Expression of Human Apolipoprotein E4 in Neurons Causes Hyperphosphorylation of Protein Tau in the Brains of Transgenic Mice (original) (raw)
Abstract
Epidemiological studies have established that the epsilon 4 allele of the ApoE gene (ApoE4) constitutes an important risk factor for Alzheimer’s disease and might influence the outcome of central nervous system injury. The mechanism by which ApoE4 contributes to the development of neurodegeneration remains unknown. To test one hypothesis or mode of action of ApoE, we generated transgenic mice that overexpressed human ApoE4 in different cell types in the brain, using four distinct gene promoter constructs. Many transgenic mice expressing ApoE4 in neurons developed motor problems accompanied by muscle wasting, loss of body weight, and premature death. Overexpression of human ApoE4 in neurons resulted in hyperphosphorylation of the microtubule-associated protein tau. In three independent transgenic lines from two different promoter constructs, increased phosphorylation of protein tau was correlated with ApoE4 expression levels. Hyperphosphorylation of protein tau increased with age. In the hippocampus, astrogliosis and ubiquitin-positive inclusions were demonstrated. These findings demonstrate that expression of ApoE in neurons results in hyperphosphorylation of protein tau and suggests a role for ApoE in neuronal cytoskeletal stability and metabolism.
The epsilon 4 allele of the apolipoprotein E gene on chromosome 19q13.2 (ApoE4) is associated with late-onset and sporadic Alzheimer’s disease (AD), as confirmed by many groups since the initial reports. 1,2 ApoE4 causes earlier onset of the disease in an allele-dose-dependent manner and increases the risk for its carrier by 1 order of magnitude. In addition, the ε4 allele of apolipoprotein E has been associated with increased plaque load 3-5 and with early onset of AD-related neurofibrillary changes in young individuals. 6 Recently, genetic studies reported polymorphisms in the promoter region of the human ApoE gene associated with AD. 7-10 These might pertain to the observation that, in the brains of AD patients, ApoE messenger RNA (mRNA) was increased. 11 In addition to its well-documented role in AD, the ApoE4 allele has been implicated in poorer neurological recovery to head injury, cerebral hemorrhage, and cognitive status after cardiac bypass surgery. 12-17 These experiments provide epidemiological evidence for the close relationship between ApoE and AD or between ApoE and the outcome in nervous system injury, but do not provide an explanation for its mechanism of action within the nervous system.
The demonstration that the mainly astrocytic protein ApoE can also be found in human neurons might be important in assessing its role within the nervous system. Immunocytochemically, ApoE was demonstrated in human hippocampal neurons of AD patients and aged individuals 18 and in cortical neurons of elderly. 19 Recently, human brain neurons have been shown to synthesize ApoE. In situ hybridization revealed ApoE mRNA in the neurons of the CA1 to CA4 region of the hippocampus, the granule cell layer of the dentate gyrus, and many neurons in the frontal cortex. 20 In addition, transgenic mice that overexpress large fragments of the human ApoE locus, including the human ApoE promoter, also showed neuronal expression of human ApoE. 21-23 It is interesting that the regions where neuronal ApoE was present are most vulnerable in developing neurofibrillary tangles. 20,24 Also, human neuroblastoma cells were shown to synthesize ApoE mRNA. 25,26 In rodent brain, ApoE has been demonstrated in central neurons only after experimental brain injury. 27-30 Numerous cell culture experiments have demonstrated receptor-mediated uptake of ApoE in neurons, and large effects on neuritic outgrowth and morphology were found. 31-37 In vitro, protein-protein interactions of ApoE with intracellular microtubule-associated proteins (MAP2c, tau) were observed. 2,38 These results support an intracellular role for ApoE in neuronal pathology in AD and suggest that ApoE might be more directly involved in disruption of the neuronal cytoskeleton.
To study the repercussions of ApoE expression in different cell types in the brain, we have generated 25 independent founder transgenic mice that overexpress human ApoE4 in neurons and/or astrocytes. We have used four different gene promoter constructs derived from either the mouse Thy1 gene, 39 the human GFAP gene, 40 the human _PDGF_-β gene, 41 or the mouse PGK gene. 42 The initial phenotypic characterization demonstrated strong neuronal expression in the hippocampus and cortex of Thy1-ApoE4 and PDGF-ApoE4 transgenic mice, whereas the PGK-ApoE4 transgenic mice showed neuronal expression over the entire brain. As expected, in the GFAP-ApoE4 transgenic mice, expression was restricted to astroglia. Surprisingly, with a panel of protein tau-specific antibodies, hyperphosphorylation of the microtubule-associated protein tau became evident in the transgenic mice that overexpressed human ApoE4 in neurons. This might provide a clue to how ApoE could influence or disturb neuronal cytoskeletal stability.
Materials and Methods
Constructs and Generation of ApoE4 Transgenic Mice
All constructs were based on a 5.5-kb _Bam_HI-_Hin_dIII fragment of the human ApoE4 gene (Figure 1) ▶ . The mouse thy1 gene, cloned as an 8.1-kb _Eco_RI genomic fragment, 39 was adapted by replacing a 1.5-kb _Ban_HI-_Xho_I fragment with a synthetic _Xho_I oligonucleotide adapter, in which the human ApoE4 _Bam_HI-_Hin_dIII fragment was ligated. The PDGF gene promoter 41,43 and the GFAP gene promoter 40,44 were combined with the 1.5-kb _Eco_RI-_Xho_I fragment containing the mouse thy1 gene 3′UTR with polyadenylation signal. For the PGK-ApoE4 construct, the 5.5-kb human ApoE4 fragment was ligated in the _Pst_I site of a PGK expression cassette. 42,45
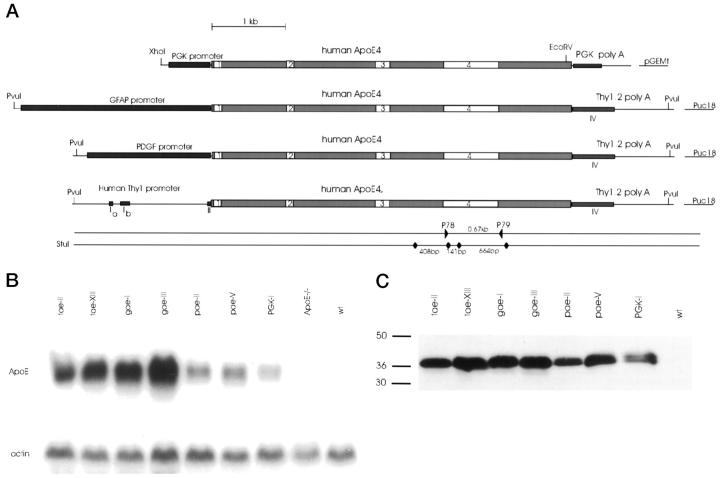
Figure 1.
Generation and characterization of different ApoE4 transgenic mouse strains. A: Schematic representation of minigene constructs synthesized as described in the text. On the lower lines are indicated the _Stu_I restriction site and size of restriction fragments used in genotyping by Southern blotting. The polymerase chain reaction primers (P78 and P79) used for routine genotyping are also indicated. B: Northern blot analysis of representative mice from the seven different ApoE4 transgenic strains. Total RNA (10 μg) was sequentially analyzed for expression of human ApoE mRNA (human-specific probe) and for actin mRNA. RNA from an ApoE knockout mouse and a wild-type mouse (wt) is included. C: Western blot of proteins extracted from the brains of the same mice as used in panel B, except for the ApoE knockout mice. A human-specific anti-human ApoE antibody was used (see Materials and Methods).
Minigenes were partially sequenced. Thy1-ApoE4, PDGF-ApoE4, and GFAP-ApoE4 vectors were linearized with _Pvu_I, and the PGK-ApoE4 vector was linearized with _Eco_RV and _Xho_I. Subsequently, purified constructs were diluted in 10 mmol/L Tris, 0.2 mmol/L ethylenediaminetetraacetic acid (EDTA), pH 7.4, to a concentration of 3 μg/ml for microinjection into 0.5-day prenuclear embryos isolated from superovulated FVB/N female mice. The injected embryos were either cultured overnight to reach two-cell stage or were immediately transferred to the oviduct of pseudopregnant CD1 foster mice, with similar results. Offspring was weaned and tail biopsies were taken for DNA isolation and Southern blotting. Genomic DNA (10 μg) was digested with _Stu_I, separated by gel-electrophoresis, transferred, and hybridized by standard procedures with a 672-bp probe generated by polymerase chain reaction from the human ApoE4 gene.
Routinely, transgenic offspring was identified by polymerase chain reaction, using primers in exon 4 of the human ApoE4 gene as follows: forward primer, 5′-GCGGGCACGGCTGTCCAAG (P78); reverse primer, 5′-GGGGTGGCGTGGGGTCGCAT (P79). Mouse tail biopsies were digested overnight at 55°C with 1 mg/μl proteinase K in 50 mmol/L Tris-HCI (pH 8.5), 1 mmol/L EDTA, 0.5% sodium dodecyl sulfate, 0.1 mol/L NaCl. The enzyme was inactivated by boiling for 10 minutes, and diluted samples were analyzed by polymerase chain reaction with the following program: 2 minutes at 95°C, followed by 30 cycles of 1 minute at 95°C, 1 minute at 60°C, 1 minute at 72°C, and a final extension of 15 minutes at 72°C.
Histology and Immunohistochemistry
Anesthetized mice were perfused with phosphate-buffered saline followed by 4% paraformaldehyde. Whole brain was fixed for 16 hours in 4% paraformaldehyde at 4°C and washed in phosphate-buffered saline, dehydrated, and embedded in paraffin for sectioning (5–7 μm). Alternatively, vibratome sections of 40 μm were cut and transferred to microtiter wells in phosphate-buffered saline containing 0.5% sodium azide at 4°C.
