Accelerated Skin Wound Healing in Plasminogen Activator Inhibitor-1-Deficient Mice (original) (raw)
Abstract
Components of the fibrinolytic system have been implicated in cell migratory events associated with tissue remodeling. Studies in plasminogen-deficient mice (PG−/−) indicated that skin wound healing is impaired, but is resolved with an additional fibrinogen deficiency. Plasminogen activator inhibitor-1 (PAI-1) expression by keratinocytes has been identified shortly after wound injury. PAI-1 expression could affect wound healing by regulating the fibrinolytic environment of the wounded area, as well as influencing events associated with cell attachment and detachment through interactions with matrix proteins. The present study directly assesses PAI-1 involvement in skin wound healing through analyses of a dermal biopsy punch model in PAI-1-deficient (PAI-1−/− mice. While the cellular events associated with the healing process are similar between wild-type (WT) and PAI-1−/− mice, the rate of wound closure is significantly accelerated in PAI-1−/− mice.
The fibrinolytic system consists of the zymogen, plasminogen (Pg), its serine protease activated form, plasmin (Pm); plasminogen activators, viz., urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA), which are also serine proteases; receptors for these proteins viz., the uPA receptor (uPAR); serine-type inhibitors, viz., plasminogen activator inhibitor-1 (PAI-1); and fibrinogen/fibrin. While the major physiological function of this system is to maintain vascular patency through fibrin surveillance, a number of in vitro and in vivo studies have implicated Pm in playing an important role in facilitating cell migration through direct proteolysis of extracellular matrix protein or indirectly through activation of other matrix degrading pathways, e.g., metalloproteases. These events are pivotal in wound healing and tissue remodeling processes. Alternatively, inhibitors of this proteolytic pathway serve to regulate the extent of tissue degradative processes and thus balance destructive and repair events. Indeed, previous studies have indicated that uPA and PAI-1 are regulated in their expression, both spatially and temporally, during the migration of keratinocytes and connective tissue cells during reepithelialization, and tissue remodeling associated with wound healing. 1 Additional studies of wound healing using the keratinocyte cell line, HaCaT, transfected with an antisense PAI-1 vector, indicated that the rate and extent of wound closure was impaired. 2 Direct studies in plasminogen-deficient (PG −/−) mice have indicated that keratinocyte migration during skin wound healing is attenuated, a phenomenon which appears to be the result of unresolved provisional fibrin matrix. 3,4 However, vascular injury models have shown that PAI-1 plays an inhibitory role during wound healing and arterial neointima formation following injury. 5-7 Therefore, the location and cell types affected play important roles in determining whether alterations in PAI-1 expression impact the wound healing responses.
Using a skin wound healing model and PAI-1 −/− mice, we have investigated the effects of alterations in the expression of PAI-1 on skin wound healing processes. The results of this investigation are reported herein.
Materials and Methods
Animals
The generation of mice homozygous for a total PAI-1 deficiency (PAI-1 −/−) has been described. 8 PAI-1 −/− mice were back-crossed to at least the F7 generation in strain C57Bl/6J (∼99% C57Bl/6J background). The animals were housed in micro-isolation cages on a constant 12 hours light/dark cycle with controlled temperature and humidity and given access to food and water ad libitum. C57Bl/6J animals (Jackson Laboratories, Bar Harbor, ME) were used as wild-type (WT) controls. All mice used in these studies were between 8 and 12 weeks of age and of mixed gender. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Induction of the Skin Wound
Mice were anesthetized by intraperitoneal injection of rodent cocktail (0.015 mg xylazine/0.075 mg ketamine/0.0025 mg aceprozamine per gram body weight). The backs of the mice were shaved and sterilized with alcohol, followed by 1% iodine solution. A full thickness wound, approximately 8 mm in diameter, was made using a dermal biopsy punch, down, but not through, the muscle fascia. Mice were singly caged, without bedding, for the first several days until a provisional matrix had formed. Wound areas (width × length) were measured every other day. Mice were sacrificed at various time points during healing or at the time when the wound appeared closed (endpoint). At least three mice were sacrificed for each time point. The wounded tissues and the surrounding skin were carefully excised, pinned to a corkboard, and fixed flat in 10% neutral buffered formalin (NBF) for 3 hours before alcoholic dehydration and paraffinization. Wounds were bisected and embedded in paraffin. Microtomy was performed at 4 μm.
