Reduced Level of CD44 and Hyaluronan Associated with Unfavorable Prognosis in Clinical Stage I Cutaneous Melanoma (original) (raw)
Abstract
The cell surface glycoprotein CD44 and its ligand, hyaluronan (HA), enhance growth and metastatic capacity of melanoma cells in vitro, but their clinical significance in primary cutaneous melanoma is still unclear. Therefore, we studied whether the levels of CD44 and HA associate with disease progression and survival of cutaneous melanoma. A series of 292 clinical stage I cutaneous melanomas was analyzed by immunohistochemistry using an anti-CD44H antibody (clone 2C5). HA was demonstrated histochemically using a biotinylated HA-specific affinity probe (bHABC). The reduced staining levels of CD44 and HA were associated with each other and indicators of progressive disease. Reduced CD44 and HA level, high tumor thickness, high pT category, high Clark’s level, bleeding, and male gender predicted short univariate recurrence free survival (RFS) and overall survival (OS). In Cox’s multivariate analysis (N = 251), the decreased level of CD44, high tumor thickness, and bleeding predicted independently short RFS. High tumor thickness and bleeding were associated with short OS. We conclude that the reduced cell surface CD44 and HA levels associate with poor prognosis in clinical stage I cutaneous melanoma. The notion that the decreased level of CD44 independently predicts short RFS suggests that reduced cell surface CD44 enhances the spreading potential in localized cutaneous melanoma and that quantification of CD44 offers a prognostic tool for its clinical evaluation.
The incidence and mortality of cutaneous malignant melanoma has increased worldwide during past decades among white populations. 1 Local disease is in most cases curable by surgical excision. 2 However, the probability of development of disseminated disease is progressively higher as the depth of the primary melanoma increases. In the worldwide multiinstitutional database of cutaneous melanoma patients, the average 5-year overall survival rate for patients with localized (AJCC stages I and II) melanoma was 79%. 3 Those patients who later develop a metastatic disease have a 2-year survival rate less than 5%. 4 As more effective adjuvant therapies, such as interferon-α, have become available it is even more important to identify the patients at high risk of developing metastatic disease. 5 More research is also needed to improve our basic understanding on the biology of cutaneous melanoma.
CD44 is a structurally variable and multifunctional cell surface glycoprotein expressed on most cell types. 6,7 Many functions of CD44 are mediated through interaction with its ligand hyaluronan (HA), 8 a ubiquitous extracellular polysaccharide. 9 HA is abundant in soft connective tissues, but also in epithelial and neural tissues. 8 HA organizes certain proteoglycans in the extracellular matrix, and facilitates cell migration and proliferation during embryogenesis, inflammation, and wound healing. 8-11
The interaction of CD44 with HA contributes to tumor cell proliferation, 8,12 migration, 6,8,13-15 invasion, 6,8,16 and formation of metastatic tumor emboli or peritoneal implants. 8,17-21 Previous experimental data suggest that CD44 and HA enhance growth and metastatic capacity of melanoma cells, 17,18,21-26 but the clinical significance of this suggestion has not been confirmed in the relatively small and divergent clinical materials reported so far. 27-35 We demonstrate here that the reduced stainings of cell surface CD44 and tumor cell associated HA are associated with each other, and with progressive disease and poor prognosis in localized cutaneous melanoma.
Materials and Methods
Patients and Histology
This retrospective study consists of primary melanomas derived from a consecutive series 369 clinical stage I cutaneous melanoma patients with sufficient clinicopathological and long-term follow-up data. 36 The patients were diagnosed and treated in the district of Kuopio University Hospital between 1974 and 1989. The histological diagnosis, Breslow thickness and Clark level were re-examined from 1 to 4 original sections of the primary tumor by the same pathologist (VMK), unaware of the clinical data. Of the original 369 cases, 292 had enough archival tumor material available for the present study (Table 1) ▶ . The most representative block was cut into new 5-μm-thick consecutive sections for CD44 and HA stainings. Depending on the availability of representative tumor material as compared with the original sections, 282 stainings with an antibody recognizing all forms of CD44 and 277 stainings for HA were eventually evaluable.
Table 1.
Patient and Tumor Characteristics of 292 Patients with Stage I Cutaneous Malignant Melanoma
| Characteristic | n | % |
|---|---|---|
| Total no. of patients | 292 | 100 |
| Age (years) | ||
| Mean (SD) | 55.9 (15.2) | |
| Range | 19.0–89.7 | |
| Anatomic site | ||
| Head and neck | 47 | 16 |
| Trunk and perineum | 145 | 50 |
| Upper limbs | 44 | 15 |
| Lower limbs | 56 | 19 |
| Bleeding* of the primary tumor | ||
| Yes | 76 | 26 |
| No | 119 | 41 |
| Unknown | 97 | 33 |
| Sex | ||
| Male | 143 | 49 |
| Female | 149 | 51 |
| Disease recurrence | ||
| Yes | 84 | 29 |
| No | 208 | 71 |
| Cause of death | ||
| Malignant melanoma | 52 | 18 |
| Other | 40 | 14 |
| Alive | 200 | 68 |
| Clark’s level | ||
| I | 12 | 4 |
| II | 54 | 18 |
| III | 82 | 28 |
| IV | 119 | 41 |
| V | 25 | 9 |
| Tumor thickness | ||
| ≤0.75 mm | 64 | 22 |
| 0.76–1.50 mm | 69 | 23 |
| 1.51–4.0 mm | 99 | 34 |
| >4.0 mm | 41 | 14 |
| Not possible to analyze | 19 | 6 |
| TNM category | ||
| pT1-T2, N0, M0 | 131 | 45 |
| pT3, N0, M0 | 115 | 39 |
| pT4, N0, M0 | 46 | 16 |
CD44 Immunostaining
CD44 was demonstrated by using a mouse IgG anti-CD44H antibody (clone 2C5) (R&D Systems, Abingdon, UK) which recognizes all forms of CD44. 37 Adjacent 5-μm sections from tumors were deparaffinized and rehydrated using xylene and graded alcohols. The sections were microwaved in a 0.01 mol/L citrate buffer (pH 6.0) for 3 × 5 minutes, incubated in a citrate buffer for 18 minutes, and washed twice for 5 minutes with phosphate-buffered saline (PBS). Endogenous peroxidase activity was blocked by 5% hydrogen peroxide for 5 minutes, followed by a wash twice for 5 minutes with PBS. The sections were incubated with 1% bovine serum albumin (BSA) and PBS for 30 minutes at 37°C. The primary antibody was diluted with 1% BSA to 1:2000 and incubated on the slides overnight at 4°C. After another washing step, the bound antibody was localized using a biotinylated secondary antibody and an avidin-biotin-peroxidase detection kit (Vectastain ABC Elite Kit, Vector Laboratories, Burlingame, CA), and the slides were developed with diaminobenzidine tetrahydrochloride (DAB) (Sigma, St. Louis, MO), counterstained with Mayer’s hematoxylin, dehydrated, cleared and mounted with DePex (BDH Poole, UK). In each batch, a melanoma specimen processed without primary antibodies served as a negative control, and one CD44-positive melanoma block served as a source for positive control sections. In addition, the adjacent normal epidermis within the tumor served as positive internal control.
