Relevance of Nuclear and Cytoplasmic von Hippel Lindau Protein Expression for Renal Carcinoma Progression (original) (raw)
Abstract
Alterations of the von Hippel-Lindau tumor-suppressor gene (VHL) on 3p25-p26 are frequent in clear-cell renal-cell carcinoma (RCC). The VHL protein (pVHL) is implicated in cell-cycle control and gene regulation, and requires transcription-dependent nuclear-cytoplasmic trafficking for its function. There are two biologically active VHL protein isoforms: pVHL30 and pVHL19. To study prevalence, subcellular expression and biological significance of pVHL in renal tumors, tissue microarrays with renal-cell carcinomas were immunohistochemically examined for pVHL expression. Antibodies against both protein isoforms (anti-pVHL30/pVHL19) and against pVHL30 (anti-pVHL30; Ig33) were used. The anti-pVHL30/pVHL19 antibody showed nuclear and cytoplasmic pVHL expression, whereas the anti-pVHL30 antibody (Ig33) detected cytoplasmic pVHL expression, suggesting that the distribution of VHL protein isoforms varies in the nuclear and cytoplasmic compartments of renal tumors. There were 175 of 398 primary clear-cell RCCs (44%) with both nuclear and cytoplasmic pVHL expression. Seventy-seven clear-cell RCCs (19%) showed only nuclear, 22 (6%) showed only cytoplasmic, and 124 tumors (31%) showed no pVHL expression. Notably, combined nuclear and cytoplasmic pVHL expression was associated with low histological grade (P < 0.0001), early tumor stage (P < 0.01), and better prognosis (P < 0.01). These results imply that alteration of subcellular pVHL trafficking is of potential relevance for the biological behavior of clear-cell RCC.
Mutations and allelic deletion of the von Hippel-Lindau tumor-suppressor gene (VHL) on chromosome 3p25-p26 are frequent in clear-cell renal-cell carcinoma (RCC), 1,2 whereas VHL gene alterations are rare in non-clear-cell renal tumors, eg, papillary or chromophobe RCC and oncocytomas. VHL inactivation appears to be an important event in clear-cell RCC initiation, but there is also evidence that VHL is involved in clear-cell RCC progression. 3,4
The VHL protein (pVHL) is indirectly involved in different cellular processes including cell-cycle control, 5 Hy-poxia Inducible Factor (HIF) target gene regulation for oxygen-dependent proteolysis, 6,7 extracellular matrix formation, 8 and regulation of microtubule stability. 9 pVHL is a component of an E3 ubiquitin-protein ligase 10 that regulates the stability of HIF transcription factor. 11,12 pVHL targets proteins for ubiquitin-mediated degradation. 10 Binding to fibronectin contributes to the ability of VHL protein to suppress tumor growth. 8
Two biologically active VHL gene products with molecular masses of 30 kd (pVHL30) and 19 kd (pVHL19) have been described. 13 The shorter variant of pVHL protein, pVHL19, appears to arise as a result of alternative translation initiation.
Few studies have determined pVHL expression in normal and neoplastic human tissue. 14-17 A comprehensive expression analysis of pVHL in RCC has not yet been performed. To study prevalence and prognostic relevance of pVHL expression in a large number of various tumors, we used our recently developed tissue microarray (TMA) technology. 18 The TMA technology allows examination of the immunohistochemical profile of hundreds of individual tumors at a time. 19-21 A recently developed polyclonal antibody raised against both pVHL isoforms (pVHL19 and pVHL30) 9 and a commercially available monoclonal antibody (Ig33) against the pVHL30 protein was used in this study. Loss of heterozygosity (LOH) at the VHL locus was also assessed to analyze the association between pVHL expression and allelic deletion.
Materials and Methods
Renal-Tumor TMAs
Renal-tumor TMAs were used for immunohistochemical analysis of pVHL expression. The construction of a renal-tumor TMA has been recently described. 21 The TMA contained 404 clear-cell RCC, 64 papillary RCC, 25 chromophobe RCC, and 17 oncocytomas. All tumors were reviewed by one pathologist (H.M.). Histological grading and tumor staging were done according to Thoenes 22 and the International Union against Cancer (UICC). 23 Tumor-specific survival data were obtained by reviewing the hospital records, by direct communication with the attending physicians, and from the Cancer Registry of Basel. To minimize false-negative immunohistochemical staining results due to tumor heterogeneity, four copies of a renal cancer TMA were constructed. Tissue cylinders for the replicate TMAs were taken from carefully selected morphologically representative regions of different tumor areas.
A second renal-cancer TMA was constructed to determine the relevance of candidate marker proteins for the metastatic process. This array contained 166 tissue cylinders; there were 50 primary tumors with 60 corresponding metastases of different sites. Five of the primary tumors were papillary RCC, 2 primary tumors were unclassified, and 43 were clear-cell RCC. In addition, tissues of 56 metastases from clear-cell RCC without available tissue of the primary tumors were included in this TMA.