Histology, Histochemistry, and Immunohistochemistry
Sections were stained with hematoxylin and eosin (H&E) to examine general tissue and cellular morphology, and with Masson’s Trichrome for identification of collagen IV. 9 The Ayoub-Shklar method was used for identification of keratin and prekeratin structures. 10 The periodic acid-Schiff procedure (PAS) was used for identification of basement membranes. 11
A number of immunohistochemical stains were performed. Fibrin was identified with a polyclonal goat-anti-mouse fibrin(ogen) antibody (Accurate Chemicals, Westbury, NY). Antigen retrieval was performed under high temperature and pressure with citrate buffer (BioGenex, San Ramon, CA), followed by endogenous peroxidase blocking with Peroxoblock (Zymed, South San Francisco, CA). Subsequent to incubation, first with rabbit serum and then with the primary antibody, the slides were incubated with the secondary rabbit anti-goat IgG (Dako, Carpinteria, CA), followed by goat peroxidase anti-peroxidase (Dako). Peroxidase activity was detected with the substrate 3-amino-9-ethylcarbazole (AEC), (Biomeda, Foster City, CA). PAI-1 antigen was detected by citrate buffer antigen retrieval and a rabbit anti-rat PAI-1 IgG (American Diagnostica, Greenwich, CT). Slides were then incubated with a swine anti-rabbit biotin F(ab)2 IgG, followed by streptavidin-HRP (BioGenex) and AEC detection. A similar procedure was used for detection of smooth muscle cell α-actin with an anti-α-actin monoclonal antibody (Sigma Chemical Co., St Louis, MO) and vascular endothelial growth factor (VEGF) with a mouse anti-human monoclonal antibody (Oncogene Research Products, Boston, MA). CD45-positive leukocytes were detected using citrate buffer antigen retrieval and a biotinylated rat anti-mouse CD45 monoclonal antibody (PharMingen, San Diego, CA). Following a streptavidin incubation (NEN, Boston, MA), detection was performed with diaminobenzidine (DAB) (Dako). Urokinase was identified using a rabbit anti-rodent antibody (American Diagnostica), followed by biotin-conjugated swine anti-rabbit F(ab)2 IgG and then streptavidin conjugated HRP. AEC chromogen was used for visualization and antigen retrieval was accomplished using Tris-HCl, pH 8.0. All immunohistochemical slides were counterstained with hematoxylin (Biomeda).
Results
Kinetics of Skin Wound Healing in WT and PAI-1−/− Mice
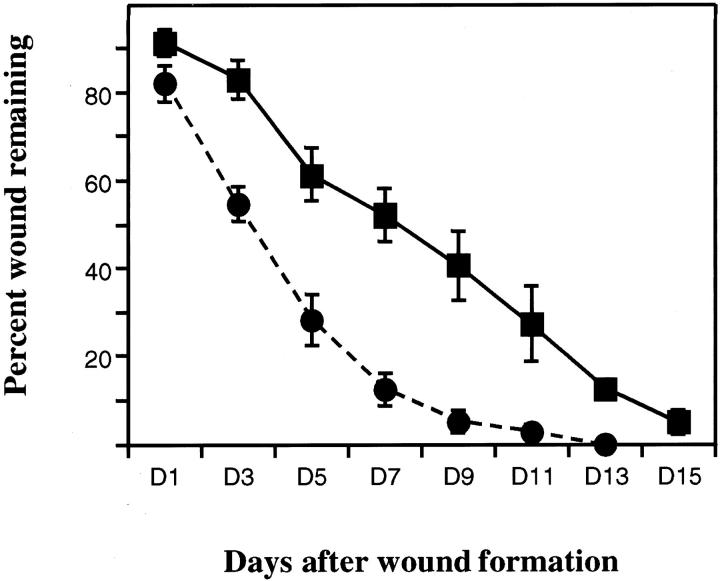
After dermal incision, wound areas were measured every other day for 15 days and expressed as percent wound remaining as a function of time. The rate of wound healing was significantly increased in PAI-1 −/− mice relative to WT mice, day 3–9 comparisons P < 0.05 (Figure 1) ▶ . By day 7, approximately 90% of the wound was closed in PAI-1 −/− mice compared to 50% in WT mice. Between days 11 and 13, the wound was completely sealed in PAI-1 −/− mice while in WT mice wound closure was still incomplete.
Figure 1.
