Inducible Costimulator Protein (Icos) Controls T Helper Cell Subset Polarization after Virus and Parasite Infection (original) (raw)
Abstract
It has been shown that certain pathogens can trigger efficient T cell responses in the absence of CD28, a key costimulatory receptor expressed on resting T cells. Inducible costimulator protein (ICOS) is an inducible costimulator structurally and functionally related to CD28. Here, we show that in the absence of CD28 both T helper cell type 1 (Th1) and Th2 responses were impaired but not abrogated after infection with lymphocytic choriomeningitis virus (LCMV), vesicular stomatitis virus (VSV), and the nematode Nippostrongylus brasiliensis. Inhibition of ICOS in CD28-deficient mice further reduced Th1/Th2 polarization. Blocking of ICOS alone had a limited but significant capacity to downregulate Th subset development. In contrast, cytotoxic T lymphocyte (CTL) responses, which are regulated to a minor and major extent by CD28 after LCMV and VSV infection, respectively, remained unaffected by blocking ICOS. Together, our results demonstrate that ICOS regulates both CD28-dependent and CD28-independent CD4+ subset (Th1 and Th2) responses but not CTL responses in vivo.
Keywords: ICOS, CD28, Th1/Th2, Nippostrongylus brasiliensis, LCMV
Introduction
Efficient induction of T cell responses requires antigen-specific triggering of TCRs together with the engagement of costimulatory molecules such as CD28 and accessory molecules such as LFA-1 and CD2. Although the generation of T cell responses against most model antigens requires CD28-mediated costimulation, many pathogens, including lymphocytic choriomeningitis virus (LCMV) 1, vaccinia virus 2, Heligmosomoides polygyrus 3, and Leishmania major 4, have been shown to trigger efficient T cell responses in CD28-deficient mice. Various factors may contribute to these CD28-independent T cell responses. Activation of the innate immune system by pathogen-associated molecular components can augment T cell responses by activation of APCs via pattern recognition receptors, which may result in enhanced antigen processing and presentation, cytokine production, and extended T cell stimulation due to prolonged APC survival 5 6. In addition, a variety of accessory receptors other than CD28 can stimulate specific T cell responses, including heat-stable antigen (HSA 7), 4-1BB 8, and OX40 9. Extended T cell stimulation by pathogens due to slow antigen clearance has also been shown to regulate the magnitude of CD28-independent CTL responses 2. Thus, activation of APCs, the presence of alternative costimulation pathways, and duration of T cell stimulation may all contribute to CD28-independent T cell responses after infection.
Inducible costimulator protein (ICOS), a surface receptor expressed on activated T cells, is a good candidate for such a CD28-independent pathway of T cell activation, since it is a close homologue of CD28 and does not interact with B7-1 and B7-2 10. Cross-linking of ICOS together with CD3/TCR has been shown to augment the production of Th1 and Th2 cytokines with the exception of IL-2 10 11. More recently, murine ICOS has also been cloned by subtractive hybridization PCR analysis of Th1 versus Th2 effector cells, since it was specifically expressed by Th2 cells (Coyle, A.J., and J.-C. Gutierrez-Ramos, manuscript submitted for publication).
To assess the role of ICOS in T cell activation in vivo, we studied the generation of parasite-induced Th2 and virus-induced Th1 and CTL responses in the absence of functional ICOS and/or CD28. Our results indicate that both ICOS- and CD28-mediated signaling contribute to CD4+ effector responses against parasite and viral antigens, although the relative requirement for these receptors depends on the nature of the immune response. In contrast, ICOS did not play a significant role in the induction of a CTL response.
Materials and Methods
Mice and Pathogens.
BALB/c and C57BL/6 mice were provided by IFFA-Credo. CD28−/− mice (1; provided by T.W. Mak, University of Toronto, Toronto, Ontario, Canada) were backcrossed for more than six generations to both C57BL/6 and BALB/c mice, and maintained in a facility free of specific pathogens at the Basel Institute for Immunology. The LCMV isolate WE was grown on L cells at a low multiplicity of infection. LCMV (200 PFU/mouse) was injected into the left footpad. Vesicular stomatitis virus (VSV) serotype Indiana (obtained from P. Ohashi, University of Toronto, Toronto, Ontario, Canada) was grown on BHK cells at a low multiplicity of infection. Mice were infected intravenously with 2 × 106 PFU. The life cycle of the nematode Nippostrongylus brasiliensis was maintained by passage in Tif Rai rats. Mice were infected subcutaneously with 750 third stage infective larvae (L3).
Generation of ICOS-Ig.
Murine ICOS has been cloned by subtractive hybridization PCR of Th1 and Th2 cells (Coyle, A.J., and J.-C. Gutierrez-Ramos, manuscript submitted for publication). The DNA sequence encoding the extracellular part of murine ICOS was amplified by PCR and cloned into an expression vector containing the CD5 signal sequence and the human IgG1 constant region (Fc). Sequencing of the resulting construct confirmed in-frame ICOS coding region and the absence of a mutation. Subsequently, COS cells were transiently transfected with the plasmid using lipofectamine (GIBCO BRL). The produced ICOS-Ig fusion molecule was purified using a protein A column. SDS-PAGE analysis demonstrated a purity >90%. Human IgG1 (Sigma-Aldrich) was used as control in all experiments.
