Pathway Pathology: Histological Differences Between ErbB/Ras and Wnt Pathway Transgenic Mammary Tumors (original) (raw)
Abstract
To study phenotype-genotype correlations, ErbB/Ras pathway tumors (transgenic for ErbB2, c-Neu, mutants of c-Neu, polyomavirus middle T antigene (PyV-mT), Ras, and bi-transgenic for ErbB2/Neu with ErbB3 and with progesterone receptor) from four different institutions were histopathologically compared with Wnt pathway tumors [transgenes Wnt1, Wnt10b, dominant-negative glycogen synthase kinase 3-β, β-Catenin, and spontaneous mutants of adenomatous polyposis coli gene (Apc)]. ErbB/Ras pathway tumors tend to form solid nodules consisting of poorly differentiated cells with abundant cytoplasm. ErbB/Ras pathway tumors also have scanty stroma and lack myoepithelial or squamous differentiation. In contrast, Wnt pathway tumors exhibit myoepithelial, acinar, or glandular differentiation, and, frequently, combinations of these. Squamous metaplasia is frequent and may include transdifferentiation to epidermal and pilar structures. Most Wnt pathway tumors form caricatures of elongated, branched ductules, and have well-developed stroma, inflammatory infiltrates, and pushing margins. Tumors transgenic for interacting genes such as protein kinase CK2α (casein kinase IIα), and the fibroblast growth factors (Fgf) Int2/Fgf3 or keratinocyte growth factor (Kgf/Fgf7) also have the Wnt pathway phenotype. Because the tumors from the ErbB/Ras and the Wnt pathway are so distinct and can be readily identified using routine hematoxylin and eosin sections, we suggest that pathway pathology is applicable in both basic and clinical cancer research.
Genetically engineered mice (GEM) have been used extensively to model human breast cancer and to dissect the molecular pathways contributing to tumorigenesis. Most mammary tumors in GEM are different from spontaneous, virus-induced mammary tumors in mice. 1 As previously reported, many transgenes in GEM induce tumors with specific, signature histological phenotypes. 2 The initial observations were based on the cases collected in one laboratory at the Harvard Medical School. They demonstrated that the Ras, Neu, and Myc transgenes, promoted by the murine mammary tumor virus long terminal repeat (MMTV-LTR), produced signature tumors with small round cells, intermediate cells, and large cells, respectively. These observations suggested that phenotype predicts genotype. 2 Subsequent studies have shown that the signature phenotypes for these three genes are similar in different laboratories even with different constructs and different promoters. 3-5
Since the initial studies, the University of California, Davis Mutant Mouse Pathology Archives have accumulated more than 3000 GEM mammary tumors that now include a sufficient variety of GEM to allow comparisons of tumors within specific signal transduction pathways. We have, therefore, undertaken a systematic study of the morphology of ErbB/Ras pathway tumors as compared with Wnt pathway tumors.
The Wnt1 gene is one of the most commonly induced genes in mice after the insertional activation by MMTV (murine mammary tumor virus). 6 The Wnt1 gene was first named “_Int1_”, or MMTV integration site 1, until the activated gene was found to be the homologue of the Drosophila wingless gene. 7 The second gene commonly activated by MMTV insertion is known as Int2 but has turned out to be a member of the Fgf family, Fgf3. 8 Although members of the Wnt and the Fgf families have been implicated in human cancer, neither has been frequently found in association with human breast cancer. A number of transgenic, mutant, and knockout mice have now been developed that involve the Wnt pathway. 9-12 Tumors in these GEM resemble the classical MMTV-induced tumors 13 suggesting that activation of the same gene by different mechanisms results in the same type of tumor. The MMTV-induced tumors have characteristic histological patterns that are not generally found in human breast cancer. 1
Interestingly, Wnt1 GEM infected with MMTV were found to have insertional activation of Fgf8, Fgf4, and Fgf3 and GEM transgenic for Fgf3 infected with MMTV were found to have activation of Wnt10b, suggesting cooperativity between Fgf and Wnt signaling. 6,9,14
On the other hand, tumors arising in ErbB2 GEM have a completely different histopathological pattern that does not resemble the MMTV-induced tumors 1 but rather do resemble some human tumors. 2 ErbB2 is a member of the epidermal growth factor receptor family and is amplified in ∼25% of human breast cancer. 15 Neu is an activated rat homologue of ErbB2. 16 When either c-ErbB2 or Neu is expressed behind the highly mammary selective MMTV-LTR promotor, a signature solid nodular tumor is generally produced. 3,4,17 Although some morphological differences may separate the Ras tumors from the Neu tumors, their phenotypes overlap to a significant degree. 2,18 Because polyoma virus middle T (PyV-mT) imitates ErbB2, it is considered a molecular surrogate for ErbB2. 19 PyV-mT protein also induces solid tumors. 19
We now report that tumors involving other members of these two pathways in GEM share one or more morphological characteristics with the better known members of the Wnt or ErbB/Ras pathways. We describe here the morphological features shared by five members of the Wnt pathway and three cooperating genes as compared to those shared by six members of the ErbB2/Ras family. These studies extend the principle that phenotype predicts genotype to demonstrate that alterations in structure and function induced by genes can also be classified by the signal transduction pathway. Because these mouse mammary tumors have such different and easily identified morphologies, they belong to distinct taxonomic groups that are related to the pathways and we suggest the term “pathway pathology” to indicate the shared morphology within each pathway.
Materials and Methods
Mice
The samples used in this study came from murine tumors that were sent to us as a part of studies of oncogene tumorigenesis in transgenic mice initiated by our collaborators. All transgenes used here were under the control of an MMTV-LTR promotor except the PR− transgenics, created by using a binary system, as described previously. 20,21 All transgenic mice were bred in the FVB background strain. In addition, spontaneous Min mutants of the adenomatous polyposis coli (Apc Min) gene in C57/BL/6J and (AKRx C57/BL/6J Min/+) F1 and N2 background were studied. Three of the six Apc Min mice were treated with ethylnitrosourea, a chemical carcinogen. 22
The animals were inspected for tumors at least once a week. Animals with tumors were necropsied between 1991 and 2001, and samples of tumors, adjacent mammary gland, and other tissues were fixed in neutral buffered 4% formalin or in Optimal Fix (American Master Tech Scientific, Inc., Lodi, CA), embedded in paraffin, cut into 4-μm sections, and stained with Mayer’s hematoxylin and eosin (H&E). Animal data, gross description, slides, and, in most cases, paraffin blocks were stored at the University of California Davis Mutant Mouse Pathology Laboratory.
Tumors
ErbB/Ras Pathway
Representative mouse mammary tumors (n = 107) transgenic for the ErbB/Ras pathway were selected for this study. The transgenes were ErbB2/Neu, 3,23,24 Neu mutants NDL1-4 and NDL2-5, 4 as well as transgenic crosses: ErbB2 with ErbB3 (Gillgrass and Muller, McMaster University, unpublished results), or with progesterone receptor α (n = 4) or β (n = 1) (G Shyamala, University of California, Berkeley, unpublished results). In addition, PyV-mT, 19,25,26 and Ras 2 tumors were examined.
Wnt Pathway
Tumors (n = 112) with one transgene or mutation activating the Wnt pathway were used. Bitransgenic tumors were not included because of the complexity of phenotypes in the Wnt pathway. The transgenes were Wnt1, 12 Wnt10b, 10 protein kinase CK2α (formerly casein kinase IIa), 11 β-Catenin, 12 dominant-negative mutant of glycogen synthase kinase 3-b (dnGSK3β) (D. C. Seldin, unpublished results). In addition, Apc Min mutants 27 were studied.