Kinetics of skin wound healing in _PAI-1_−/− and WT mice. The percentage area of the wound (width × length) remaining as a function of time after skin incision in PAI-1 −/− (•) and WT (▪) mice. At least 4 mice/genotype/time point are graphically represented and expressed as the average ± SE. P values at days 3–9 < 0.05.
Histological Analyses of Skin Wound Healing in WT and PAI-1−/− Mice 5 Days after Incision
At day 5, it was already evident that wound healing was accelerated in PAI-1 −/− mice relative to WT mice. The newly formed neoepithelial layer from the marginal edges of the wound were thickened and connected underneath the provisional matrix in PAI-1 −/− mice (Figure 2a) ▶ . This is in contrast to WT mice, at this time interval, where the neoepithelial layer was still associated with the incisional edges of the wound, marginal to the matrix (Figure 2b) ▶ . Additionally, weak PAI-1 immunoreactivity was observed in WT wounds at the marginal edges and no differences in uPA localization were observed in wounds from PAI-1 −/− and WT mice (data not shown). While the underlying dermal layer in PAI-1 −/− mice was not yet developed, organization of collagen fiber formation was initiated at this time in PAI-1 −/− mice, with little evidence of this occurring in WT mice (Figure 2, c and d) ▶ . A small amount of bleeding was still observed in PAI-1 −/− mice wounds in unconnected areas under the matrix but was much more evident in WT mice wounds where there was a larger open area of injury (Figure 2, e and f) ▶ . While wound-associated fibrin(ogen) was observed to be diffuse in PAI-1 −/− animals, it was much more evident in wounds from WT mice (Figure 3, a and b) ▶ . This supports observations made in PG −/− mice and PG −/−/FG −/− double deletions, where it was demonstrated that a lack of Pm activity resulted in diminished skin wound healing which was resolved with a fibrinogen deficiency. 3,4 Inflammation appeared to be much more prominent in WT wounds at day 5 than in PAI-1 −/− wounds consistent with the different stages of wound healing in these mice (Figure 3, c and d) ▶ . VEGF, a growth factor known to be up-regulated during skin wound healing, 12 was evident in the granulation tissue in WT mice wounds but very poorly expressed in _PAI-1_−/− skin wounds.
Figure 2.
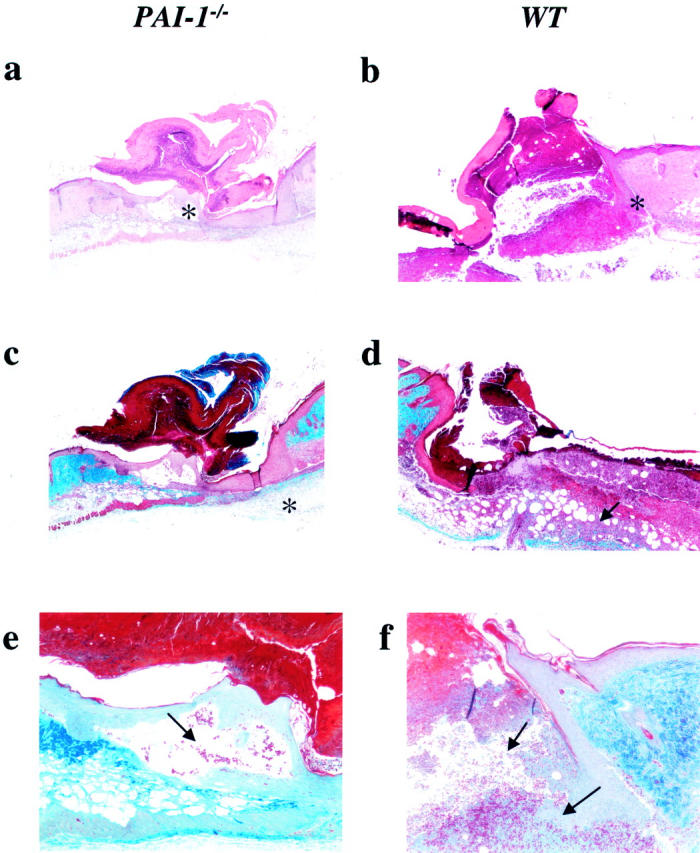
Histological analysis of a skin lesion 5 days following administration of the wound in _PAI-1_−/− and WT mice (×40–100). H&E staining of a newly connected and thickened neoepithelial layer underneath the formed matrix, which is now attached only at a small, single point (*) in PAI-1 −/− mice (a) (×40). Under the same magnification, the leading edges of the migrating epithelium (*) have not yet associated in WT mice skin wounds (b). The underlying dermal layer in PAI-1 −/− mice (c) is not yet developed although Masson’s trichrome staining indicates the initiation of collagen deposition (*) in this area (×40). Some collagen is also present under the granulation tissue (arrow) of WT mice lesions (×40) (d). Ayoub-Shklar staining demonstrates that some erythrocytes (arrow) are still present in the immature and unconnected area in PAI-1 −/− mice (e) (×100) but is much more substantial in the WT mice wound (f) (arrows ×100).