Preparation of bHABC and Staining of HA
The biotinylated complex of hyaluronan binding region and link protein (bHABC) was prepared from bovine articular cartilage as described previously. 38,39 Briefly, the proteoglycans were extracted from the cartilage with 4 mol/L guanidinium chloride. The extract was dialyzed against distilled water in the presence of high molecular weight hyaluronan. The C-terminus of the proteoglycan molecule was cleaved off with trypsin, and the resultant complex of hyaluronan binding region and link protein (HABC) with HA was purified using hydroxylapatite chromatography and gel filtration. The complex was biotinylated, and the bHABC was separated from HA using gel filtration under dissociative conditions. The purity of the preparation was tested by polyacrylamide gel electrophoresis and Western blotting.
The sections were deparaffinized in xylene, rehydrated with graded alcohols and washed with PB. Endogenous peroxidase was blocked with 1% hydrogen peroxide for 5 minutes and nonspecific binding was blocked with 1% BSA in PB for 30 minutes. The sections were incubated in bHABC (2.5 μg/ml, diluted in 1% BSA) overnight at 4°C. The slides were washed with PB and treated with avidin-biotin-peroxidase kit (1:200 dilution) for 1 h at room temperature. Following wash with PB the color was developed with 0.05% DAB and 0.03% hydrogen peroxide in PB at room temperature. The slides were counterstained with Mayer’s hematoxylin for 2 minutes, washed, dehydrated, and mounted in Depex. The specificity of the staining was controlled by digesting some sections with 100 TRU/ml of Streptomyces hyaluronidase (Seikagaku Kogyo Co., Tokyo, Japan) in the presence of protease inhibitors before the staining, or preincubating the bHABC-probe with hyaluronan oligosaccharides to block the specific binding site (Figure 1A) ▶ . 39
Figure 1.
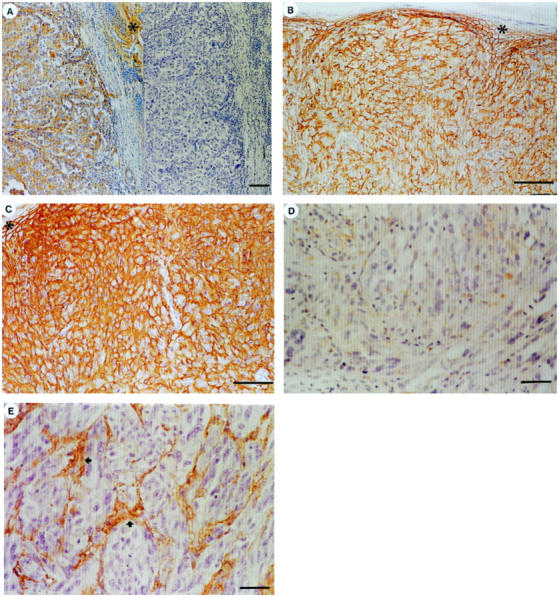
HA (A, C, E) and CD44 (B, D) stainings of cutaneous melanoma. A: Left, HA-positive tumor. Note HA-positive dermal stroma (asterisk). Right, a cons3ecutive section digested by Streptomyces hyaluronidase before HA staining (negative control). Scale bar, 100 μm. B and C: Consecutive sections, strong CD44 and HA positivity. Asterisk, epidermis. Scale bars, 100 μm. D and E: Consecutive sections without cell surface CD44 or cellular HA signal. Tumor stroma (arrows) is HA positive. Scale bars, 50 μm.
Evaluation and Scoring of the CD44 and HA Stainings
Throughout the evaluations, the observers were unaware of the clinical data. In all stainings, scoring was performed with a dual head microscope (field diameter 490 μm) by two observers (JMK and VMK) in the whole tumor area on the slide. In both CD44 and HA stainings, the positivities were assessed qualitatively, ie, the tumor cells were considered as positive when there was a homogeneous and clearly visible signal present, and negative if the signal was absent. According to this principle, the fraction of positively stained cancer cells in the entire slide was evaluated within each specimen. The frequency distribution and percentiles for the HA- and CD44-positive cancer cell fractions within each specimen were analyzed within the whole series (Figure 2) ▶ . For both stainings, the tumors were eventually categorized as high (91 to 100% of positively stained cancer cells for CD44 and 71 to 100% of positively stained cancer cells for HA) or reduced (0 to 90% of positively stained cancer cells for CD44 and 0 to 70% of positively stained cancer cells for HA) expressors according to the median percentage of positively stained cancer cells. 40 The staining intensity of hyaluronan in the intratumoral stroma was compared qualitatively to the adjacent normal HA-positive epidermis and categorized as follows: +, weaker than epidermis; ++, as strong as epidermis; and +++, stronger than epidermis.
Figure 2.