Immunohistochemistry
Two antibodies were used for the detection of pVHL: a recently generated polyclonal rabbit anti-pVHL antibody 10 and a commercially available mouse monoclonal antibody (Ig33; Neomarkers, Fremont, CA). Our affinity-purified polyclonal rabbit anti-pVHL recognizes both the long (pVHL30) and the short forms (pVHL19) of pVHL. 9 Specificity of the antibody for both pVHL isoforms was demonstrated in human U2-OS cells by Western blotting. 9 The mouse monoclonal antibody Ig33 (Neomarkers, Fremont, CA) was raised against amino acids 1–54 and recognizes exclusively human pVHL30. Standard indirect immunoperoxidase procedures were used for immunohistochemistry (VECTASTAIN Elite ABC kit; Vector Laboratories, Burlingame, CA). The optimal titer for pVHL expression was defined as the dilution that gave clearly identifiable cytoplasmic and/or nuclear staining and negligible background on conventional histological samples of normal renal tissue and breast cancer tissue. Breast cancer was used as positive control in accordance with previous reports, showing frequent pVHL expression in breast cancer tissue. 17 Primary antibodies were omitted in negative controls. After determination of the optimal antibody dilution on large tissue sections, the immunohistochemical procedure was adopted on renal TMA sections. The mouse monoclonal antibody Ig33, which we termed anti-pVHL30, was used at a dilution of 1:100 after microwave pretreatment. The polyclonal anti-pVHL30/pVHL19 antibody was used at a dilution of 1:200 after heat pretreatment (steam).
Determination of pVHL expression of renal tumors was done on all four replicates of the renal cancer TMA and on the renal cancer metastases TMA. Five-μm-thick sections were cut from the TMAs. Each TMA contained normal renal tissue as positive internal control. Tumors were considered pVHL-positive if cytoplasmic and/or nuclear expression was found in at least one of the four tumor samples. Nuclear and cytoplasmic pVHL expression were independently assessed.
Loss of Heterozygosity (LOH)
A subset of 113 consecutive formalin-fixed, paraffin-embedded clear-cell RCC was selected for VHL locus-specific LOH analysis to compare pVHL expression with VHL deletion. Clinicopathological data of this tumor set have been previously published. 24 Tumor tissue was defined on the basis of hematoxylin and eosin (H&E)-stained sections to ensure a minimum of 75% tumor cells in the samples. Deparaffinizing of the tissues, DNA extraction, and polymerase chain reaction (PCR) were performed according to protocols of Qiagen (Basel, Switzerland). For LOH analysis, genomic DNA of matched normal/tumor samples were examined at D3S1597 and D3S1307 (3p25-p26). The microsatellite primer sequences were obtained directly from the Genome DataBase. PCR was performed as described. 25 Forward primers were labeled with IRD-800 (MWG-Biotech, Ebersberg, Germany). Microsatellites were analyzed on a LICOR DNA Sequencer. Allelic loss was defined if one allele was more than 40% reduced in the tumor DNA than in the corresponding normal DNA.
Statistics
Contingency table analysis was used to analyze the association between pVHL expression and tumor grade, stage, and allelic loss. Survival analyses were performed using the Kaplan-Meier method. Statistical differences between groups were determined with log-rank test. A Cox proportional hazard analysis was used to test for independent prognostic information.
Results
pVHL Expression in Primary Tumors
Staining of large normal renal sections with the anti-pVHL30/pVHL19 antibody and the anti-pVHL30 antibody (Ig33) showed moderate to strong cytoplasmic pVHL expression on renal tubular epithelial cells. In addition to the cytoplasmic staining, the anti-pVHL30/pVHL19 antibody revealed nuclear staining in normal renal epithelial cells. A nuclear pVHL expression was not detected with the anti-pVHL30 antibody, even if different pretreatment conditions (microwave, steam, protease) were tried.
Renal tumors were considered positive in the cytoplasm if moderate or strong staining was seen. Some clear-cell RCCs showed membranous accentuation, which was also considered as cytoplasmic positivity. Since a very weak nuclear staining of single tumor cells was seen in some renal tumors with the anti-pVHL30/pVHL19 antibody, the definition applied for nuclear positivity was crucial. A conservative cut-off of 20% tumor cells with nuclear pVHL expression was used to define nuclear positivity to avoid false-positive results. Examples of pVHL expression patterns in different renal-tumor subtypes are illustrated in Figure 1 ▶ .
Figure 1.
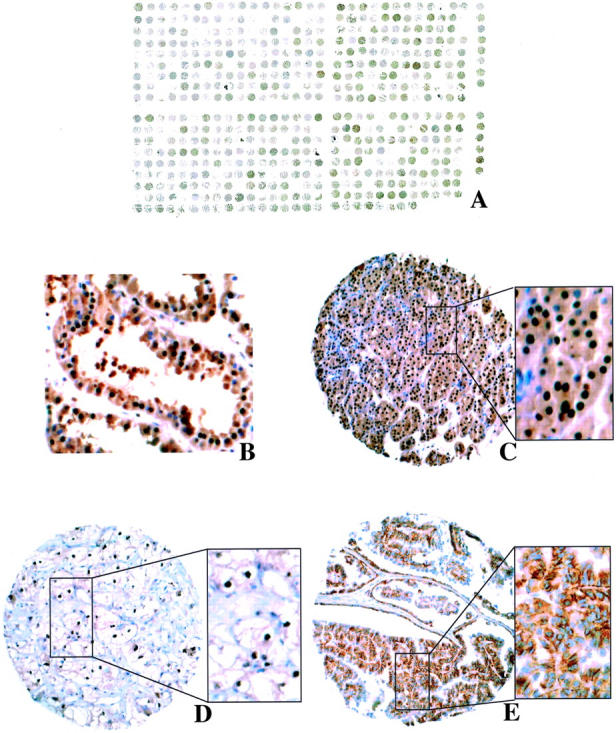
A: Renal-tumor TMA after immunostaining with anti-pVHL30/pVHL19-antibody. B to E: Examples of pVHL expression on a renal-tumor TMA. B: Nuclear and cytoplasmic pVHL in normal renal tubules. C: Nuclear and cytoplasmic pVHL in chromophobe RCC. D: Nuclear pVHL in clear-cell RCC. E: Cytoplasmic pVHL in papillary RCC.