Figure 3.
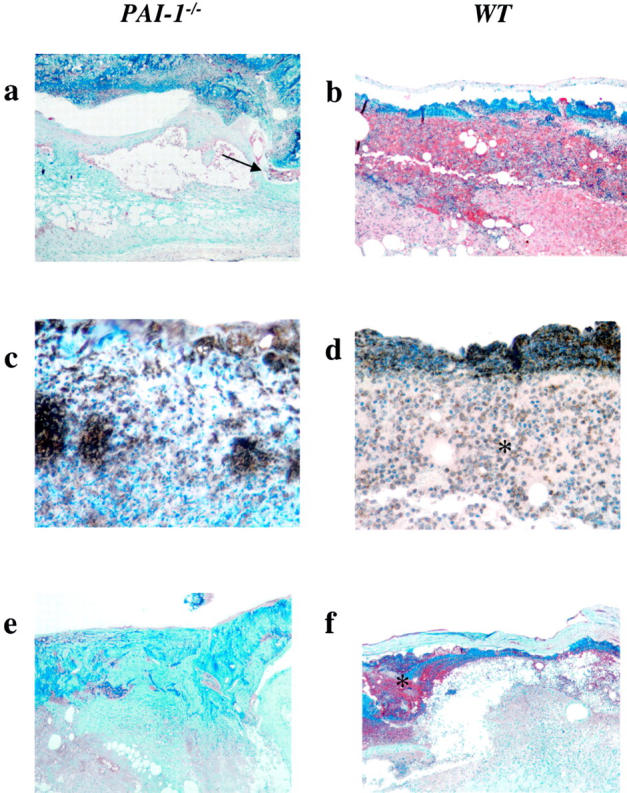
Immunohistochemical analysis of a skin lesion 5 days following wound placement in _PAI-1_−/− and WT mice. Anti-fibrin(ogen) immunostaining shows diffuse fibrin deposition (brown) within the healing lesion in PAI-1 −/− mice (a) and between the wound and the provisional matrix (arrow), whereas fibrin deposition in the WT mice skin lesions (b) remains generally unresolved (×100). Similarly, CD45-positive leukocytes are still prevalent in the granulation tissue (*) under the matrix of the WT mice lesions, (d) but not as prevalent in lesions from PAI-1 −/− mice (c) (×400). VEGF expression was prominent in the granulation tissue (*) of skin wounds from WT mice (f) but scarce in wounds from PAI-1 −/− mice (e) (×100).
Histological Analyses of Healed Skin Wounds in PAI-1−/− Mice
Healed wounds in PAI-1 −/− mice demonstrated a thickened epidermal layer within the newly healed lesion that is not seen in uninjured skin (Figure 4, a and b) ▶ . There is also evidence of hair follicle structure development that is represented by areas of invagination of the epidermis (Figure 4a) ▶ . The dermal layer contains diffuse deposition of collagen fibers that are not organized as in uninjured skin (Figure 4, c and d) ▶ . A thickened keratinized layer is more evident above the epidermal layer of the healed lesion relative to non-wounded skin (Figure 4, e and f) ▶ . Reticular fiber structures of a developing basement membrane are unorganized and, for the most part, cellular in the healed lesion as compared to that observed in non-wounded skin (Figure 4, g and h) ▶ . The finding of localized α-actin positive cells within the developing basement membrane indicates that this region is fibroblast-rich (Figure 5,a and b) ▶ . Additionally, the lack of significant accumulation of fibrin(ogen) (Figure 5, c and d) ▶ or inflammatory cells (Figure 5, e and f) ▶ within the lesion is indicative of a resolved wound and is similar in appearance to nonwounded skin. These findings are similar to those observed in healed WT skin lesions.
Figure 4.
