Tumor-Associated Macrophages Express Lymphatic Endothelial Growth Factors and Are Related to Peritumoral Lymphangiogenesis (original) (raw)
Abstract
Formation of lymphatic metastasis is the initial step of generalized spreading of tumor cells and predicts poor clinical prognosis. Lymphatic vessels generally arise within the peritumoral stroma, although the lymphangiopoietic vascular endothelial growth factors (VEGF)-C and -D are produced by tumor cells. In a carefully selected collection of human cervical cancers (stage pT1b1) we demonstrate by quantitative immunohistochemistry and in situ hybridization that density of lymphatic microvessels is significantly increased in peritumoral stroma, and that a subset of stromal cells express large amounts of VEGF-C and VEGF-D. The density of cells producing these vascular growth factors correlates with peritumoral inflammatory stroma reaction, lymphatic microvessel density, and indirectly with peritumoral carcinomatous lymphangiosis and frequency of lymph node metastasis. The VEGF-C- and VEGF-D-producing stroma cells were identified in situ as a subset of activated tumor-associated macrophages (TAMs) by expression of a panel of macrophage-specific markers, including CD68, CD23, and CD14. These TAMs also expressed the VEGF-C- and VEGF-D-specific tyrosine kinase receptor VEGFR-3. As TAMs are derived from monocytes in the circulation, a search in peripheral blood for candidate precursors of VEGFR-3-expressing TAMs revealed a subfraction of CD14-positive, VEGFR-3-expressing monocytes, that, however, failed to express VEGF-C and VEGF-D. Only after in vitro incubation with tumor necrosis factor-α, lipopolysaccharide, or VEGF-D did these monocytes start to synthesize VEGF-C de novo. In conclusion VEGF-C-expressing TAMs play a novel role in peritumoral lymphangiogenesis and subsequent dissemination in human cancer.
Life expectancy of patients with malignant tumors is determined by metastatic dissemination that proceeds initially via lymphatic vessels to lymph nodes, and eventually via blood vessels to distant organs. Thus, targeted disruption of established lymphatic vessels, or inhibition of local lymphatic neoangiogenesis, promises to reduce metastasis. 1 Interference with lymphatic angiogenesis has become possible only recently with the identification of vascular endothelial growth factors (VEGF)-C and –D as specific growth factors for lymphatic endothelia, and their corresponding tyrosine kinase receptor, VEGFR-3 2-4 that, however, is also expressed in peritumoral blood capillaries. 5 Transgene overexpression of VEGF-C and VEGF-D in experimental tumors has established a direct relation between lymph vessel density and rate of lymphatic metastasis. 6-8 These studies were performed in human mammary carcinomas and melanomas implanted into mice, and in murine sarcomas, 6,9,10 using novel markers for localization of lymphatic vessels, such as the membrane mucoprotein podoplanin, 11 and the CD44-related hyaluronan receptor LYVE-1. 12 In contrast to intratumoral blood vessels that receive their growth stimulus from intratumoral VEGF, 13 lymphatic capillaries were encountered almost exclusively within the peritumoral stroma at the tumor surface, but not within tumors. 9,10,14,15 This study on human squamous carcinomas of the uterine cervix provides evidence that a subfraction of tumor-associated macrophages (TAMs) are a major source of VEGF-C and VEGF-D, that their density correlates with regional lymph vessel proliferation, and that they are presumably derived from a subfraction of circulating monocytes.
Materials and Methods
Tissues
Archival paraffin-embedded or frozen tissue samples of 7 normal human cervixes, 5 cases of low-grade squamous intraepithelial lesions (LSILs), 7 cases of high-grade squamous intraepithelial lesions (HSIL), and 32 cases of International Union against Cancer (UICC) Stage pT1b1 cervical squamous carcinomas treated by radical hysterectomy and pelvic lymph node dissection were included into this study. All procedures were performed in compliance with Austrian legislation.
Immunohistochemistry and Confocal Microscopy
Immunohistochemistry and immunofluorescence were performed on formalin-fixed, paraffin-embedded cervical tissue samples, acetone-fixed cryostat sections, or slides with cultured cells. For immunohistochemical detection of protein expression, antibodies with the following specificities were used: VEGF-C (Zymed Inc., South San Francisco, CA), VEGF-C 15-meric peptide (VEGF-C amino acids 260 to 274) prepared with an automated peptide synthesizer (model 430A; Applied Biosystems Inc., Foster City, CA), CD1, CD2, CD3, CD8, CD14, CD16, CD19, CD20, CD23, CD34, CD45, CD45RA, CD56, CD68, CD123, HLA-DR (all from Research Diagnostics Inc., Flanders, NJ), VEGFR-3, 2 podoplanin (IgG raised in rabbits, 11 or sera produced in mice), LYVE-1 (affinity-purified rabbit anti-IgG, kindly provided by Dr. David Jackson, Oxford, UK), tryptase (Chemicon International Inc., Temecula, CA), and VEGF-D (Santa Cruz Biotechnology Inc., Santa Cruz, CA). For immunohistochemistry, a biotin-streptavidin-horseradish peroxidase-based method was used. For immunofluorescence, Alexa 488- and Alexa 633-labeled secondary antibodies were used and for nuclear counterstaining, propidium iodide was used (all from Molecular Probes, Eugene, OR). Triple-channel confocal laser-scanning microscopy analysis was performed on a Zeiss LSM 510 (Oberhochen, Germany).