Int2 Pathway
Ninety tumors transgenic for Int2/Fgf3 28,29 or for keratinocyte growth factor (Kgf/Fgf7) 30 were studied.
The tumors were initially classified using the taxonomy recommended by the Annapolis Pathology Panel. 1 However, the remarkable phenotypes in the Wnt pathway tumors required the development of new taxonomic groups and terms (section results). Images were captured with ×10 and ×20 objectives using a Carl Zeiss (Thornwood, NY) Axiocam camera and were processed using Adobe Photoshop (Adobe Systems Incorporated) software.
Immunohistochemistry (IHC)
IHC was performed on 35 tumors transgenic for ErbB2, Neu mutants, and PyV-mT, and on 40 tumors transgenic for the Wnt pathway to assess myoepithelial differentiation, further on 5 tumors transgenic for the Wnt pathway to demonstrate ductular architecture, and on skin and 5 mammary pilar tumors to study pilar differentiation. Four-μm paraffin sections were placed onto Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA), deparaffinized, and cleared. IHC was performed after inhibition of endogenous peroxidase activity in a solution of 3% hydrogen peroxide (H2O2) in methanol and hydration in graded alcohol to distilled water. Before antibody incubations, antigen retrieval was performed by high temperature (microwave) incubation in 0.01 mol/L of citric acid buffer (pH 6.0) for 3 × 4 minutes. Slides were allowed to cool for 10 minutes in citric acid buffer then transferred to phosphate-buffered saline (pH 7.4) (2 × 5 minutes each). Ten percent normal horse serum (Vector Laboratories, Burlingame, CA) was applied to sections and incubated for 20 minutes in a humidified chamber at room temperature.
IHC for smooth muscle actin (SMA) was performed using a 1:1000 diluted mouse monoclonal primary antibody (Sigma, St. Louis, MO). IHC for hard (hair) keratin was performed using a 1:20 diluted cell culture supernatant with the mouse monoclonal primary antibody AE-13 31 (a kind gift from T.-T. Sun, New York University). The Animal Research Kit (DAKO, Carpinteria, CA) with peroxidase was used as amplification system according to the manufacturer’s instructions.
To exclude SMA-positive myofibroblasts, all 12 questionable SMA-positive ErbB/Ras pathway tumors, two adenomyoepitheliomas, and 5 spindle cell tumors were stained for cytokeratin 14 (CK14). Staining for cytokeratin 8 (CK8) was performed to illustrate the ductular organization of Wnt pathway tumors. We used 1:200 (CK14) and 1:300 (CK8) diluted polyclonal sheep primary antibodies (Binding Site, San Diego, CA). Slides were covered with primary antibody solution and were incubated overnight at room temperature. The Vectastain ABC Elite Kit (Vector Laboratories) was used as amplification system according to the manufacturer’s instructions. Slides were counterstained in Mayer’s hematoxylin, dehydrated, cleared, and coverslipped. Negative control slides were run without primary antibody. Control slides known to be positive for each antibody were incorporated into each run.
Results
ErbB/Ras Pathway Tumors
ErbB2 and Ras transgenic mammary tumors have recognizable signature phenotypes as previously described. 1 Ras tumors consist of uniform cells with abundant eosinophilic cytoplasm and small ovoid nucleus with dense chromatin structure. ErbB2/Neu tumor cells are larger than Ras tumor cells, and have larger nuclei and paler but abundant cytoplasm (Figure 1E) ▶ . ErbB2/Neu transgenic tumors are solid and nodular (Table 1) ▶ . Solid ErbB/Ras pathway tumors (Figure 1, A and E) ▶ have characteristic concentric zones of cell populations: I, one to two peripheral layers of pallisading cells; II, several, more internal layers of larger cells with larger nuclei and more open chromatin structure (vesicular in the ErbB2/Neu tumors); and III, small, tightly packed central cells with smaller, elongated nuclei and less cytoplasm than in the other zones. Some tumors have central necrosis surrounded by bigger tumor cells than the zone III type cells. PyV-mT (Figure 1C) ▶ and Ras transgenic tumors have more variable phenotypes than ErbB2 tumors (Table 1) ▶ . Some solid PyV-mT tumors have minor components of glandular differentiation or cystic spaces. Although ErbB/Ras pathway tumors can have histological types other than solid (Table 1) ▶ , they all contain solid components. With few exceptions, ErbB/Ras pathway tumors share common morphological characteristics.
Figure 1.
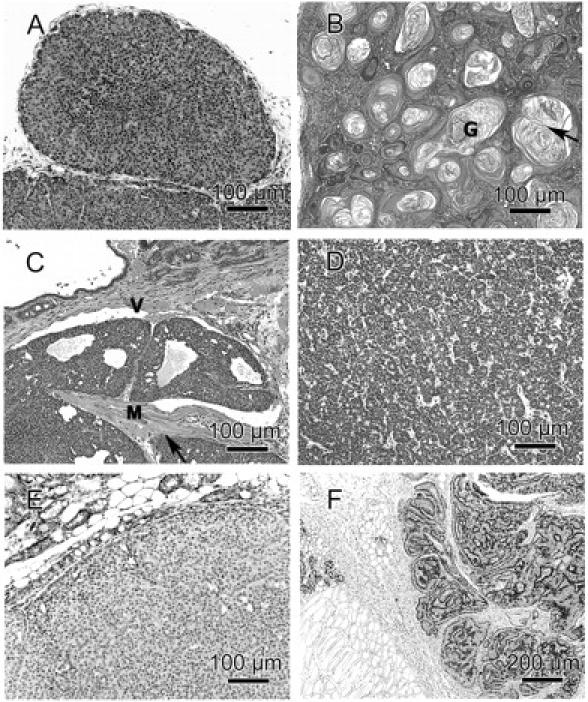
Representative histomorphology of ErbB/Ras pathway (A, C, E) and Wnt pathway (B, D, F) tumors. A: Solid nodular tumor with scanty stroma (transgene: ErbB2/Neu mutant Ndl1-4). B: Pilar mammary tumor with inflammatory infiltrates in well-developed stroma. Note central dilated neoplastic ducts filled with ghost cells (G) and laminar keratin, which forms concentric, and sometimes confluent, swirls (arrow). This tumor has both epidermal and hair-specific characteristics (mutated gene: Apc). C: Cystic-glandular tumor with solid components and scanty stroma invading into a dilated, thin-walled vessel (V) and into (arrow) the skeletal muscle (M) (transgene: PyV-mT). D: Acinar tumor with well-developed stroma (transgene_: β-Catenin_). E: SMA-negative solid nodular tumor with scanty stroma and no visible inflammatory infiltrate. Note that the myoepithelium in the adjacent dilated ductal and alveolar structures (top left) is SMA-positive (transgenes: Neu and Progesterone receptor). F: Stroma-rich type P tumor with inflammatory infiltrates. In both, tumor (right) and normal alveoli (left), the SMA-positive myoepithelium is located between luminal epithelium and stroma and forms a continuous line (transgene: Wnt1). A–D: H&E. E and F: Anti-SMA counterstained with hematoxylin. All photographs were taken with a ×10 or a ×20 objective, the exact scale is given in each picture.
Table 1.