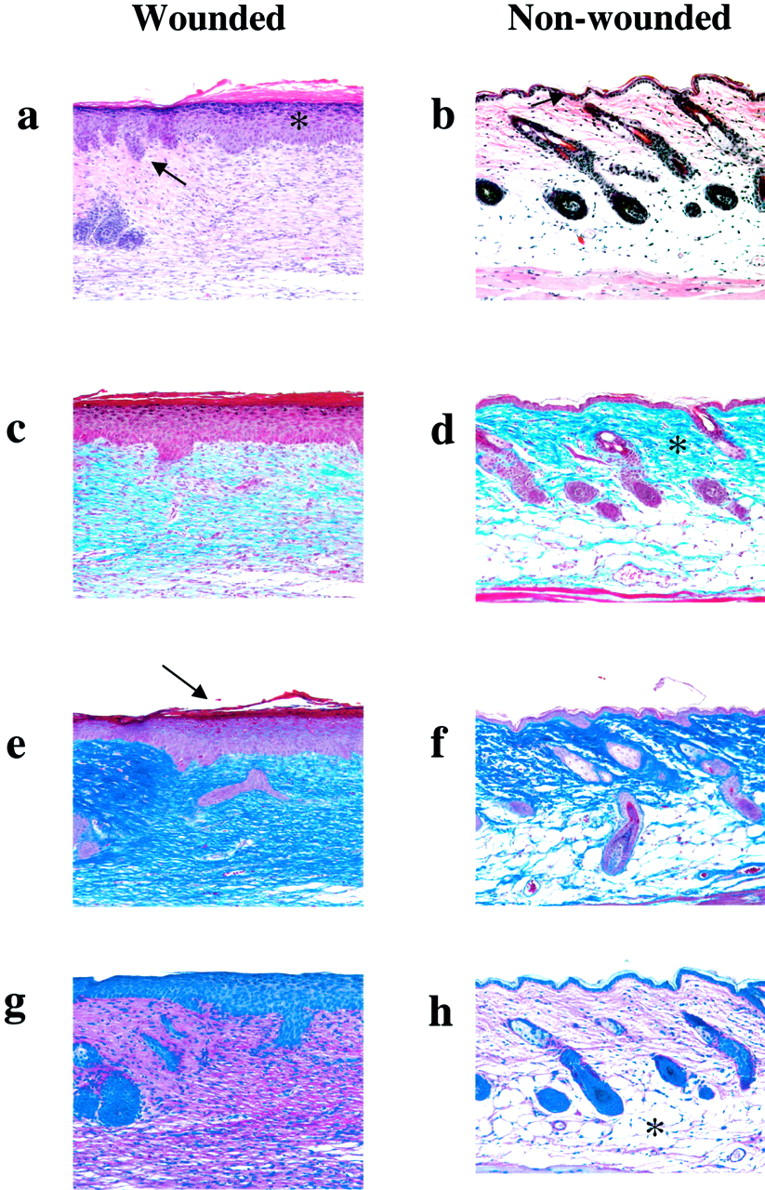
Histological comparisons of healed skin wounds (a,c,e,g) versus non-wounded skin (b,d,f,h) in _PAI-1_−/− mice (×200). H&E staining demonstrating a significantly thickened epidermal layer (*) within the healed lesion (a) as compared to uninjured skin (b). Invaginations of epidermal epithelium (arrow), which will ultimately result in the development of hair follicle structure, are observed. Masson’s Trichrome staining indicates premature, diffuse deposition of collagen (blue) (c) as compared to the rich organization of collagen fibers (*) in non-wounded skin (d). Ayoub Shklar staining demonstrates a thickened keratinized layer above the epidermis (arrow) (e). PAS staining demonstrates that the reticular fiber structure (pink) is still cellular and does not yet demonstrate the organized basement membrane (g) as compared to the more non-cellular reticular layer of fibrous network (*) seen in the non-wounded skin (h). These findings are similar to those observed in healed WT skin lesions.
Figure 5.