Bronchoalveolar Lavage.
_N. brasiliensis_–infected mice were killed by CO2 7 d after infection, and the tracheae were cannulated with a 22-G needle surrounded by a plastic tubing (Polyethylene [PE] 60 Intramedic Tubing; Becton Dickinson). PBS (0.3 ml) was injected and withdrawn repeatedly (four times) with a syringe. Bronchoalveolar lavage (BAL) cells were harvested and used for flow cytometry analysis.
Expression of Intracellular Cytokines.
To analyze intracellular cytokine expression, cells were stimulated with PMA (10−7 M) and ionomycin (1 μg/ml) for 4 h. 2 h before harvesting, brefeldin A (10 μg/ml) was added to cultures to retain cytokines in the cytoplasm. Subsequently, cells were incubated with anti-CD32 to block Fc binding followed by staining with allophycocyanin-labeled anti-CD4 and FITC-labeled anti-CD8 antibodies for 15 min at 4°C. Subsequently, cells were washed once with PBS, then fixed with 2% paraformaldehyde for 30 min at room temperature followed by intracellular staining in permeabilization buffer containing 0.5% saponin, 1% BSA in PBS, and either PE-labeled anti–IL-4, PE-labeled anti–IL-10, or PE-labeled anti–IFN-γ antibodies (all from BD PharMingen). Cells were washed and resuspended in 1% BSA/PBS solution and analyzed by flow cytometry. Data were analyzed using CellQUEST™ software (Becton Dickinson).
Proliferation and Cytokine Production of Virus-specific CD4+ T Cells.
Mice were killed at day 12 after LCMV infection, or at day 6 after VSV infection. Spleens were removed, and subsequently CD4+ T cells were purified by MACS® according to the instructions of the supplier (Miltenyi Biotec). Purity was >95%. CD4+ T cells (105) were stimulated with irradiated splenocytes (105) pulsed with LCMV (highest concentration = multiplicity of infection = 0.3) or UV light–inactivated VSV (highest concentrations = 3 × 107 PFU/ml). After 4 d, an aliquot of the supernatant was removed for measurement of IFN-γ levels, and proliferation was assessed by [3H]thymidine incorporation.
Footpad Swelling and Measurement of CTL Responses.
Mice were immunized with LCMV (200 PFU) into one footpad, and footpad size was assessed daily using a spring-loaded caliper. 12 d after infection, spleen cell suspensions were prepared and tested directly in a 51Cr-release assay, essentially as described 12 using peptide p33 (derived from the LCMV glycoprotein, amino acids 33–42) labeled EL-4 cells as target cells. In brief, EL-4 target cells were pulsed with peptide p33 (KAVYNFATM, amino acids 33–42 derived from the LCMV glycoprotein) at a concentration of 10−7 M for 90 min at 37°C in the presence of [51Cr]sodium chromate in IMDM supplemented with 10% FCS. Ex vivo–isolated spleen cell suspensions from infected mice were serially diluted and mixed with peptide-pulsed target cells. 51Cr release was determined after 5 h in a gamma counter.
Alternatively, mice were infected intravenously with 2 × 106 PFU VSV, and spleen cells were isolated 6 d later and tested in a 51Cr-release assay on EL-4 cells pulsed with peptide NP derived from VSV nucleoprotein 12.
Generation and Use of Tetramers.
Soluble, biotinylated class I monomers, comprising the murine Db molecule, human β2-microglobulin, and LCMV peptide p33, were generated as described previously 13. Tetramer complexes were subsequently generated by stepwise addition of PE-labeled extravidin (Sigma-Aldrich) to the biotinylated monomers at a 1:4 molar ratio. Single cell suspensions were prepared from spleens and incubated with PE-conjugated tetramers at 37°C for 15 min. Allophycocyanin-conjugated anti-CD8 antibodies were then added on ice for 30 min. Cells were washed and analyzed on a FACScan™ (Becton Dickinson) using CellQUEST™ software.
Detection of Antibodies by ELISA.
Antibodies against the NP of LCMV were detected by an ELISA. Recombinant NP expressed by baculovirus (14; a gift from Rolf Zinkernagel (Institute of Experimental Immunology, Zürich, Switzerland), was coated onto a 96-well plate (Maxisorp; Nunc) in a concentration of 200 ng/well in 50 μl PBS overnight at 4°C. Subsequent steps were performed at room temperature and followed by three washing procedures with PBS. Coated plates were blocked with 1% BSA for 1–2 h, and sera serially diluted in PBS containing 0.1% BSA were then added for 3–5 h. Alkaline phosphatase–labeled goat anti–mouse antibodies to IgG1, IgG2a, or IgG2b (Southern Biotechnology Associates, Inc.) were added for 1–2 h followed by the addition of _p_-nitrophenyl phosphate (Sigma-Aldrich) as substrate before reading the OD at 405 nm. Positive titers were defined as three standard deviations above mean values of negative controls.
Results
ICOS Mediates CD28-independent Priming of Th2 Cells.