Morphometry
Microvessel density was determined by two independent observers who were blind to the clinical course of the patient. Mean values of microvessel densities scored by both investigators for each patient were entered into further calculations. For determination of lymphatic microvessel density (LMVD), the area directly adjacent to tumor formations with the greatest number of distinctly highlighted microvessels (hot spot) 16 was selected. LMVD was then determined by counting all decorated vessels at a total magnification of ×200 within an examination area of 0.25 mm2 using an eye grid. Each stained lumen was regarded as a single countable microvessel. Lymphangiosis carcinomatosa was considered positive if at least one tumor cell cluster was detected within a podoplanin-labeled lymphovascular lumen. The number of VEGF-C- and VEGF-D-positive stroma cells was determined in the area of their highest density (hot spot) at a magnification of ×400 (field of view, 0.08 mm2). The ratio of CD68- and VEGF-C-expressing stroma cells was determined in identical areas in consecutive tissue sections. Immunostaining intensity of VEGF-C in cancer cells was graded as strong (+++), medium (++), and weak expression (+). Peritumoral inflammation was graded as: +, when inflammatory reaction was sparse; ++, with moderate/inhomogeneous reaction; and +++, with dense, homogenous inflammatory infiltrate, as described. 17
In Situ Hybridization
Human VEGF-C anti-sense and sense RNA probes were generated from linearized (_Apa_I, _Kpn_I) pCR2.1 topo vector (Invitrogen, San Diego, CA), corresponding to nucleotides 1033 to 1593 of human VEGF-C cDNA (sense 5′-TTCCCTGCCAGCAACACTACCA-3′, anti-sense 5′-CCAATATGAAGGGACACAACGACA-3′). Digoxigenin-labeled anti-sense mRNA was synthesized using T7 RNA polymerase and [DIG]UTP, and sense mRNA, using SP6 RNA polymerase and [DIG]UTP (Boehringer, Mannheim, Germany). In situ hybridization for VEGF-C mRNA expression was performed on 5-μm thick formalin-fixed, paraffin-embedded tissue samples of four different cases, as described. 18
Isolation and Stimulation of Blood Mononuclear Cells
Blood-borne mononuclear cells were isolated from heparinized peripheral blood of normal healthy donors by density gradient centrifugation with Ficoll-Paque (Pharmacia, Uppsala, Sweden). T cells and monocytes were separated by magnetic sorting using the MACS technique (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) as described. 19 Monocytes were enriched using biotinylated CD14 monoclonal antibody VIM13 (purity >95%) (kindly provided by O Majdic, Institute of Immunology, Vienna, Austria). The relative number of VEGFR-3 surface-positive CD14+ monocytes was determined by cell sorting (FACSCalibur; Becton-Dickinson. Mountain View, CA), starting with 106 CD14+ cells in phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 10% fetal calf serum. Incubation was performed with monoclonal antibody toward human VEGFR-3 20 for 30 minutes at 4°C, followed by three washes in PBS and Alexa 488-conjugated goat anti-mouse IgG (Molecular Probes Inc.). Controls were performed with irrelevant first antibodies. Alternatively, CD14+ cells were centrifuged onto slides by cytospinning, labeled for immunofluorescence with the same antibodies, and the relative fraction of VEGFR-3+/CD14+ cells was visually counted. Samples from four healthy individuals were analyzed and the relative number of VEGFR-3+ cells were expressed as mean value with SE.
For in vitro activation, CD14+ monocytes (1 × 106/ml) were cultured in RPMI 1640 (Life Technologies, Inc., Grand Island, NY), supplemented with 2 mmol/L of l-glutamine, 10% fetal calf serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin in 24-well plates (Costar, Cambridge, MA) in the presence of lipopolysaccharide (LPS) from Escherichia coli (1 μg/ml) (serotype 0127-B8; Sigma Chemie GmbH, Deisenhofen, Germany), tumor necrosis factor (TNF)-α (50 U/ml) (provided by Dr. GR Adolf, Boehringer Ingelheim, Vienna, Austria), recombinant human VEGF-D (0.3 μg/ml) (R&D Systems Inc., Minneapolis, MN), or medium alone at 37°C. Freshly isolated monocytes were kept at 4°C to avoid activation.
Qualitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA of monocytes was isolated with TriReagent (Molecular Research Center, Cincinnati, OH). Total RNA (3 μg) was used for cDNA synthesis (total volume, 20 μl), and the reverse transcriptase reaction mixture (2 μl) was used for PCR reaction with a DNA thermal cycler (Perkin Elmer Cetus) for 40 cycles (60 seconds at 94°C, 60 seconds at 57°C, and 60 seconds at 72°C), with following primers: G3PDH sense 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′, anti-sense 5′-CATGTGGGCCATGAGGTCCACAC–3; VEGF-C sense 5′-GATGTGGGGAAGGA-GTTTGGAGTC-3′, VEGF-C anti-sense 5′-TTGGCTGGGG-AAGAGTTTGTTTTT-3′; VEGF-D sense 5′-CTAGAGAAACGTGCGTGGAGGTG-3′, VEGF-D anti-sense 5′-AGTTTTTGGGGTGCTGGATTAGAT-3′; VEGFR-3 sense 5′-CAGACGGGCAGGAGGTGGTGTG-3′, VEGFR-3 anti-sense 5′-CGGCTGTGACGCGAGTAGATGC. The amplified PCR products (G3PDH, 452 bp; VEGF-C, 567 bp; VEGF-D, 525 bp; and VEGFR-3, 787 bp) were electrophoresed in 1% agarose gels and stained with ethidium bromide.
Immunoblotting
U937 cells (CRL-2367; American Type Culture Collection, Manassas, VA) were cultured as described, lysed in reducing sodium dodecyl sulfate sample buffer, and proteins were electrophoresed by 5 to 15% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes (BioRad, Richmond, CA). Membranes were cut and strips were incubated with monoclonal anti-VEGFR-3 IgG, rabbit anti-human VEGF-C, and rabbit anti-human VEGF-D antibodies. A lysate of isolated cultured human blood vessel endothelial cells 21 was processed and used as control. Strips were washed and binding of primary antibodies was developed as described. 21
Statistics
The Mann-Whitney test, Kruskal-Wallis-test, Spearman’s coefficient of correlation, and chi-square test were used as indicated in the figure legends. Numbers given are mean values ± SE. A P value of ≤0.05 was considered as significant.