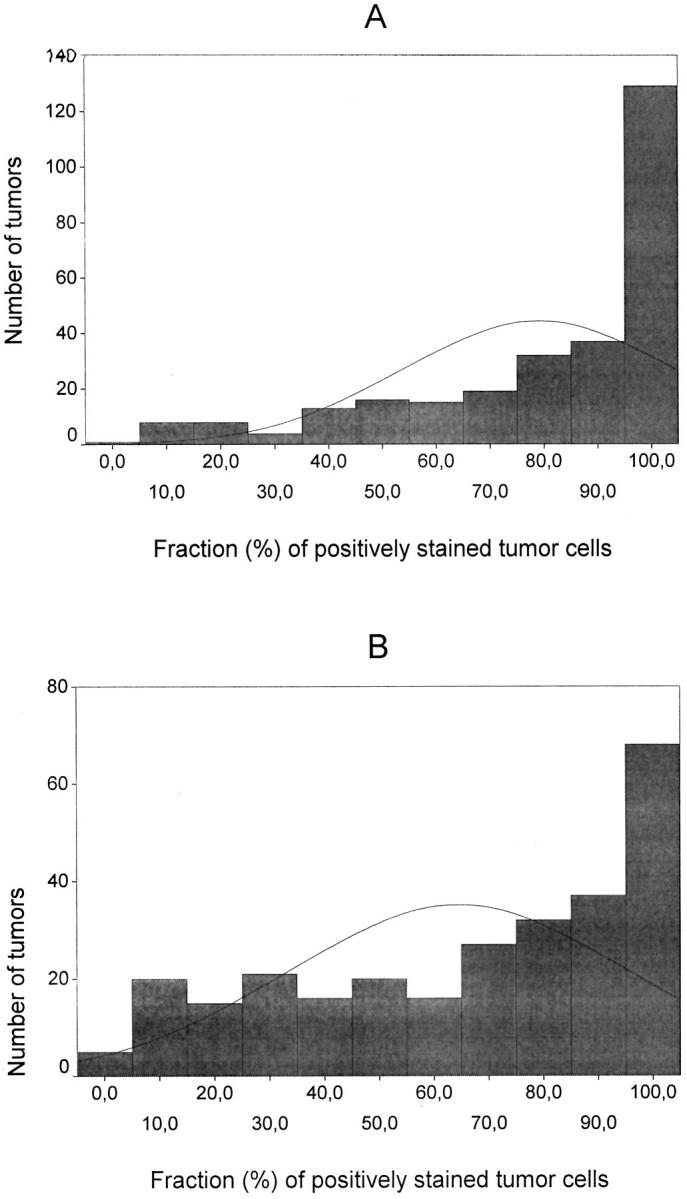
Distributions of the cutaneous melanomas according to the percentage of CD44 (A) and HA (B) positivity among the malignant cells (n = 282 for CD44 and 277 for HA).
Statistical Analyses
The SPSS-Win 7.5 program package was used in a PC computer for basic statistical calculations. The χ 2 test was used to compare the frequency distributions of clinicopathological features between the original database (n = 369) and the population available for the current study. A Spearman correlation coefficient was used to test the relationships between continuous variables. Nonparametric tests (Kruskal-Wallis) were applied for the comparisons of staining levels between different clinicopathological categories. Frequency tables were analyzed using a 2-sided Fisher’s exact test. In univariate survival analyses the EGRET statistical software package was used for calculation of Kaplan-Meier estimates of survival rates and the log rank analysis 41 to test the differences between the survival curves. Cox’s multivariate survival analysis was done using the Log likelihood ratio significance test in a forward stepwise manner. 42 The adequacy of the proportional hazards assumption was tested by logminlog plots. Overall survival (OS) analysis included as an event only the deaths due to malignant melanoma. Deaths due to postoperative complications within 30 days were excluded. Recurrence-free survival (RFS) was defined as the time elapsed between the primary treatment and the recurrent melanoma. For all statistical tests, probability values less than 0.05 were regarded as significant.
Results
Patient and Tumor Characteristics
The frequency distributions of the clinicopathological characteristics, as well as the follow-up and survival times between the original database (N = 369) 36 and the patients with valid material available for CD44 and HA stainings were almost identical (Table 1) ▶ . The mean follow-up time of all 292 patients was 6.3 ± 3.3 (SD) years (median, 5.4 years; range, 0.5–18 years).
Staining Patterns of CD44 and HA
The CD44 positivity (median value 90%) was confined to tumor cell membranes (Figure 1B) ▶ . Most tumors showed intense membranous and cytoplasmic HA-positivity (median value 70%), as did the consistently positive epidermis (Figure 1C) ▶ . The dermal stroma within and outside the tumor was always HA positive (Figure 1A) ▶ . The histograms of the cellular CD44 and HA levels are shown in Figure 2 ▶ and the cellular CD44 and HA levels and stromal HA intensity in different categories in Table 2 ▶ .
Table 2.
Expression of CD44 and Hyaluronan in Clinical Stage I Cutaneous Malignant Melanoma
| Staining | n | % |
|---|---|---|
| CD44 | ||
| 0–90% | 153 | 54 |
| 91–100% | 129 | 46 |
| Total | 282 | 100 |
| Cellular HA | ||
| 0–70% | 140 | 51 |
| 71–100% | 137 | 49 |
| Total | 277 | 100 |
| Stromal HA intensity | ||
| + | 43 | 15 |
| ++ | 218 | 79 |
| +++ | 16 | 6 |
| Total | 277 | 100 |
Correlations of CD44 and HA Stainings with Each Other and Markers of Disease Progression
CD44 positivity was strongly associated with cellular HA according to Spearman’s statistics (r = 0.309; P < 0.00005; N = 267) and a 2-sided Fisher’s exact test (P = 0.005). Decreasing levels of cancer cell-associated CD44 (Figure 1D) ▶ and HA (Figure 1E) ▶ were both related to increasing Breslow thickness (χ 2 = 20.5, P < 0.00005 for CD44 and χ 2 = 16.4, P = 0.001 for HA), increasing Clark level (χ 2 = 35.1, P < 0.00005 for CD44 and χ 2 = 26.5, P < 0.00005 for HA), and increasing pT category (χ2=34.8, P < 0.00005 for CD44 and χ2=19.1, P < 0.00005 for HA), and this trend was evenly distributed within Breslow’s thickness, Clark’s level and pT categories. Decreasing CD44 and HA levels also associated with bleeding (χ 2 = 7.4, P = 0.024 for CD44 and χ 2 = 9.0, P = 0.011 for HA) and with recurrent disease (χ 2 = 18.2, P < 0.00005 for CD44 and χ2=6.7, P = 0.01 for HA). Stromal HA intensity did not show statistically significant correlation with CD44 level or with any of the clinicopathological variables.