Using the anti-pVHL30 antibody (Ig33) cytoplasmic pVHL expression was more frequently detected in papillary (88%) and chromophobe RCC (96%) than in clear-cell RCC (72%), although this trend did not reach statistical significance. Oncocytomas displayed cytoplasmic pVHL expression in 76%. There was no association between pVHL30 expression and histological grade and tumor stage (data not shown).
The anti-pVHL30/pVHL19 antibody detected cytoplasmic, nuclear, or combined nuclear and cytoplasmic pVHL expression in 69% clear-cell, 68% papillary, and 76% chromophobe RCCs and in 94% of oncocytomas (Table 1) ▶ . Cytoplasmic pVHL expression without nuclear expression was rare (Table 1) ▶ . The majority of pVHL-positive renal-cell carcinomas showed combined nuclear and cytoplasmic pVHL expression. In clear-cell RCC nuclear and cytoplasmic pVHL expression was detected in 48% of pT1/2 clear-cell RCC and in 41% pT3 tumors (P < 0.05; Table 2 ▶ ). There was pVHL30/pVHL19 expression in 67% grade 1, 49% grade 2, but only in 26% grade 3 clear-cell RCC (P < 0.0001; Table 2 ▶ ). Notably, exclusive nuclear pVHL expression was more frequent in chromophobe RCC (64%) and oncocytomas (53%) than in clear-cell RCC (19%) and papillary RCC (24%) (P < 0.001).
Table 1.
pVHL Expression in Renal Tumors Analyzed by the Anti-pVHL30/pVHL19 Antibody
| Tumor type (n) | pVHL expression (% positive tumors) | |||
|---|---|---|---|---|
| Negative (%) | Cytoplasmic (%) | Nuclear (%) | Nuclear/cytoplasmic (%) | |
| Clear-cell RCC (398) | 31 | 6 | 19 | 44 |
| Papillary RCC (62) | 32 | 0 | 24 | 44 |
| Chromophobe RCC (25) | 24 | 0 | 64 | 12 |
| Oncocytoma (17) | 6 | 0 | 53 | 41 |
Table 2.
pVHL30/pVHL19 Expression, Tumor Stage and Grade in Clear-Cell RCC
| Number of tumors (n) | pVHL expression (% positive tumors) | P value | |||
|---|---|---|---|---|---|
| Negative (%) | Nuclear (%) | Cytoplasmic (%) | Nuclear/cytoplasmic (%) | ||
| pT1/T2 (173) | 24 | 23 | 5 | 48 | |
| pT3 (225) | 36 | 16 | 6 | 41 | <0.05 |
| Grade 1 (46) | 13 | 17 | 2 | 67 | |
| Grade 2 (225) | 24 | 21 | 5 | 49 | |
| Grade 3 (127) | 50 | 17 | 8 | 26 | <0.0001 |
Clinical Outcome and pVHL Expression
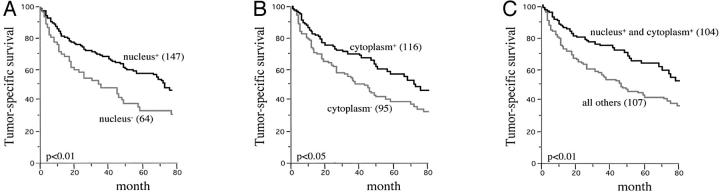
Clinical follow-up data were available from 211 clear-cell RCC included in the TMA. There was no association between pVHL30 expression and patient prognosis (data not shown). In contrast, the subcellular pVHL30/pVHL19 expression was related to prognosis in clear-cell RCC patients. When nuclear and cytoplasmic pVHL expression were separately analyzed (Figure 2, A to B) ▶ , a significantly worse prognosis was observed for patients with loss of pVHL expression in both subcellular compartments (P < 0.05). Combined nuclear and cytoplasmic pVHL expression was associated with good patient outcome (P < 0.01) (Figure 2C) ▶ . The Cox proportional hazard analysis with the variables tumor stage, histological grade, and pVHL expression showed that combined nuclear and cytoplasmic pVHL expression may be an independent prognostic parameter (P < 0.05) (Table 3) ▶ .
Figure 2.
Nuclear and cytoplasmic pVHL expression and tumor-specific survival in clear-cell RCC. A: All clear-cell RCC with and without nuclear staining. B: All clear-cell RCC with and without cytoplasmic staining. C: All clear-cell RCC with and without combined nuclear and cytoplasmic pVHL expression and its association with tumor-specific survival in clear-cell RCC.