Results
LMVD Is Increased in Peritumoral Stroma and Correlates with Regional Lymphangiosis Carcinomatosa
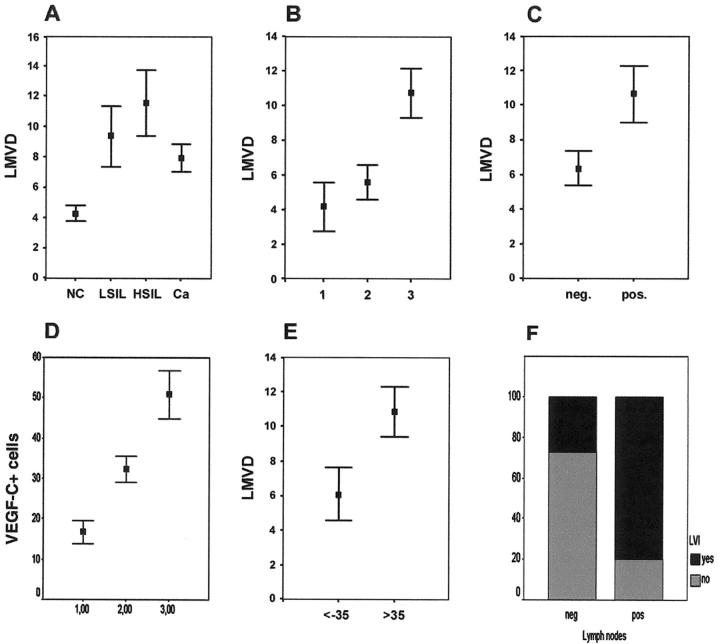
Podoplanin and LYVE-1 double-positive lymphatic microvessels were found exclusively within the peritumoral stroma, but not between tumor cells, both in noninvasive and pT1b1-stage invasive carcinomas (Figure 1, B and G) ▶ . In contrast, CD34+ blood capillaries were also encountered between tumor cell sheets without intervening stroma in consecutive sections (Figure 1A) ▶ . Double immunofluorescence further confirmed that all vessels labeled with podoplanin also expressed LYVE-1 (Figure 1; D, E, and F) ▶ . Morphometric analysis indicated that in normal cervical tissue the average density of lymphatic vessels (LMVD) amounted to 4.3 ± 0.5 microvessels/high-power field (HPF), whereas it was markedly elevated in LSILs (9.4 ± 2 microvessels/HPF), HSILs (11.6 ± 2.2 microvessels/HPF), and invasive cancers (8.3 ± 1.1 microvessels/HPF; Figure 2A ▶ ). Inflammatory stroma reaction was rated + in 9.4%, ++ in 43.8%, and +++ in 46.9% of the cases. A significant correlation was encountered between LVMD and inflammatory stroma reaction (P = 0.012) (Figure 2B) ▶ , and a trend toward increased LMVD with higher dysplasia was observed (Figure 2A) ▶ . Median LMVD significantly correlated with lymphangiosis carcinomatosa (11.3 ± 1.6 microvessels/HPF in cases with lymphovascular tumor invasion versus 6.1 ± 1.4 in cases without; P = 0.014) (Figure 2C) ▶ . Invasion of tumor cells into peritumoral lymphatic vessels was observed in 44% of cases, and was statistically significantly associated with lymph node metastasis (P = 0.008) (Figure 2F) ▶ . Expression of VEGF-C in cancer cells was graded as +++ in 37.5%, ++ in 25%, and + in 37.5%. There was no association of VEGF-C expression of tumor cells with any other parameter, including LMVD and lymph node status (P > 0.05).
Figure 1.
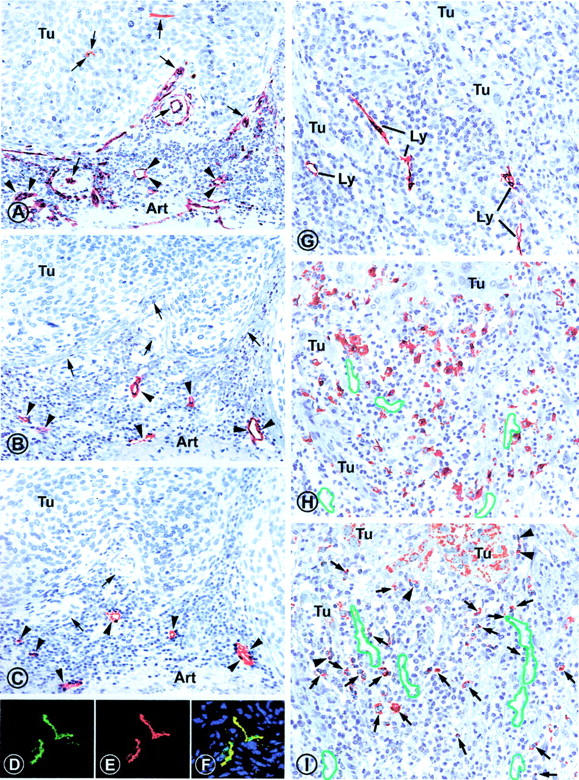
Identification of lymphatic vessels (A–F) in consecutive serial sections of a representative case of stage pT1b1 squamous cell carcinoma of the uterine cervix. A: All vessels are immunolabeled (arrowheads) with anti-CD34 antibody that does not discriminate between lymphatic and blood vessel endothelial cells. Besides several vessels, including small arteries (arrows) within the tumor stroma, two vessels are also labeled within the tumor (Tu). B: Consecutive section labeled with rabbit anti-podoplanin antibody, revealing six sections through lymphatic vessels (arrowheads), whereas other vessels (arrows) that were marked by CD34 antibody in the previous section were podoplanin-negative and are blood vessels. C: Next consecutive section immunostained with rabbit anti-LYVE 1 IgG that marks the same lymphatic vessels (arrowheads) as podoplanin in the previous level. Blood vessels are not marked (arrows). D–F: Double immunofluorescence on a squamous cell carcinoma with mouse antibodies to podoplanin (green, D) and rabbit anti-LYVE 1 IgG (red, E) reveals perfect overlap of both markers on the same lymphatic vessel (yellow, F). G–I: Association of peritumoral lymphatic vessels (G), CD68-positive TAMs (H), and VEGF-C-producing cells (I) on consecutive sections of cervix squamous epithelial carcinoma. G: Lymphatic vessels (Ly) are localized rabbit anti-podoplanin antibody within the peritumoral mononuclear infiltrate surrounding infiltrative extensions of the invasive carcinoma (Tu). H: CD68+ TAMs are localized close to the tumor and surround the lymphatic vessels (outlined in green; position was deduced from the preceding section and indicates vessels that were free of erythrocytes in higher magnification in this section). I: VEGF-C is expressed in tumor cells (Tu) in a granular pattern, as well as in peritumoral inflammatory cells (arrows), some of which are associated with the surface of the invading tumor (arrowheads). Original magnifications: ×350 (A–C); ×600 (D–F); ×420 (G–I).
Figure 2.
Correlations between lymphatic vessel density and clinical parameters. A: There was a clear trend toward higher LMVD in low-grade squamous intraepithelial lesions (LSILs), high-grade squamous intraepithelial lesions (HSILs), and squamous cell cervical cancers (Ca) compared to normal cervical tissue that, however, failed to reach statistical significance. (P = 0.078, Kruskal Wallis test). B: There was a significant correlation between LMVD and the grade of inflammatory stroma reaction (1, absent; 2, moderate; 3, dense homogenous inflammatory infiltrate) (P = 0.012, Kruskal-Wallis test). C: LMVD was significantly increased in cervical cancers with invasion of peritumoral lymphatic vessels by tumor cells (lymphangiosis carcinomatosa) (pos.) when compared to those without (neg.) (11.39 ± 1.62 versus 6.06 ± 1.36) (P = 0.014, Mann-Whitney test). D: The number of VEGF-C-expressing peritumoral cells correlated with the grade of inflammatory stroma reaction in invasive cancers as well as in LSILs (P = 0.043, Kruskal Wallis test). E: LMVD in squamous carcinomas, stage pT1b1, with ≤35 VEGF-C-positive stroma cells was significantly lower than in those cancer samples with >35 VEGF-C-positive cells (6.11 ± 1.53 versus 10.87 ± 1.45) (P = 0.014, Mann-Whitney test), using the median value of 35 VEGF-C-positive stroma cells as cutoff score. F: Lymphatic vessel invasion of tumor cells was highly associated with the presence of lymph node metastases (P = 0.008, chi-square test).