Histological Types of ErbB/Ras Pathway Transgenic Tumors
| Transgene | Acinar | Glandular | Papillary | Solid | Adenosquamous | Pilar | Type P | Myoepithelial |
|---|---|---|---|---|---|---|---|---|
| ErbB2/Neu (n = 12) | 0 | 0 | 1 | 11 | 0 | 0 | 0 | 0 |
| activated Neu (n = 5) | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 |
| Neu mutants (n = 25) | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 |
| ErbB2 and PR (n = 5) | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 |
| ErbB2 and ErbB3 (n = 5) | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 |
| PyV-mt (n = 49) | 2 | 8 | 5 | 29 | 2 | 2 | 1 | 0 |
| Ras (n = 6) | 1 | 0 | 0 | 5 | 0 | 0 | 0 | 0 |
| (n = 107) | 3 | 8 | 6 | 85 | 2 | 2 | 1 | 0 |
| (100%) | (3%) | (7%) | (6%) | (79%) | (2%) | (2%) | (1%) | (0%) |
The signature characteristics of the ErbB/Ras pathway tumors (Table 3) ▶ are: solid pattern (Table 1 ▶ ; Figure 1, A and E ▶ ), scanty stroma (Figure 1 ▶ ; A, C, and E), invasive growth (Figure 1C) ▶ , no myoepithelium (Figure 1E) ▶ , and no squamous metaplasia (Figure 1 ▶ ; A, C, and E). The PyV-mT tumors tend to have more stroma than Ras and ErbB2 tumors. Except for one tumor, ErbB/Ras pathway tumors do not have any evidence of milk or lipid secretion, even when the adjacent mammary gland is lactating. The connective tissue adjacent to the tumor has either no response or is edematous, but not fibrous (Figure 1E) ▶ . Inflammatory infiltrates are limited to necrotic zones.
Table 3.
Morphologic Criteria to Distinguish ErbB/Ras from Wnt Pathway Tumors
| Criterion | ErbB/Ras pathway | Wnt pathway |
|---|---|---|
| Histological pattern | Solid | Branched ductules/acinar component |
| Keratinization | Rarely present | Frequently present |
| Myoepithelium | Not present | Frequently present;* |
| Stroma | Scanty | Dense |
| Inflammatory infiltrates | Present only in necrosis | Frequently present |
The ErbB2/Neu phenotype is consistently found in combinations of ErbB2/c-Neu transgenes with progesterone receptor or ErbB3 transgenes.
Wnt Pathway Tumors
Mammary tumors induced by mutations in genes of the canonical Wnt pathway, or of the Wnt pathway interactors CK2a, Int2 (Fgf-3), or Kgf (Fgf-7) (in the following referred to as Wnt pathway tumors) exhibit a variety of morphological patterns. Despite the variety of histological types (Table 2) ▶ , these transgenic tumors have common histological characteristics, which are different from the ErbB/Ras pathway phenotype (Table 3) ▶ . The key features of Wnt pathway tumors are branched ductular architecture (Figure 2, A and B) ▶ , dense stroma with lymphocytic infiltrates (Figure 1, B and D ▶ , and Figure 2, A to D ▶ ), and differentiation into acinar (Figure 1D) ▶ , squamous (Figures 1B and 2D) ▶ ▶ , and myoepithelial (Figure 1F ▶ and Figure 2, E and F ▶ ) components. Each of these characteristics was found in more than 50% of the Wnt pathway tumors studied, and each Wnt pathway tumor had at least one of these characteristics.
Table 2.
Histological Types of Wnt Pathway and CK2α and Fgf Transgenic Tumors
| Transgene | Acinar | Glandular | Papillary | Solid | Adenosquamous | Pilar | Type P | Myoepithelial |
|---|---|---|---|---|---|---|---|---|
| Wnt1 (n = 7) | 0 | 0 | 1 | 0 | 0 | 0 | 6 | 0 |
| Wnt10b (n = 23) | 7 | 4 | 1 | 1 | 0 | 1 | 9 | 0 |
| CK2α (n = 29) | 2 | 1 | 6 | 0 | 1 | 8 | 1 | 10 |
| dnGSK-3β (n = 36) | 1 | 6 | 8 | 0 | 2 | 11 | 1 | 7 |
| Apc (n = 6) | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 |
| β-Catenin (n = 11) | 2 | 2 | 5 | 0 | 0 | 1 | 1 | 0 |
| Int2 (n = 34) | 6 | 6 | 7 | 0 | 5 | 7 | 1 | 2 |
| Kgf (n = 56) | 2 | 10 | 6 | 1 | 1 | 19 | 5 | 12 |
| (n = 202) | 20 | 29 | 34 | 2 | 9 | 53 | 24 | 31 |
| (100%) | (10%) | (14%) | (17%) | (1%) | (4%) | (27%) | (12%) | (15%) |
Figure 2.
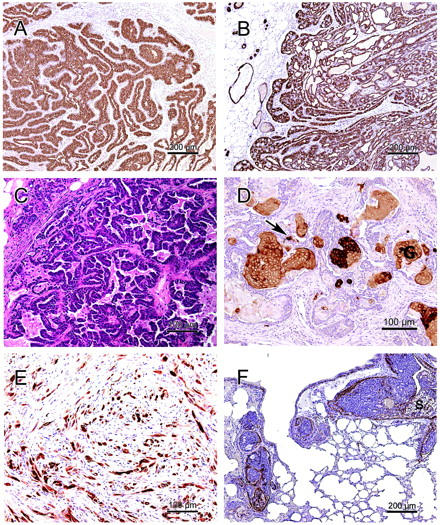
Characteristics of Wnt pathway tumors: Ductular architecture (A, B) with glandular (B), papillary (C), pilar (D), and myoepithelial differentiation (E, F), and dense stroma with lymphocytic infiltrates (A–D). A: Type P tumor. Note the well-differentiated branching neoplastic ductules (transgene: Wnt10b). B: Type P tumor with glandular differentiation (transgene: Wnt1). C: Papillary tumor with micropapillary components. The ductular architecture is less prominent than in type P tumors (transgene: CK2a). D: Pilar tumor with squamous metaplasia and ghost cells (G) embedded in fibrous stroma with moderate inflammatory infiltrates. Staining with antibody AE-13 shows that expression of hair keratin in viable cells is limited to few single cells (arrow) (transgene: Int2). E: Myoepithelial differentiation in a spindle cell tumor (transgene: CK2a). F: Lung metastasis of a glandular mammary tumor with secretory activity (S) and maintained myoepithelium. In mice, maintained myoepithelium does not exclude tumor emboli or metastasis (transgene: Kgf). All photographs were taken with a ×10 or a ×20 objective, the exact scale is given in each picture. A and B: Anti-cytokeratin-8; C: H&E; D: AE13 (anti-hair-keratin); E: anti-cytokeratin-14; F: anti-SMA counterstained with hematoxylin.
Within the spectrum of Wnt tumor phenotypes are 1) better differentiated tumors exhibiting a ductular architecture (Figure 1, B and F ▶ , and Figure 2 ▶ ; A, B, and D); 2) less differentiated tumors without a ductular architecture (Figures 1D and 2E) ▶ ▶ . Tumors with hints of ductular organization, but predominance of less differentiated components (Figure 2C) ▶ , are classified with group 2.
The better-differentiated Wnt tumors have structures resembling elongated, branched ductules. The more peripheral portions of the tumor may have several patterns of terminal differentiation that may be classified according to the predominant pattern. The two types of well-differentiated tumors in this pathway are designated, here, as P-type and pilar tumors.
P-type tumors are characterized by ductules lined by single or multilayered epithelium and surrounded by dense stroma. The basal layer of myoepithelium is maintained (Figure 1F) ▶ . The intraductal cells, especially in the periphery of the tumor, may differentiate into acinar, glandular, and papillary patterns or undergo minimal squamous metaplasia. However, the terminal ends of the ducts may also have masses of undifferentiated cells. Because this type of tumor phenotype has previously been described as “type P tumor” in conjunction with pregnancy-dependent, plaque-like tumors, 32 we chose to keep the term.