Table 3.
Univariate and Multivariate Analysis of pVHL Expression, Histological Grade, and pT Stage
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Risk ratio | 95% CI | P value | Risk ratio | 95% CI | P value | |
| pVHL expression (nuclear and cytoplasm vs others) | 1.75 | 1.2–2.54 | 0.004 | 1.49 | 1.02–2.19 | 0.04 |
| Grade (G1/G2 vs G3) | 0.48 | 0.33–0.69 | <0.0001 | 0.57 | 0.39–0.83 | 0.003 |
| Stage (pT1/pT2 versus pT3) | 0.47 | 0.32–0.69 | 0.0001 | 0.52 | 0.35–0.78 | 0.0015 |
pVHL Expression in Metastases
pVHL expression could be evaluated in tissue cylinders of 102 metastases from clear-cell RCC. The prevalence of pVHL expression in metastases was similar to primary clear-cell RCC. There were 28 (27%) metastases with nuclear, 17 (17%) with cytoplasmic and 42 (41%) with combined nuclear and cytoplasmic pVHL expression. To further investigate pVHL expression during metastatic progression of RCC, nuclear pVHL was compared between primary clear-cell RCC and their corresponding metastases; this was possible in 39 tumor pairs. A different distribution of nuclear pVHL expression in primary tumors and their metastases was found in 20 pairs (51%). Nuclear pVHL expression in metastatic tissue but not in the corresponding primary tumors was found in 11 pairs. There were 8 tumors with nuclear pVHL expression in the primary but not in the corresponding metastasis.
Allelic Deletion and pVHL Expression
The results obtained from two microsatellite loci D3S1597 and D3S1307 were combined because both microsatellites encompass the VHL locus (NCBI, Human Genome Resources). LOH at 3p25-p26 was detected in 48 of 95 informative clear-cell RCC (51%). Representative examples of allele losses at the VHL locus are depicted in Figure 3 ▶ . There was no correlation between tumor stage and histological grade with 3p25-p26 deletions (data not shown). VHL loss was not associated with pVHL30/pVHL19 expression or pVHL30 expression, even if nuclear and cytoplasmic pVHL expression were separately analyzed. Notably, pVHL expression was frequently observed in clear-cell RCC with VHL loss. Nuclear pVHL expression was seen in 8 (19%), cytoplasmic in 2 (5%), and combined expression in 22 (52%) of 42 clear-cell RCC with LOH at the VHL locus.
Figure 3.
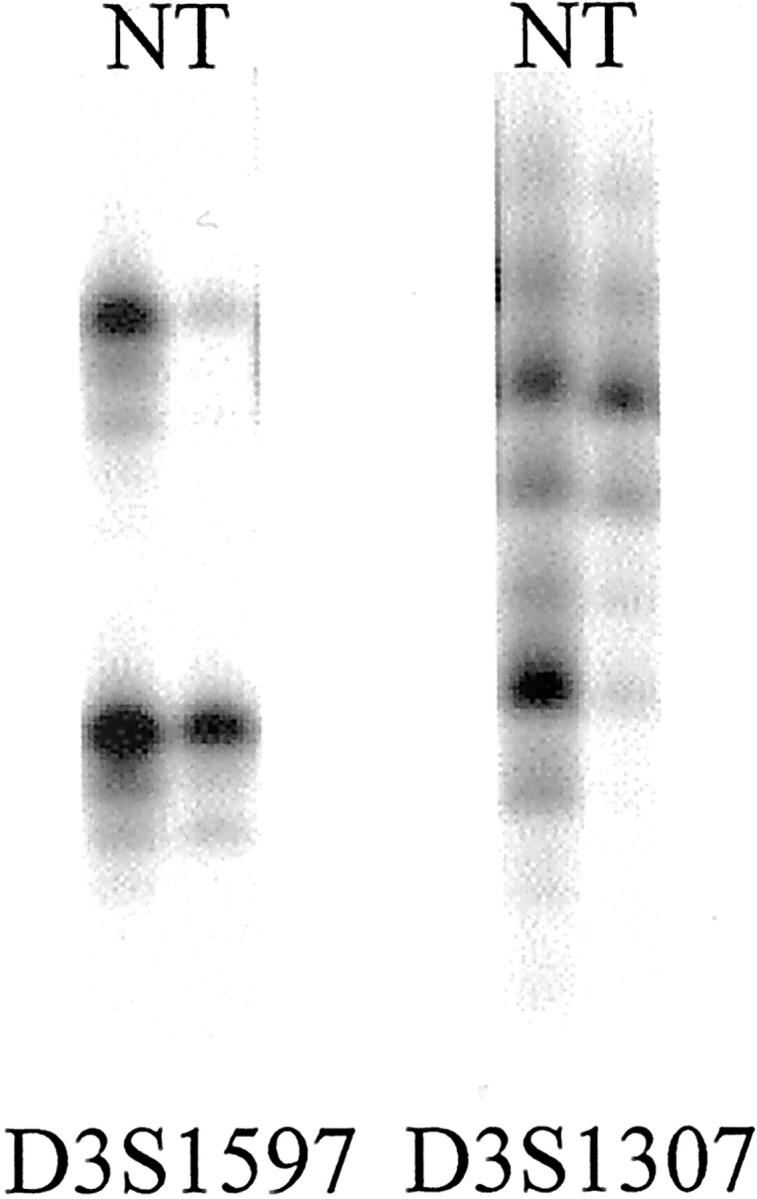
LOH at microsatellite loci D3S1597 and D3S1307 on chromosome 3p25-p26 in two clear-cell RCCs.