VEGF-C and VEGF-D Are Expressed in Mononuclear Cells in the Peritumoral Inflammatory Infiltrate
Expression of VEGF-C and VEGF-D was observed in a subset of mononuclear cells of the peritumoral inflammatory stroma by immunohistochemistry (Figures 1I and 3B) ▶ ▶ , and in four cases also by in situ hybridization (Figure 3A) ▶ , using a digitonin-labeled anti-sense probe, and a corresponding sense probe as negative control (data not shown). Frequently, these mononuclear cells formed small clusters close to lymphatic microvessels (Figures 1C and 3B) ▶ ▶ and were in close contact to the tumor surfaces (Figures 1I and 3A) ▶ ▶ . Relatively smaller amounts of the growth factors and of their specific mRNA were also detected within tumor cells (Figures 1I and 3A) ▶ ▶ . The mean number of VEGF-C-expressing cells in invasive carcinomas (39.5 ± 3.7/HPF) significantly correlated with the grade of inflammatory stroma reaction (P = 0.001) (Figure 2D) ▶ , and was highest in carcinomas (39.5 ± 3.7/HPF), followed by HSILs (23.3 ± 5.8/HPF) and LSILs (8.4 ± 3.1/HPF) (P = 0.001). A significant correlation was found between the number of VEGF-C-expressing peritumoral cells, and median LMVD (Figure 2E) ▶ .
Figure 3.
Localization of VEGF-C-expressing cells by in situ hybridization (A) and immunofluorescence (B). A: Expression of VEGF-C mRNA is found by in situ hybridization in highest concentration in cells within the peritumoral stroma, some of which directly sit on the surfaces (arrowheads) of the infiltrating tumor extensions (T). Small amounts of VEGF-C mRNA are also expressed by tumor cells (arrows). These results are representative for four different patients. For negative control, hybridization with sense probe was performed and no signal was found (data not shown). B: When VEGF-C and lymphatic vessels are co-localized by double immunofluorescence and confocal laser-scanning microscopy, individual and clusters of VEGF-producing cells (green channel) frequently adjoin the lymphatic vessel wall marked by podoplanin (red channel). Nuclear counterstaining was performed with propidium iodide (blue channel). Original magnifications: ×600 (A); ×2500 (B).
VEGF-C-Expressing Stroma Cells Are TAMs
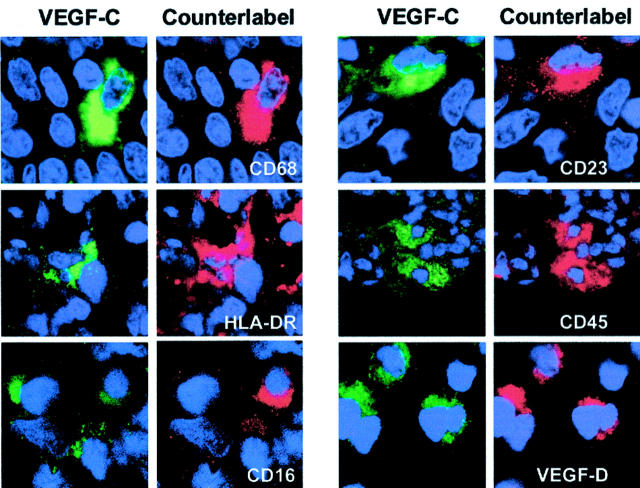
The VEGF-C-expressing peritumoral stroma cells were characterized in situ, using double immunofluorescence and confocal microscopy, keeping anti-VEGF-C antibody labeling constant, and varying the antibodies in the other channel (Figure 4) ▶ . All VEGF-C-producing cells expressed the markers CD68, CD14, CD23, HLA-DR, and CD45 (Figure 4) ▶ , while ∼50% also produced CD16 (Figure 4) ▶ . The VEGF-C-expressing cells failed to produce CD1a, CD2, CD3, CD8, CD19, CD20, CD34, CD56, CD45-RA, CD123, and tryptase. All VEGF-C-positive cells also expressed equally VEGF-D (Figure 4) ▶ . These results indicated that the VEGF-C-positive cells are a subset of TAMs, comprising ∼25% of all CD68-positive cells in the peritumoral inflammatory infiltrate (Figure 1H) ▶ .
Figure 4.
Identification of VEGF-C- and VEGF-D-producing peritumoral cells as TAMs. Analysis was performed on paraffin sections of cervical carcinomas by double-labeling confocal laser-scanning microscopy. VEGF-C-expressing cells (green channel) are aligned in the left columns, and the counterlabeling antibodies are displayed in the red channel. This analysis indicates that VEGF-C-expressing cells are also labeled with antibodies specific for CD68, HLA-DR, partially CD16, CD23, CD45, and VEGF-D. Nuclei were counterstained with propidium iodide (blue channel). Original magnifications, ×2500.
VEGF-C-Expressing Macrophages Produce VEGFR-3
Double-immunofluorescence analysis revealed that all peritumoral VEGF-C- and VEGF-D-expressing cells were also labeled by anti-VEGFR-3 antibodies in a granular intracellular pattern (Figure 5) ▶ , but not at cell surface membranes, although VEGFR-3 is a tyrosine kinase receptor. 2 Stromal cells that failed to express VEGF-C and VEGF-D were also consistently devoid of VEGFR-3, and also the tumor cells failed to express the receptor (data not shown).
Figure 5.

Co-expression of VEGFR-3 and VEGF-C in peritumoral stroma and in CD14 affinity-purified circulating human monocytes. In the peritumoral tissue, both VEGF-C (green channel) and VEGFR-3 (red channel) are co-expressed simultaneously by the same TAMs, and co-localize within the cytoplasm in a granular compartment, presumably in endosomes or lysosomes. By contrast, round CD14 affinity-purified monocytes express VEGFR-3 on their surface membrane, and in a perinuclear compartment, presumably the endoplasmic reticulum, but they do not express VEGF-C. The number of these CD14+ cells that express VEGFR-3 was determined in four different patients by fluorescence-activated cell sorting, and amounted on average to ∼60%. Incubation of these cells with exogenous VEGF-D and TNF-α induced de novo synthesis of VEGF-C. While incubated with VEGF-D (1 μg/ml, 30 minutes, 37°C) they remained round, TNF-α (50 U/ml, 30 minutes, 37°C) induced cell flattening and endocytosis of receptor-ligand complexes, similar to TAMs observed in vivo. Original magnifications, ×2500.