Paraffin sections were routinely stained with hematoxylin-eosin (H&E), cresylviolet (Nissl stain), and Garvey silver stain by standard procedures. Immunohistochemical staining of sections was performed by standard procedures with commercially available antibodies: ApoE (Dako, Glostrup, Denmark), GFAP (Dako), ubiquitin (Dako), AT8 (Innogenetics, Ghent, Belgium), PHF1 and Alz50 (P. Davies), and B19 (J. P. Brion). Vibratome sections were treated with 0.6% (w/v) hydrogen peroxide to quench the endogenous peroxidase activity. After rinsing, sections were incubated in Tris-buffered saline, containing 10% goat serum and 0.2% Triton-X-100 for 2 hours, before incubation with antibodies against ApoE (1:10,000, overnight at room temperature). For immunohistochemical staining with B19 (1:500), the detergent was omitted. Secondary antibody was biotinylated goat-anti-rabbit immunoglobulin G (IgG) followed by StreptABComplex/HRP (Dako). Final staining was developed with 0.075% 3,3-diaminobenzidine and 0.01% (w/v) hydrogen peroxide. Staining with the monoclonal antibodies AT8 (1:80), PHF1 (1:500), and Alz50 (1:50) was performed with the Dako ARK staining method, by the instructions of the manufacturer. Sections were mounted on gelatin-coated glass slides, counterstained with hematoxylin, dehydrated, and mounted.
In Situ Hybridization
The sense and antisense human ApoE4 probes were synthesized from a pBluescript (SK-) vector (Stratagene, La Jolla, CA) in which we cloned a 161-bp _Eco_NI/_Eco_47III restriction fragment from the human ApoE4 gene. The plasmid was linearized with either _Eco_RI or _Cla_I and transcribed with T7 and T3 RNA polymerase, respectively, in the presence of [35S]UTP. Paraffin sections (6 μm) were transferred on silanylated glass slides, dewaxed, and rehydrated through an ethanol series. Sections were digested with proteinase K (20 μg/ml), postfixed in 4% paraformaldehyde, and treated with 0.25% acetic anhydride in 0.1 mol/L triethanolamine-HCl. Sections were hybridized overnight in 50% deionized formamide, 0.3 mol/L NaCl, 20 mmol/L Tris-HCl, 5 mmol/L EDTA (pH 8.0) with 10% dextran sulfate, 1× Denhardt’s solution, 0.5 mg/ml yeast RNA, and 10 mmol/L dithiothreitol and supplemented with the appropriate radiolabeled riboprobe. After stringency washes and ribonucleaseA treatment, sections were dehydrated, and dipped in photographic emulsion (LM-1, Amersham) and exposed for 1 week.
Analysis of RNA and Protein
Brains of 6-week-old transgenic offspring were removed from the skulls as quickly as possible; for each, one hemisphere was used for analysis of ApoE mRNA, and the other was used for analysis of ApoE protein expression. Analysis of RNA and protein was done as previously described. 39 ApoE protein was detected with a polyclonal antibody to human ApoE4 (Dako).
In brains from 3- to 24-month-old transgenic offspring, one hemisphere was fixed in 4% paraformaldehyde and processed for immunohistochemistry, and the other was used for Western blot analysis of protein tau. Samples were homogenized in 10 vol of buffer consisting of 0.1 mol/L 2-(_N_-morpholino)ethanesulfonic acid (Mes), pH 6.4, 0.5 mmol/L MgCl2, 0.1 mmol/L EDTA, 1 mmol/L ethylene glycol bis(β-aminoethyl ether)-_N,N,N′,N′_-tetraacetic acid. Just before use, the following proteinase and phosphatase inhibitors were added: 1 mmol/L dithiothreitol, 5 μg/ml leupeptin, 5 μg/ml pepstatin, 200 μmol/L phenylmethylsulfonyl fluoride, 20 mmol/L NaF, 100 mmol/L Na3VO4, 1 μmol/L okadaic acid, 1 mmol/L EDTA, 5 μg/ml soybean trypsin inhibitor, 1% sodium deoxycholate, 1% Trition-X-100, and 0.1% sodium dodecyl sulfate (final concentrations). The homogenized suspension was centrifuged for 30 minutes at 100,000 × g at 4°C. Supernatants were stored at −70°C until use. Protein concentration was measured with the BioRad Dc protein Assay (BioRad, Hercules, CA). Before loading on the gel, 2% sodium chloride and 5% β-mercaptoethanol were added. Samples were heated at 100°C for 10 minutes in tightly capped tubes, chilled on ice for 30 minutes, and centrifuged for 15 minutes at 12,000 × g at 4°C. To the supernatants, 2% sodium dodecyl sulfate, 1% β-mercaptoethanol, and 10% glycerol blue (final concentrations) were added. After heating at 95°C for 10 minutes, proteins were separated by denaturing sodium dodecyl sulfate-polyacrylamide gels (NOVEX, Frankfurt-au-Main, Germany). Western blotting was performed with monoclonal antibodies PHF1, AT8, AT180, and TAU5. The monoclonal antibodies AT8 and AT180 (Innogenetics) recognize protein tau phosphorylated at serine-202 and threonine-205 (AT8), and threonine-231 (AT180). Monoclonal antibody PHF-1 (kindly provided by P. Davies) is specific for protein tau phosphorylated at serine-396 and serine-404. Total tau protein was detected with monoclonal antibody TAU5 (Pharmingen, San Diego, CA), which recognizes a phosphorylation-independent epitope of tau protein. Dephosphorylation of protein tau was performed with 300 U/ml alkaline phosphatase (Boehringer Mannheim, Mannheim, Germany), incubated at 37°C for 1 h.
Quantitative analysis of Western blots, assessed by densitometric scanning, was normalized, and 3 to 4 mice per transgenic strain and per age group were analyzed.
Results
Generation of Transgenic Mice Expressing Human ApoE4 with Four Different Gene Promoters
Transgenic mice were generated that overexpress human ApoE4 under the control of well-characterized promoters, derived from the following four genes: the mouse Thy1 gene, the human PDGF gene, the human GFAP gene, and the mouse PGK gene. The constructs are schematically represented in Figure 1A ▶ , and the known expression patterns of the gene promoters are summarized in Table 1 ▶ .
Table 1.
Expression Patterns of Promoters According to the Literature
| Promotor | Expression in | Time of expression | References | |
|---|---|---|---|---|
| Embryonal | Postnatal | |||
| Thy1 | Neurons | − | p15, onset; p22, full expression | 46 |
| Hippocampus | 47 | |||
| Cortex | 50 | |||
| 49 | ||||
| GFAP | Astroglia | From E12.5–E13.5 | + | 40 |
| 44 | ||||
| PDGF | Neurons Hippocampus | E9.5–E10.5, periaortic mesenchym; E12.5–E16.5, mesenchym | + | 43 41 |
| Cortex | ||||
| Hypothalamus | ||||
| Cerebellum | ||||
| Also in other tissues | ||||
| PGK | All tissues in brain | E3.5–E4.5, blastocyst | Variable expression | 51 |
| Large neurons | 52 | |||
| Cortex | ||||
| Hippocampus | ||||
| Dentate gyrus |
The linearized constructs were injected into mouse prenuclear embryos isolated from superovulated FVB female mice. Fifteen independent Thy1-ApoE4, five PDGF-ApoE4, five GFAP-ApoE4, and two PGK-ApoE4 founders were obtained. For each construct, two independent transgenic founders with high expression of ApoE4 and with good breeding performance were selected, except for PGK-ApoE4, for which only one strain was retained. The following notations are used to designate individual lines of the different constructs: tae-II (Thy1-ApoE4 line 2), tae-XIII (Thy1-ApoE4 line 13), pae-II (PDGF-ApoE4 line 2), pae-V (PDGF-ApoE4 line 5), pgk-I (PGK-ApoE4 line 1), gae-I (GFAP-ApoE4 line 1), and gae-III (GFAP-ApoE4 line 3). Wt is used to designate wild-type mice and ApoE−/− to designate ApoE knockout mice.
Analysis of Expression of Human ApoE4
Expression of human ApoE4 in brain was demonstrated and measured by Northern and Western blotting in F1 offspring (2 months old), from all founders. Comparative analysis of expression of human ApoE4 mRNA and protein in brain from the selected transgenic mouse strains is shown in Figure 1, B and C ▶ , respectively. Northern blotting demonstrated expression in all tissues examined in the PGK-ApoE4 transgenic mice, whereas in situ hybridization showed expression already in postimplantation embryos at E5.5.
Quantitation of relative levels of ApoE4 mRNA by densitometric scanning, showed that expression levels in the tae-II and tae-XIII transgenic mice were, respectively, 3- and 4.4-fold higher than in pae-II transgenic mice. In the gae-I transgenic mice, ApoE4 expression levels were 4.5-fold higher than in pae-II transgenic mice.
The spatial distribution of human ApoE4 mRNA was visualized by in situ hybridization in brain of adult transgenic mice. Expression patterns resulting from the different gene promoters were as expected. 40,41,43,44,46-52 Strong hybridization signals in pyramidal cells of the hippocampus, in the granular cells of the dentate gyrus, and in the deeper layers of the cerebellar cortex were detected in the brains of Thy1-ApoE4 mice (Figure 2, A and C) ▶ . Expression was further evident in neurons of the amygdala and striatum. Signals were most restricted to neuronal cell bodies, with minor hybridization signals in the proximal ends of some neurites. PDGF-ApoE4 transgenic mice strongly expressed in the neuronal cell bodies of the cortex, in pyramidal cells of the hippocampus, and in granular cells of the dentate gyrus, but only weakly in amygdala (Figure 2, F and H) ▶ . The PGK-ApoE4 mice showed diffuse expression in cells over the entire brain, with pyramidal and granular cells of the hippocampus and cells in the choroid plexus displaying stronger signals (Figure 2, G and I) ▶ . In contrast, but as expected, GFAP-ApoE4 mice expressed the transgene strongly in astrocytes (Figure 2, B and D) ▶ . The presence of weak signals in layer II and V neurons in the cortex could not be excluded.