Univariate Survival Analysis
During the follow-up, 84/292 patients (29%) had a recurrence, 52 patients (18%) died of melanoma, and 40 patients (14%) died of other causes. The 5-year rates for crude, overall, and recurrence-free survivals were 78%, 86%, and 75%, respectively.
Reduced levels of CD44 (0 to 90% positively stained cancer cells) and cancer cell-associated HA (0 to 70% positively stained cancer cells) predicted short OS (P = 0.0077 and P = 0.0146, Figure 3 ▶ ) and short RFS (P = 0.0001 and P = 0.0141, Figure 4 ▶ ) (Table 3) ▶ . Reduced CD44 level predicted poor RFS also within the low-risk (≤1.5 mm) subgroup, (P = 0.0147, N = 127, other data not shown). Stromal HA intensity was not significantly related to OS or RFS. The conventional parameters predicting poor RFS and OS were high tumor thickness and high pT category (P < 0.00005 for both RFS and OS, respectively), high Clark’s level of invasion (P < 0.00005 for RFS and P = 0.0001 for OS), bleeding (P = 0.0001 for both RFS and OS) and male gender (P = 0.0292 for RFS and P = 0.0339 for OS) (Table 3) ▶ . In addition, the reduction of CD44 and HA staining associated fairly well with unfavorable prognosis also by using the 33rd and 66th percentiles of frequency distribution as cut off points (RFS: P = 0.0027 for HA and P = 0.001 for CD44; OS: P = 0.0632 for HA and P = 0.0112 for CD44, other data not shown).
Figure 3.

A: OS according to cell surface CD44 expression. N = 282. B: OS according to tumor cell associated HA expression. N = 277.
Figure 4.
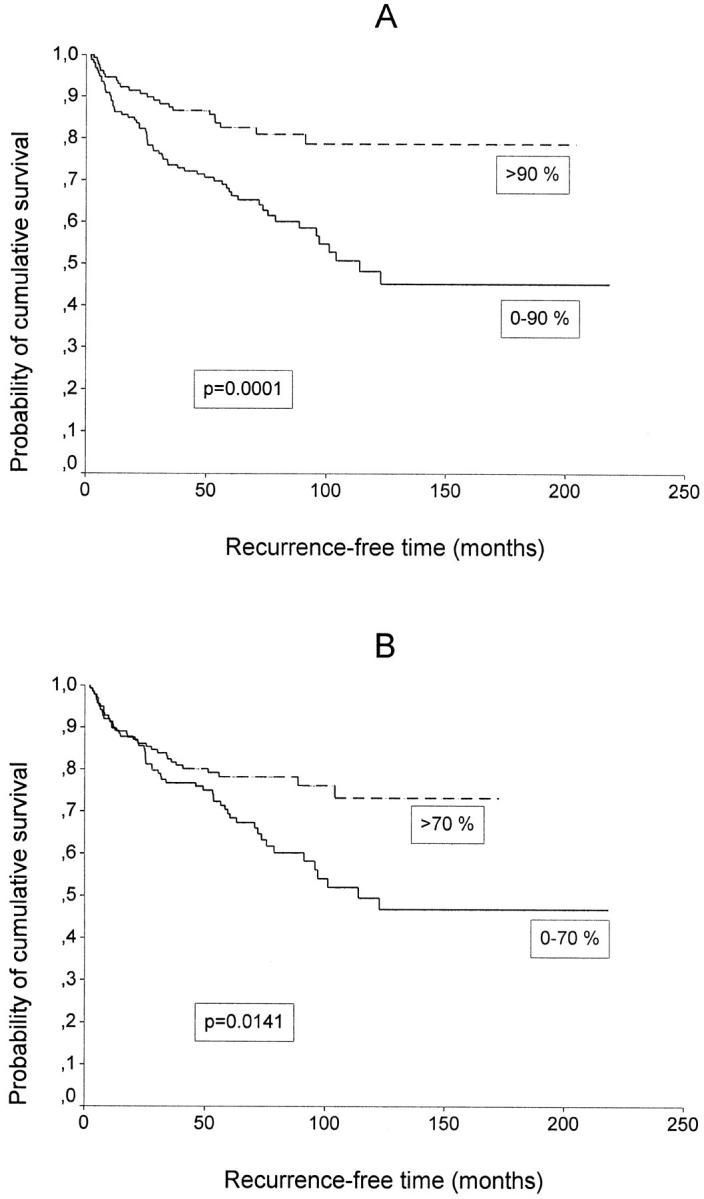
A: RFS according to cell surface CD44 expression. N = 282. B: RFS according to tumor cell associated HA expression. N = 277.
Table 3.