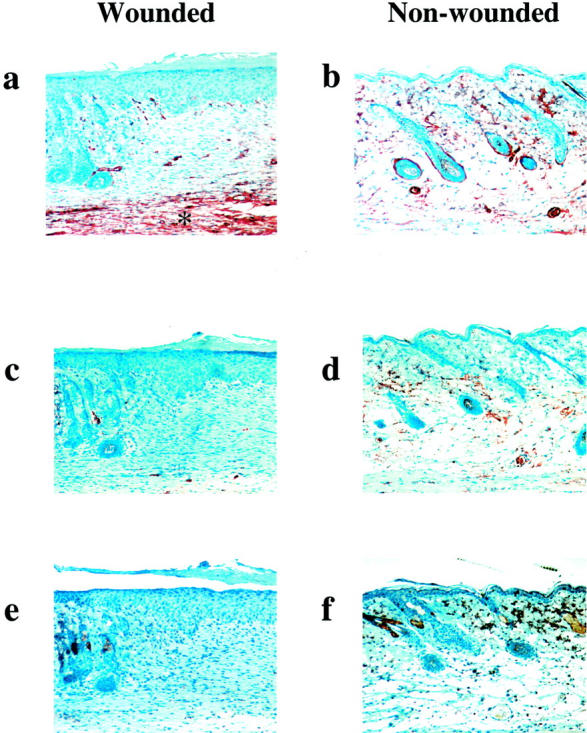
Immunohistochemical analysis of healed (a, c, e) and non-wounded skin (b, d, f) in _PAI-1_−/− mice (×200). Anti-α-actin immunostaining demonstrates a reorganization of α-actin positive cells with particular staining in the deep fibroblast-rich reticular region (*) of the healed lesion (a) compared to a more scattered and less localized presence in the dermis of non-wounded skin (b). There is no longer a significant presence of extravascular fibrin(ogen) (c) or CD45-positive leukocytes (e) within the healed area although nonspecific staining of the sebaceous glands is typical for the latter stain as demonstrated in f. Fibrinogen in non-wounded skin is primarily associated within the vasculature (d).
Discussion
Wound healing is a multifactorial process involving 3 distinct yet interrelated phases that temporally overlap. The inflammatory phase, or exudative phase, is involved in removal of the damaged tissue and cleansing of the wound site. The proliferative phase involves formation of the granulation tissue which acts as a provisional replacement tissue. Finally, the differentiation or regeneration phase supports scar formation and reepithelialization of the wound site. Components of the fibrinolytic system have been implicated in a number of the events associated with wound healing and direct studies in PG −/− mice have indicated a significantly attenuated rate of wound healing which is resolved in a fibrinogen deficient state. While wound healing is impaired in PG −/− mice, it eventually occurs, indicating the involvement of other proteolytic pathways in these events. Additional studies support a functional overlap between the fibrinolytic system and the MMP family of matrix degrading proteases in skin wound healing processes 13 and have shown that inhibition of both pathways leads to a complete arrest of wound healing and closure. Therefore, imbalances in either proteolytic system could manifest itself in altered wound healing events.
An important physiological regulator for the generation of plasmin is PAI-1. While PAI-1 is not normally expressed by keratinocytes in the epidermis, it has been shown to be increased in expression following in vitro and in vivo wound injury 1,14 and localized to the hyperproliferative migrating zone of epithelial cells. Clinically, it has been demonstrated that elevated PAI-1 levels occur in diabetic patients who also suffer from delayed wound healing responses resulting in skin ulcerations and other serious clinical complications. 15,16 The current study supports a role for PAI-1 in wound healing and indicates that a loss of PAI-1 function results in accelerated wound healing. Interestingly, as observed in other models in our laboratory7,17, PAI-1 expression appears to regulate VEGF expression. Further studies to characterize the mechanisms associated with this observation are ongoing.
PAI-2 is also expressed in the epidermis and could play a role in skin wound healing. 18 Surprisingly, a wound healing model using PAI-2 −/− and PAI-2 −/− /PAI-1 −/− double deficient mice failed to show an effect on the healing process. 19 PAI-2 is a member of the ovalbumin family of serpins and it has been demonstrated that mice produce a much larger array of these serpins relative to humans. 20 Perhaps a loss of PAI-2 expression is compensated for by increased expression of one or more of these other inhibitors. Indeed, the ovalbumin serpin, proteinase inhibitor 6, PI-6, is up-regulated during keratinocyte differentiation 21 and hurpin, proteinase inhibitor 13 (PI13), is up-regulated in keratinocytes during psoriasis. 22
Additional studies using artificially wounded keratinocytes, in cell culture, have indicated that healing events are impaired after transfection with an antisense PAI-1 vector. 2 However, these studies were performed on human transformed cells and with a single population of cell type, keratinocytes. Normal wound healing involves a number of different cells, i.e., leukocytes, endothelial cells, and fibroblasts.