Figure 2.
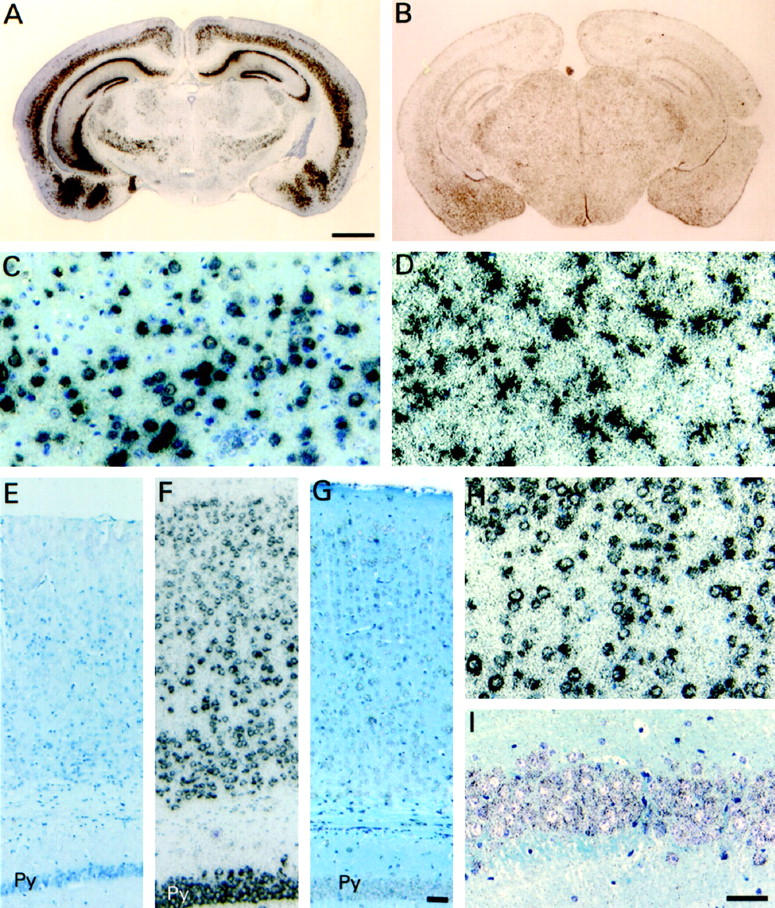
In situ hybridization of brains of ApoE4 transgenic and Wt mice. Sections were probed with the antisense human-specific ApoE probe and counterstained with toluidine blue. Coronal brain sections of Thy1-ApoE4 (A), and GFAP-ApoE4 (B) mice. Scale bar, 1 mm. Bar indicated in A also applies to B. C: Detail of the cortex of Thy1-ApoE4 (C) and GFAP-ApoE4 (D) mice. Parietal associative cortex of Wt (E), PDGF-ApoE4 (F), and PGK-ApoE4 (G) mice. Detail of the cortex of PDGF-ApoE4 (H) and of the pyramidal cells of the CA1 region of the hippocampus of PGK-ApoE4 (I) mice. Scale bar, 50 μm. Bar indicated in G also applies to E and F. Bar indicated in I also applies to C, D, and H. Py, pyramidal cell layer of the hippocampus.
The distribution of the human ApoE4 protein in transgenic mouse brain as analyzed immunohistochemically on vibratome sections (Figure 3) ▶ was similar to the distribution of its mRNA in all mice analyzed from the seven transgenic strains. In the Thy1-ApoE4, PDGF-ApoE4, and PGK-ApoE4 transgenic mice, we observed somatodendritic staining of neurons (Figure 3, A, C, D, F, G, and J) ▶ , whereas in the GFAP-ApoE4 transgenic mice, ApoE immunoreaction was restricted to astrocytes (Figure 3, H and I) ▶ . In the Thy1-ApoE4 transgenic mice, a granular staining pattern, probably corresponding to vesicular structures, was present in some dendrites (Figure 3D) ▶ .
Figure 3.
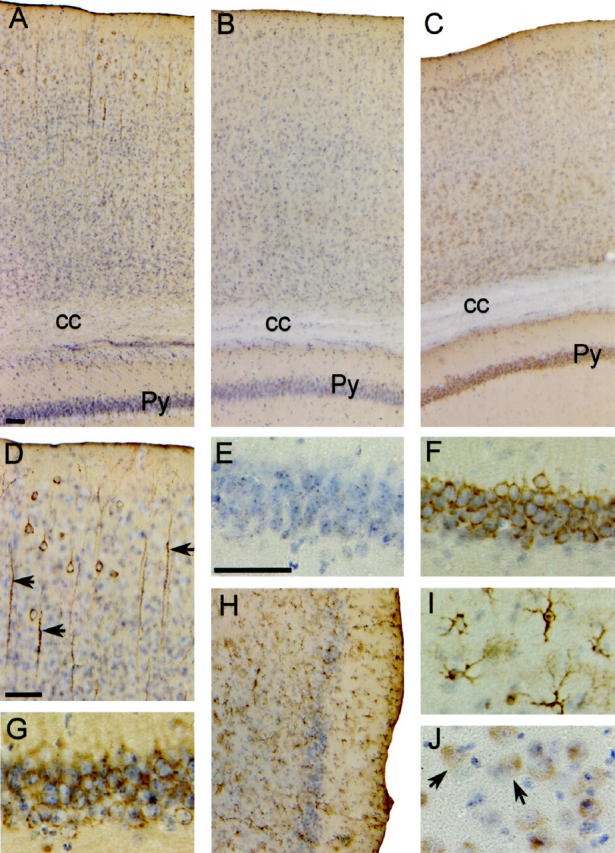
Immunohistochemistry for human ApoE4 in brains of transgenic and Wt mice. Sections were counterstained with hematoxylin. A–C: Cortical region of Thy1-ApoE4, Wt, and PDGF-ApoE4 mice, respectively. D: Detail of dendrites in cortex of a Thy1-ApoE4 mouse. E–G: Details of the pyramidal cells in the CA1 region of the hippocampus in Wt , PDGF-ApoE4, and Thy1-ApoE4 transgenic mice, respectively. H and I: Astrocytes in the entorhinal cortex of a GFAP-ApoE4 mouse. J: Astrocytes of neuronal cell bodies in the neocortex of a PGK-ApoE4 mouse. Scale bars, 50 μm. Scale bar indicated in A also applies to B, C, and H. Scale bar indicated in E also applies to G, F, I, and J. Py, pyramidal cells of the hippocampus; cc, corpus callosum.
Spontaneous Behavior of ApoE4 Transgenic Mice
All transgenic mice from all constructs appeared normal during the first 2 to 3 months of life. Thereafter, many mice progressively manifested motor problems accompanied by muscle wasting, loss of total body weight, and premature death. These signs were evident in the five transgenic strains with Thy1-ApoE4 (3 to 12 months old), PDGF-ApoE4, and PGK-ApoE4 constructs (12 months or older).
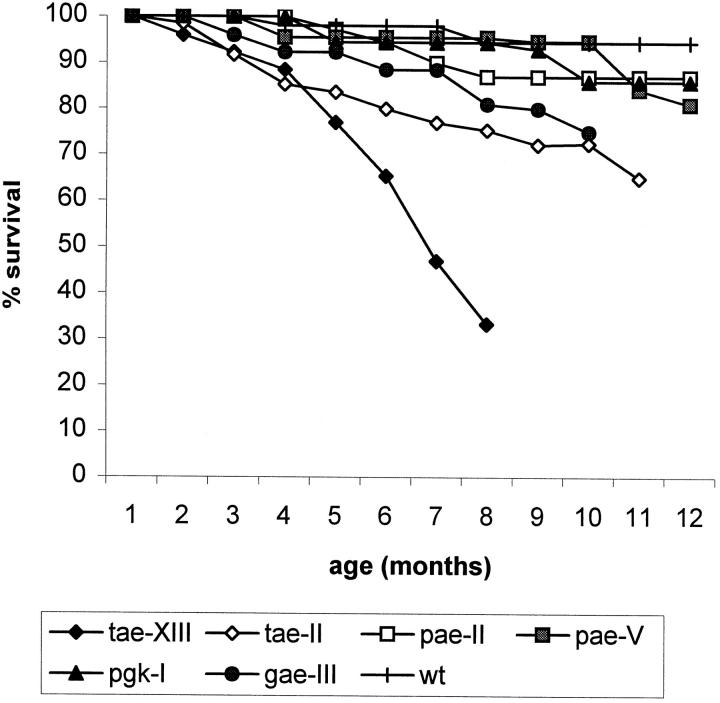
Thy1-ApoE4 transgenic mice with highest expression levels progressively developed the most severe phenotype. At around 6 months of age, about 60% of the mice in strain tae-II displayed bleeding excoriations on the eyelids, which appeared swollen. Close observation revealed these to result from scratching with the hind legs. Homozygous animals developed the phenotype at an earlier age than heterozygous animals of the same strain, housed in the same conditions. Muscle wasting and loss of bodyweight (30%) accompanied these symptoms, most likely due to the progressive inability to climb to the roof of the cage for feeding. This was further reflected in the increased mortality of the Thy1-ApoE4 transgenic mice: at 6 months, already 66% of transgenic mice in the highest expressing strain (tae-XIII) and 25% in the lower expressing Thy1-ApoE4 strain (tae-II) succumbed (Figure 4) ▶ .
Figure 4.
Survival of ApoE4 transgenic mice: tae-XIII, n = 15; tae-II, n = 43; pae-II, n = 31; pae-V, n = 19; pgk-I, n = 14; gae-III, n = 21; wt, n = 30.
Similar but much less intense or severe phenotypic characteristics appeared in a limited number of transgenic PDGF-ApoE4, PGK-ApoE4, and GFAP-ApoE4 animals, when more than 12 months old. In these transgenic mouse strains, the mortality was only marginally higher than in nontransgenic mice of the same age and gender housed under identical conditions (Figure 4) ▶ .