Clinical, Histological, Immunohistochemical, and Histochemical Factors Related to Survival in 292 Clinical Stage I Cutaneous Malignant Melanoma
| Category (variable) | No. of patients | 5-year RFS (95% CI) | P* | 5-year OS (95% CI) | P* |
|---|---|---|---|---|---|
| Sex | 0.0292 | 0.0339 | |||
| Male | 143 | 0.69 (0.60–0.76) | 0.81 (0.73–0.87) | ||
| Female | 149 | 0.80 (0.73–0.86) | 0.90 (0.83–0.94) | ||
| Bleeding | 0.0001 | 0.0001 | |||
| Yes | 76 | 0.65 (0.52–0.75) | 0.80 (0.68–0.88) | ||
| No | 119 | 0.86 (0.78–0.91) | 0.93 (0.86–0.97) | ||
| Unknown | 97 | 0.69 (0.59–0.78) | 0.81 (0.71–0.88) | ||
| Clark’s level | <0.00005 | 0.0001 | |||
| I | 12 | 0.88 (0.39–0.98) | 0.88 (0.39–0.98) | ||
| II | 54 | 0.98 (0.88–1.0) | 0.98 (0.87–1.0) | ||
| III | 82 | 0.79 (0.68–0.87) | 0.93 (0.84–0.97) | ||
| IV | 119 | 0.63 (0.54–0.71) | 0.79 (0.70–0.86) | ||
| V | 25 | 0.61 (0.38–0.78) | 0.63 (0.39–0.80) | ||
| Tumor thickness (mm) | <0.00005 | <0.00005 | |||
| ≤0.75 | 64 | 0.97 (0.87–0.99) | 0.96 (0.86–0.99) | ||
| 0.76–1.50 | 69 | 0.79 (0.66–0.87) | 0.93 (0.82–0.97) | ||
| 1.51–4.0 | 99 | 0.67 (0.57–0.76) | 0.82 (0.72–0.88) | ||
| >4.0 | 41 | 0.48 (0.31–0.63) | 0.60 (0.40–0.75) | ||
| TNM category | <0.00005 | <0.00005 | |||
| pT1-T2, N0, M0 | 131 | 0.91 (0.85–0.95) | 0.96 (0.90–0.99) | ||
| pT3, N0, M0 | 115 | 0.66 (0.56–0.74) | 0.81 (0.73–0.88) | ||
| pT4, N0, M0 | 46 | 0.52 (0.36–0.66) | 0.63 (0.44–0.77) | ||
| CD44 | 0.0001 | 0.0077 | |||
| ≤90% | 153 | 0.67 (0.59–0.74) | 0.81 (0.74–0.87) | ||
| >90% | 129 | 0.83 (0.75–0.88) | 0.89 (0.82–0.94) | ||
| Cellular HA | 0.0141 | 0.0146 | |||
| ≤70% | 140 | 0.69 (0.61–0.76) | 0.82 (0.74–0.87) | ||
| >70% | 137 | 0.78 (0.70–0.84) | 0.88 (0.80–0.93) |
Multivariate Survival Analysis
Cox’s multivariate analysis of 251 patients with a complete set of data included variables that significantly predicted univariate survival, except pT category because it includes Clark’s level and tumor thickness. Tumor thickness (P = 0.0008) and bleeding (P = 0.0411) were associated with short OS. Reduced CD44 level was an independent predictor of short RFS (P = 0.0308). Other independent predictors of short RFS were tumor thickness (P = 0.001) and bleeding (P = 0.0346) (Table 4) ▶ .
Table 4.
Independent Predictors of Overall Survival and Recurrence-Free Survival in Cox’s Analysis
| Category | Hazard rate | 95% CI | P* |
|---|---|---|---|
| Overall survival | |||
| Tumor thickness (>1.5 mm vs. ≤1.5 mm) | 3.41 | 1.67–7.01 | 0.0008 |
| Bleeding (yes vs. no) | 2.45 | 1.04–5.78 | 0.0411 |
| Recurrence-free survival | |||
| Tumor thickness (>1.5 mm vs. ≤1.5 mm) | 2.46 | 1.44–4.21 | 0.001 |
| CD44 expression (≤90 vs. >90%) | 1.74 | 1.05–2.87 | 0.0308 |
| Bleeding (yes vs. no) | 1.93 | 1.05–3.56 | 0.0346 |
Discussion
The clinical role of CD44 and HA in primary cutaneous melanoma has remained obscure in previous clinical reports due to divergent results and relatively small materials that do not enable multivariate analysis. 27-35 As far as we know, the current series consisting of patients with localized disease is decisively larger than any previous report on the CD44 level and prognosis of cutaneous melanoma. Further, we have included novel data on the level of HA, the main ligand of CD44. Our findings demonstrate that a reduced level of cell surface CD44 and associated HA within the primary tumor correlates with subsequent disease progression and short survival. In addition, reduced level of CD44 acts as an independent, unfavorable prognostic factor.
There are no presettled criteria for selecting the cut off points for CD44 and HA positivities in cutaneous melanoma. We chose the median values, because they can be used without introducing a statistical bias, 40 and because they easily divided the material into two groups of equivalent size. Dichotomizing continuous variables according to a single more or less arbitrary percentile may discard important relationships. However, using the 33rd and 66th percentiles of frequency distribution as HA and CD44 positivity cut off points separated the present series into significantly different prognostic groups almost as effectively as the median (data not shown), thus strengthening our results. The conventional parameters that predicted poor RFS and OS in this series have also been established in most prognostic studies worldwide, 3,43-45 indicating that the current material was comparable with previous ones. Thus, we believe that the present findings significantly contribute to the current understanding in this area.
The present results on clinical tumors apparently contrast with those of previous experimental studies, in which the growth or metastatic capacity of melanoma cells is inhibited by breaking the CD44-HA interaction with CD44 antibodies, 23 soluble IgG fusion proteins 22 or hyaluronan oligomers. 26 Considering that cutaneous melanomas evolve in basal epidermis, surrounded by keratinocytes with high levels of CD44 46,47 and an environment rich in HA, 47 results on CD44 and HA metabolism in melanoma cell lines may not be comparable to the clinical behavior of human melanoma. The genetically labile and heterogeneous nature of cells within a single tumor and between tumors may further lead to selection of cell lines that do not represent the expression pattern in vivo. 48,49
Both high 50-52 and reduced 53-55 CD44 expression levels have been associated with cancer growth and adverse prognosis in various malignant tumors, supporting the concept that the growth regulation of cancer cells by CD44 is highly dependent on the cellular background. 53,56 Supporting our findings, CD44 expression diminished with increasing invasiveness of primary tumors 30,31,34 and in metastatic melanomas. 33 However, in the only prognostic study concerning CD44 expression in cutaneous melanoma, high CD44 level in primary malignant melanomas (n = 92) associated with progressive disease and poor univariate survival. 32
Although elevated concentrations of HA have been found in several human cancers, 8,57 there are relatively few studies in which HA has been detected in specific locations of the cancer tissue architecture. 58-63 Two categories have arisen among the malignancies studied with this kind of assay. In one of them, comprising malignancies of hyaluronan-poor monolayered epithelia such as that of colon, 58 breast 59 and ovary, 60 abnormally increased hyaluronan is strongly associated with unfavorable prognosis. The other category with normally hyaluronan positive squamous epithelia, like that in the esophageal, 61 laryngeal 62 and lung 63 carcinoma, show an opposite trend where loss of cell surface HA is associated with poor differentiation, 61,63 metastasis and poor survival. 62 The present study indicates that cutaneous melanoma also belongs to the latter category.