A second well-differentiated tumor also has caricatures of branched mammary ductules, but squamous metaplasia is present at the blind buds of the ductules, forming keratin-filled neoplastic ductules (abortive hair shafts) embedded in a fibrous stroma (Figures 1B and 2D) ▶ ▶ . In most cases, ghost cells (Figures 1B and 2D) ▶ ▶ , a typical component of pilomatricomas, are present. Pilar mammary tumors express hair-specific hard keratins as assessed by AE-13 antibody 31 (Figure 2D) ▶ . The strong resemblance to hair structures and hair matrix-derived tumors has led us to designate these mammary tumors as “pilar tumors.” The ductules of pilar tumors are filled with variable amounts of ghost (shadow) cells and keratin. These lumina are surrounded by basaloid cells (Figure 2D) ▶ and in some cases by an additional layer of myoepithelium. A subset of pilar tumors has been referred to earlier as “molluscoid tumor.” 13 The periphery of this molluscoid subtype consists of abortive hair shafts. The centers of molluscoid tumors are filled with confluent swirls of laminar keratin (Figure 1B) ▶ . These swirls allow the distinction from other keratin cysts. The swirls suggest that the keratin cysts of a pilar tumor are derived from individual neoplastic ductules that fuse together as they continue to produce keratin.
Pilar tumors are defined as mammary tumors composed of radially arranged hair shaft-like neoplastic ductules, or composed of a keratin cyst containing keratin swirls. In most cases, both components and ghost cells are present. The stroma of pilar tumors often has an intense inflammatory reaction. Pilar tumors may resemble squamous nodules, but they are larger and can metastasize. In other classifications, subsets of pilar tumors were referred to as adenosquamous carcinoma, 1 as squamous cell carcinoma 1 (the molluscoid subtype), or as intraductal squamous cell carcinoma. 33 These categories were not used to describe pilar tumors here because they include tumors other than pilar tumors. In Table 2 ▶ , adenosquamous carcinoma 1 and squamous cell carcinoma 1 refer only to the nonpilar tumors of this category.
The less differentiated tumors found in the Wnt pathway are generally composed of one or more predominant histological patterns such as microacinar (Dunn type A) (Figure 1D) ▶ , solid cords (Dunn type B), glandular, papillary (Figure 2C) ▶ , squamous, or myoepithelial (Figure 2E) ▶ . Tumors primarily composed of myoepithelial cells (Table 2) ▶ include adenomyoepitheliomas and spindle cell tumors (Figure 2E) ▶ and represent 15% of the Wnt pathway tumors. Some papillary tumors had components with pure micropapillary pattern. Some of the less differentiated tumors were adjacent to well-differentiated type P tumors.
The most characteristic histological pattern in the Wnt pathway tumors is squamous differentiation (Table 3) ▶ . Squamous metaplasia may be extensive as in the pilar tumor or may be scattered as in other tumors. In addition, the majority of tumors also have acinar components. However, pure acinar tumors are rare. The majority of Wnt pathway tumors have some myoepithelial differentiation (Table 3) ▶ as confirmed by IHC for SMA (33 of 45 positive, Figures 1E and 2F ▶ ▶ ) or for CK14 (7 of 7 positive, Figure 2E ▶ ). The myoepithelium is either limited to a basal layer as in the better differentiated tumors (Figure 1F) ▶ , or is the predominant population in the myoepithelial tumors (Figure 2E) ▶ . Myoepithelium is also present in some pulmonary emboli and metastases (Figure 2F) ▶ . Many Wnt pathway tumors have pushing tumor margins.
As might be expected from neoplasms with such complex phenotypes, Wnt pathway tumor cells have no one characteristic cytological feature. However, the cytoplasm of Wnt pathway tumor cells appears to be less abundant than in the ErbB/Ras pathway tumors with an inverted nuclear/cytoplasmic ratio. Thirty percent of the tumors had cytoplasmic lipid droplets corresponding to secretion, even though the adjacent mammary gland had involuted.
Discussion
We previously reported that GEM mammary tumors were frequently different from spontaneous MMTV-induced mammary tumors and many had recognizable signature phenotypes. 2 These observations led to the recognition that phenotype predicts genotype. 2 Currently, the University of California, Davis Mutant Mouse Pathology Laboratory archives include examples from more than 100 different GEM lines. Therefore, comparison of numerous genes involved in the same signal transduction pathways was possible. In the course of such studies, it became apparent that tumors from the members of the Wnt pathway could be distinguished from those affecting the ErbB/Ras pathway. Our observations expand the genotype predicts phenotype concept 2 to pathway pathology.
We previously described distinctive or signature phenotypes for Erbb2/Neu (Figure 1, A and E) ▶ and Ras transgenic tumors. 2 The ErbB2 phenotype has been found to be remarkably consistent in a number of subtypes of the gene. 2,24,34 We found here that ErbB2 and Ras tumors share morphological characteristics with the PyV-mT transgenic tumors (Table 3) ▶ . Because PyV-mT acts as a surrogate for activated ErbB2, all three genes are members of the ErbB signaling pathway 35 (Figure 3) ▶ . Some investigators have suggested that ErbB2 tumors resemble human lobular carcinoma and originate from lobular hyperplasia. 36 Likewise, PyV-mT GEM have mammary glands with normal ducts and abortive lobules. 26 Both PyV-mT and ErbB2 tumors completely lack myoepithelium (Table 3 ▶ , Figure 1E ▶ ). In fact, this myoepithelium is lost when _PyV-mT-_induced hyperplasia first becomes detectable. 26 Most ErbB pathway tumors consist of solid nests and cords. Tumor cell populations are frequently organized in concentric zones (see Results) and, in the predominant zone II, they have large nuclei with an open chromatin structure and abundant but undifferentiated cytoplasm.
Figure 3.