PAI-1, through its ability to alter the fibrinolytic environment, could manifest its effects during wound healing by temporally regulating the extent of the fibrin-rich provisional matrix allowing for fibroblast migration into the wound and eventual replacement of fibrin with collagen. Other studies have indicated that vitronectin receptor and plasminogen activators are increased in expression in migrating cells during wound healing. 1,23-25 PAI-1 has been shown to inhibit cell migration by blocking the vitronectin receptor, αVβ3, interaction with vitronectin. 26 PAI-1 interaction with plasminogen activators, in this setting, results in a loss of affinity of PAI-1 for vitronectin, which restores cell migration. In this context, PAI-I exerts its effects by regulating cell adhesion and detachment during cell migratory events of wound healing. A role for vitronectin in wound healing was confirmed using a skin wound healing model in vitronectin deficient animals in which it was demonstrated that healing was delayed. 27 This would indicate that vitronectin/receptor interactions facilitate cell migration during wound healing and that blocking that interaction, through PAI-I, could alter the healing process.
Acknowledgments
We thank Ms. Mayra Sandoval-Cooper for assistance with histological and immunohistological stains, Ms. Stacey Raje for the care and maintenance of the animal colony, and Drs. Harm HogenEsch and Mark Suckow for assistance in the histopathology.
Footnotes
Address reprint requests to Dr. Victoria A. Ploplis, W. M. Keck Center for Transgene Research and the Department of Chemistry and Biochemistry, 229 Nieuwland Science Hall, University of Notre Dame, Notre Dame, IN 46556. E-mail: ploplis.3@nd.edu.
Supported in part by an American Heart Association Midwest Postdoctoral Fellowship (to J. C. Y. C.), National Institutes of Health grants HL-13423 (to F. J. C.) and HL-63682 (to V. A. P.), a grant from the W. M. Keck Foundation (to F. J. C.), and by the Kleiderer-Pezold Family Endowed Professorship (to F. J. C.).
References
- 1.Romer J, Lund LR, Eriksen J, Ralfkiaer E, Zeheb R, Gelehrter TD, Dano K, Kristensen P: Differential expression of urokinase-type plasminogen activator and its type-1 inhibitor during healing of mouse skin wounds. J Invest Dermatol 1991, 97:803-811 [DOI] [PubMed] [Google Scholar]
- 2.Li F, Goncalves J, Faughnan K, Steiner MG, Pagan-Charry I, Esposito D, Chin B, Providence KM, Higgins PJ, Staiano-Coico L: Targeted inhibition of wound-induced PAI-1 expression alters migration and differentiation in human epidermal keratinocytes. Exp Cell Res 2000, 258:245-253 [DOI] [PubMed] [Google Scholar]
- 3.Romer J, Bugge TH, Pyke C, Lund LR, Flick MJ, Degen JL, Dano K: Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med 1996, 2:287-292 [DOI] [PubMed] [Google Scholar]
- 4.Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL: Loss of fibrinogen rescues mice from pleiotropic effects of plasminogen deficiency. Cell 1996, 87:709-719 [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Moons L, Lijnen R, Janssens S, Lupu F, Collen D, Gerard RD: Inhibitory role of plasminogen activator inhibitor-1 in arterial wound healing and neointima formation: a gene targeting and gene transfer study in mice. Circulation 1997, 96:3180-3191 [DOI] [PubMed] [Google Scholar]
- 6.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D: Plasminogen activator inhibitor-1 deficiency protects against atherosclerosis progression in the mouse carotid artery. Blood 2000, 96:4212-4215 [PubMed] [Google Scholar]
- 7.Ploplis VA, Cornelissen I, Sandoval-Cooper MJ, Weeks L, Noria FA, Castellino FJ: Remodeling of vessel wall after copper-induced injury is highly attenuated in mice with a total deficiency of plasminogen activator inhibitor-1. Am J Pathol 2001, 158:107-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmeliet P, Kieckens L, Schoonjans L, Ream B, van Nuffelen A, Prendergast G, Cole M, Bronson R, Collen D, Mulligan RC: Plasminogen activator inhibitor-1 gene deficient mice. I: generation by homologous recombination. J Clin Invest 1993, 92:2746-2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masson P: Trichrome stainings and their preliminary technique. J Tech Methods 1929, 12:75-90 [Google Scholar]