The mechanism by which CD44 (and HA) influence the clinical outcome of cutaneous melanoma patients in the present series remains to be solved. In vitro, hypoxia leads to down-regulation of CD44 and subsequent melanoma cell detachment, which, on reoxygenation, is followed by up-regulation of CD44, cell reattachment, and growth. 64 Melanoma cells may thus modulate their CD44/HA function to enable initial cell detachment (down-regulation) 64 and later formation of metastatic deposits (up-regulation), 6,21-23 depending on environmental factors such as oxygen content. 64 Cell surface CD44 and HA maintain adhesive restraints between the cells in normal epidermis, 46,47,65,66 and may mediate a tumor suppressive effect also in primary cutaneous melanoma by the same mechanism. It should be noticed that the metastasis suppression by the standard CD44 isoform can be independent of its ability to bind to hyaluronate and may require also other ligands. 67 In addition, cell surface CD44, with its ligand HA, may suffer as victims of enhanced proteolysis, and diffuse out of the tissue or be subjects of premature endocytosis and degradation, as discussed earlier. 62
Restoration of CD44H expression in colon carcinomas has been observed to reduce tumorigenicity in vitro and in vivo. 68 Because of the possibility that the biological role of CD44 may change depending on the cellular environment, the use of CD44 in gene transfection treatments might be complicated in melanoma. Defining how the malignant cells regulate their CD44 expression and its affinity for HA or other ligands could provide alternative ways to interfere with tumor cell spreading. Meanwhile, quantification of CD44 in the primary cutaneous melanoma offers a reproducible and available prognostic tool for clinical practice, for instance to select patients for more aggressive therapy.
Acknowledgments
The authors thank Ms Aija Parkkinen, Ms Riikka Eskelinen, Ms Tiina Räsänen, Ms Eija Rahunen, Mr Kari Kotikumpu, and Mr Alpo Pelttari for their skillful technical assistance. The statistical assistance from Ms Pirjo Halonen is gratefully acknowledged.
Footnotes
Address reprint requests to Veli-Matti Kosma, M.D., Ph.D., Department of Pathology and Forensic Medicine, University of Kuopio, P.O. Box 1627, FIN-70211 Kuopio, Finland. E-mail: velimatti.kosma@uku.fi.
Supported by grants from the Cancer Fund of North Savo (Savon Syöpärahasto), The Paavo Koistinen Foundation, The Finnish Cancer Foundation, The Finnish Medical Society Duodecim, and by Special Government Funding of Kuopio University Hospital, Kuopio, Finland.
References
- 1.Berwick M, Halpern A: Melanoma epidemiology. Curr Opin Oncol 1997, 9:178-182 [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood JM, Resnick GD, Cole BF: Efficacy, safety and risk-benefit analysis of adjuvant interferon α-2b in melanoma. Semin Oncol 1997, 24:(Suppl 4):S16–23 [PubMed]
- 3.Balch CM, Cascinelli N, Drzewiecki KT, Eldh J, MacKie RM, McCarthy WH, McLeod GR, Morton DL, Seigler HF, Shaw HM, Sim FH, Sober AJ, Soong S, Takematsu H, Tonak J, Wong J: A Comparison of Prognostic Factors Worldwide: Cutaneous Melanoma, ed 2 Balch CM Houghton AN Milton GW Sober AJ Soong S-J eds. 1992, :pp 188-199 Lippincott, Philadelphia [Google Scholar]
- 4.Mellado B, Colomer D, Castel T, Munoz M, Carballo E, Galan M, Mascaro JM, Vives-Corons JL, Grau JJ, Estape J: Detection of circulating neoplastic cells by reverse-transcriptase polymerase chain reaction in malignant melanoma: association with clinical stage and prognosis. J Clin Oncol 1996, 14:2091-2097 [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH: Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the eastern cooperative oncology group trial EST 1684. J Clin Oncol 1996, 14:7-17 [DOI] [PubMed] [Google Scholar]
- 6.Naor D, Sionov RV, Ish-Shalom D: CD44: Structure, function, and association with the malignant process. Adv Cancer Res 1997, 71:241-319 [DOI] [PubMed] [Google Scholar]
- 7.Haynes BF, Liao H-X, Patton KL: The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells (Cold Spring Harbor) 1991, 3:347–350 [PubMed]
- 8.Catterall JB, Garner MJ, Turner GA: Hyaluronic acid, cell adhesion and metastasis. Cancer J 1995, 8:320-330 [DOI] [PubMed] [Google Scholar]
- 9.Laurent TC, Fraser JRE: Hyaluronan. FASEB J 1992, 6:2397-2404 [PubMed] [Google Scholar]
- 10.Toole B: Glycosaminoglycans in morphogenesis. Hay E eds. Cell Biology of the Extracellular Matrix. 1981, :pp 259-294 Plenum Press, New York [Google Scholar]
- 11.Mast B, Diegelmann R, Krummel T, Cohen I: Scarless wound healing in the mammalian fetus. Surg Gynecol Obstet 1992, 174:441-451 [PubMed] [Google Scholar]
- 12.Kosaki R, Watanabe K, Yamaguchi Y: Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res 1999, 59:1141-1145 [PubMed] [Google Scholar]
- 13.Pauli BU, Knudson W: Tumor invasion: a consequence of destructive and compositional matrix alterations. Hum Pathol 1988, 19:628-639 [DOI] [PubMed] [Google Scholar]
- 14.Thomas L, Etoh T, Stamenkovic I, Mihm MC, Byers HR: Migration of human melanoma cells on hyaluronate is related to CD44 expression. J Invest Dermatol 1993, 100:115-120 [DOI] [PubMed] [Google Scholar]
- 15.Peck D, Isacke CM: CD44 phosphorylation regulates melanoma cell and fibroblast migration on, but not attachment to, a hyaluronan substratum. Curr Biol 1996, 6:884-890 [DOI] [PubMed] [Google Scholar]