Hyperphosphorylation of Protein tau in Thy1-ApoE4 Transgenic Mice
We investigated protein tau phosphorylation in mice transgenic for human apolipoprotein E4 by Western blotting of brain homogenates. tau proteins are separated as a complex set of protein species reflecting both the expression of several isoforms and the presence of differentially phosphorylated species. 53
Protein tau phosphorylation was comparatively analyzed with four specific monoclonal antibodies, ie, AT8, AT180, PHF1, and TAU5. Western blot analysis of brain homogenates of transgenic mice showed that the microtubule-associated protein tau became hyperphosphorylated in mice expressing ApoE4 in neurons, which was demonstrated in two independent Thy1-ApoE4 transgenic lines. An increase in protein tau phosphorylation appeared in Thy1-ApoE4 line 13 (tae-XIII) transgenic mice of 3 months and was very prominent in mice of 18 months (Figure 5A) ▶ . In line tae-II, with lower neuronal expression levels, increased protein tau phosphorylation appeared when mice were 7 months old, indicating that hyperphosphorylation of protein tau correlated with neuronal ApoE4 expression levels. The marked decrease in electrophoretical mobility of the immunoreactive protein tau isoforms, following immunoblotting with TAU5 antibody, confirmed the increase in phosphorylation of protein tau in the brain of Thy1-ApoE4 transgenic mice, demonstrated with antibodies AT8, AT180, and PHF1 (Figure 5A) ▶ . Dephosphorylation of protein tau by pretreatment of brain extracts with alkaline phosphatase reduced or abolished the immunoreaction of the slower-migrating isoforms, detected with antibodies AT8 and TAU5 (Figure 7) ▶ . Phosphorylation of protein tau was variable at all age groups, but was always higher in transgenic mice relative to age-matched Wt mice.
Figure 5.
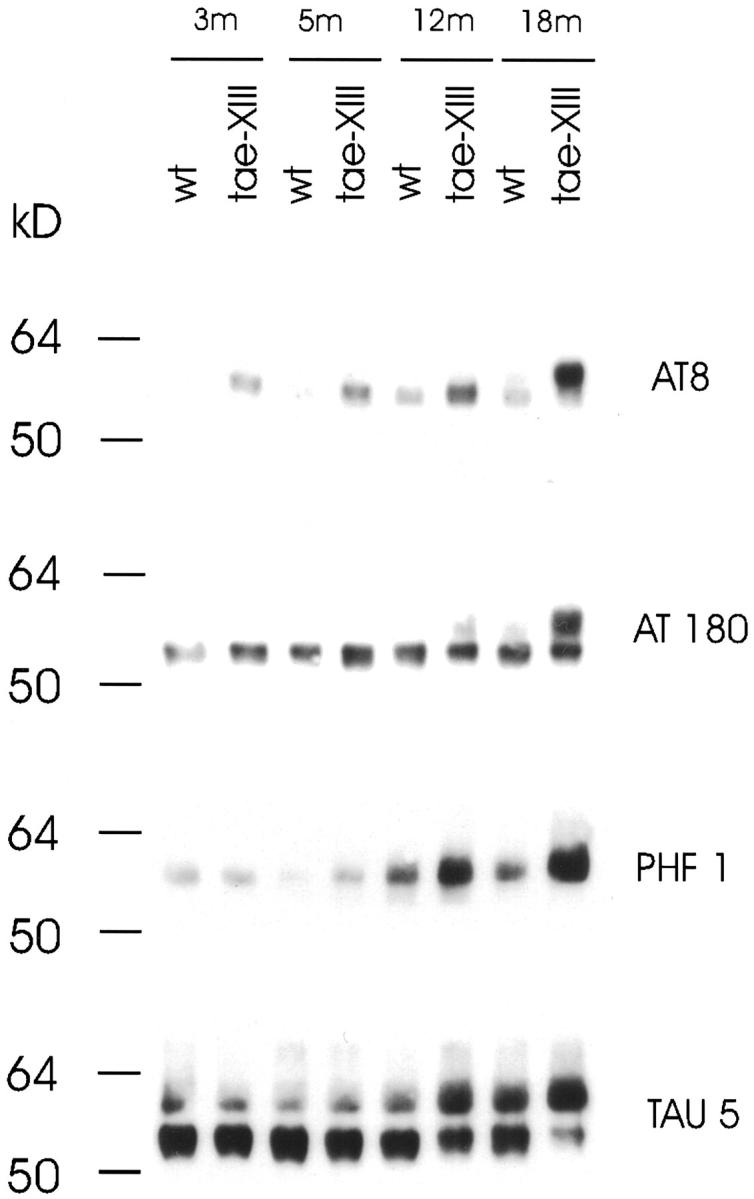
Comparison of protein tau hyperphosphorylation between Thy1-ApoE4 (line tae-XIII) and wild-type (wt) mice at ages 3 to 18 months. Western blots shown are representative examples. Four independent tau-specific monoclonal antibodies (AT8, AT180, PHF1, and TAU5) were used. Goat-anti-mouse peroxidase was used as secondary antibody.
Figure 7.
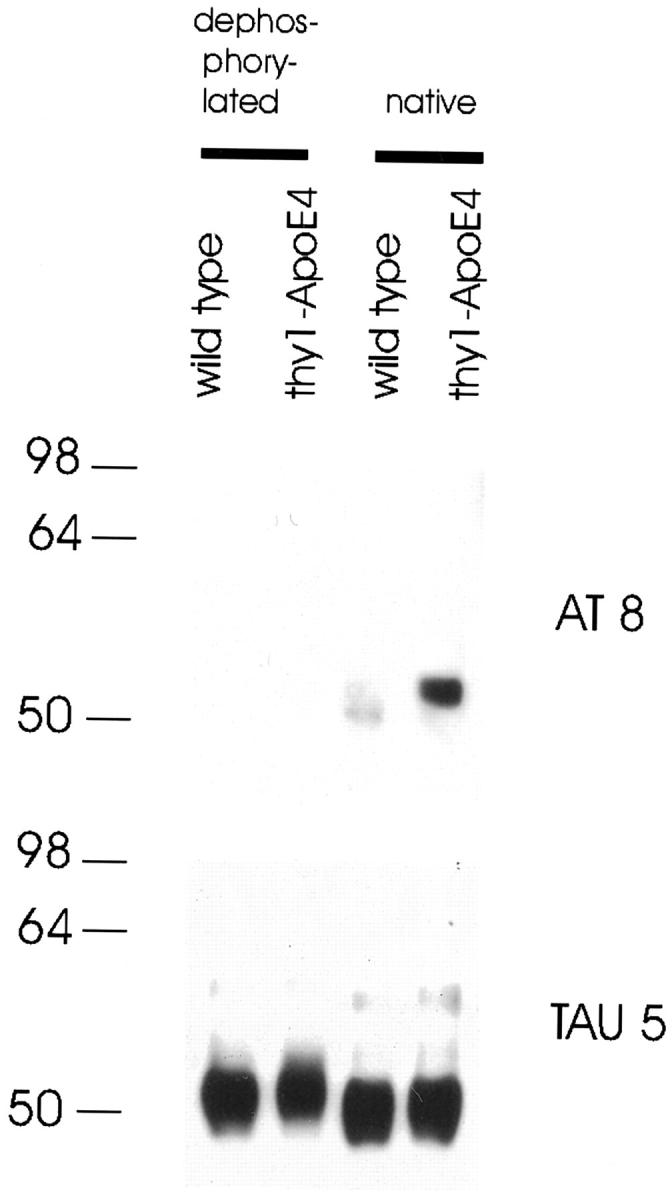
Dephosphorylated protein extract of a Thy1-ApoE4 and wild-type mouse with alkaline phosphatase, compared with untreated (native) samples. AT8 and TAU5 monoclonal antibodies were used to detect bands.
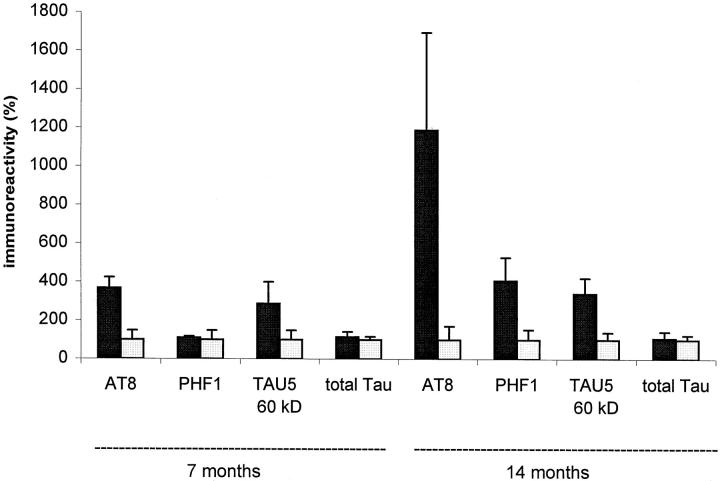
Protein tau phosphorylation was quantified by densitometric analysis of Western blots. In Thy1-ApoE4 line 2 (tae-II) transgenic mice, AT8 staining was 3.7-fold higher at 7 months and 12-fold higher at 14 months compared with age-matched Wt mice. PHF-1 staining was not significantly increased at 7 months, but was 4.1-fold higher at 14 months in tae-II transgenic mice relative to age-matched Wt mice (Figure 8) ▶ . The 60-kd protein tau band, detected by the monoclonal antibody TAU5, was threefold more intensive in tae-II transgenic mice relative to age-matched Wt mice, when mice were 7 or 14 months old (Figure 8) ▶ , whereas total amounts of protein tau were not different (Figure 8) ▶ .
Figure 8.