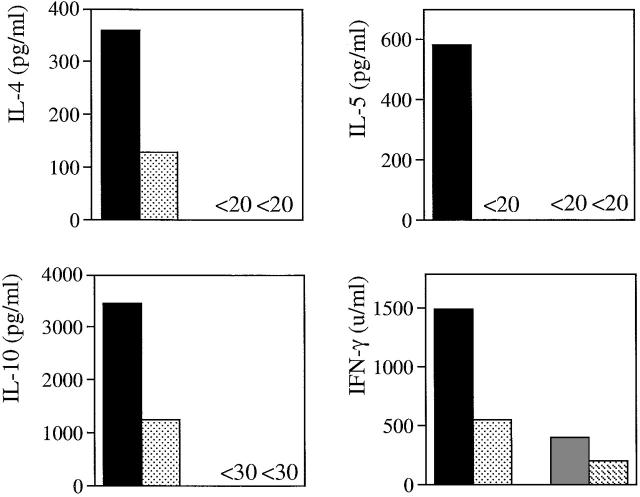
Infection with the gastrointestinal nematode N. brasiliensis is a well-characterized model used to study Th2 responses 15. Clearance of worms is critically dependent on the IL-4/IL-13 pathway 16 17 18. Reduced Th2 responses have been reported in N. _brasiliensis_–infected mice treated with CTLA-4-Ig, whereas Th2 responses were completely normal in CD28-deficient mice infected with H. polygyrus, another gastrointestinal nematode 3. To study the role of ICOS for the generation of Th subset responses in the presence and absence of CD28, both wild-type BALB/c and CD28-deficient (BALB/c) mice were infected with third stage (L3) larvae of N. brasiliensis and were additionally treated with an ICOS-Ig fusion molecule or control human IgG1 antibodies. Mesenteric lymph node cells were isolated at day 7 and restimulated in vitro with a protein extract derived from mature worms to measure cytokine production. As expected, BALB/c mice produced the Th2 cytokines IL-4, IL-5, and IL-10, and also the Th1 cytokine IFN-γ. Inhibition of ICOS in BALB/c mice resulted in reduced Th2 and Th1 cytokine production (Fig. 1). In the absence of CD28, levels of IL-4, IL-5, IL-10, and IFN-γ were either absent or strongly reduced (Fig. 1). Therefore, no conclusion could be reached as to the potential of ICOS to mediate CD28-independent T cell responses. Nevertheless, these results indicate that both ICOS and CD28 promote the generation of Th2 and Th1 responses to nematode-specific antigens.
Figure 1.
ICOS and CD28 contribute to nematode-induced Th1 and Th2 cytokine production in draining lymph node cells. CD28−/− and BALB/c control mice (n = 8/group) were infected subcutaneously with N. brasiliensis (750 larvae L3) and treated intravenously with 100 μg ICOS-Ig (n = 4) or control IgG1 (n = 4) at days 0, 2, 4, and 6. At day 7, mice were killed and mesenteric lymph node cells were isolated and stimulated in pools at a density of 5 × 105/microwell with specific antigen (N. brasiliensis protein extract, 75 μg/well) for 4 d. Levels of IL-4, IL-5, IL-10, and IFN-γ in the supernatants were measured by ELISA using paired mAbs (BD PharMingen). Data are representative of two separate experiments.
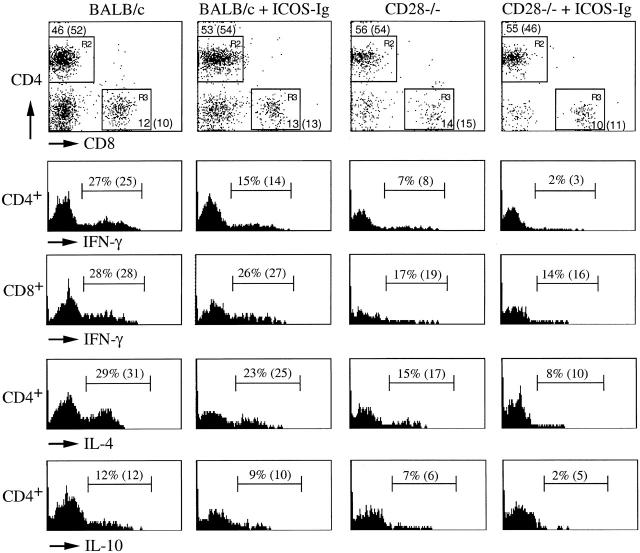
After penetration (or infection) through the skin during the first 2 d, L3 larvae migrate to the lungs, where a strong eosinophilic inflammatory response is induced 19. From there, the larvae reach the esophagus and finally the intestines, where they mature into egg-laying adults (at days 5–8). To measure Th subset development more directly in vivo, we performed a BAL at day 7, and studied CD4+ and CD8+ T cell cytokine production at the single cell level by intracellular staining and flow cytometry. Blocking of ICOS in BALB/c mice reduced the frequency of pulmonary Th1 cells (IFN-γ+ CD4+ cells) by ∼50%, whereas the frequency of Th2 cells (IL-4+ and IL-10+) was reduced by only 20–25% (Fig. 2). Absence of CD28 had more of an effect, since the number of both Th1 and Th2 cells was reduced by 50–75% (Fig. 2). Importantly, residual Th1 and Th2 cells in CD28-deficient mice were further reduced upon inhibition of ICOS. The frequency of IFN-γ–producing CD8+ T cells was reduced by 35% in CD28-deficient mice compared with wild-type BALB/c mice. In contrast, inhibition of ICOS did not show an effect on CD8+ T cells in either wild-type BALB/c or CD28-deficient mice (Fig. 2). Together, our results demonstrate that CD28 is the main costimulator of Th1/Th2 polarization to nematode infection with a limited but significant role of ICOS. However, ICOS mediated CD28-independent Th responses, indicating functionally redundant pathways. Importantly, CD28- and ICOS-mediated signals augmented not only Th2 but also Th1 responses to N. brasiliensis.