- 16.Knudson CB, Knudson W: Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J 1993, 7:1233-1241 [PubMed] [Google Scholar]
- 17.Zhang L, Underhill CB, Chen L: Hyaluronan on the surface of tumor cells is correlated with metastatic behavior. Cancer Res 1995, 55:428-433 [PubMed] [Google Scholar]
- 18.van Muijen GNP, Danen EHJ, Veerkamp JH, Ruiter DJ, Lesley J, van den Heuwel LPW: Glycoconjugate profile and CD44 expression in human melanoma cell lines with different metastatic capacity. Int J Cancer 1995, 61:241-248 [DOI] [PubMed] [Google Scholar]
- 19.Cannistra S, Kansas G, Niloff J, DeFranzo B, Kim Y, Ottensmeier C: Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res 1993, 53:3830-3838 [PubMed] [Google Scholar]
- 20.Gardner M, Catterall J, Jones L, Turner G: Human ovarian tumor cells can bind hyaluronic acid via membrane CD44: a possible step in peritoneal metastasis. Clin Exp Metastasis 1996, 14:325-334 [DOI] [PubMed] [Google Scholar]
- 21.Birch M, Mitchell S, Hart IR: Isolation and characterization of human melanoma cell variants expressing high and low levels of CD44. Cancer Res 1991, 51:6660-6667 [PubMed] [Google Scholar]
- 22.Bartolazzi A, Peach R, Aruffo A, Stamenkovic I: Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med 1994, 180:53-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y, Ma J, Wang J-H, Che X, Narula J, Bigby M, Wu M, Sy M-S: Inhibition of human melanoma growth and metastasis in vivo by anti-CD44 monoclonal antibody. Cancer Res 1994, 54:1561-1565 [PubMed] [Google Scholar]
- 24.Price EA, Coombe DR, Murray JC: Endothelial CD44H mediates adhesion of a melanoma cell line to quiescent human endothelial cells in vitro. Int J Cancer 1996, 65:513-518 [DOI] [PubMed] [Google Scholar]
- 25.Zawadzki V, Perschl A, Rösel L, Hekele A, Zöller M: Blockade of metastasis formation by CD44-receptor globulin. Int J Cancer 1998, 75:919-924 [DOI] [PubMed] [Google Scholar]
- 26.Zeng C, Toole BP, Kinney SD, Kuo J-w, Stamenkovic I: Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer 1998, 77:396–401 [DOI] [PubMed]
- 27.Manten-Horst E, Danen EHJ, Smit L, Snoek M, Le Poole C, Van Muijen GNP, Pals ST, Ruiter DJ: Expression of CD44 splice variants in human cutaneous melanoma and melanoma cell lines is related to tumour progression and metastatic potential. Int J Cancer 1995, 64:182-188 [DOI] [PubMed] [Google Scholar]
- 28.Simon JC, Heider K-H, Dietrich A, Wuttig C, Schöpf E, Adolf GR, Ponta H, Herrlich P: Expression of CD44 isoforms in human skin cancer. Eur J Cancer 1996, 32A:1394-1400 [DOI] [PubMed] [Google Scholar]
- 29.Seiter S, Schadendorf D, Herrman K, Schneider M, Rösel M, Arch R, Tilgen W, Zöller M: Expression of CD44 variant isoforms in malignant melanoma. Clin Cancer Res 1996, 2:447-456 [PubMed] [Google Scholar]
- 30.Leigh CJ, Palechek PL, Knutson JR, McCarthy JB, Cohen MB, Argenyi ZB: CD44 expression in benign and malignant nevomelanocytic lesions. Hum Pathol 1996, 27:1288-1294 [DOI] [PubMed] [Google Scholar]
- 31.Harwood CA, Green MA, Cook MG: CD44 expression in melanocytic lesions: a marker of malignant progression? Br J Dermatol 1996, 135:876-882 [DOI] [PubMed] [Google Scholar]
- 32.Dietrich A, Tanczos W, Vansceidt W, Schöpf E, Simon JC: High CD44 surface expression of primary tumours of malignant melanoma correlates with increased metastatic risk and reduced survival. Eur J Cancer 1997, 33:926-930 [DOI] [PubMed] [Google Scholar]
- 33.Seelentag WKF, Böni R, Günthert U, Futo E, Burg G, Heitz PU, Roth J: Expression of CD44 isoforms and beta1,6-branched oligosaccharides in human malignant melanoma is correlated with tumour progression but not with metastatic potential. J Cutan Pathol 1997, 24:206-211 [DOI] [PubMed] [Google Scholar]
- 34.Ichikawa T, Masumoto J, Kaneko M, Saida T, Sagara J, Taniguchi S: Moesin and CD44 expression in cutaneous melanocytic tumours. Br J Dermatol 1998, 138:763-768 [DOI] [PubMed] [Google Scholar]
- 35.Schaider H, Soyer HP, Heider K-H, Hofmann-Wellenhof R, Zatloukal K, Smolle J, Kerl H: CD44 variants in melanocytic skin neoplasms. J Cutan Pathol 1998, 25:199-203 [DOI] [PubMed] [Google Scholar]
- 36.Karjalainen JM, Kellokoski JK, Eskelinen MJ, Alhava EM, Kosma V-M: Downregulation of transcription factor AP-2 predicts poor survival in stage I cutaneous malignant melanoma. J Clin Oncol 1998, 16:3584-3591 [DOI] [PubMed] [Google Scholar]
- 37.Fox S, Fawcett J, Jackson D, Collins I, Gatter KC, Harris AL, Gearing A, Simmons DL: Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res 1994, 54:4539-4546 [PubMed] [Google Scholar]
- 38.Tammi R, Ågren U, Tuhkanen AL, Tammi M: Hyaluronan metabolism in skin. Prog Histochem Cytochem 1994, 29:1–77 [DOI] [PubMed]
- 39.Tammi R, Ripellino JA, Margolis RU, Tammi M: Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol 1988, 90:412-414 [DOI] [PubMed] [Google Scholar]
- 40.Simon R, Altman DG: Statistical aspects of prognostic factor studies in oncology. Br J Cancer 1994, 69:979-985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958, 53:457-481 [Google Scholar]
- 42.Cox DR: Regression models and life tables with discussion. J Stat Soc B 1972, 34:187-192 [Google Scholar]
- 43.Buzaid AC, Ross MI, Balch CM, Soong S, McCarthy WH, Tinoco L, Mansfield P, Lee JE, Bedikian A, Eton O, Plager C, Papadopoulos N, Legha SS, Benjamin RS: Critical analysis of the current American Joint Committee on Cancer staging system for cutaneous melanoma and proposal of a new staging system. J Clin Oncol 1997, 15:1039-1051 [DOI] [PubMed] [Google Scholar]