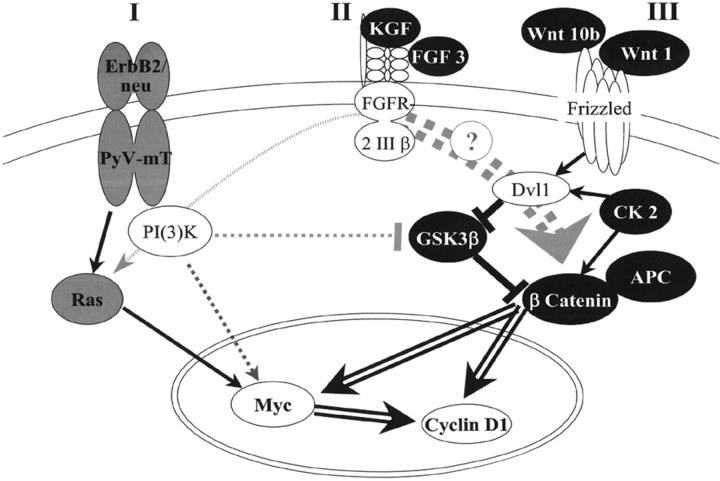
ErbB/Ras pathway and Wnt pathway. The morphological phenotypes of transgenic tumors reflect the signaling pathway activated by the transgene. The color of the studied transgenes indicates the observed phenotype: gray for ErbB2/Ras pathway-like, and black for Wnt pathway-like. Black links between genes indicate activation or inhibition. Gray dotted links show interpathway links, and open arrows symbolize transcriptional activation. I: ErbB2/neu and its intracytoplasmic substitute PyV-mT activate the phosphoinositol-(3) kinase [PI(3)K] 19 and the Ras pathways, 35 leading to accumulation of Myc and cyclin D1 in the nucleus. 53,63,64 II: Fibroblast growth factor 3 (FGF3) [synonym, MMTV integration site 2 gene (Int2)] and keratinocyte growth factor (KGF) [synonym, fibroblast growth factor 7 (FGF7)] both bind to the same receptor, fibroblast growth factor receptor 2 III β (FGFR 2 III β). 65 The intracellular signal transduction varies depending on organ, tissue maturity, and species. 66-68 Our results suggest an activation of the Wnt pathway in FGF transgenic mouse mammary tumors. III: Wnt1 or Wnt10b bind to Frizzled, 69 and activate the mouse dishevelled homolog 1 (Dvl1). Protein kinase CK2 (CK2) also phosphorylates and stabilizes β-catenin and Dvl1. 37 Dvl1 inhibits the activity of glycogen synthase kinase-3β (GSK3β), leading to an accumulation of β-catenin. 70 The degradation of β-catenin depends on complex formation with several proteins including the adenomatous polyposis of the colon gene product (APC). If β-catenin accumulates, it translocates into the nucleus and induces the transcription of target genes, including Myc and Cyclin D1. 37,54
As examples for Wnt pathway tumors, slides from four transgenic models of the Wnt pathway and from the spontaneous Apc Min mutation (Figure 3) ▶ were studied. In addition, we analyzed tumors from a CK2α and two Fgf transgenic models. Our morphological study supports recently published data that CK2 is capable of promoting activation of the Wnt pathway through phosphorylation and stabilization of β-catenin and disheveled. 37 It is an important finding that tumors induced by Kgf and Int2, two members of the Fgf family, have the same phenotype as Wnt pathway tumors, referred to as Wnt pathway phenotype. We hypothesize that these two genes not only cooperate with the Wnt pathway 6,9,14 but may also activate this pathway. This hypothesis is supported by the observation that MMTV infection activates Fgf family members in Wnt GEM, and activates Wnt family members in Fgf GEM. 9,14
Several features were identified that were characteristic for the Wnt pathway tumors, and were rarely, if ever, observed in the ErbB/Ras pathway tumors (Table 3 ▶ ; Figure 1, A to E ▶ ). These include 1) branched ductal architecture; 2) differentiation into squamous, acinar or glandular, solid, and/or myoepithelial components; and 3) well-developed stroma and host response. Because these patterns have not been emphasized or grouped in previous publications, they are discussed in more detail below.
Branched Ductal Architecture
The characteristic feature of well-differentiated Wnt pathway tumors is the organization of the tissue around irregularly branched, elongated ductules. Since this phenomenon has previously been described, 13 we used the old term “type P tumor” (see Results and Figure 1F ▶ and Figure 2, A and B ▶ ). However, the branching morphogenesis could also be identified in association with less differentiated tumors with predominant glandular, papillary, or pilar differentiation. The fact that some poorly differentiated tumors were in direct continuity with well-differentiated tumors suggests that the less differentiated tumors arose from subpopulations of cells within the originating tumor.
The Wnt pathway is critical for tissue and organ differentiation. 38 Different components influence cell fate decisions. The ductal dysmorphogenesis that seems to be so characteristic of the Wnt pathway tumors is in contrast to the lobular dysmorphogenesis observed in the ErbB pathway. However, the reader is to be reminded that the classical consequence of MMTV infection is the hyperplastic alveolar nodule, which is clearly the result of lobulo-alveolar differentiation. In our experience, the MMTV-induced hyperplastic alveolar nodule is associated with Wnt1 activation whereas the type P tumor is primarily associated with Int-2. 39
Terminal Differentiation
The aberrant ductules described above terminate in masses of cells that appear to differentiate along several different pathways. As suggested, the same tumor may have foci that have differentiated along different pathways.
Squamous metaplasia and keratinization was found in, but not limited to, pilar tumors (see Results). The frequent presence of squamous metaplasia in the transgenic Wnt pathway tumors may be related to the dysregulation of β-Catenin 40 (Figure 3) ▶ . The spontaneous mouse mammary tumors, that are primarily associated with Wnt1 activation, 39 seldom have squamous metaplasia. 13 As observed here, ErbB/Ras pathway tumors rarely have squamous metaplasia. Squamous differentiation was less frequent in Wnt1 and Wnt10b GEM than in GEM with mutations in genes downstream from Wnt (Table 2 ▶ and Figure 3 ▶ ). It is possible that the Wnt glycoproteins activate noncanonical pathways 41 that promote acinar or glandular differentiation and prevent keratinization.
Pilar tumors (see Results and Figure 1B ▶ ), a distinct histological type with squamous differentiation, represent the most common histological type in the Wnt pathway tumors (Table 3) ▶ . Pilar tumors may have ghost cells (Figures 1B and 2D) ▶ ▶ , neoplastic ductules resembling abortive hair shafts, or squamous cysts that appear to be derived from confluent ductules (Figure 2B) ▶ characterized by swirls of laminar keratin on the cross-section. Pilar tumors are an unusual phenotype for the mammary gland, but closely resemble trichoepitheliomas or pilomatricomas, skin or hair matrix-derived tumors (Figure 2, C and D) ▶ .
Accumulation of β-Catenin induces de novo hair morphogenesis and trichoepitheliomas in the skin. 42-44 In the mammary gland, a skin appendix, the Wnt/β-Catenin pathway promotes both epidermal transdifferentiation 71 and hair-specific features (Table 2 ▶ , Figures 1B and 2D ▶ ▶ ).
All mammary tumors in mice bearing a mutation of the Apc Min gene were pilar (Table 2) ▶ . Mammary and intestinal tumors in Apc Min mice were extensively studied in various backgrounds regarding spontaneous and chemical carcinogenesis. 22 Treatment with chemical carcinogens in mice frequently induces squamous mammary tumors. However, the pilar phenotype was also observed in spontaneous Apc Min tumors and in various backgrounds. Interestingly, chemically induced adenocarcinomas with squamous metaplasia in nontransgenic mice have Ras mutations in 20%. 45,46 However, the six Ras transgenic tumors included in our study had no keratinization.
Squamous metaplasia of the lactiferous ducts, in humans, is related to smoking (just another chemical carcinogen), and is often associated with periductal inflammatory infiltrates 47 and with inflammatory pseudocapsules ofsilicon prosthesis implants. 48 We observed a similar association of inflammatory infiltrates and squamous metaplasia in the murine Wnt pathway tumors (Table 3 ▶ and Figure 1B ▶ ). Squamous metaplasia is also found in 4% of human breast carcinomas. 48
Microacinar and glandular differentiation and secretory activity were frequent in the Wnt pathway tumors (Tables 2 and 3 ▶ ▶ ; Figure 1, D and F ▶ ; and Figure 2, B and F ▶ ). Although only a few tumors were pure classical MMTV-induced microacinar type A tumors as described by Dunn, minor microacinar components were characteristic for many Wnt pathway tumors. This supports the concept of pathway pathology (Table 3 ▶ , Figure 1D ▶ ). Although some ErbB/Ras pathway tumors are papillary, they rarely have significant glandular differentiation.
Solid cords of cells with peripheral palisades of SMA-positive cells are characteristic of the classical type B tumor. 13 Solid tumors are rare in transgenic Wnt pathway tumors (Table 2) ▶ . However, the ends of some of the terminal ducts contain solid masses of undifferentiated cells that do not express the epithelial marker CK8.