Figure 2.
Inhibition of ICOS reduces the frequency of pulmonary Th1 and Th2 cells but not CD8+ IFN-γ+ T cells in CD28−/− mice. Mice (n = 4/group) were infected with N. brasiliensis and treated as described in the legend to Fig. 1. At day 7, BAL was performed and BAL cells were stimulated with PMA and ionomycin for 4 h in the presence of brefeldin A for the last 2 h to retain cytokines in the cytoplasm. Cells were stained with allophycocyanin-labeled anti-CD4 and FITC-labeled anti-CD8, followed by fixation, permeabilization, and intracellular staining with PE-labeled IL-4, PE-labeled anti–IL-10, or PE-labeled anti–IFN-γ. Subsequently, cells were analyzed by three-color flow cytometry. Values indicate percentage of CD4+ T cells expressing IL-4, IFN-γ, and IL-10, and CD8+ T cells expressing IFN-γ of BAL from individual mice. Shown in brackets are averages of groups of mice. Data are representative of three separate experiments.
ICOS Costimulates Antiviral Th1 Responses.
LCMV induces a strong Th1 response in mice 20. To assess the role of ICOS in costimulating Th1 responses, groups of CD28-deficient and C57BL/6 control mice were infected with LCMV and treated with the ICOS-Fc fusion molecule or control human IgG1. 12 d after infection, splenocytes were isolated and purified CD4+ T cells were stimulated in vitro with LCMV-infected APCs. Proliferation (Fig. 3 A) and IFN-γ production (Fig. 3 B) of LCMV-specific CD4+ T cells derived from CD28-deficient mice were strongly reduced. Nevertheless, remaining CD4+ T cell responses to LCMV in the absence of CD28 were significant (Fig. 3A and Fig. B). Neutralization of ICOS abrogated proliferation and IFN-γ secretion in CD28-deficient mice (Fig. 3A and Fig. B), whereas it did not significantly inhibit the generation of Th1 responses in wild-type C57BL/6 mice. Thus, ICOS mediates the residual CD28-independent component of the LCMV-specific Th1 response.
Figure 3.
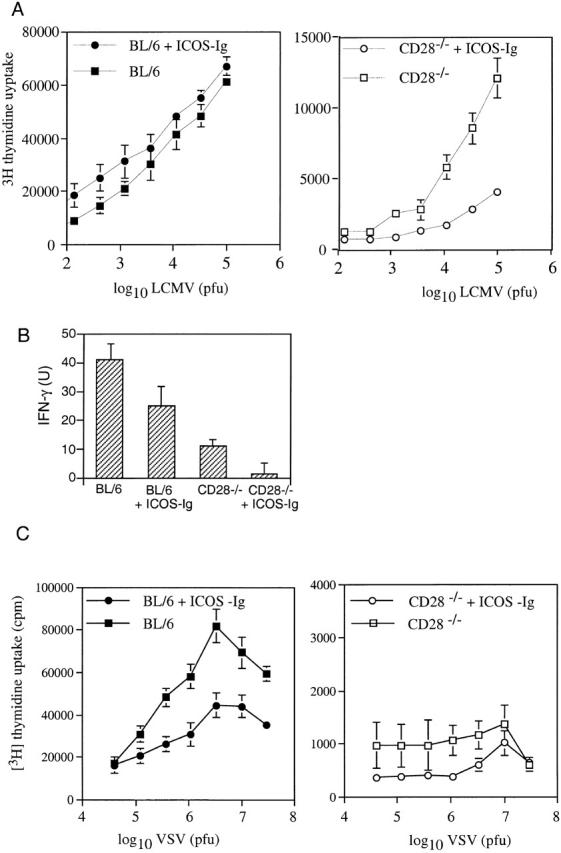
ICOS costimulates antiviral Th1 responses. CD28−/− mice (open symbols) and C57BL/6 control mice (BL/6, filled symbols) were infected with LCMV (200 PFU) into the footpad (A and B) or with VSV intravenously (C). Groups of mice (n = 4) were injected intraperitoneally with ICOS-Ig (circles) or control IgG1 (squares) starting at day 0 every 2 d until day 10 (A and B) or until day 4 (C). CD4+ T cells were isolated at day 12 (A and B) or at day 6 (C) and restimulated with splenic APCs pulsed with viral particles. Proliferation (A and C) and secretion of IFN-γ (B) were assessed 4 d after stimulation. Data are representative of two separate experiments.