VEGF-C Synthesis Is Induced in Vitro in VEGFR-3-Expressing Monocytes
CD14+ monocytes were affinity purified from the blood of four normal humans, immunolabeled with anti-VEGFR-3 antibodies, and analyzed by fluorescence-activated cell sorting or by morphometry on cytospin preparations. Consistently, a subfraction of 61.4 ± 8.9% of VEGFR-3+ cells was identified in prepurified CD14+ monocytes (Figure 5) ▶ . These cells expressed VEGFR-3 on their surface, and also in perinuclear location, presumably in the endoplasmic reticulum (Figure 5) ▶ , however, they failed to produce VEGF-C (Figures 5 and 6) ▶ ▶ . Incubation with TNF-α, LPS, and also with human recombinant VEGF-D induced VEGF-C production, and interiorization of VEGFR-3 into a granular intracellular compartment (Figures 5 and 6) ▶ ▶ , similar to VEGF-C-producing TAMs in tissue (Figure 5) ▶ . These immunohistochemical results were confirmed by RT-PCR (Figure 6) ▶ .
Figure 6.
A and B: Activation of monocytes with TNF-α and LPS to produce VEGF-C and VEGF-D mRNA, detected by RT-PCR. Monocytes were isolated from peripheral blood by CD14 affinity purification (A) or by elutriation (B) at 4°C to avoid activation. These cells express constitutively VEGFR-3 (787 bp), but not VEGF-C (567 bp) and VEGF-D (525 bp). Incubation with TNF-α (50 U/ml) or LPS (50 U/ml for 30 minutes) causes rapid initiation of VEGF-C and VEGF-D mRNA production. C: The macrophage-related tumor cell line U937 constitutively produces CD68 and CD23, and also VEGF-C and VEGFR-3 without stimulation.
The Cell Line U937 Expresses Intact VEGF-C, VEGF-D, and VEGFR-3
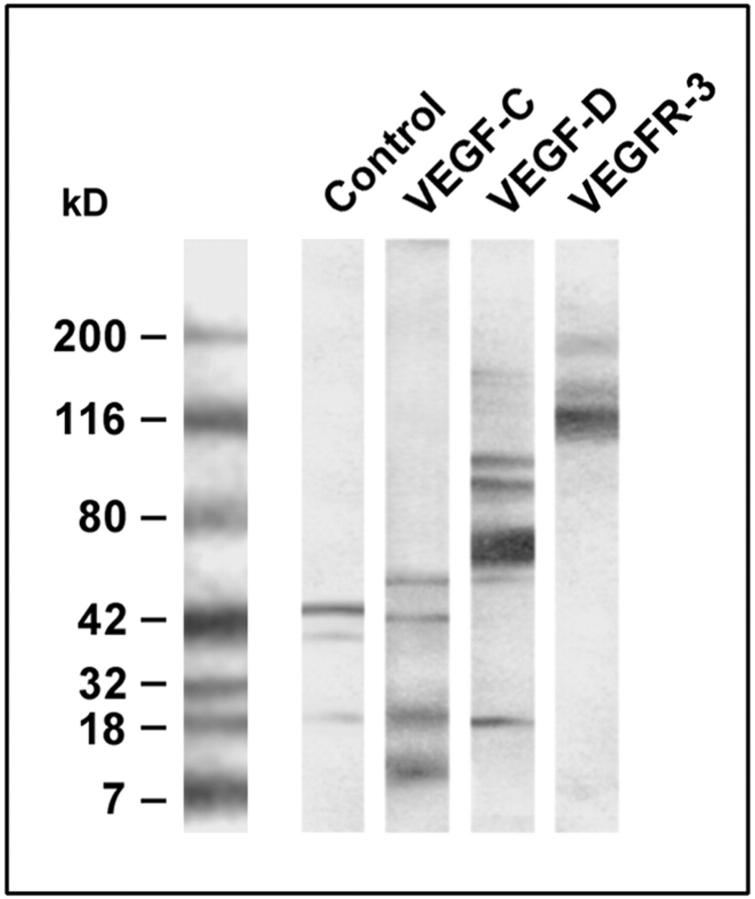
The macrophage-related tumor cell line U937 constitutively expressed VEGFR-3, as well as VEGF-C and VEGF-D (Figure 7) ▶ , suitable for identification of the respective proteins, whereas it was impossible to isolate phenotypically stable TAMs in sufficient numbers from the surgical specimens of cervical carcinomas. Western blotting revealed that U937 cells produced the receptor and both ligands in their respective active, mature forms, as well as in typical processed products (Figure 7) ▶ .
Figure 7.
Expression of VEGF-C, VEGF-D, and VEGFR-3 protein in a lysate of isolated, cultured lymphatic endothelial cells (control) and in the human macrophage-related cell line U937. Tissue and cells were lysed in sodium dodecyl sulfate buffer, the proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose, and immunoblotted with polyclonal antibodies specific for VEGF-C, VEGF-D, and VEGFR-3. VEGF-C is detected in the control and in U937 cells as an ∼21-kd protein, along with typical dimers and a degradation products. Also VEGF-D is encountered in U937 cells as an ∼21-kd band, in addition to oligomers. VEGFR-3 is expressed in U937 cells in its mature 195-kd form, and as a proteolytically processed 125-kd product.
Discussion
Tumor dissemination starts with the migration of tumor cells through lymphatic vessels to develop a metastasis in the regional sentinel lymph node. This places center stage the intra- and/or peritumoral lymphatic vessels, and the growth factors VEGF-C and VEGF-D that secure their survival, 20 and support their proliferation. 22 Their significance was recently highlighted by experiments in which rapid development of lymph node metastasis was achieved in immunodeficient mice that were implanted with transgenically VEGF-C-overexpressing tumors and that developed large peritumoral lymphatic vessels. 6,7,9,10 However, results obtained on experimental animals are limited by the fact that their tumor vasculature may differ unpredictably from that in humans, as demonstrated for example by the dual specificity of VEGF-D for both blood and lymphatic endothelial cells in mice, contrasted by restriction to lymphatics in humans. 24
To gain insight into the mechanisms of (peri)tumoral lymphangiogenesis in natural human tumors and to provide a basis for functional studies, descriptive analysis of histopathologically well-defined, preferentially early-stage tumors without necrosis and other complicating secondary changes are required. Human squamous epithelial carcinomas of the uterine cervix are advantageous because of their unique anatomical simplicity. They originate from stratified, nonkeratinized squamous epithelium that rests on a collagen-rich stroma with only few equidistant blood and lymphatic vessels, and sparse inflammatory cells. Neoplasia progresses via histopathologically exactly defined, preinvasive, intraepithelial precursors of low-grade (LSIL) and high-grade (HSIL) squamous intraepithelial lesion, to stroma invasive carcinomas. Invariably, tumors of all stages are demarcated by a mixed inflammatory stroma infiltrate. For this study we have selected 32 patients with early-stage cervical squamous cell carcinoma that was restricted to the cervix and did not exceed a diameter of 4 cm (stage pT1b1, UICC), with complete clinical follow-up.