Quantitative comparison of the levels of hyperphosphorylated protein tau immunoreactivities of 7- and 14-month-old Thy1-ApoE4 transgenic and age-matched control mice. Data are means ± SEMs (bars) of wild type (□, n = 3) and Thy1-ApoE4 transgenic (▪, n = 3) mice. Data were obtained by densitometric analysis of immunoblots and normalized.
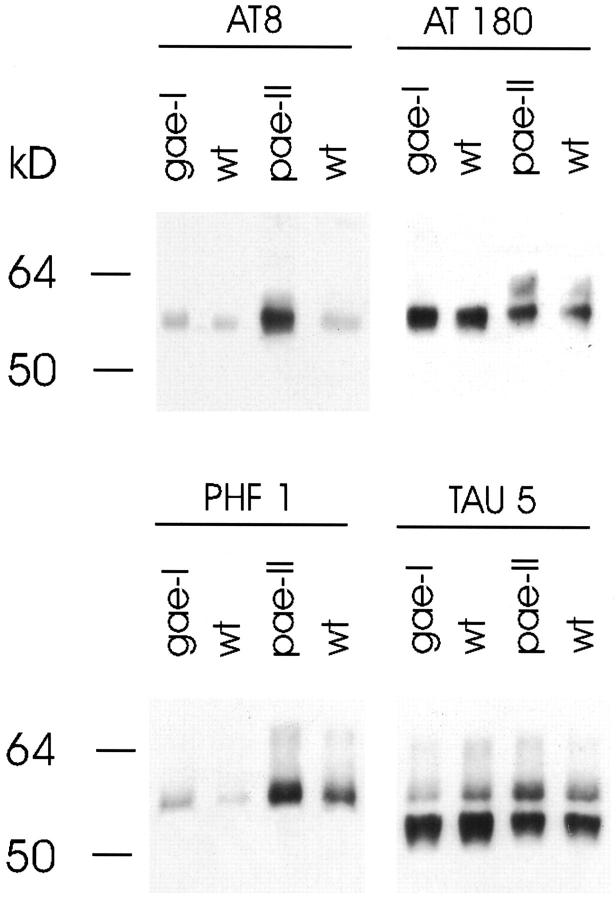
Hyperphosphorylation of protein tau became evident only in brain of PDGF-ApoE4 mice when 2 years old (Figure 6) ▶ . Neither in GFAP-ApoE4 mice of two years old (Figure 6) ▶ nor in PGK-ApoE4 mice of 12 to 17 months old was an increase in protein tau phosphorylation ever observed.
Figure 6.
Comparison of protein tau hyperphosphorylation between 2-year-old PDGF-ApoE4 (pae-II), GFAP-ApoE4 (gae-I), and wild-type (wt) mice. Goat-anti-mouse peroxidase was used as secondary antibody.
Brain Histology
Histological and immunohistochemical analysis was performed on 28 transgenic and 11 Wt mice between 3 and 23 months old. The histological repercussion of ApoE4 overexpression was minimal as judged by H&E staining of brain sections of transgenic mice and age-matched Wt mice. The brain appeared microscopically normal and did not show signs of deviating architecture or neuronal loss.
Astrogliosis, assessed by immunostaining for GFAP, was evident in the brain of older ApoE4-transgenic mice (Figure 9, A–F) ▶ . Gliosis was most prominent in the neocortex, hippocampus, and amygdala of transgenic mice with highest neuronal expression levels, ie, in the Thy1-ApoE4 and PDGF-ApoE4 mice (Figure 9, A, B, and F) ▶ . In the GFAP-ApoE4 mouse, only minimal gliosis was present (Figure 9D) ▶ .
Figure 9.
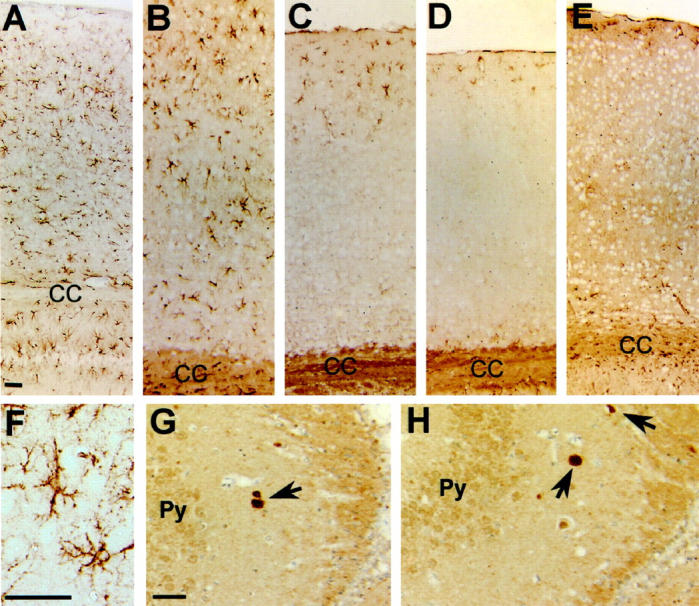
A–E: GFAP staining of the parietal associative cortex of Thy1-ApoE4 (A), PDGF-ApoE4 (B), PGK-ApoE4 (C), GFAP-ApoE4 (D), and Wt (E) mice. F: Detail of the hippocampus of a Thy1-ApoE4 mouse showing reactive astrogliosis. G and H: Ubiquitin positive inclusions (arrows) can be seen in the CA3 region of the hippocampus of Thy1-ApoE4 (G) and PDGF-ApoE4 (H) mice. Scale bar, 50 μm. Scale bar indicated in A also applies to B, C, D, and E. Scale bar indicated in G also applies to H. Py, pyramidal cells of the hippocampus; CC, corpus callosum.
Ubiquitin-containing inclusions were present in the neuropil of the stratum oriens of the hippocampus and in the fimbria hippocampi of Thy1-ApoE4 and PDGF-ApoE4 transgenic mice, but not in Wt mice (Figure 9, G–H) ▶ . The inclusions were rounded or more angular in shape.
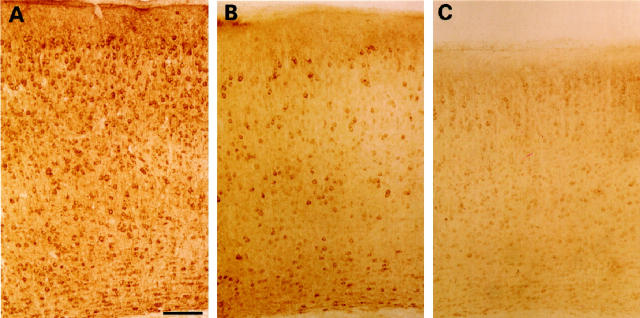
Immunohistochemical staining with B19, a polyclonal antiserum recognizing protein tau independent of its phosphorylation state, showed widespread axonal and somatodendritic staining in white matter fiber tracts, neocortex, hippocampus, and thalamus, which was not different in Thy1-ApoE4 transgenic mice relative to Wt mice (Figure 10A) ▶ . This staining pattern was also similar to the one observed in rat brain. 54 Staining with the phosphate-dependent monoclonal antibody AT8 revealed reactivity mainly in the neocortex, hippocampus, and amygdala of Thy1-ApoE4 transgenic mice. Staining was most intense in old Thy1-ApoE4 transgenic mice (12–18 months), compared with age-matched Wt mice (Figure 10, B and C) ▶ . The phosphorylation-dependent monoclonal antibody PHF1 stained neurons in the neocortex, thalamic nuclei, and the CA3 and CA4 regions of the hippocampus. Staining was again most intense in old Thy1-ApoE4 transgenic mice compared with age-matched Wt mice. Staining with the conformation-dependent monoclonal antibody Alz50 remained negative both in Wt and Thy1-ApoE4 transgenic mice. Neither immunohistochemistry nor silver staining revealed any neurofibrillary tangles or other types of neurofibrillary inclusions.
Figure 10.
A: Staining with the polyclonal anti-tau antibody B19 shows a widespread somatodendritic localization in the parietal associative cortex of a Thy1-ApoE4 transgenic mouse 18 months old. B: Staining with the monoclonal phosphorylation-dependent antibody AT8 recording a slightly more intense somatodendritic staining in the parietal associative cortex of the Thy1-ApoE4 mice18 months old, compared with aged-matched wild-type mice (C). Scale bar, 100 μm.
Immunohistochemistry for βA4 or silver staining did not reveal amyloid deposition in any of the ApoE4 transgenic mice.
Discussion
The impact of expression of human apolipoprotein E4 in different cell types in the brain was examined by generating transgenic mice expressing human ApoE4 in neurons or glial cells. Mice transgenic for human ApoE4 expressed the transgene exclusively in neurons when under control of the Thy1 or PDGF gene promoters and in astrocytes when under control of the GFAP gene promoter, as expected. Remarkably, the expression in neurons resulted in hyperphosphorylation of protein tau, in addition to behavioral disturbances and morphological neuronal changes in the brain, such as ubiquitin-positive inclusions and astrogliosis.
In three independent transgenic mouse strains, neuronal expression of human ApoE4 resulted in protein tau hyperphosphorylation. The level of neuronal expression, in combination with aging, appeared to be the most important determining factors. Offspring of the highest expressing Thy1-ApoE4 transgenic line (line tae-XIII) displayed protein tau hyperphosphorylation when 3 months old, whereas mild overexpression, ie, in line pae-II, resulted in hyperphosphorylation only when mice were 2 years old. Within a particular age group of Thy1-ApoE4 transgenic mice, protein tau hyperphosphorylation was somewhat variable, as reflected by the levels of the immunoreactive isoforms detected in Western blotting with specified monoclonal antibodies. Using the polyclonal phosphorylation-independent antibody B19, the somatodendritic localization of protein tau in cortical and hippocampal neurons was similar in old Thy1-ApoE4 transgenic and Wt mice. This result is in agreement with a previous study in rats, showing phosphorylated tau in the soma and dendrites of neurons in adult brain. 54 In addition, the brain of Thy1-ApoE4 transgenic mice, shown to exhibit increased protein tau phosphorylation on Western blotting, also showed increased immunoreactivity for phosphorylated tau with antibodies AT8 and PHF1 on brain sections.