Discussion
Our data give a detailed description of the pVHL expression in renal tumors and provide evidence that nuclear-cytoplasmic pVHL trafficking is of potential relevance for the biological behavior of clear-cell RCC.
There are few reports with pVHL expression analysis in human tissues. 14-17 Corless et al 17 detected strong cytoplasmic staining for VHL protein in lung, prostate, colon, breast, bladder, and thyroid carcinoma and in 4 of 5 sporadic clear-cell RCC. Immunohistochemically, pVHL was expressed in about 70% of the RCCs on our renal-tumor TMA, which is in agreement with a recent observation of frequent VHL mRNA expression in clear-cell RCC. 26
Importantly, all recent studies of human tissues have exclusively described cytoplasmic pVHL expression. Only some immunofluorescence analyses have shown that pVHL can be expressed, to a lesser extent, in the nucleus or in association with cell membranes. 8,13,15,27 Most of the previously used pVHL antibodies are directed against full-length pVHL30. However, it is known that the cell-line 293 cells produce a shorter variant of the pVHL protein, pVHL19. 13 pVHL19 appears to arise as a result of alternative translation initiation. In this study, we used our recently developed polyclonal pVHL antibody, which is also directed against pVHL19. 9 In contrast to the monoclonal anti-pVHL30 antibody (Ig33), which detected only cytoplasmic pVHL expression, the polyclonal anti-pVHL30/pVHL19 antibody showed nuclear and cytoplasmic pVHL expression in many renal tumors. This finding suggests that pVHL30 is primarily localized in the cytoplasm whereas the shorter variant pVHL19 is mainly expressed in the nucleus. This is consistent with cell fractionation experiments and our recent study showing that pVHL19 is primarily detected in the cell nucleus. 9,13
It is of note that there was heterogeneity in the expression of the two pVHL isoforms in RCC. For example, 92 clear-cell RCC showed cytoplasmic pVHL30 expression detected by Ig33 but were negative when analyzed with the polyclonal anti-pVHL30/pVHL19 antibody. In contrast, 36 tumors that were Ig33-negative showed nuclear and cytoplasmic staining with the polyclonal antibody. This observation may be explained by the different epitopes recognized by the two antibodies. Polyclonal anti-pVHL30/pVHL19 antibody is directed against the last 16 amino acids of pVHL, whereas Ig33 recognizes the first 54 amino acids of pVHL, which are lacking in pVHL19. As a consequence, RCCs with VHL mutations leading to truncated pVHL protein forms cannot be recognized by this antibody. On the other hand, there may also be tumors that exclusively express pVHL19 both in the nucleus and in the cytoplasm.
The overall goal of this study was to determine the associations of pVHL expression with pathological and prognostic parameters. High-density tissue microarrays are extremely useful for profiling protein expression in large numbers of samples (“protein profiling”) but their use for clinical biomarker studies may be limited in heterogeneous tumors like RCC because of inadequate tissue sampling. Recent studies have demonstrated the value of TMAs to discover and then validate candidate biomarkers. Studies comparing whole section and tissue microarray results have already shown a high concordance between TMA and large section data if multiple tissue cores were used. According to these studies, 3 to 4 TMA cores are optimal for evaluating biomarker expression on TMAs. 20,28,29
Using four replicate arrays of our renal cancer TMA, we have shown an association of pVHL expression with tumor stage, histological grade, and patient outcome. Functional inactivation of pVHL may contribute to metastasis and poor prognosis in RCC because pVHL plays an important role in different cellular processes including cell-cycle control, 5 regulation of gene transcription 11 and microtubule stability, 9 and extracellular matrix formation. 8,30 Interestingly, especially loss of nuclear pVHL expression was related to poor prognosis in clear-cell RCC, suggesting that the transcription-dependent subcellular pVHL trafficking is of potential relevance for the biological behavior. pVHL solely expressed in the nucleus was frequently seen in chromophobe RCC and oncocytomas. As oncocytomas are benign tumors and chromophobe RCC are associated with a better prognosis than clear-cell and papillary RCC, 31 it is tempting to speculate that nuclear pVHL expression generally correlates with less aggressive behavior. This hypothesis is supported by in vitro observations that pVHL is accumulated in the nucleus during G1/G0-phase, 15 in response to inhibition of RNA polymerase II-mediated transcription, 27 and in sparsely growing cells. 32
Nuclear-cytoplasmic trafficking seems to be important for the physiological function of pVHL. 15,27,32 The nuclear-cytoplasmic trafficking is transcription- and cell-cycle-dependent. Lee et al 27 showed that specific deletions affecting exon 2 abrogate transcription-dependent trafficking between nucleus and cytoplasm. Recently, we performed a VHL mutation analysis of 113 clear-cell RCCs. 4 Forty-eight different VHL sequence alterations were found, but only 6 mutations affected exon 2. Nineteen VHL mutations were nonsense or frameshift mutations, predicted to change the open reading frame of VHL and we could show that only these “loss-of-function” mutations were associated with poor patient prognosis. Theoretically, such mutations might also have influence on the nuclear-cytoplasmic trafficking of pVHL.