Next, we wanted to assess the roles of ICOS and CD28 for the stimulation of CD4+ T cells during a less virulent infection than LCMV. To this end, we used VSV, which replicates only abortively in mice, and infected C57BL/6 and CD28-deficient mice treated repeatedly with ICOS-Fc or control human (h)IgG1. Splenic CD4+ T cells were purified on day 6 after infection and restimulated in vitro using splenic APCs pulsed with UV light–inactivated virus particles. As previously shown for the VSV-specific CTL response 2, CD28-deficient mice failed to mount a measurable proliferative CD4+ T cell response to VSV (Fig. 3 C). In addition, inhibition of ICOS triggering in C57BL/6 mice substantially inhibited the proliferation of VSV-specific CD4+ T cells (Fig. 3 C), indicating that ICOS may be involved in the costimulation of weak (VSV) but not strong (LCMV) Th1 responses.
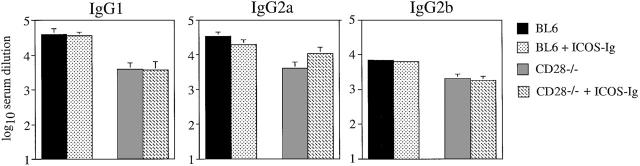
ICOS has been reported to be expressed by germinal center T cells 10. Therefore, it was conceivable that it is critically involved in the regulation of Th cell–dependent B cell responses. To assess this question, we measured specific IgG antibody levels in response to LCMV infection in the various groups of mice. IgG1, IgG2a, and IgG2b antibody responses were reduced 5–10-fold in CD28-deficient mice, but were not affected in C57BL/6 mice treated with ICOS-Fc (Fig. 4). Surprisingly, neutralization of ICOS in CD28-deficient mice did not further reduce specific antibody levels (Fig. 4), despite reduced CD4+ T cell proliferation and IFN-γ production in this situation.
Figure 4.
Blocking ICOS does not affect antibody isotype switching after infection with LCMV. CD28−/− mice and C57BL/6 control mice (BL6) were infected with 200 PFU LCMV) and treated with ICOS-Ig or control IgG1 (solid bars) starting at day 0 every 2 d until day 10. Blood was taken 12 d after infection, and LCMV-specific IgG1, IgG2a, and IgG2b titers were determined by ELISA. Data are representative of two separate experiments.
Blocking ICOS Does Not Affect Antiviral CTL Responses.
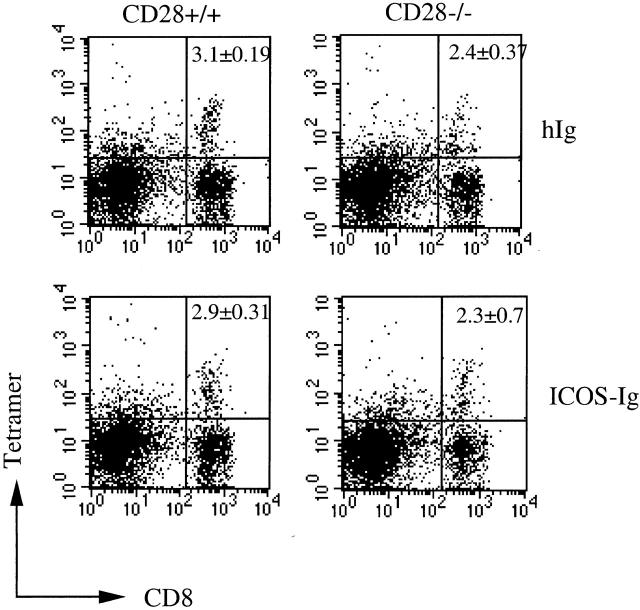
It has been reported that LCMV induces an efficient CTL response in the absence of CD28 1 2. Therefore, the role of ICOS in the regulation of CTL responses against LCMV was assessed next. C57BL/6 and CD28-deficient mice were infected locally in the footpads with LCMV and treated repeatedly every 2 d with ICOS-Fc or control hIgG1. LCMV induces an immunopathological footpad swelling reaction upon local injection of live virus 21. This swelling reaction is mediated exclusively by CD8+ T cells in the early phase of an immune response, and therefore serves as an excellent in vivo read-out for CTL activity 22. LCMV-induced footpad swelling reaction was clearly impaired in the CD28-deficient mice. However, blocking ICOS showed no effect on footpad size in either control or CD28-deficient mice, indicating that it was not significantly involved in the induction of this CD8+ T cell–mediated immunopathology (Fig. 5 A). In agreement with these results, the lytic activity of LCMV-specific CTLs, as determined by classical 51Cr-release assay, was slightly reduced in CD28-deficient mice, but remained unaffected in control and CD28-deficient mice treated with ICOS-Fc (Fig. 5 B). To directly assess the frequency of LCMV-specific CTLs by flow cytometry, we stained spleen cells from infected mice with CD8 and MHC class I tetramers loaded with specific peptide 13. Consistent with the lytic activity, CD28-deficient mice showed a frequency of specific CD8+ T cells that was reduced by ∼25% compared with control mice, whereas ICOS in the presence and absence of CD28 appeared to play no significant role in the regulation of CTL proliferation after infection with LCMV (Fig. 6).
Figure 5.