The remarkable phenotypic parameter of all transgenic mice that express ApoE4 in neurons, ie, protein tau hyperphosphorylation, relates to in vitro studies illustrating isoform-specific interactions between ApoE and protein tau. 2 Our results demonstrate that, under specified conditions, ApoE4 can cause protein tau to become hyperphosphorylated directly, in vivo. Whether this is through a direct molecular interaction or indirectly, remains at present unclear.
Transgenic mice expressing ApoE4 in neurons under control of the neuron-specific enolase promoter were reported to have difficulties with learning in water maze tasks and with exploratory behavior at 6 months. 55 Humanized transgenic mice carrying genomic sequences for ApoE have been generated, showing neuronal expression of ApoE in addition to glial expression. 21-23 It would be interesting to know whether these mice would also show protein tau hyperphosphorylation at an older age. In the PDGF-ApoE4 transgenic mice, which have lower expression levels, tau hyperphosphorylation and motor impairments appeared only when mice were around 2 years old.
Other transgenic mice generated in our laboratory, expressing very high levels of unrelated proteins under control of the same Thy1 gene promoter, did not show protein tau hyperphosphorylation and remained completely normal throughout life. Therefore, we exclude the possibility of a general neurotoxic effect of overexpressing high levels of any protein in central neurons, under control of the Thy1 gene promoter.
Protein tau hyperphosphorylation has been demonstrated in mice lacking ApoE, 56,57 although conflicting results have been reported. 58 Because brain endogenous mouse ApoE levels remained unchanged in transgenic mice expressing human ApoE4 in neurons, the observed protein tau hyperphosphorylation cannot be attributed to a down-regulation or absence of endogenous mouse ApoE.
In apolipoprotein E transgenic mice generated with the same GFAP promoter as used here, ApoE proteins were secreted in high-density like lipoprotein particles. 59 The present results suggest that expression of ApoE4 in glial cells, secretion, and eventual uptake by neurons were not a sufficient mechanism to cause protein tau hyperphosphorylation in neurons. Expression in neurons in combination with aging appeared to be the important factors.
Ubiquitin immunoreactivity has been associated with hyperphosphorylated protein tau in AD. 60 In our experimental models, ubiquitin-positive inclusions were demonstrated in the brain of transgenic Thy1-ApoE4 and PDGF-ApoE4 mice, which were not found in Wt or GFAP-ApoE4 mice. However, silver staining or immunohistochemistry for protein tau did not reveal intraneuronal tangles or abnormal inclusions. In agreement with these results, neurofibrillary tangle formation could not be demonstrated in mouse models showing hyperphosphorylation of protein tau, not even when mice were 19 months old 61-63 or in mice overexpressing and hyperphosphorylating human protein tau isoforms. 64-66 In addition, it has been shown that AT8 immunoreactivity precedes tangle formation in AD and natural animal models such as aging sheep and goats. 67,68
Reactive astrocytes reflect general central nervous system injury. 69 Astrogliosis in the neocortex, hippocampus, and amygdala was strongest in Thy1-ApoE4, PDGF-ApoE4, and PGK-ApoE4 mice and absent in Wt mice. Only mild astrogliosis was present in GFAP-ApoE4 mice, and, despite higher expression levels than in the Thy1-ApoE4 mice, no signs of protein tau hyperphosphorylation were ever noted, at any age.
To explain the genetic association of ApoE4 to AD, two types of mechanisms have been proposed. First, ApoE could function as a “pathological chaperone,” affecting clearance of β-amyloid and causing amyloid deposition. 70-72 We obtained no evidence for the presence of amyloid plaques in any of our ApoE4 transgenic mice, which of course might be because only endogenous mouse APP is present, which is less amyloidogenic. 39 The second hypothesis states that ApoE interacts with the microtubule-associated protein tau, thereby altering its phosphorylation state, and hence is involved in stabilizing the neuronal cytoskeleton. 2,73 The results presented here support this hypothesis. However, the route by which ApoE gains access to the neuronal cytoplasm has been the major criticism against this hypothesis and subject of much speculation. Most recently, it was shown that direct expression of ApoE in the cytosol of Neuro-2a cells is toxic. 74 The ApoE4 transgenic mice are, however, not expected to express ApoE directly in the cytosol, and we also did not observe obvious signs of neurotoxicity. In addition, the hyperphosphorylation of protein tau was not restricted to brain regions expressing human ApoE as shown by immunohistochemistry. Therefore, we favor a mechanism involving an indirect interaction between ApoE and protein tau. In addition, this implies that hyperphosphorylation of protein tau is not simply a nonspecific downstream event marking degeneration.
Although great caution must be taken in extrapolating findings from transgenic mice to complex human neurodegenerative diseases such as AD, the results presented here might be relevant for the pathological process in AD. An obvious question is whether expression of the ApoE3 isoform in neurons, to the same levels as ApoE4, would have the same or other effects. Reconciling two different hypotheses, we propose that the phenotype we observed is not typical for ApoE4, but will be observed with all ApoE isoforms. Recent in situ hybridization experiments on human brain tissue and brain tissue of humanized transgenic lines carrying genomic sequences for ApoE demonstrated that low levels of human ApoE are expressed in neurons, independently of the ApoE isotype, 20-23 suggesting that neuronal synthesis of ApoE is typical for and caused by regulatory sequences in the human ApoE gene. In addition, polymorphisms in the promoter region of the ApoE gene have been associated with AD, 7-10,75-77 and many of them were correlated with increased ApoE expression. 10,11,76,78 It is important that tight linkage of ApoE gene promoter polymorphisms with the ε4 allele was demonstrated. 7,8,79 Although direct evidence is lacking at this moment, combined with our observation that neuronal expression of ApoE results in hyperphosphorylation of protein tau, it is tempting to speculate that quantitative differences in ApoE expression in neurons might be related to promoter polymorphisms and that altered neuronal expression of ApoE would be genetically associated with the ε4 allele. This not only points to an important function for neuronal ApoE but could also provide an alternative explanation for the observed association of the ε4 allele of ApoE with AD. To prove this last hypothesis, a quantitative analysis is needed of ApoE expression in neurons, related to the different ApoE promoter polymorphisms and different ApoE alleles in AD and controls. Obviously, this hypothesis does not exclude additional effects, ie, a different extracellular role of the different ApoE isoforms on neurons, as indicated by cell culture experiments. 31-37,80-82
In conclusion, we demonstrated that expression of human ApoE4 in neurons resulted in hyperphosphorylation of the microtubule-associated protein tau in vivo, as opposed to expression in non-neuronal cells. We also showed that the neuronal expression levels of human ApoE, as well as aging, are major factors for hyperphosphorylation of protein tau to become evident. Although many different aspects still need to be addressed, as discussed, the current transgenic mice offer the opportunity to investigate the interactions between ApoE and protein tau.
Acknowledgments
We thank Dr. M. Brenner for the pGfazlac-1 plasmid, Dr. P. Davies for donating PHF1 and Alz50 antibody, and Dr. J. P. Brion for donating B19 antibody. We thank T. Boon for technical assistance. We also thank A. M. Kelles for advise with in situ hybridization and V. Baekelandt and K. Lorent for practical advise with immunohistochemistry. We thank K. Bruynseels for computer assistance.
Footnotes
Address reprint requests to Fred Van Leuven, Ph.D., Dr.Sc., Experimental Genetics Group (EGG), Vlaams Instituut voor Biotechnologie (VIB), Center for Human Genetics (CME)-K.U. Leuven, Campus Gasthuisberg O&N 06, B-3000 Leuven, Belgium. E-mail: fredvl@med.kuleuven.ac.be.
Supported by the Fonds voor Wetenschappelijk Onderzoek (FWO), the Interuniversity-Network for Fundamental Research (IUAP), by the special Action Program for Biotechnology of the Flemish government (VLAB/IWT, COT-008), by the Rooms fund, and by KULeuven Research fund.