Myoepithelial differentiation is also characteristic of Wnt pathway tumors (Figure 1F ▶ ; Figure 2, E and F ▶ ; Table 3 ▶ ). In contrast, the loss of myoepithelium marks PyV-mT and ErbB2 atypia and neoplasia 26 and most human breast cancers. 49,50 The presence of myoepithelium in the Wnt pathway tumors may be significant in that the myoepithelium is considered a natural tumor suppressor. 51,52 The myoepithelium appears as a distinctive basal layer in many Wnt pathway tumors. However, the Wnt pathway induced spindle cell tumors also proved to be myoepithelial (Figure 2E ▶ and Table 2 ▶ ). Because spindle cell tumors are concentrated in the Kgf, CK2a, and dnGSK3b genotypes, this variation of phenotype may be because of additional pathways activated by these genes (Figure 3) ▶ .
Stroma
In contrast to the ErbB/Ras pathway tumors, the stroma of most Wnt pathway tumors was well developed and contained inflammatory infiltrates, predominantly lymphocytes (Table 3 ▶ ; Figure 1, B and F ▶ ; Figure 2, A to D ▶ ). The host-tumor interface had a pushing margin in many Wnt pathway tumors (Figure 1F) ▶ in contrast to the more invasive growth of the ErbB/Ras pathway tumors (Figure 1C) ▶ . 3,25
Two target genes activated by both pathways are frequently amplified in human breast cancer: myc and cyclin D1 (Figure 3) ▶ . The dependence of Wnt- and ErbB2-induced tumorigenesis on cyclin D1 has recently been documented by Yu and co-workers. 53 The phenotype of tumors induced by these target genes will be discussed elsewhere (A. Rosner, R. D. Cardiff, and J. P. Gregg, unpublished data).
Conclusions
We compared the histology of ErbB/Ras and Wnt pathway transgenic mammary tumors. Wnt pathway tumors frequently show combinations of acinar, glandular, myoepithelial, or pilar differentiation. Despite the complexity of differentiation patterns in the Wnt pathway tumors, the morphological criteria given in Table 3 ▶ distinguish Wnt pathway tumors from ErbB/Ras pathway tumors. We should emphasize that the lesions described here are only signature lesions that are closely related to the genotype. As is recorded in the tables presented here and elsewhere, any given GEM genotype can give rise to a range of tumor phenotypes. However, the tumors described here are so characteristic of the pathway that, when identified in histological sections, they are pathognomonic of the pathway and, thus, should be placed in a separate part of the taxonomic nomenclature related to the pathway. Hence, pathway pathology should result in a specific taxonomic category for mouse tumors.
Pathway pathology should have applications in both basic and clinical research. Histomorphological criteria can help to identify unexpected activated signaling pathways in transgenic tumors. For example, the relationship between the morphology of Fgf and Wnt pathway tumors implies the utilization of the same molecular processes. Our studies also suggest that a subset of keratinizing or myoepithelial human breast tumors might have activation of the Wnt pathway.
In human disease, the diagnosis of the oncogenic genetic aberrations is increasingly important for predicting prognostic and therapeutic strategies. Overexpression of ErbB2 is a well-established example of molecular profiling. The subgroup of breast cancer patients with tumors expressing ErbB2 benefits from treatment with a humanized anti-Her2/Neu monoclonal antibody (Herceptin). 55,56 The need for genetic profiling is growing because of the rapid development of strategies based on gene therapy. 57-59
Studies in human breast cancer, based on standard histological classifications, have found several correlations between the tumor phenotype and genotype. Ductal carcinoma in situ often has an amplification or overexpression of ErbB2. 15 Mutations of the BRCA1 gene are especially frequent in medullary carcinoma. 60,61 Lobular carcinomas are associated with deletion of E-cadherin. 62
Our research now suggests that these concepts need to be expanded to include entire pathways. The correlations should become even stronger as additional criteria are recognized and as pathways and phenotypes are compared with gene expression signatures. Furthermore, as targeted molecular therapeutics become more widely available in the future, these correlations will have increasing clinical importance.
Acknowledgments
We thank Dr. R. J. Munn for help with the imaging; J. E. Walls for assistance with the immunohistochemistry; Drs. P. Leder and W. J. Muller for providing tissues from transgenic mammary tumors; Dr. T.-T. Sun for kindly providing the antibody AE-13 and for discussion of keratinization markers; Drs. G. W. Robinson, L. Hennighausen, and G. B. Baretton for stimulating discussions; and Drs. E. V. Schmidt, J. J. Galvez, and A. D. Borowsky for critical reading of the manuscript.
Note Added in Proof
Since the submission of this paper, an article has appeared that documents hierarchical clustering of tumor types at the level of gene expression. (Desai KV, Xiao N, Wang W, Gangi L, Greene J, Powell JI, Dickson R, Furth P, Hunter K, Kucherlapati R, Simon R, Liu ET, Green JE. Initiating oncogenic event determines gene-expression patterns of human breast cancer models. Proc Natl Acad Sci USA, 2002 99:6967–6972.)
Footnotes
Address reprint requests to Robert D. Cardiff, M.D., Center for Comparative Medicine, University of California, Davis, County Rd. 98 and Hutchison Dr., Davis, CA 95616. E-mail: rdcardiff@ucdavis.edu.
Supported in part by the German Academic Exchange Service (to A. R.), the State of California Breast Cancer Research Program (grant 5JB-0014 to R. D. C.), the National Institutes of Health (CA64843 to A. R. M. and CA81376 to C. M. and R. D. C.), the National Center for Research Resources (U42 RR14905 to R. D. C.), the National Institute of Environmental Health Sciences (ES11624 to D. C. S.), the Massachusetts Department of Public Health Breast Cancer Research Grant Program (to E. L.-B.), the National Cancer Institute (CA665401 to G. S.), the Canadian Breast Cancer Initiative (to A. E. G.), and the Clayton Foundation (C.M. is a Clayton Foundation Investigator).
Current address of X. X.: McLaughlin Research Institute, Great Falls, MT.