- 44.Balch C: Cutaneous melanoma: Prognosis and treatment results worldwide. Semin Surg Oncol 1992, 8:400-414 [DOI] [PubMed] [Google Scholar]
- 45.Vollmer R: Malignant melanoma. A multivariate analysis of prognostic factors. Pathol Annu 1989, 24:383-407 [PubMed] [Google Scholar]
- 46.Seelentag WKF, Günthert U, Saremaslani P: CD44 standard and variant isoform expression in normal human skin appendages and epidermis. Histochem Cell Biol 1996, 106:283-289 [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Tammi M, Tammi R: Distribution of hyaluronan and its CD44 receptor in the epithelia of human skin appendages. Histochemistry 1992, 98:105-112 [DOI] [PubMed] [Google Scholar]
- 48.Levesque MC, Haynes BF: In vitro culture of human peripheral blood monocytes induces hyaluronan binding and up-regulates monocyte variant CD44 isoform expression. J Immunol 1996, 156:1557-1565 [PubMed] [Google Scholar]
- 49.Haramaki M, Yano H, Fukuda K, Momosaki S, Ogasawara S, Kojiro M: Expression of CD44 in human hepatocellular carcinoma cell lines. Hepatology 1995, 21:1276-1284 [PubMed] [Google Scholar]
- 50.Mayer B, Jauch KW, Günthert U, Figdor CG, Schildberg FW, Funke I, Johnson JP: De-novo expression of CD44 and survival in gastric cancer. Lancet 1993, 342:1019-1022 [DOI] [PubMed] [Google Scholar]
- 51.Speiser P, Wanner C, Tempfer C, Mittelböck M, Hanzal E, Bancher-Todesca D, Gitsch G, Reinthaller A, Kainz C: CD44 is an independent prognostic factor in early-stage cervical cancer. Int J Cancer 1997, 74:185-188 [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi A, Urano T, Goi T, Saito M, Takeuchi K, Hirose K, Nakagawara G, Shiku H, Furukawa K: Expression of CD44 variant containing exons 8 to 10 is a useful independent factor for the prediction of prognosis in colorectal cancer patients. J Clin Oncol 1996, 14:1122-1127 [DOI] [PubMed] [Google Scholar]
- 53.Noordzij MA, van Steenbrugge G-J, Verkaik NS, Schröder FH, van der Kwast TH: The prognostic value of CD44 isoforms in prostate cancer patients treated by radical prostatectomy. Clin Cancer Res 1997, 3:805-815 [PubMed] [Google Scholar]
- 54.Friedrichs K, Franke F, Lisboa B-W, Kügler G, Gille I, Terpe H-J, Hölzel F, Maass H, Günthert U: CD44 isoforms correlate with cellular differentiation but not with prognosis in human breast cancer. Cancer Res 1995, 55:5424-5433 [PubMed] [Google Scholar]
- 55.Favrot MC, Combaret V, Lasset C: CD44—a new prognostic marker for neuroblastoma (letter). N Engl J Med 1993, 329:1965. [DOI] [PubMed] [Google Scholar]
- 56.Goodison S, Tarin D: Current status of CD44 variant isoforms as cancer diagnostic markers. Histopathology 1998, 32:1-6 [DOI] [PubMed] [Google Scholar]
- 57.Knudson W: Tumor-associated hyaluronan: providing an extracellular matrix that facilitates invasion. Am J Pathol 1996, 148:1721-1726 [PMC free article] [PubMed] [Google Scholar]
- 58.Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, Ågren U, Alhava E, Kosma V-M: Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res 1998, 58:342–347 [PubMed]
- 59.Auvinen P, Tammi R, Parkkinen J, Tammi M, Ågren U, Johansson R, Hirvikoski P, Eskelinen M, Kosma V-M: Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol 2000, 156:529–536 [DOI] [PMC free article] [PubMed]
- 60.Anttila M, Tammi R, Tammi M, Syrjänen K, Saarikoski S, Kosma V-M: High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res 2000, 60:150-155 [PubMed] [Google Scholar]
- 61.Wang C, Tammi M, Guo H, Tammi R: Hyaluronan distribution in the normal epithelium of esophagus, stomach, and colon and their cancers. Am J Pathol 1996, 148:1861-1869 [PMC free article] [PubMed] [Google Scholar]
- 62.Hirvikoski P, Tammi R, Kumpulainen E, Virtaniemi J, Parkkinen J, Tammi M, Johansson R, Ågren U, Karhunen J, Kosma V-M: Irregular expression of hyaluronan and its CD44 receptor is associated with metastatic phenotype in laryngeal squamous cell carcinoma. Virchows Arch 1999, 434:37–44 [DOI] [PubMed]
- 63.Pirinen R, Tammi R, Tammi M, Pääkkö P, Parkkinen J, Ågren U, Johansson R, Viren M, Törmänen U, Soini Y, Kosma V-M: Expression of hyaluronan in normal and dysplastic bronchial epithelium and in squamous cell carcinoma of the lung. Int J Cancer 1998, 79:251–255 [DOI] [PubMed]
- 64.Hasan NM, Adams GE, Joiner MC, Marshall JF, Hart IR: Hypoxia facilitates tumour cell detachment by reducing expression of surface adhesion molecules and adhesion to extracellular matrices without loss of cell viability. Br J Cancer 1998, 77:1799-1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soukka T, Salmi M, Joensuu H, Häkkinen L, Sointu P, Koulu L, Kalimo K, Klemi P, Grenman R, Jalkanen S: Regulation of CD44v6-containing isoforms during proliferation of normal and malignant epithelial cells. Cancer Res 1997, 57:2281-2289 [PubMed] [Google Scholar]
- 66.Milstone LM, Hough-Monroe L, Kugelman LC, Bender JR, Haggerty JG: Epican, a heparan/chondroitin sulfate proteoglycan form of CD44, mediates cell-cell adhesion. J Cell Sci 1994, 107:3183-3190 [DOI] [PubMed] [Google Scholar]
- 67.Gao AC, Lou W, Sleeman JP, Isaacs JT: Metastasis suppression by the standard CD44 isoform does not require the binding of prostate cancer cells to hyaluronate. Cancer Res 1998, 58:2350-2352 [PubMed] [Google Scholar]
- 68.Tanabe KK, Stamenkovic I, Cutler M, Takahashi K: Restoration of CD44H expression in colon carcinomas reduces tumorigenicity. Ann Surg 1995, 222:493-503 [DOI] [PMC free article] [PubMed] [Google Scholar]