D. Moechars’ present adress: Janssens Research Foundation, B-2340 Beerse, Belgium.
References
- 1.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261:921-923 [DOI] [PubMed] [Google Scholar]
- 2.Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong LM, Jakes R, Huang DY, Pericak-Vance M, Schmechel D, Roses AD: Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci USA 1994, 91:11183-11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olichney JM, Hansen LA, Galasko D, Saitoh T, Hofstetter CR, Katzman R, Thal LJ: The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer’s disease and Lewy body variant. Neurology 1996, 47:190-196 [DOI] [PubMed] [Google Scholar]
- 4.Nagy Z, Esiri MM, Jobst KA, Johnston C, Litchfield S, Sim E, Smith AD: Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer’s disease. Neuroscience 1995, 69:757-761 [DOI] [PubMed] [Google Scholar]
- 5.Gearing M, Mori H, Mirra SS: A beta-peptide length and apolipoprotein E genotype in Alzheimer’s disease. Ann Neurol 1996, 39:395-399 [DOI] [PubMed] [Google Scholar]
- 6.Ghebremedhin E, Schultz C, Braak E, Braak H: High frequency of apolipoprotein E epsilon4 allele in young individuals with very mild Alzheimer’s disease-related neurofibrillary changes. Exp Neurol 1998, 153:152-155 [DOI] [PubMed] [Google Scholar]
- 7.Ahmed AR, MacGowan SH, Culpan D, Jones RW, Wilcock GK: The −491 A/T polymorphism of the apolipoprotein E gene is associated with the ApoEepsilon4 allele, and Alzheimer’s disease. Neurosci Lett 1999, 263:217-219 [DOI] [PubMed] [Google Scholar]
- 8.Lambert JC, Pasquier F, Cottel D, Frigard B, Amouyel P, Chartier-Harlin MC: A new polymorphism in the ApoE promoter associated with risk of developing Alzheimer’s disease. Hum Mol Genet 1998, 7:533-540 [DOI] [PubMed] [Google Scholar]
- 9.Bullido MJ, Artiga MJ, Recuero M, Sastre I, Garcia MA, Aldudo J, Lendon C, Han SW, Morris JC, Frank A, Vazquez J, Goate A, Valdivieso F: A polymorphism in the regulatory region of ApoE associated with risk for Alzheimer’s dementia. Nat Genet 1998, 18:69-71 [DOI] [PubMed] [Google Scholar]
- 10.Artiga MJ, Bullido MJ, Sastre I, Recuero M, Garcia MA, Aldudo J, Vazquez J, Valdivieso F: Allelic polymorphisms in the transcriptional regulatory region of apolipoprotein E gene. FEBS Lett 1998, 421:105-108 [DOI] [PubMed] [Google Scholar]
- 11.Lambert JC, Berr C, Pasquier F, Delacourte A, Frigard B, Cottel D, Perez-Tur J, Mouroux V, Mohr M, Cecyre D, Galasko D, Lendon C, Poirier J, Hardy J, Mann D, Amouyel P, Chartier-Harlin MC: Pronounced impact of Th1/E47cs mutation compared with −491 AT mutation on neural APOE gene expression and risk of developing Alzheimer’s disease. Hum Mol Genet 1998, 7:1511-1516 [DOI] [PubMed] [Google Scholar]
- 12.Alberts MJ, Graffagnino C, McClenny C, DeLong D, Strittmatter W, Saunders AM, Roses AD: ApoE genotype, and survival from intracerebral haemorrhage. Lancet 1995, 346:575. [DOI] [PubMed] [Google Scholar]
- 13.Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S: Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. J Am Med Assoc 1997, 278:136-140 [PubMed] [Google Scholar]
- 14.Mayeux R, Ottman R, Maestre G, Ngai C, Tang MX, Ginsberg H, Chun M, Tycko B, Shelanski M: Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology 1995, 45:555-557 [DOI] [PubMed] [Google Scholar]
- 15.Nicoll JA, Roberts GW, Graham DI: Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid β-protein following head injury. Nat Med 1995, 1:135-137 [DOI] [PubMed] [Google Scholar]
- 16.Reed T, Carmelli D, Swan GE, Breitner JC, Welsh KA, Jarvik GP, Deeb S, Auwerx J: Lower cognitive performance in normal older adult male twins carrying the apolipoprotein E epsilon 4 allele. Arch Neurol 1994, 51:1189-1192 [DOI] [PubMed] [Google Scholar]
- 17.Tardiff BE, Newman MF, Saunders AM, Strittmatter WJ, Blumenthal JA, White WD, Croughwell ND, Davis RD, Jr, Roses AD, Reves JG: Preliminary report of a genetic basis for cognitive decline after cardiac operations. The Neurologic Outcome Research Group of the Duke Heart Center. Ann Thorac Surg 1997, 64:715-720 [DOI] [PubMed] [Google Scholar]
- 18.Han SH, Hulette C, Saunders AM, Einstein G, Pericak-Vance M, Strittmatter WJ, Roses AD, Schmechel DE: Apolipoprotein E is present in hippocampal neurons without neurofibrillary tangles in Alzheimer’s disease, and in age-matched controls. Exp Neurol 1994, 128:13-26 [DOI] [PubMed] [Google Scholar]
- 19.Metzger RE, LaDu MJ, Pan JB, Getz GS, Frail DE, Falduto MT: Neurons of the human frontal cortex display apolipoprotein E immunoreactivity: implications for Alzheimer’s disease. J Neuropathol Exp Neurol 1996, 55:372-380 [DOI] [PubMed] [Google Scholar]
- 20.Xu PT, Gilbert JR, Qiu HL, Ervin J, Rothrock-Christian TR, Hulette C, Schmechel DE: Specific regional transcription of apolipoprotein E in human brain neurons. Am J Pathol 1999, 154:601-611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu PT, Gilbert JR, Qiu HL, Rothrock-Christian T, Settles DL, Roses AD, Schmechel DE: Regionally specific neuronal expression of human APOE gene in transgenic mice. Neurosci Lett 1998, 246:65-68 [DOI] [PubMed] [Google Scholar]
- 22.Xu PT, Schmechel D, Rothrock-Christian T, Burkhart DS, Qiu HL, Popko B, Sullivan P, Maeda N, Saunders AM, Roses AD, Gilbert JR: Human apolipoprotein E2, E3, and E4 isoform-specific transgenic mice: human-like pattern of glial and neuronal immunoreactivity in central nervous system not observed in wild-type mice. Neurobiol Dis 1996, 3:229-245 [DOI] [PubMed] [Google Scholar]
- 23.Roses AD, Gilbert J, Xu PT, Sullivan P, Popko B, Burkhart DS, Christian-Rothrock T, Saunders AM, Maeda N, Schmechel DE: cis-acting human ApoE tissue expression element is associated with human pattern of intraneuronal ApoE in transgenic mice. Neurobiol Aging 1998, 19:S53-S58 [DOI] [PubMed] [Google Scholar]
- 24.Einstein G, Patel V, Bautista P, Kenna M, Melone L, Fader R, Karson K, Mann S, Saunders AM, Hulette C, Mash D, Roses AD, Schmechel DE: Intraneuronal ApoE in human visual cortical areas reflects the staging of Alzheimer disease pathology. J Neuropathol Exp Neurol 1998, 57:1190-1201 [DOI] [PubMed] [Google Scholar]
- 25.Soulié Cathia VM, Dupont-Wallois L, Chartier-Harlin M-C, Beauvillain JC, Delacourte A, Caillet-Boudin ML: Synthesis of apolipoprotein E (ApoE) mRNA by human neuronal-type SK N SH-SY 5Y cells and its regulation by nerve growth factor and ApoE. Neurosci Lett 1999, 265:147–150 [DOI] [PubMed]
- 26.Dupont-Wallois L, Soulie C, Sergeant N, Wavrant-de Wrieze N, Chartier-Harlin M-C, Delacourte A, Caillet-Boudin ML: ApoE synthesis in human neuroblastoma cells. Neurobiol Dis 1997, 4:356–364 [DOI] [PubMed]
- 27.Horsburgh K, Nicoll JA: Selective alterations in the cellular distribution of apolipoprotein E immunoreactivity following transient cerebral ischaemia in the rat. Neuropathol Appl Neurobiol 1996, 22:342-349 [DOI] [PubMed] [Google Scholar]
- 28.Ali SM, Dunn E, Oostveen JA, Hall ED, Carter DB: Induction of apolipoprotein E mRNA in the hippocampus of the gerbil after transient global ischemia. Brain Res Mol Brain Res 1996, 38:37-44 [DOI] [PubMed] [Google Scholar]
- 29.Ishimaru H, Ishikawa K, Ohe Y, Takahashi A, Maruyama Y: Cystatin C, and apolipoprotein E immunoreactivities in CA1 neurons in ischemic gerbil hippocampus. Brain Res 1996, 709:155-162 [DOI] [PubMed] [Google Scholar]
- 30.Zarow C, Victoroff J: Increased apolipoprotein E mRNA in the hippocampus in Alzheimer disease and in rats after entorhinal cortex lesioning. Exp Neurol 1998, 149:79-86 [DOI] [PubMed] [Google Scholar]
- 31.DeMattos RB, Curtiss LK, Williams DL: A minimally lipidated form of cell-derived apolipoprotein E exhibits isoform-specific stimulation of neurite outgrowth in the absence of exogenous lipids or lipoproteins. J Biol Chem 1998, 273:4206-4212 [DOI] [PubMed] [Google Scholar]
- 32.Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL: Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci USA 1995, 92:9480-9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathan BP, Chang KC, Bellosta S, Brisch E, Ge N, Mahley RW, Pitas RE: The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J Biol Chem 1995, 270:19791-19799 [DOI] [PubMed] [Google Scholar]
- 34.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE: Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science 1994, 264:850-852 [DOI] [PubMed] [Google Scholar]
- 35.Bellosta S, Nathan BP, Orth M, Dong LM, Mahley RW, Pitas RE: Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem 1995, 270:27063-27071 [DOI] [PubMed] [Google Scholar]
- 36.Puttfarcken PS, Manelli AM, Falduto MT, Getz GS, LaDu MJ: Effect of apolipoprotein E on neurite outgrowth and β-amyloid-induced toxicity in developing rat primary hippocampal cultures. J Neurochem 1997, 68:760-769 [DOI] [PubMed] [Google Scholar]
- 37.Fagan AM, Bu G, Sun Y, Daugherty A, Holtzman DM: Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth, and is a ligand for the low density lipoprotein receptor-related protein. J Biol Chem 1996, 271:30121-30125 [DOI] [PubMed] [Google Scholar]
- 38.Huang DY, Goedert M, Jakes R, Weisgraber KH, Garner CC, Saunders AM, Pericak-Vance MA, Schmechel DE, Roses AD, Strittmatter WJ: Isoform-specific interactions of apolipoprotein E with the microtubule-associated protein MAP2c: implications for Alzheimer’s disease. Neurosci Lett 1994, 182:55-58 [DOI] [PubMed] [Google Scholar]
- 39.Moechars D, Lorent K, De Strooper B, Dewachter I, Van Leuven F: Expression in brain of amyloid precursor protein mutated in the α-secretase site causes disturbed behavior, neuronal degeneration and premature death in transgenic mice. EMBO J 1996, 15:1265-1274 [PMC free article] [PubMed] [Google Scholar]