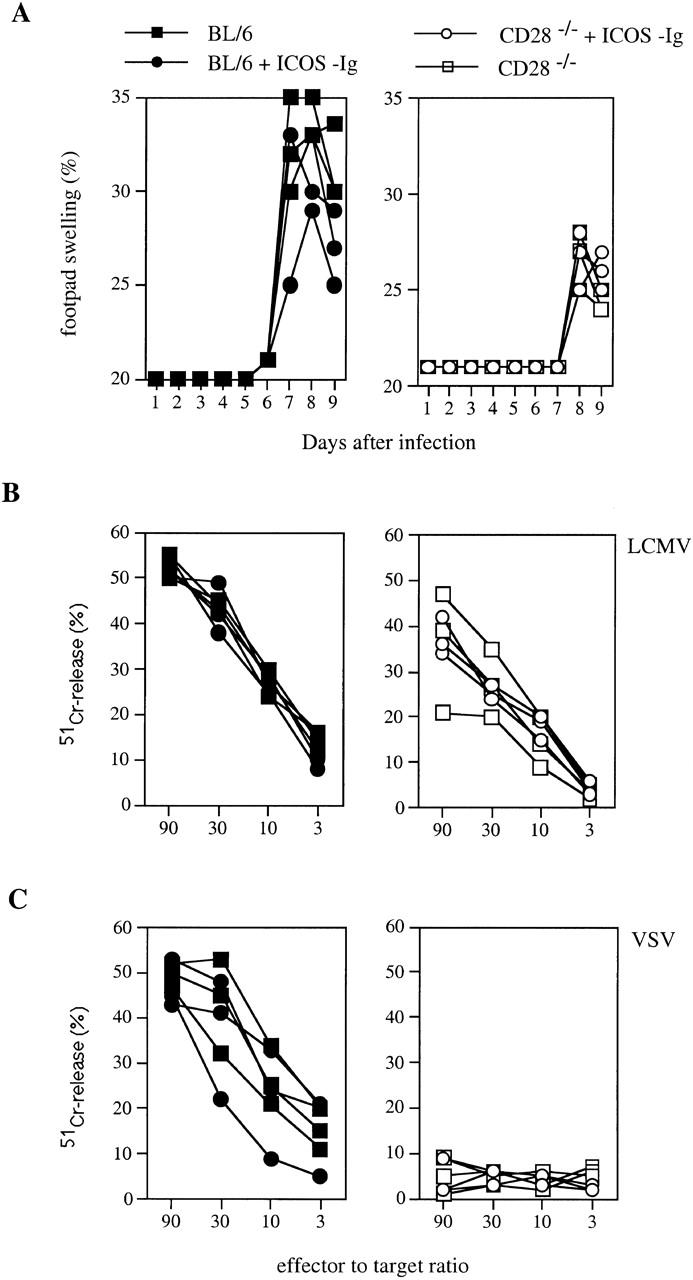
Blocking ICOS does not affect antiviral CTL responses. CD28−/− mice (open symbols) and C57BL/6 control mice (BL/6, filled symbols) were infected with LCMV (200 PFU) into the footpad (A and B) or with VSV intravenously (C). Groups of mice were injected intraperitoneally with ICOS-Ig (circles) or control IgG1 (squares) starting at day 0 every 2 d until day 10 (A and B) or until day 4 (C). (A) CD8+ T cell–mediated footpad swelling was assessed daily with a spring-loaded caliper. (B) LCMV-specific CTL activity was assessed 12 d after infection in a 51Cr-release assay on peptide-pulsed target cells. (C) VSV-specific CTL activity was assessed 6 d after infection in a 51Cr-release assay on peptide-pulsed target cells. Data are representative of two separate experiments.
Figure 6.
Blocking ICOS does not affect frequencies of LCMV-specific CTLs. CD28−/− mice (right) and C57BL/6 control mice (left) were infected with LCMV and treated with ICOS-Ig or control IgG1. Spleen cells were isolated 12 d after infection, and specific T cells were stained using H-2Db tetramers pulsed with peptide p33.
We have shown above that ICOS was more important for CD4+ T cell responses to VSV than to LCMV infection. It remained possible that this was also the case for CTL responses. To assess this possibility, we studied the role of CD28 and ICOS in the generation of CTL responses to VSV. In agreement with a previous report 2, we found that CTL responses to VSV were abrogated in the absence of CD28 (Fig. 5 C). In contrast, inhibition of ICOS did not significantly affect the VSV-specific CTL response in the presence of CD28 (not shown). Due to the profound suppression of CTL induction, it was impossible to assess the contribution of ICOS in the absence of CD28. Nevertheless, these data further indicate that ICOS plays a minor role in costimulation of CTL responses.
Discussion
This study addressed the role of ICOS-mediated T cell costimulation in the presence and absence of CD28 in vivo. Our results suggest that ICOS plays a minor role, if any, in the regulation of CTL responses but contributes to the generation of Th1 and Th2 cell responses, in particular in the absence of CD28.