Localization of lymphatic vessels, isolation of lymphatic endothelial cells, and unraveling of their function has become possible only recently, with the discovery of markers specific for the lymphatic endothelium. The current inventory comprises molecules of widely diverse functions, such as the VEGF-C- and VEGF-D-specific tyrosine kinase receptor VEGFR-3, 2-4 the CD44-related hyaluronan receptor LYVE-1, 12 the transcription factor Prox-1, 25 and the membrane mucoprotein podoplanin, 11 that contributes to cell adhesion (D. Kerjaschki, manuscript in preparation). Although all these molecules are useful tools for the localization of lymphatics in tissue sections, they also have their shortcomings; 26 for example, antibodies specific for VEGRF-3 also immunolabel blood microvessels in the vicinity of tumors, 5,10 LYVE-1 is expressed in liver sinusoidal cells, 27 and only in a subpopulation of isolated cultured lymphatic endothelial cells, 20 Prox-1 is found also in the nuclei of nonendothelial cells, 26,28 and podoplanin is located in neoplastic endothelial cells presumably derived from blood vessels, such as high-grade angiosarcomas and Kaposi sarcoma, 11 and in nonvascular cells, such as renal glomerular podocytes, 11,29 and myofibroblasts (D. Kerjaschki, unpublished observation). Thus, it was appropriately suggested that use of combinations of the markers at hand could compensate for their individual deficits and may yield more reliable immunohistochemical results. 26 The use of podoplanin as a lymphatic endothelial tag in this study was warranted because it was previously 1) excluded from dermal blood vessels that express the specific marker PAL-E; 30 2) co-expressed in most, but not all VEGFR-3+ vessels; 3) coincided with LYVE-1 labeling of peritumoral lymphatic cysternae in VEGF-C-overexpressing pancreatic β-cell carcinomas in Rip-TAG mice (D Kerjaschki and G Christofori G, unpublished observation); and 4) co-distributed with LYVE-1 in the present study. Thus, the combined use of podoplanin and LYVE-1 in this study validated the conclusion that double-positive vessels were considered as lymphatics.
Lymphatic vessels were absent from all cervical squamous cell carcinomas examined and were concentrated within the peritumoral stroma, whereas CD34+ blood microvessels were also encountered within tumors. These results are similar to previous findings in several other human tumors, such as melanomas, 9 colorectal and hepatocellular carcinoma, 27 and others. 15,31 However, they are in contrast to recent findings in squamous cell carcinomas of the head and neck region, in which intratumoral lymphatic vessels were observed by LYVE-1 labeling. 32 This discrepancy cannot be simply explained by the use of different immunohistochemical markers because LYVE-1 was also used in the present study. Presumably, squamous cell carcinomas of different anatomical origin also vary in their lymphangiogenic properties that may be also influenced by organ- and region-specific, as yet unidentified, factors. 26
It is of importance for potential future anti-metastatic therapies to know whether the density of lymphatic capillaries within and around tumors is increased by lymphangiogenesis, and supports tumor cell spreading. Direct evidence for neoangiogenesis was provided by immunostaining with the proliferation marker Ki67 so far only for squamous epithelial cell carcinomas of the head and neck region. 32 In cervical squamous carcinomas examined in this study the local density of peritumoral lymphatic vessels was significantly increased over normal tissues, both in intraepithelial noninvasive and invasive tumors. Because noninvasive intraepithelial tumors do not compress the underlying stroma, this provides indirect evidence that the focal peritumoral increase in lymphatic vessel density was because of neoangiogenesis, rather than passive aggregation of pre-existing vessels by pushing of the tumor, as found in intradermally inoculated experimental rat sarcoma. 10
In our series of cervical carcinomas the density of lymphatic vessels was directly related to peritumoral chronic inflammation. 17 A link to clinical relevance was established by the statistical association of increase in peritumoral lymph vessel density with the number of lymphatic vessels containing carcinoma cells (lymphangiosis carcinomatosa), that correlates with the occurrence of lymph node metastasis. 14 Lymphangiosis carcinomatosa and lymph node metastases are established prognostic factors in early-stage cervical cancer, indicating unfavorable outcome. 31
Human tumors of different organs were previously found to express VEGF-C that was related to the lymph node status and eventual clinical outcome of the patients. 32-36 These studies mainly used RT-PCR of tissue samples that is not suited to distinguish between sites of VEGF-C production by tumor and/or stroma cells. Intriguingly, immunohistochemical investigations have revealed that lymphatic microvessels were localized primarily within the peritumoral stroma. 9,14,15,17,23,27,31 This raised the question whether intratumoral lymphatics were either not present at all, or were actually induced by VEGF-C of tumor cells, but not detected with the currently available probes, for example because these lymphatic marker proteins could be down-regulated within tumors, but not in their surroundings. Alternatively, tumor cells could produce anti-lymphangiogenic factor(s) or degrade VEGF-C proteolytically to fragments that interact both with VEGFR-3 and VEGFR-2, and thus promote angiogenesis of blood vessels. 9,37 Eventually, VEGF-C and VEGF-D could be produced and released by as yet unidentified cells in the peritumoral stroma, and account for the peritumoral proliferation of lymphatic vessels. In this study we have localized VEGF-C and VEGF-D by antibodies specific for different epitopes and by in situ hybridization. Similar to previous studies, we have found VEGF-C expression within tumor cells in high-grade noninvasive and in invasive carcinomas, however, this failed to correlate statistically with any parameter related to peritumoral lymphangiogenesis. Labeling for VEGF-C and VEGF-D protein and mRNA was concentrated in mononuclear cells within the peritumoral inflammatory infiltrates. These cells were identified in situ as a subset of activated TAMs based on their expression of CD68, CD14, CD23, HLA-DR, and CD45, but not CD123, excluding plasmocytic dendritic cells. 38 They also failed to produce CD1a, CD2, CD3, CD8, CD19, CD20, CD34, CD56, CD45-RA, and tryptase, thus excluding endothelial cells, platelets that produce VEGF-C, 39 basophilic granulocytes, lymphocytes, and dendritic cells. All VEGF-C-producing TAMs co-expressed VEGF-D, indicating that both growth factors are synthesized in parallel. Approximately 25% of CD68-positive TAMs expressed VEGF-C, and their number correlated significantly with peritumoral inflammation and density of lymphatic microvessels, that in turn was linked to peritumoral lymphangiosis carcinomatosa.