- 40.Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A: GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci 1994, 14:1030-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Nadsworth S, Wolozin B, Zhao J: Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 1995, 373:523-527 [DOI] [PubMed] [Google Scholar]
- 42.te Riele H, Maandag ER, Berns A: Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs. Proc Natl Acad Sci USA 1992, 89:5128-5132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasahara M, Fries JW, Raines EW, Gown AM, Westrum LE, Frosch MP, Bonthron DT, Ross R, Collins T: PDGF B-chain in neurons of the central nervous system, posterior pituitary, and in a transgenic model. Cell 1991, 64:217-227 [DOI] [PubMed] [Google Scholar]
- 44.Johnson WB, Ruppe MD, Rockenstein EM, Price J, Sarthy VP, Verderber LC, Mucke L: Indicator expression directed by regulatory sequences of the glial fibrillary acidic protein (GFAP) gene: in vivo comparison of distinct GFAP-lacZ transgenes. Glia 1995, 13:174-184 [DOI] [PubMed] [Google Scholar]
- 45.Umans L, Serneels L, Overbergh L, Lorent K, Van Leuven F, Van den Berghe H: Targeted inactivation of the mouse α 2-macroglobulin gene. J Biol Chem 1995, 1978, 270:19778-19785 [DOI] [PubMed] [Google Scholar]
- 46.Evans GA, Ingraham HA, Lewis K, Cunningham K, Seki T, Moriuchi T, Chang HC, Silver J, Hyman R: Expression of the Thy-1 glycoprotein gene by DNA-mediated gene transfer. Proc Natl Acad Sci USA 1984, 81:5532-5536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ingraham HA, Lawless GM, Evans GA: The mouse Thy-1.2 glyco-protein gene: complete sequence and identification of an unusual promoter. J Immunol 1986, 136:1482-1489 [PubMed] [Google Scholar]
- 48.Morris R: Thy-1, the enigmatic extrovert on the neuronal surface. BioEssays 1992, 14:715-722 [DOI] [PubMed] [Google Scholar]
- 49.Kelley KA, Friedrich VL, Jr, Sonshine A, Hu Y, Lax J, Li J, Drinkwater D, Dressler H, Herrup K: Expression of Thy-1/lacZ fusion genes in the CNS of transgenic mice. Brain Res Mol Brain Res 1994, 24:261-274 [DOI] [PubMed] [Google Scholar]
- 50.Gordon JW, Chesa PG, Nishimura H, Rettig WJ, Maccari JE, Endo T, Seravalli E, Seki T, Silver J: Regulation of Thy-1 gene expression in transgenic mice. Cell 1987, 50:445-452 [DOI] [PubMed] [Google Scholar]
- 51.Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, Huarte J: Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 1994, 13:1017-1030 [DOI] [PubMed] [Google Scholar]
- 52.Latham KE, Rambhatla L: Expression of X-linked genes in androgenetic, gynogenetic, and normal mouse preimplantation embryos. Dev Genet 1995, 17:212-222 [DOI] [PubMed] [Google Scholar]
- 53.Bahr BA, Vicente JS: Age-related phosphorylation and fragmentation events influence the distribution profiles of distinct tau isoforms in mouse brain. J Neuropathol Exp Neurol 1998, 57:111-121 [DOI] [PubMed] [Google Scholar]
- 54.Tashiro K, Hasegawa M, Ihara Y, Iwatsubo T: Somatodendritic localization of phosphorylated tau in neonatal and adult rat cerebral cortex. Neuroreport 1997, 8:2797-2801 [DOI] [PubMed] [Google Scholar]
- 55.Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L: Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci USA 1998, 95:10914-10919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Genis I, Gordon I, Sehayek E, Michaelson DM: Phosphorylation of tau in apolipoprotein E-deficient mice. Neurosci Lett 1995, 199:5-8 [DOI] [PubMed] [Google Scholar]
- 57.Genis I, Fisher A, Michaelson DM: Site-specific dephosphorylation of tau of apolipoprotein E-deficient and control mice by M1 muscarinic agonist treatment. J Neurochem 1999, 72:206-213 [DOI] [PubMed] [Google Scholar]
- 58.Mercken L, Brion JP: Phosphorylation of tau protein is not affected in mice lacking apolipoprotein E. Neuroreport 1995, 6:2381-2384 [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, Wu S, Bu G, Onifade MK, Patel SN, LaDu MJ, Fagan AM, Holtzman DM: Glial fibrillary acidic protein-apolipoprotein E (apoE) transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J Neurosci 1998, 18:3261-3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM: Tau and ubiquitin immunoreactivity at different stages of formation of Alzheimer neurofibrillary tangles. Prog Clin Biol Res 1989, 317:837-848 [PubMed] [Google Scholar]
- 61.Brownlees J, Irving NG, Brion JP, Gibb BJ, Wagner U, Woodgett J, Miller CC: Tau phosphorylation in transgenic mice expressing glycogen synthase kinase-3β transgenes. Neuroreport 1997, 8:3251-3255 [DOI] [PubMed] [Google Scholar]
- 62.James ND, Davis DR, Sindon J, Hanger DP, Brion JP, Miller CC, Rosenberg MP, Anderton BH, Propst F: Neurodegenerative changes including altered tau phosphorylation and neurofilament immunoreactivity in mice transgenic for the serine/threonine kinase Mos. Neurobiol Aging 1996, 17:235-241 [DOI] [PubMed] [Google Scholar]
- 63.Kayyali US, Zhang W, Yee AG, Seidman JG, Potter H: Cytoskeletal changes in the brains of mice lacking calcineurin A α. J Neurochem 1997, 68:1668-1678 [DOI] [PubMed] [Google Scholar]
- 64.Brion JP, Tremp G, Octave JN: Transgenic expression of the shortest human tau affects its compartmentalization and its phosphorylation as in the pretangle stage of Alzheimer’s disease. Am J Pathol 1999, 154:255-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gotz J, Probst A, Spillantini MG, Schafer T, Jakes R, Burki K, Goedert M: Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J 1995, 14:1304-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spittaels K, Van den Haute C, Van Dorpe J, Bruynseels K, Vandezande K, Laenen I, Geerts H, Mercken M, Sciot R, Van Lommel A, Loos R, Van Leuven F: Prominent axonopathy in the brain and spinal cord of transgenic mice overexpressing four-repeat human tau protein. Am J Pathol 1999, 155:2153-2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braak H, Braak E: Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 1995, 16:271-284 [DOI] [PubMed] [Google Scholar]
- 68.Braak H, Braak E: Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl 1996, 165:3-12 [DOI] [PubMed] [Google Scholar]
- 69.Ridet JL, Malhotra SK, Privat A, Gage FH: Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 1997, 20:570-577 [DOI] [PubMed] [Google Scholar]
- 70.Rebeck GW, Reiter JS, Strickland DK, Hyman BT: Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 1993, 11:575-580 [DOI] [PubMed] [Google Scholar]
- 71.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD: Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA 1993, 90:1977-1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wisniewski T, Frangione B: Apolipoprotein E: A pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett 1992, 135:235-238 [DOI] [PubMed] [Google Scholar]
- 73.Strittmatter WJ, Weisgraber KH, Goedert M, Saunders AM, Huang D, Corder EH, Dong LM, Jakes R, Alberts MJ, Gilbert JR, Hans H, Hulette C, Einstein G, Schmechel DE, Pericak-Vance MA, Roses AD: Hypothesis: microtubule instability and paired helical filament formation in the Alzheimer disease brain are related to apolipoprotein E genotype. Exp Neurol 1994, 125:163-174 [DOI] [PubMed] [Google Scholar]
- 74.DeMattos RB, Thorngate FE, Williams DL: A test of the cytosolic apolipoprotein E hypothesis fails to detect the escape of apolipoprotein E from the endocytic pathway into the cytosol and shows that direct expression of apolipoprotein E in the cytosol is cytotoxic. J Neurosci 1999, 19:2464-2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chartier-Harlin MC, Parfitt M, Legrain S, Perez-Tur J, Brousseau T, Evans A, Berr C, Vidal O, Roques P, Gourlet V, Fruchart JC, Delacourte A, Rosser M, Amoyel P. Apolipoprotein E, epsilon 4 allele as a major risk factor for sporadic early and late-onset forms of Alzheimer’s disease: analysis of the 19q13.2 chromosomal region. Hum Mol Genet 1994, 3:569–574 [DOI] [PubMed]
- 76.Lambert JC, Perez-Tur J, Dupire MJ, Galasko D, Mann D, Amouyel P, Hardy J, Delacourte A, Chartier-Harlin MC: Distortion of allelic expression of apolipoprotein E in Alzheimer’s disease. Hum Mol Genet 1997, 6:2151-2154 [DOI] [PubMed] [Google Scholar]
- 77.Poduslo SE, Neal M, Schwankhaus J: A closely linked gene to apolipoprotein E may serve as an additional risk factor for Alzheimer’s disease. Neurosci Lett 1995, 201:81-83 [DOI] [PubMed] [Google Scholar]
- 78.Laws SM, Taddei K, Martins G, Paton A, Fisher C, Clarnette R, Hallmayer J, Brooks WS, Gandy SE, Martins RN: The −491 AA polymorphism in the APOE gene is associated with increased plasma apoE levels in Alzheimer’s disease. Neuroreport 1999, 10:879-882 [DOI] [PubMed] [Google Scholar]
- 79.Mui S, Briggs M, Chung H, Wallace RB, Gomez-Isla T, Rebeck GW, Hyman BT: A newly identified polymorphism in the apolipoprotein E enhancer gene region is associated with Alzheimer’s disease and strongly with the epsilon 4 allele. Neurology 1996, 47:196-201 [DOI] [PubMed] [Google Scholar]
- 80.Marques MA, Tolar M, Harmony JA, Crutcher KA: A thrombin cleavage fragment of apolipoprotein E exhibits isoform-specific neurotoxicity. Neuroreport 1996, 7:2529-2532 [DOI] [PubMed] [Google Scholar]
- 81.Tolar M, Marques MA, Harmony JAK, Crutcher KA: Neurotoxicity of the 22 kd thrombin-cleavage fragment of apolipoprotein E and related synthetic peptides is receptor-mediated. J Neurosci 1997, 17:5678-5686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crutcher KA, Clay MA, Scott SA, Tian X, Tolar M, Harmony JA: Neurite degeneration elicited by apolipoprotein E peptides. Exp Neurol 1994, 130:120-126 [DOI] [PubMed] [Google Scholar]