The prevalence of nuclear and cytoplasmic VHL expression in our renal-cancer metastasis TMA was similar to that observed in primary tumors. However, there were tumor pairs with nuclear pVHL expression in metastatic tissue but not in the corresponding primary tumors and vice versa. Although this result is probably due to intratumoral heterogeneity, the findings are consistent with previously reported Comparative Genomic Hybridization, LOH, and FISH analyses observing cases with allelic deletions on 3p in primary tumors but not in the corresponding metastases. 33-35
Several mechanisms lead to loss of pVHL function in the majority of clear-cell RCC, including deletion, mutation, and hypermethylation of VHL. It is likely that the anti-pVHL30/pVHL19 antibody used in this study may also bind to mutant and truncated forms of pVHL. Therefore, no mechanistic conclusions can be drawn from the observed immunohistochemical stainings regarding the mechanism of pVHL inactivation. Nevertheless, pVHL expression was completely absent in a subset of papillary and chromophobe RCC. Although chromosome 3p deletions have been described in both chromophobe and papillary RCC, 36,37 this finding was not suspected, because VHL mutations and/or deletions are rare in these RCC subtypes. 3,36,38-42 Our result of absent pVHL expression in papillary and chromophobe RCCs suggests that VHL down-regulation can occur without VHL deletions and/or mutations. Hypermethylation of a normally unmethylated CpG island in the promoter may provide an additionally important mechanism for inactivation of the VHL gene in these renal tumor subtypes. 43 However, Brauch et al 3 found no VHL hypermethylation in 17 chromophobe RCC analyzed. As the VHL promoter contains putative transcription-factor binding sites for SP1, nuclear respiratory factor 1, and PAX, 44,45 down-regulation of transcription factors or specific mutations abrogating these binding sites might be other explanations for the observed absence of pVHL in chromophobe and papillary RCC.
In summary, our data suggest that the distribution of VHL protein isoforms varies in the nuclear and cytoplasmic compartments of renal tumors and that nuclear-cytoplasmic pVHL trafficking is important for prognosis in RCC. Clarification of the complex pVHL pathways is a major challenge for the understanding of the biological behavior of renal tumors and cancer progression.
Acknowledgments
We thank R. Epper, S. Grieshaber, Y. Knecht, M. Mirlacher, and the technical staff of the Institute for Pathology Basel for excellent technical support.
Footnotes
Address reprint requests to Holger Moch, M.D., Institute of Pathology, University of Basel, Schönbeinstrasse 40, CH-4031 Basel, Switzerland. E-mail: hmoch@uhbs.ch.
Supported by Swiss National Science Foundation (31–63923.00).
References
- 1.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L, Schmidt L, Zhou F, Li H, Wei MH, Chen F, Glenn G, Choyke P, Walther MM, Weng Y, Duan D-SR, Dean M, Glavac D, Richards F, Crossey P, Ferguson-Smith MA, LePaslier D, Chumakov I, Cohen D, Chinault AC, Maher ER, Lineham WM, Zbar B, Lerman MI: Identification of the von Hippel-Lindau disease tumor-suppressor gene Science 1993, 260:1317-1320 [DOI] [PubMed] [Google Scholar]
- 2.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, Lubensky J, Duan DR, Florence C, Potatti R, Walther MM, Bander NH, Grossman HB, Brach H, Pomer S, Brooks JD, Issacs WB, Lerman MI, Zber B, Lineham WM: Mutations of the VHL tumour suppressor gene in renal carcinoma Nat Genet 1994, 7:85-90 [DOI] [PubMed] [Google Scholar]
- 3.Brauch H, Weirich G, Brieger J, Glavac D, Rodl H, Eichinger M, Feurer M, Weidt E, Puranakanitstha C, Neuhaus C, Pomer S, Brenner W, Schirmacher P, Storkel S, Rotter M, Masera A, Gugeler N, Decker HJ: VHL alterations in human clear-cell renal-cell carcinoma: association with advanced tumor stage and a novel hot spot mutation Cancer Res 2000, 60:1942-1948 [PubMed] [Google Scholar]
- 4.Schraml P, Struckmann K, Hatz F, Sonnet S, Kully C, Gasser T, Sauter G, Mihatsch MJ, Moch H: VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear-cell renal-cell carcinoma J Pathol 2002, 196:186-193 [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Kishida T, Duh FM, Renbaum P, Orcutt ML, Schmidt L, Zbar B: Suppression of growth of renal carcinoma cells by the von Hippel-Lindau tumor-suppressor gene Cancer Res 1995, 55:4804-4807 [PubMed] [Google Scholar]
- 6.Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ: Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth Proc Natl Acad Sci USA 1997, 94:8104-8109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ: The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis Nature 1999, 399:271-275 [DOI] [PubMed] [Google Scholar]