All VEGF-C- and VEGF-D-producing TAMs co-expressed also the corresponding tyrosine kinase receptor VEGFR-3, whereas growth factor-negative TAMs were consistently also devoid of this receptor. VEGFR-3-expressing TAMs were recently also observed in human melanomas implanted into immunodeficient mice, and evidence was provided that VEGF-C serves as chemoattractant in vitro for mouse peritoneal macrophages, 9 however their role as source for VEGF-C and VEGF-D was not appreciated. Co-expression of VEGF-C, VEGF-D, and their receptor by the same cell provides the elements for an autocrine regulatory system, as recently also postulated for endothelial cells of human lymphangiomas. 40 Taken together, these results argue for a novel role of a subclass of TAMs as major producers of VEGF-C and VEGF-D within the peritumoral stroma and as a potential cause for peritumoral lymphatic neoangiogenesis.
Because TAMs are derived from circulating monocytes, we searched for a monocyte subpopulation that expressed VEGFR-3 on their surfaces, and could qualify as precursor for the VEGFR-3 and VEGF-C- and VEGF-D-expressing TAM population. By direct morphometry, as well as by fluorescence-activated cell sorting, 61.4 ± 8.9% of CD14-purified monocytes in the peripheral blood of normal humans were found to express VEGFR-3 in their cell membranes and in their perinuclear endoplasmic reticulum. However, they failed to produce VEGF-C, both by immunofluorescence and RT-PCR. Only after incubation in vitro with TNF-α, LPS, and also withrecombinant human VEGF-D. VEGF-C production and interiorization of VEGFR-3 into a granular intracellular compartment commenced, similar to TAMs in situ. These results point to a novel VEGFR-3-expressing subclass of monocytes that do not synthesize VEGF-C or VEGF-D, unless activated via different receptor-ligand systems, including the VEGFR-3 by exogenous VEGF-D. It is thus possible to induce in CD14-purified monocytes in vitro a phenotype similar to that of VEGF-C- and VEGF-D-producing TAMs in situ. The in situ location of TAMs close to the tumor surfaces could provide a milieu of various activating factors, including TNF-α, causing the induction of VEGF-C and VEGF-D synthesis in freshly immigrated monocytes. Previously, LPS was also found to promote VEGF expression in human monocytes/macrophages. 41
VEGF-C and VEGF-D, as well as VEGFR-3, are subject to alternative splicing and postranslational modifications that may also significantly alter their biological activities, 9 and this raises the question which type of VEGF-C and VEGF-D are produced and released by TAMs. Because phenotypically stable TAMs could not be purified in large enough quantities from the available surgical specimens of cervical carcinomas, we have examined as surrogate for TAMs the macrophage-related tumor cell line U937. 42 These cells produced constitutively VEGF-C, VEGF-D, and VEGFR-3 in amounts sufficient for analysis by Western blotting that revealed expression of both growth factors and their receptor in their respective active, mature forms, along with typical processed forms. This suggests that biologically active forms of VEGF-C and VEGF-D, as well as of VEGFR-3, are generated at least in this surrogate TAM cell line.
Collectively, the results of this investigation provide evidence for a hypothesis of peritumoral lymphatic neoangiogenesis that assigns a role to a novel subfraction of VEGFR-3-expressing monocytes. We assume that these cells are chemoattracted toward the tumor and are exposed to activators in the peritumoral stroma, such as TNF-α. Here they are converted to TAMs, and switch on de novo synthesis of VEGF-C and VEGF-D that cause proliferation of lymphatic microvessels to launch anti-tumoral immune responses by providing conduits for antigen-presenting cells toward secondary lymphatic organs. A potential role for VEGFR-3-positive dendritic cells derived from CD14+ monocytes 43 remains to be established. However, tumor cells may use the same routes for spreading and formation of lymph node metastasis. As preliminary studies on several other types of human carcinomas reveal similar scenarios (our unpublished observations), the connection between VEGF-C- and VEGF-D-producing TAMs and lymphatic spreading of tumors may be of more widespread significance.
Acknowledgments
We thank Dr. H. Budka, Institute of Neurology, University of Vienna, for the permission to use the confocal microscope; and Dr. David Jackson, John Radcliffe Hospital, University of Oxford, UK, for rabbit anti-LYVE-1 antibody.
Footnotes
Address reprint requests to Dontscho Kerjaschki, M.D. Department of Pathology, University of Vienna, AKH, Wahringer Gurtel 18-20, Vienna, Austria 1090. E-mail: dontscho.kerjaschki@akh-wien.ac.at.
Supported by the Fonds zur Förderung der Wissenschaftlichen Forschung (SFB 05, project 007 to D. K.).