Human ICOS has been observed to stimulate the production of IFN-γ (Th1) and IL-10 (Th2) in vitro upon cross-linking 10. Consistent with this finding, inhibition of ICOS during infection with the nematode N. brasiliensis resulted in reduced ex vivo Th1 and Th2 cytokine production upon restimulation with N. brasiliensis antigens. Visualization of T cells and their cytokine patterns infiltrating the lungs in response to N. _brasiliensi_s infection showed that frequencies of both lung Th1 and Th2 cells were reduced in the absence of CD28. Blocking of ICOS in CD28-deficient mice showed a further reduction, whereas ICOS inhibition in the presence of CD28 played a limited role. Remarkably, CD28 and ICOS costimulation did not differentially regulate Th subset development. Parasite-induced Th2 but also Th1 cell development was affected in the absence of either costimulatory pathway. Moreover, inhibition of ICOS attenuated VSV-specific Th1 responses in the presence of CD28 and LCMV-specific Th1 responses in the absence of CD28. Expulsion of N. brasiliensis is critically dependent on Th2 responses mediated by the IL-4R–signal transducer and activator of transcription 6 (STAT6) pathway 16 17 18. Remarkably, we found that CD28-deficient mice treated with ICOS-Ig expelled worms normally by day 13 (not shown), despite a massive inhibition of Th2 development (Fig. 2). This successful expulsion in the absence of effective Th2 responses may be the consequence of similarly impaired Th1 responses, which inhibit worm expulsion 15.
Triggering of CD28 has been suggested to be required for Th2 rather than for Th1 responses in vitro 23 and in vivo 24 25 26. Similarly, we recently found that inhibition of ICOS reduced Th2- but not Th1-dependent lung inflammation and airway hyperresponsiveness (Coyle, A.J., and J.-C. Gutierrez-Ramos, manuscript submitted for publication). It is important to note that this selective inhibition of Th2 responses by blocking of ICOS was observed after adoptive transfer of in vitro–polarized Th1 versus Th2 effector cells and intranasal aeroallergen challenge. In contrast, in the experiments presented here, Th subset differentiation was inhibited de novo, since mice were treated with ICOS-Ig before and during pathogen infection. Therefore, it is possible that shortly after T cell activation ICOS is expressed on both Th1 and Th2 precursor populations, whereas a Th2-specific expression of ICOS and hence a discrete regulatory function is only apparent on highly polarized effector Th2 cells.
The importance of ICOS-mediated costimulation in the presence of CD28 seems to vary with the antigen. Although blocking ICOS in the presence of CD28 had no measurable effect on LCMV-specific Th1 responses, it reduced VSV-specific Th1 responses. Similarly, Th1 responses to VSV compared with LCMV were more strongly dependent on the presence of CD28. This may suggest that “weak” T cell responses (e.g., VSV) depend to a relatively high degree on CD28 and ICOS signaling, with partial functional overlap of the two pathways, whereas “strong” T cell responses (e.g., LCMV) depend partially on CD28 (Fig. 3 A) and OX40 9, with no or little contribution of the ICOS pathway. As pointed out previously, the duration of antigenic stimulation may be a critical factor for the costimulatory dependence of the various antigens 2.
ICOS is expressed at high levels on germinal center T cells 10. In addition, as shown here, ICOS mediates residual Th cell responses in CD28−/− mice. It was therefore surprising that reduced CD4+ T cell proliferation and Th subset differentiation in ICOS-Ig–treated CD28−/− mice did not diminish specific antibody responses to LCMV below levels in CD28−/− mice. Even in the absence of CD28, when proliferation was reduced by ∼85%, there was still a considerably strong LCMV-specific IgG response, although it was reduced 5–10-fold compared with wild-type mice. Recently, we reported that OX40−/− mice show normal B cell responses despite impaired ex vivo proliferation and differentiation after virus infection 9. These examples demonstrate that impaired CD4+ proliferation ex vivo does not necessarily translate into reduced T help available for B cells in vivo. Distinct populations of T cells may mediate in vitro proliferative responses and in vivo isotype switching. Alternatively, it may be particularly difficult to neutralize ICOS on germinal center T cells, which express ICOS at very high levels. Nevertheless, after immunization with protein antigen (OVA) in alum, specific antibody response (e.g., IgG1, IgG2a, and IgE) levels were inhibited by ICOS-Ig treatment, in particular in a secondary immune response (Coyle, A.J., and J.-C. Gutierrez-Ramos, manuscript submitted for publication). Likewise, although CTLA-4-Ig is effective in inhibiting a primary immune response 27, some studies have shown that secondary immune responses cannot be fully suppressed by administration of CTLA-4-Ig 28, consistent with B7-independent activation of effector and memory cells 29. Taken together, it appears that the requirement of different costimulatory molecules is dependent on both (a) the nature of the antigen and (b) the phase of the immune response (e.g., induction versus maintenance of primary versus memory responses).