References
- 1.Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE: The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene 2000, 19:968-988 [DOI] [PubMed] [Google Scholar]
- 2.Cardiff RD, Sinn E, Muller W, Leder P: Transgenic oncogene mice. Tumor phenotype predicts genotype. Am J Pathol 1991, 139:495-501 [PMC free article] [PubMed] [Google Scholar]
- 3.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ: Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA 1992, 89:10578-10582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel PM, Dankort DL, Hardy WR, Muller WJ: Novel activating mutations in the neu proto-oncogene involved in induction of mammary tumors. Mol Cell Biol 1994, 14:7068-7077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrechek ER, Hardy WR, Siegel PM, Rudnicki MA, Cardiff RD, Muller WJ: Amplification of the neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis. Proc Natl Acad Sci USA 2000, 97:3444-3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callahan R: MMTV-induced mutations in mouse mammary tumors: their potential relevance to human breast cancer. Breast Cancer Res Treat 1996, 39:33-44 [DOI] [PubMed] [Google Scholar]
- 7.Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, Moon R, Varmus H: A new nomenclature for int-1 and related genes: the Wnt gene family. Cell 1991, 64:231. [DOI] [PubMed] [Google Scholar]
- 8.Dickson C, Peters G: Potential oncogene product related to growth factors. Nature 1987, 326:833. [DOI] [PubMed] [Google Scholar]
- 9.Lee FS, Lane TF, Kuo A, Shackleford GM, Leder P: Insertional mutagenesis identifies a member of the Wnt gene family as a candidate oncogene in the mammary epithelium of int-2/Fgf-3 transgenic mice. Proc Natl Acad Sci USA 1995, 92:2268-2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane TF, Leder P: Wnt-10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene 1997, 15:2133-2144 [DOI] [PubMed] [Google Scholar]
- 11.Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC: Protein kinase CK2 in mammary gland tumorigenesis. Oncogene 2001, 20:3247-3257 [DOI] [PubMed] [Google Scholar]
- 12.Michaelson JS, Leder P: Beta-catenin is a downstream effector of Wnt-mediated tumorigenesis in the mammary gland. Oncogene 2001, 20:5093-5099 [DOI] [PubMed] [Google Scholar]
- 13.Dunn T: Morphology of mammary tumors in mice. Homburger F eds. The Physiopathology of Cancer. 1959:pp 38-84 Hoeber-Harper, New York
- 14.Kapoun AM, Shackleford GM: Preferential activation of Fgf8 by proviral insertion in mammary tumors of Wnt1 transgenic mice. Oncogene 1997, 14:2985-2989 [DOI] [PubMed] [Google Scholar]
- 15.Barnes DM, Bartkova J, Camplejohn RS, Gullick WJ, Smith PJ, Millis RR: Overexpression of the c-erbB-2 oncoprotein: why does this occur more frequently in ductal carcinoma in situ than in invasive mammary carcinoma and is this of prognostic significance? Eur J Cancer 1992, 28:644-648 [DOI] [PubMed] [Google Scholar]
- 16.Bargmann CI, Hung MC, Weinberg RA: The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 1986, 319:226-230 [DOI] [PubMed] [Google Scholar]
- 17.Webster MA, Muller WJ: Mammary tumorigenesis and metastasis in transgenic mice. Semin Cancer Biol 1994, 5:69-76 [PubMed] [Google Scholar]
- 18.Cardiff RD, Wellings SR: The comparative pathology of human and mouse mammary glands. J Mammary Gland Biol Neoplasia 1999, 4:105-122 [DOI] [PubMed] [Google Scholar]
- 19.Webster MA, Hutchinson JN, Rauh MJ, Muthuswamy SK, Anton M, Tortorice CG, Cardiff RD, Graham FL, Hassell JA, Muller WJ: Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol 1998, 18:2344-2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E: Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci USA 1998, 95:696-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shyamala G, Yang X, Cardiff RD, Dale E: Impact of progesterone receptor on cell-fate decisions during mammary gland development. Proc Natl Acad Sci USA 2000, 97:3044-3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moser AR, Mattes EM, Dove WF, Lindstrom MJ, Haag JD, Gould MN: ApcMin, a mutation in the murine Apc gene, predisposes to mammary carcinomas and focal alveolar hyperplasias. Proc Natl Acad Sci USA 1993, 90:8977-8981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P: Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 1988, 54:105-115 [DOI] [PubMed] [Google Scholar]
- 24.Guy CT, Cardiff RD, Muller WJ: Activated neu induces rapid tumor progression. J Biol Chem 1996, 271:7673-7678 [DOI] [PubMed] [Google Scholar]
- 25.Guy CT, Cardiff RD, Muller WJ: Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 1992, 12:954-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maglione JE, Moghanaki D, Young LJ, Manner CK, Ellies LG, Joseph SO, Nicholson B, Cardiff RD, MacLeod CL: Transgenic polyoma middle-T mice model premalignant mammary disease. Cancer Res 2001, 61:8298-8305 [PubMed] [Google Scholar]
- 27.Moser AR, Hegge LF, Cardiff RD: Genetic background affects susceptibility to mammary hyperplasias and carcinomas in Apc(min)/+ mice. Cancer Res 2001, 61:3480-3485 [PubMed] [Google Scholar]
- 28.Muller WJ, Lee FS, Dickson C, Peters G, Pattengale P, Leder P: The int-2 gene product acts as an epithelial growth factor in transgenic mice. EMBO J 1990, 9:907-913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwan H, Pecenka V, Tsukamoto A, Parslow TG, Guzman R, Lin TP, Muller WJ, Lee FS, Leder P, Varmus HE: Transgenes expressing the Wnt-1 and int-2 proto-oncogenes cooperate during mammary carcinogenesis in doubly transgenic mice. Mol Cell Biol 1992, 12:147-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitsberg DI, Leder P: Keratinocyte growth factor induces mammary and prostatic hyperplasia and mammary adenocarcinoma in transgenic mice. Oncogene 1996, 13:2507-2515 [PubMed] [Google Scholar]
- 31.Lynch MH, O’Guin WM, Hardy C, Mak L, Sun TT: Acidic and basic hair/nail (“hard”) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to “soft” keratins. J Cell Biol 1986, 103:2593-2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Nie R, Dux A: Biological and morphological characteristics of mammary tumors in GR mice. J Natl Cancer Inst 1971, 46:885-897 [PubMed] [Google Scholar]
- 33.Rehm S, Liebelt AG: Nonneoplastic and neoplastic lesions of the mammary gland. Mohr U Dungworth DL Capen CC Carlton WW Sundberg JP Ward JM eds. Pathobiology of the Aging Mouse. 1996:pp 381-398 ILSI Press, Washington DC
- 34.Weinstein EJ, Kitsberg DI, Leder P: A mouse model for breast cancer induced by amplification and overexpression of the neu promoter and transgene. Mol Med 2000, 6:4-16 [PMC free article] [PubMed] [Google Scholar]
- 35.Malaney S, Daly RJ: The ras signaling pathway in mammary tumorigenesis and metastasis. J Mammary Gland Biol Neoplasia 2001, 6:101-113 [DOI] [PubMed] [Google Scholar]
- 36.Di Carlo E, Diodoro MG, Boggio K, Modesti A, Modesti M, Nanni P, Forni G, Musiani P: Analysis of mammary carcinoma onset and progression in HER-2/neu oncogene transgenic mice reveals a lobular origin. Lab Invest 1999, 79:1261-1269 [PubMed] [Google Scholar]
- 37.Song DH, Sussman DJ, Seldin DC: Endogenous protein kinase CK2 participates in Wnt signaling in mammary epithelial cells. J Biol Chem 2000, 275:23790-23797 [DOI] [PubMed] [Google Scholar]
- 38.Cadigan KM, Nusse R: Wnt signaling: a common theme in animal development. Genes Dev 1997, 11:3286-3305 [DOI] [PubMed] [Google Scholar]