S. F. S. and P. B. both contributed equally to this work.
References
- 1.Karpanen T, Alitalo K: Lymphatic vessels as targets of tumor therapy? J Exp Med 2001, 194:37-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K: Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 1995, 92:3566-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K: A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996, 15:290-298 [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada Y, Nezu J, Shimane M, Hirata Y: Molecular cloning of a novel vascular endothelial growth factor, VEGF-D. Genomics 1997, 42:483-488 [DOI] [PubMed] [Google Scholar]
- 5.Partanen TA, Alitalo K, Miettinen M: Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer 1999, 86:2406-2412 [PubMed] [Google Scholar]
- 6.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M: Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001, 7:192-198 [DOI] [PubMed] [Google Scholar]
- 7.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K: Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 2001, 61:1786-1790 [PubMed] [Google Scholar]
- 8.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS: Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 2001, 20:672-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M: Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol 2001, 159:893-903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK: Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res 2000, 60:4324-4327 [PubMed] [Google Scholar]
- 11.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D: Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999, 154:385-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG: LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 1999, 144:789-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmeliet P, Jain RK: Angiogenesis in cancer and other diseases. Nature 2000, 407:249-257 [DOI] [PubMed] [Google Scholar]
- 14.Birner P, Schindl M, Obermair A, Breitenecker G, Kowalski H, Oberhuber G: Lymphatic microvessel density as a novel prognostic factor in early-stage invasive cervical cancer. Int J Cancer 2001, 95:29-33 [DOI] [PubMed] [Google Scholar]
- 15.Schoppmann SF, Birner P, Studer P, Breiteneder-Geleff S: Lymphatic microvessel density and lymphovascular invasion assessed by anti-podoplanin immunostaining in human breast cancer. Anticancer Res 2001, 21:2351-2355 [PubMed] [Google Scholar]
- 16.Weidner N: Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 1995, 36:169-180 [DOI] [PubMed] [Google Scholar]
- 17.Schoppmann SF, Schindl M, Breiteneder-Geleff S, Soleiman A, Breite-necker G, Karner B, Birner P: Inflammatory stroma reaction correlates with lymphatic microvessel density in early-stage cervical cancer. Anticancer Res 2001, 21:3419-3423 [PubMed] [Google Scholar]
- 18.Breitschopf H, Suchanek G, Gould RM, Colman DR, Lassmann H: In situ hybridization with digoxigenin-labeled probes: sensitive and reliable detection method applied to myelinating rat brain. Acta Neuropathol 1992, 84:581-587 [DOI] [PubMed] [Google Scholar]
- 19.Pickl WF, Majdic O, Kohl P, Stockl J, Riedl E, Scheinecker C, Bello-Fernandez C, Knapp W: Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol 1996, 157:3850-3859 [PubMed] [Google Scholar]
- 20.Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K: Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J 2001, 20:4762-4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kriehuber E, Breiteneder-Geleff S, Groeger M, Soleiman A, Schoppmann SF, Stingl G, Kerjaschki D, Maurer D: Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med 2001, 194:797-808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K: Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997, 276:1423-1425 [DOI] [PubMed] [Google Scholar]
- 23.Schoppmann SF, Horvat R, Birner P: Lymphatic vessels and lymphangiogenesis in female cancer: mechanisms, clinical impact and possible implications for anti-lymphangiogenic therapies. Oncol Rep 2001, 9:455-466 [PubMed] [Google Scholar]
- 24.Baldwin ME, Catimel B, Nice EC, Roufail S, Hall NE, Stenvers KL, Karkkainen MJ, Alitalo K, Stacker SA, Achen MG: The specificity of receptor binding by vascular endothelial growth factor-d is different in mouse and man. J Biol Chem 2001, 276:19166-19171 [DOI] [PubMed] [Google Scholar]
- 25.Wigle JT, Oliver G: Prox1 function is required for the development of the murine lymphatic system. Cell 1999, 98:769-778 [DOI] [PubMed] [Google Scholar]
- 26.Jain RK, Fenton BT: Intratumoral lymphatic vessels: a case of mistaken identity or malfuntion. J Natl Cancer Inst 2002, 94:417-421 [DOI] [PubMed] [Google Scholar]
- 27.Carreira CM, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK: LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res 2001, 61:8079-8084 [PubMed] [Google Scholar]
- 28.Papoutsi M, Siemeister G, Weindel K, Tomarev SI, Kurz H, Schachtele C, Martiny-Baron G, Christ B, Marme D, Wilting J: Active interaction of human A375 melanoma cells with the lymphatics in vivo. Histochem Cell Biol 2000, 144:373-385 [DOI] [PubMed] [Google Scholar]
- 29.Matsui K, Breiteneder-Geleff S, Kerjaschki D: Epitope-specific antibodies to the 43-kD glomerular membrane protein podoplanin cause proteinuria and rapid flattening of podocytes. J Am Soc Nephrol 1998, 9:2013-2026 [DOI] [PubMed] [Google Scholar]
- 30.Schlingemann RO, Dingjan GM, Emeis JJ, Blok J, Warnaar SO, Ruiter DJ: Monoclonal antibody PAL-E specific for endothelium. Lab Invest 1985, 52:71-76 [PubMed] [Google Scholar]
- 31.Birner P, Obermair A, Schindl M, Kowalski H, Breitenecker G, Oberhuber G: Selective immunohistochemical staining of blood and lymphatic vessels reveals independent prognostic influence of blood and lymphatic vessel invasion in early-stage cervical cancer. Clin Cancer Res 2001, 7:93-97 [PubMed] [Google Scholar]
- 32.Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG: Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 2002, 62:1315-1320 [PubMed] [Google Scholar]
- 33.Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP: High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res 2000, 6:1900-1908 [PubMed] [Google Scholar]
- 34.Bunone G, Vigneri P, Mariani L, Buto S, Collini P, Pilotti S, Pierotti MA, Bongarzone I: Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol 1999, 155:1967-1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akagi K, Ikeda Y, Miyazaki M, Abe T, Kinoshita J, Maehara Y, Sugimachi K: Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer 2000, 83:887-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsurusaki T, Kanda S, Sakai H, Kanetake H, Saito Y, Alitalo K, Koji T: Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br J Cancer 1999, 80:309-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K: Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 1997, 16:3898-3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moretti S, Lanza F, Dabusti M, Tieghi A, Campioni D, Dominici M, Castoldi GL: CD123 (interleukin 3 receptor alpha chain). J Biol Regul Homeost Agents 2001, 15:98-100 [PubMed] [Google Scholar]
- 39.Wartiovaara U, Salven P, Mikkola H, Lassila R, Kaukonen J, Joukov V, Orpana A, Ristimaki A, Heikinheimo M, Joensuu H, Alitalo K, Palotie A: Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost 1998, 80:171-175 [PubMed] [Google Scholar]
- 40.Huang HY, Ho CC, Huang PH, Hsu SM: Co-Expression of VEGF-C and its receptors, VEGFR-2 and VEGFR-3, in endothelial cells of lymphangioma implication in autocrine or paracrine regulation of lymphangioma. Lab Invest 2001, 81:1729-1734 [DOI] [PubMed] [Google Scholar]
- 41.Itaya H, Imaizumi T, Yoshida H, Koyama M, Suzuki S, Satoh K: Expression of vascular endothelial growth factor in human monocyte/macrophages stimulated with lipopolysaccharide. Thromb Haemost 2001, 85:171-176 [PubMed] [Google Scholar]
- 42.Koren HS, Anderson SJ, Larrick JW: In vitro activation of a human macrophage-like cell line. Nature 1979, 279:328-331 [DOI] [PubMed] [Google Scholar]
- 43.Fernandez Pujol B, Lucibello FC, Zuzarte M, Lutjens P, Muller R, Havemann K: Dendritic cells derived from peripheral monocytes express endothelial markers and in the presence of angiogenic growth factors differentiate into endothelial-like cells. Eur J Cell Biol 2001, 80:99-110 [DOI] [PubMed] [Google Scholar]