- 8.Ohh M, Yauch RL, Lonergan KM, Whaley JM, Stemmer-Rachamimov AO, Louis DN, Gavin BJ, Kley N, Kaelin WG, Jr, Iliopoulos O: The von Hippel-Lindau tumor-suppressor protein is required for proper assembly of an extracellular fibronectin matrix Mol Cell 1998, 1:959-968 [DOI] [PubMed] [Google Scholar]
- 9.Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W: Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL Nat Cell Biol 2003, 5:64-70 [DOI] [PubMed] [Google Scholar]
- 10.Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W: The von Hippel-Lindau tumor-suppressor protein is a component of an E3 ubiquitin-protein ligase activity Genes Dev 1999, 13:1822-1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG: Ubiquitination of hypoxia-inducible factor requires direct binding to the β-domain of the von Hippel-Lindau protein Nat Cell Biol 2000, 2:423-427 [DOI] [PubMed] [Google Scholar]
- 12.Tanimoto K, Makino Y, Pereira T, Poellinger L: Mechanism of regulation of the hypoxia-inducible factor-1α by the von Hippel-Lindau tumor-suppressor protein EMBO J 2000, 19:4298-4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iliopoulos O, Ohh M, Kaelin WG, Jr: pVHL19 is a biologically active product of the von Hippel-Lindau gene arising from internal translation initiation Proc Natl Acad Sci USA 1998, 95:11661-11666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakashita N, Takeya M, Kishida T, Stackhouse TM, Zbar B, Takahashi K: Expression of von Hippel-Lindau protein in normal and pathological human tissues Histochem J 1999, 31:133-144 [DOI] [PubMed] [Google Scholar]
- 15.Ye Y, Vasavada S, Kuzmin I, Stackhouse T, Zbar B, Williams BR: Subcellular localization of the von Hippel-Lindau disease gene product is cell cycle-dependent Int J Cancer 1998, 78:62-69 [DOI] [PubMed] [Google Scholar]
- 16.Los M, Jansen GH, Kaelin WG, Lips CJ, Blijham GH, Voest EE: Expression pattern of the von Hippel-Lindau protein in human tissues Lab Invest 1996, 75:231-238 [PubMed] [Google Scholar]
- 17.Corless CL, Kibel AS, Iliopoulos O, Kaelin WG, Jr: Immunostaining of the von Hippel-Lindau gene product in normal and neoplastic human tissues Hum Pathol 1997, 28:459-464 [DOI] [PubMed] [Google Scholar]
- 18.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP: Tissue microarrays for high-throughput molecular profiling of tumor specimens Nat Med 1998, 4:844-847 [DOI] [PubMed] [Google Scholar]
- 19.Simon R, Nocito A, Hubscher T, Bucher C, Torhorst J, Schraml P, Bubendorf L, Mihatsch MM, Moch H, Wilber K, Schotzau A, Kononen J, Sauter G: Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer J Natl Cancer Inst 2001, 93:1141-1146 [DOI] [PubMed] [Google Scholar]
- 20.Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Kochli OR, Mross F, Dieterich H, Moch H, Mihatsch M, Kallioniemi OP, Sauter G: Tissue microarrays for rapid linking of molecular changes to clinical endpoints Am J Pathol 2001, 159:2249-2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moch H, Schraml P, Bubendorf L, Mirlacher M, Kononen J, Gasser T, Mihatsch MJ, Kallioniemi OP, Sauter G: High-throughput tissue microarray analysis to evaluate genes uncovered by cDNA microarray screening in renal-cell carcinoma Am J Pathol 1999, 154:981-986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thoenes W, Stoerkel S, Rumpelt H: Histopathology and classification of renal cell tumors (adenomas, oncocytomas, and carcinomas): the basic cytological and histopathological elements and their use for diagnostics Pathol Res Pract 1986, 181:125-143 [DOI] [PubMed] [Google Scholar]
- 23.: UICC: TNM Classification of Malignant Tumours 1997. Wiley-Liss New York, Chichester, Weinheim, Brisbane, Singapore, Toronto
- 24.Schraml P, Struckmann K, Bednar R, Fu W, Gasser T, Wilber K, Kononen J, Sauter G, Mihatsch MJ, Moch H: CDKNA2A mutation analysis, protein expression, and deletion mapping of chromosome 9p in conventional clear-cell renal carcinomas: evidence for a second tumor-suppressor gene proximal to CDKN2A Am J Pathol 2001, 158:593-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schraml P, Muller D, Bednar R, Gasser T, Sauter G, Mihatsch MJ, Moch H: Allelic loss at the D9S171 locus on chromosome 9p13 is associated with progression of papillary renal cell carcinoma J Pathol 2000, 190:457-461 [DOI] [PubMed] [Google Scholar]
- 26.Brieger J, Weidt EJ, Schirmacher P, Storkel S, Huber C, Decker HJ: Inverse regulation of vascular endothelial growth factor and VHL tumor-suppressor gene in sporadic renal cell carcinomas is correlated with vascular growth: an in vivo study on 29 tumors J Mol Med 1999, 77:505-510 [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Neumann M, Stearman R, Stauber R, Pause A, Pavlakis GN, Klausner RD: Transcription-dependent nuclear-cytoplasmic trafficking is required for the function of the von Hippel-Lindau tumor-suppressor protein Mol Cell Biol 1999, 19:1486-1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nocito A, Bubendorf L, Maria Tinner E, Suess K, Wagner U, Forster T, Kononen J, Fijan A, Bruderer J, Schmid U, Ackermann D, Maurer R, Alund G, Knonagel H, Rist M, Anabitarte M, Hering F, Hardmeier T, Schoenenberger AJ, Flury R, Jager P, Luc Fehr J, Schraml P, Moch H, Mihatsch MJ, Gasser T, Sauter G: Microarrays of bladder cancer tissue are highly representative of proliferation index and histological grade J Pathol 2001, 194:349-357 [DOI] [PubMed] [Google Scholar]
