubMed] [Google Scholar]
- 9.Matano T, Shibata R, Siemon C, Connors M, Lane HC, Martin MA: Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus monkeys. J Virol 1998, 72:164-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Looney JE, Willinger A, Lin G, Rieber EP, Riethmüller G, Ghrayeb J: Expression and characterization of cMT-413, a chimeric anti-CD4 antibody with in vitro immunosuppressive activity. J Immunother Emphasis Tumor Immunol 1994, 16:36-46 [DOI] [PubMed] [Google Scholar]
- 11.Rieber EP: T-cell section report. Edited by Knapp W, Doerken B, Gilks WR, Rieber EP, Schmidt RE, Stein H, von dem Borne AEGKr: Leucocyte Typing IV. Oxford University Press, Oxford, 1989, pp 229–249
- 12.Miller MD, Yamamato H, Hughes AL, Watkins DI, Letvin NL: Definition of an epitope and MHC class I molecule recognized by Gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J Immunol 1991, 147:320-329 [PubMed] [Google Scholar]
- 13.Kuroda MJ, Schmitz JE, Barouch DH, Craiu A, Allen TM, Sette A, Watkins DA, Forman MA, Letvin NL: Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J Exp Med 1998, 187:1373-1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seth A, Ourmanov I, Kuroda MJ, Schmitz JE, Carroll MW, Wyatt LS, Moss B, Forman MA, Hirsch VM, Letvin NL: Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide teramer. Proc Natl Acad Sci USA 1998, 95:10112-10116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel C-W, Muller-Eberhard HJ: Cobra venom factor: improved method for purification and biochemical characterization. J Immunol Meth 1984, 73:203-220 [DOI] [PubMed] [Google Scholar]
- 16.Schmitz JE, Lifton MA, Reimann KA, Montefiori DC, Shen L, Racz P, Tenner-Racz K, Ollert MW, Forman MA, Gelman RS, Vogel C-W, Letvin NL: Effect of complement consumption by cobra venom factor on the course of primary infection with simian immunodeficiency virus in rhesus monkeys. AIDS Res Hum Retroviruses 1999, 15:195-202 [DOI] [PubMed] [Google Scholar]
- 17.Giclas PC: Complement tests. Manual of Clinical Laboratory Immunology. Fifth ed. Edited by NR Rose, E Conway de Macario, JD Folds, HC Lane, RM Nakamura. Washington, DC, AMS Press, 1997, pp 181–202
- 18.Sasseville VG, Rottman JB, Du Z, Veazey R, Knight HL, Caunt D, Desrosiers RC, Lackner AA: Characterization of the cutaneous exanthem in macaques infected with a Nef gene variant of SIVmac239. J Invest Dermatol 1998, 110:894-901 [DOI] [PubMed] [Google Scholar]
- 19.O’Rourke AM, Mescher MF: The roles of CD8 in cytotoxic T lymphocyte function. Immunol Today 1993, 14:183-188 [DOI] [PubMed] [Google Scholar]
- 20.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM, Wexler LH, Gress RE: Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy N Engl J Med 1995, 332:143-149 [DOI] [PubMed] [Google Scholar]
- 21.Neville DM, Jr, Scharff J, Hu HZ, Rigaut K, Shiloach J, Slingland W, Jonker M: A new reagent for the induction of T cell depletion, anti-CD3-CRM9. J Immunother Emphasis Tumor Immunol 1996, 19:85-92 [DOI] [PubMed] [Google Scholar]
- 22.Vogel C-W: Cobra venom factor: the complement-activating protein of cobra venom. Tu AT eds. Handbook of Natural Toxins, 1991, vol. 5.:pp 147-188 Marcel Dekker, New York [Google Scholar]
- 23.Frew AJ, Kay AB: Eosinophils and T-lymphocytes in late-phase allergic reactions. J Allergy Clin Immunol 1990, 85:533-539 [DOI] [PubMed] [Google Scholar]
- 24.Colditz IG, Watson DL: The effect of cytokines and chemotactic agonists on the migrations of T lymphocytes into skin. Immunology 1992, 76:272-278 [PMC free article] [PubMed] [Google Scholar]
- 25.Baldridge JR, Ward JR: Effective adjuvants for the induction of antigen-specific delayed-type hypersensitivity. Vaccine 1997, 15:395-401 [DOI] [PubMed] [Google Scholar]
- 26.Bianchi ATJ, Moonen-Leusen HWM, van Milligen FJ, Savelkoul HFJ, Zwart RJ, Kimman TG: A mouse model to study immunity against pseudorabies virus infection: significance of CD4+ and CD8+ cells in protective immunity. Vaccine 1998, 16:1550-1558 [DOI] [PubMed] [Google Scholar]
- 27.Rice JC, Bucy RP: Difference in degree of depletion, rate of recovery, and the preferential elimination of naive CD4+ T cells by anti-CD4 monoclonal antibody (GK1.5) in young and aged mice. J Immunol 1995, 154:6644-6654 [PubMed] [Google Scholar]
- 28.Mackall CL, Gress RE: Thymic aging and T-cell regeneration. Immunol Rev 1997, 160:91-102 [DOI] [PubMed] [Google Scholar]
- 29.Wyand MS, Manson KH, Garcia-Moll M, Montefiori D, Desrosiers RC: Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol 1996, 70:3724-3733 [DOI] [PMC free article] [PubMed] [Google Scholar]