- 10.Ayoub P, Shklar G: Ayoub-Shklar method for keratin and prekeratin. J Oral Surg 1963, 16:580-581 [DOI] [PubMed] [Google Scholar]
- 11.McManus JFA: Histological and histochemical uses of periodic acid. Stain Technol 1948, 23:99-108 [DOI] [PubMed] [Google Scholar]
- 12.Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L: Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 1992, 176:1375-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund L, Romer J, Bugge TH, Nielsen BS, Frandsen TL, Degen JL, Stephens RW, Dano K: Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J 1999, 18:4645-4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staiano-Coico I, Carano K, Allan VM, Steiner MG, Pagan-Charry I, Bailey BB, Babaar P, Rigas B, Higgins PJ: PAI-1 gene expression is growth state-regulated in cultured human epidermal keratinocytes during progression to confluence and post-wounding. Exp Cell Res 1996, 227:123-134 [DOI] [PubMed] [Google Scholar]
- 15.McGill JB, Schneider DJ, Arfken CL, Lucore CL, Sobel BE: Factors responsible for impaired fibrinolysis in obese subjects and NIDDM patients. Diabetes 1994, 43:104-109 [DOI] [PubMed] [Google Scholar]
- 16.Meyer JS: Diabetes and wound healing. Crit Care Nurs Clin North Am 1996, 8:195-201 [PubMed] [Google Scholar]
- 17.Gutierrez LS, Schulman A, Brito-Robinson T, Noria F, Ploplis VA, Castellino FJC: Tumor development is retarded in mice lacking the gene for urokinase-type plasminogen activator or its inhibitor, plasminogen activator inhibitor-1. Cancer Res 2000, 60:5839-5847 [PubMed] [Google Scholar]
- 18.Hibino T, Izaki S, Ohkuma M, Kon S, Thorsen S, Astedt B: Epidermal plasminogen activator inhibitor (PAI) is immunologically identical to placental-type PAI-2. FEBS Lett 1988, 231:202-206 [DOI] [PubMed] [Google Scholar]
- 19.Dougherty KM, Pearson JM, Yang AY, Westrick RJ, Baker MS, Ginsburg D: The plasminogen activator inhibitor-2 gene is not required for normal murine development or survival. Proc Natl Acad Sci USA 1999, 96:686-691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, Ooms L, Bird CH, Sutton VR, Trapani JA, Bird PI: A new family of 10 murine ovalbumin serpins includes two homologs of proteinase inhibitor 8 and two homologs of the granzyme B inhibitor (proteinase inhibitor 9). J Biol Chem 1997, 272:15434-15441 [DOI] [PubMed] [Google Scholar]
- 21.Scott FL, Paddle-Ledinek JE, Cerruti L, Coughlin PB, Salem HH, Bird PI: Proteinase inhibitor 6 (PI-6) expression in human skin: induction of PI-6 and a PI-6/proteinase complex during keratinocyte differentiation. Exp Cell Res 1998, 245:263-271 [DOI] [PubMed] [Google Scholar]
- 22.Abts HF, Weiss T, Scheuring S, Scott FL, Irving JA, Michel G, Bird PI, Ruzicka T: Sequence, organization, chromosomal localization, and alternative splicing of the human serine protease inhibitor gene hurpin (PI13) which is up-regulated in psoriasis. DNA Cell Biol 2001, 20:123-131 [DOI] [PubMed] [Google Scholar]
- 23.Clark RA, Tonnesen MG, Gailit J, Cheresh DA: Transient functional expression of alpha V beta 3 on vascular cells during wound repair. Am J Pathol 1996, 148:1407-1421 [PMC free article] [PubMed] [Google Scholar]
- 24.Grondahl-Hansen J, Lund LR, Ralfkaier E, Ottevanger V, Dano K: Urokinase- and tissue-type plasminogen activators in keratinocytes during wound reepithelialization in vivo. J Invest Dermatol 1988, 90:790-795 [DOI] [PubMed] [Google Scholar]
- 25.Nakajima S, Morioka S: Effects of plasminogen activator on epidermal cell migration. Nippon Hifuka Gakkai Zasshi 1990, 100:1199-1201 [PubMed] [Google Scholar]
- 26.Stefansson S, Lawrence DA: The serpin PAI-1 inhibits cell migration by blocking integrin alpha V beta 3 binding to vitronectin. Nature 1996, 383:441-443 [DOI] [PubMed] [Google Scholar]
- 27.Jang YC, Tsou R, Gibran NS, Isik FF: Vitronectin is associated with increased wound fibrinolysis and decreased microvascular angiogenesis in mice. Surgery 2000, 127:696-704 [DOI] [PubMed] [Google Scholar]