- 29.Rubin MA, Dunn R, Strawderman M, Pienta KJ: Tissue microarray sampling strategy for prostate cancer biomarker analysis Am J Surg Pathol 2002, 26:312-319 [DOI] [PubMed] [Google Scholar]
- 30.Los M, Zeamari S, Foekens JA, Gebbink MF, Voest EE: Regulation of the urokinase-type plasminogen activator system by the von Hippel-Lindau tumor-suppressor gene Cancer Res 1999, 59:4440-4445 [PubMed] [Google Scholar]
- 31.Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ: Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors Cancer 2000, 89:604-614 [PubMed] [Google Scholar]
- 32.Lee S, Chen DY, Humphrey JS, Gnarra JR, Linehan WM, Klausner RD: Nuclear/cytoplasmic localization of the von Hippel-Lindau tumor-suppressor gene product is determined by cell density Proc Natl Acad Sci USA 1996, 93:1770-1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gronwald J, Storkel S, Holtgreve-Grez H, Hadaczek P, Brinkschmidt C, Jauch A, Lubinski J, Cremer T: Comparison of DNA gains and losses in primary renal clear-cell carcinomas and metastatic sites: importance of 1q and 3p copy number changes in metastatic events Cancer Res 1997, 57:481-487 [PubMed] [Google Scholar]
- 34.Gronwald J, Hadaczek P, Storkel S, Holtgreve-Grez H, Rabbitts P, Cremer T, Lubinski J: Molecular evidence for derivation of metastatic cells from minor subclones of primary clear renal cell carcinomas Cancer Detect Prev 1999, 23:479-484 [DOI] [PubMed] [Google Scholar]
- 35.Bissig H, Richter J, Desper R, Meier V, Schraml P, Schäffer A, Sauter G, Mihatsch M, Moch H: Evaluation of the clonal relationship between primary and metastatic renal cell carcinoma by comparative genomic hybridization Am J Pathol 1999, 155:267-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velickovic M, Delahunt B, Grebe SK: Loss of heterozygosity at 3p14.2 in clear-cell renal-cell carcinoma is an early event and is highly localized to the FHIT gene locus Cancer Res 1999, 59:1323-1326 [PubMed] [Google Scholar]
- 37.Speicher M, Schoell B, Du Manoir S, Schröck E, Ried T, Cremer T, Stoerkel S, Kovacs A, Kovacs G: Specific loss of chromosomes 1, 2, 6, 10, 13, 17, and 21 in chromophobe renal cell carcinomas revealed by comparative genomic hybridization Am J Pathol 1994, 145:356-364 [PMC free article] [PubMed] [Google Scholar]
- 38.Kenck C, Wilhelm M, Bugert P, Staehler G, Kovacs G: Mutation of the VHL gene is associated exclusively with the development of non-papillary renal cell carcinomas J Pathol 1996, 179:157-161 [DOI] [PubMed] [Google Scholar]
- 39.Shuin T, Kondo K, Torigoe S, Kishida T, Kubota Y, Hosaka M, Nagashima Y, Kitamura H, Latif F, Zbar B, Lerman MI, Yao M: Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor-suppressor gene in primary human renal cell carcinomas Cancer Res 1994, 54:2852-2855 [PubMed] [Google Scholar]
- 40.Jiang F, Moch H, Richter J, Gasser T, Gschwind R, Egenter C, Bubendorf L, Sauter G, Mihatsch M: Comparative genomic hybridization reveals frequent chromosome 13q and 4q losses in renal cell carcinomas with sarcomatoid transformation J Pathol 1998, 185:382-388 [DOI] [PubMed] [Google Scholar]
- 41.Presti J, Reuter V, Cordon-Cardo C, Mazumdar M, Fair W, Jhanwar S: Allelic deletions in renal tumors: histopathological correlations Cancer Res 1993, 53:5780-5783 [PubMed] [Google Scholar]
- 42.Velickovic M, Delahunt B, Storkel S, Grebem SK: VHL and FHIT locus loss of heterozygosity is common in all renal cancer morphotypes but differs in pattern and prognostic significance Cancer Res 2001, 61:4815-4819 [PubMed] [Google Scholar]
- 43.Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, Samid D, Duan DS, Gnarra JR, Linehan WM, Baylin SB: Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma Proc Natl Acad Sci USA 1994, 91:9700-9704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukhopadhyay D, Knebelmann B, Cohen HT, Ananth S, Sukhatme VP: The von Hippel-Lindau tumor-suppressor gene product interacts with Sp1 to repress vascular endothelial growth factor promoter activity Mol Cell Biol 1997, 17:5629-5639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuzmin I, Duh FM, Latif F, Geil L, Zbar B, Lerman MI: Identification of the promoter of the human von Hippel-Lindau disease tumor-suppressor gene Oncogene 1995, 10:2185-2194 [PubMed] [Google Scholar]