- 39.Morris DW, Barry PA, Bradshaw HD, Jr: Cardiff RD: Insertion mutation of the int-1 and int-2 loci by mouse mammary tumor virus in premalignant and malignant neoplasms from the GR mouse strain. J Virol 1990, 64:1794-1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyoshi K, Shillingford JM, Le Provost F, Gounari F, Bronson R, von Boehmer H, Taketo MM, Cardiff RD, Hennighausen L, Khazaie K: Activation of beta-catenin signaling in differentiated mammary secretory cells induces transdifferentiation into epidermis and squamous metaplasias. Proc Natl Acad Sci USA 2002, 99:219-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huelsken J, Birchmeier W: New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev 2001, 11:547-553 [DOI] [PubMed] [Google Scholar]
- 42.Gat U, DasGupta R, Degenstein L, Fuchs E: De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998, 95:605-614 [DOI] [PubMed] [Google Scholar]
- 43.Chan EF, Gat U, McNiff JM, Fuchs E: A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet 1999, 21:410-413 [DOI] [PubMed] [Google Scholar]
- 44.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W: Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105:533-545 [DOI] [PubMed] [Google Scholar]
- 45.Cardiff RD, Gumerlock PH, Soong MM, Dandekar S, Barry PA, Young LJ, Meyers FJ: c-H-ras-1 expression in 7,12-dimethyl benzanthracene-induced Balb/c mouse mammary hyperplasias and their tumors. Oncogene 1988, 3:205-213 [PubMed] [Google Scholar]
- 46.Swanson SM, Guzman RC, Tsukamoto T, Huang TT, Dougherty CD, Nandi S: N-Ethyl-N-nitrosourea induces mammary cancers in the pituitary-isografted mouse which are histologically and genotypically distinct from those induced by N-methyl-N-nitrosourea. Cancer Lett 1996, 102:159-165 [DOI] [PubMed] [Google Scholar]
- 47.Furlong AJ, al-Nakib L, Knox WF, Parry A, Bundred NJ: Periductal inflammation and cigarette smoke. J Am Coll Surg 1994, 179:417-420 [PubMed] [Google Scholar]
- 48.Krech RH, Brunnert K, Neumann H: Primary squamous cell carcinoma of female mammary gland. Pathologe 1998, 19:373-378 [DOI] [PubMed] [Google Scholar]
- 49.Guelstein VI, Tchypysheva TA, Ermilova VD, Ljubimov AV: Myoepithelial and basement membrane antigens in benign and malignant human breast tumors. Int J Cancer 1993, 53:269-277 [DOI] [PubMed] [Google Scholar]
- 50.Nagle RB, Bocker W, Davis JR, Heid HW, Kaufmann M, Lucas DO, Jarasch ED: Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem 1986, 34:869-881 [DOI] [PubMed] [Google Scholar]
- 51.Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH: The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res 1997, 3:1949-1958 [PubMed] [Google Scholar]
- 52.Sternlicht MD, Barsky SH: The myoepithelial defense: a host defense against cancer. Med Hypotheses 1997, 48:37-46 [DOI] [PubMed] [Google Scholar]
- 53.Yu Q, Geng Y, Sicinski P: Specific protection against breast cancers by cyclin D1 ablation. Nature 2001, 411:1017-1021 [DOI] [PubMed] [Google Scholar]
- 54.Smalley MJ, Dale TC: Wnt signaling and mammary tumorigenesis. J Mammary Gland Biol Neoplasia 2001, 6:37-52 [DOI] [PubMed] [Google Scholar]
- 55.Neve RM, Lane HA, Hynes NE: The role of overexpressed HER2 in transformation. Ann Oncol 2001, 12:S9-S13 [DOI] [PubMed] [Google Scholar]
- 56.Piccart MJ: Proposed treatment guidelines for HER2-positive metastatic breast cancer in Europe. Ann Oncol 2001, 12:S89-S94 [DOI] [PubMed] [Google Scholar]
- 57.Harris JD, Gutierrez AA, Hurst HC, Sikora K, Lemoine NR: Gene therapy for cancer using tumour-specific prodrug activation. Gene Ther 1994, 1:170-175 [PubMed] [Google Scholar]
- 58.Hortobagyi GN, Ueno NT, Xia W, Zhang S, Wolf JK, Putnam JB, Weiden PL, Willey JS, Carey M, Branham DL, Payne JY, Tucker SD, Bartholomeusz C, Kilbourn RG, De Jager RL, Sneige N, Katz RL, Anklesaria P, Ibrahim NK, Murray JL, Theriault RL, Valero V, Gershenson DM, Bevers MW, Huang L, Lopez-Berestein G, Hung MC: Cationic liposome-mediated E1A gene transfer to human breast and ovarian cancer cells and its biologic effects: a phase I clinical trial. J Clin Oncol 2001, 19:3422-3433 [DOI] [PubMed] [Google Scholar]
- 59.Pandha HS, Martin LA, Rigg A, Hurst HC, Stamp GW, Sikora K, Lemoine NR: Genetic prodrug activation therapy for breast cancer: a phase I clinical trial of erbB-2-directed suicide gene expression. J Clin Oncol 1999, 17:2180-2189 [DOI] [PubMed] [Google Scholar]
- 60.Eisinger F, Jacquemier J, Charpin C, Stoppa-Lyonnet D, Bressac-de Paillerets B, Peyrat JP, Longy M, Guinebretiere JM, Sauvan R, Noguchi T, Birnbaum D, Sobol H: Mutations at BRCA1: the medullary breast carcinoma revisited. Cancer Res 1998, 58:1588-1592 [PubMed] [Google Scholar]
- 61.Lakhani SR, Gusterson BA, Jacquemier J, Sloane JP, Anderson TJ, van de Vijver MJ, Venter D, Freeman A, Antoniou A, McGuffog L, Smyth E, Steel CM, Haites N, Scott RJ, Goldgar D, Neuhausen S, Daly PA, Ormiston W, McManus R, Scherneck S, Ponder BA, Futreal PA, Peto J, Stoppa-Lyonnet D, Bignon YJ, Stratton MR: The pathology of familial breast cancer: histological features of cancers in families not attributable to mutations in BRCA1 or BRCA2. Clin Cancer Res 2000, 6:782-789 [PubMed] [Google Scholar]
- 62.Rasbridge SA, Gillett CE, Sampson SA, Walsh FS, Millis RR: Epithelial (E-) and placental (P-) cadherin cell adhesion molecule expression in breast carcinoma. J Pathol 1993, 169:245-250 [DOI] [PubMed] [Google Scholar]
- 63.Muller WJ, Neville MC: Introduction: signaling in mammary development and tumorigenesis. J Mammary Gland Biol Neoplasia 2001, 6:1-5 [DOI] [PubMed] [Google Scholar]
- 64.Hynes NE, Lane HA: Myc and mammary cancer: myc is a downstream effector of the ErbB2 receptor tyrosine kinase. J Mammary Gland Biol Neoplasia 2001, 6:141-150 [DOI] [PubMed] [Google Scholar]
- 65.Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M: Receptor specificity of the fibroblast growth factor family. J Biol Chem 1996, 271:15292-15297 [DOI] [PubMed] [Google Scholar]
- 66.Chandrasekher G, Kakazu AH, Bazan HE: HGF- and KGF-induced activation of PI-3K/p70 s6 kinase pathway in corneal epithelial cells: its relevance in wound healing. Exp Eye Res 2001, 73:191-202 [DOI] [PubMed] [Google Scholar]
- 67.Carballada R, Yasuo H, Lemaire P: Phosphatidylinositol-3 kinase acts in parallel to the ERK MAP kinase in the FGF pathway during Xenopus mesoderm induction. Development 2001, 128:35-44 [DOI] [PubMed] [Google Scholar]
- 68.Mehta PB, Robson CN, Neal DE, Leung HY: Keratinocyte growth factor activates p38 MAPK to induce stress fibre formation in human prostate DU145 cells. Oncogene 2001, 20:5359-5365 [DOI] [PubMed] [Google Scholar]
- 69.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R: A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996, 382:225-230 [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A: Phosphorylation of axin, a Wnt signal-negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem 1999, 274:10681-10684 [DOI] [PubMed] [Google Scholar]
- 71.Miyoshi K, Rosner A, Nozawa M, Byrd C, Morgan F, Landesman-Bollag E, Xu X, Seldin DC, Schmidt EV, Taketo MM, Robinson GW, Cardiff RD, Henninghausen L: Activation of different Wnt/β-catenin signaling components in mammary epithelium induces transdifferentiation and the formation of pilar tumors. Oncogene 2002, 21:5548-5556 [DOI] [PubMed] [Google Scholar]