ICOS was observed to be more important for the induction of Th cell responses than CTL responses. This finding is compatible with the notion that CTL responses are generally less dependent on costimulatory molecules than Th cell responses. Indeed, LCMV-specific CTL responses are poorly affected in CD28−/− mice (Fig. 5 B; references 1, 2), whereas CD4+ T cell responses against the same virus are reduced more strikingly (Fig. 3A and Fig. B). Similarly, although maximal Th cell responses have been observed to depend on functional CD40–CD40L and OX40–OX40L interactions, the corresponding primary CTL responses were induced normally in the respective gene-deficient mice 9 30 31 32 33. In this respect, the 4-1BB–4-1BBL interaction is an exception, since it is thought to affect CTL rather than Th cell responses 34. In light of these previous findings, it may not be surprising that ICOS-mediated costimulation affected Th cell rather than CTL responses in vivo. For immune responses, this difference for costimulatory requirements between CTLs and Th cells remains speculative. It has been known for some time that tolerance at the Th cell level is much more stringent than tolerance at the B cell level 35 36. This observation was explained by the notion that optimal induction of Th cells is crucial for B cell isotype switching. In fact, in the absence of functional T cell help, antibody production remains transient and no B cell memory is generated. Hence, strict control of CD4+ T cell responses may be sufficient to avoid B cell–mediated autoimmunity. By analogy, CD4+ T cells may serve as critical regulators of CTL responses. Although the CD4+ T cell dependence of many CTL responses is certainly less obvious than the CD4+ T cell dependence of most B cell responses, it is nevertheless striking that (a) weak CTL responses against, e.g., tumor antigens are dependent on the presence of CD4+ T cells 37 38 39; (b) memory CTL responses to viruses fade away in the absence of CD40–CD40L interaction and T cell help 31 32; and (c) the presence of functional Th inhibits the exhaustion and/or peripheral deletion of CTL 40. Taken together, CD4+ T cells are involved in regulation of acute and long-term CTL responses under some circumstances. As observed previously for B cells, stringent control of Th cells may be sufficient to avoid CD8+ T cell–mediated autoimmunity in many cases. Thus, CTLs may be less dependent on the presence of costimulation than Th cells, since their long-term responsiveness is controlled by the continuous presence of specific T help.
Although we have titrated the amounts of ICOS-Ig in vitro and in vivo using another model antigen (not shown), we cannot entirely exclude that ICOS-L blocking was incomplete. In our experiments, Th cell generation was more affected by blocking of ICOS in CD28-deficient mice than in wild-type mice. This may indicate that ICOS does not deliver a unique signal, but may costimulate T cell responses using signaling pathways similar to CD28. Thus, CD28 may be able to compensate relatively well for lack of ICOS. In contrast, ICOS seems to be able to replace CD28 only under certain circumstances. Specifically, pathogens are often able to induce CD28-independent T cell responses, whereas model antigens fail to do so 6. The distinct expression patterns of CD28 and ICOS and their respective ligands B7 and B7Rp (B7h) may explain the difference 11 41. The relatively broad tissue distribution of ICOS ligand(s) and the upregulation in nonlymphoid compartments in an inflammatory setting suggest a role for these new costimulators that is different from the CD28–B7 interactions 11 41. CD28 is constitutively expressed on T cells, whereas ICOS is only expressed on activated T cells 10. Consequently, ICOS cannot initiate a T cell response. Pathogens usually stimulate T cells for extended time periods, which has been shown to be critical for their ability to induce T cell responses in the absence of CD28 2. Moreover, pathogens often activate APCs via innate mechanisms, further increasing T cell stimulation by APCs 2 5. This extensive and long-lived TCR triggering may be able to activate T cells sufficiently for the expression of alternative costimulatory molecules such as ICOS. By contrast, model antigens may often fail to activate T cells sufficiently for ICOS expression in the absence of CD28. Consequently, these types of antigens depend to a higher degree on the presence of CD28 for T cell priming. In this respect, CD28 and ICOS may behave similarly to CD40 and receptor activator of nuclear factor κB (RANK). Only pathogens, but not proteins in adjuvants, were found to stimulate APCs and Th cell responses via the RANK pathway in the absence of CD40 42. CD28 and CD40 are expressed constitutively, whereas ICOS 10 and RANK ligand 43 are expressed only after cell activation. Hence, neither ICOS nor RANK ligand can initiate T cell responses. However, they can sustain T cell responses that have been initiated by a costimulation-independent mechanism.
Thus, this study demonstrates that ICOS costimulates both Th1 and Th2 responses to various pathogens. Although CD28 appears to be more essential than ICOS, both pathways are partially overlapping and ICOS may primarily stimulate T cells in the absence of CD28–B7 interactions.
Acknowledgments
We thank Barbara Ecabert and Karin Lefrang for excellent technical assistance, and Theo Staehelin and Marco Colonna for critically reading the manuscript.
The Basel Institute was founded and is supported by Hoffmann-LaRoche, Ltd.
Footnotes
Abbreviations used in this paper: BAL, bronchoalveolar lavage; ICOS, inducible costimulator protein; LCMV, lymphocytic choriomeningitis virus; RANK, receptor activator of nuclear factor κB; VSV, vesicular stomatitis virus.
References
- Shahinian A., Pfeffer K., Lee K.P., Kündig T.M., Ohashi P.S., Thompson C.B., Mak T.W. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- Kündig T.M., Shahinian A., Kawai K., Mittrücker H.-W., Sebzda E., Bachmann M.F., Mak T.W., Ohashi P.S. Duration of TCR stimulation determines costimulatory requirements. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- Gause W.C., Chen S.J., Greenwald R.J., Halvorson M.J., Lu P., Zhou X.D., Morris S.C., Lee K.P., June C.H., Finkelman F.D. CD28 dependence of T cell differentiation to IL-4 production varies with the particular type 2 immune response J . Immunol. 1997;158:4082–4087. [PubMed] [Google Scholar]
- Brown D.R., Green J.M., Moskowitz N.H., Davis M., Thompson C.B., Reiner S.L. Limited role of CD28-mediated signals in T helper subset differentiation. J. Exp. Med. 1996;184:803–810. doi: 10.1084/jem.184.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr. Innate immunitythe virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Zinkernagel R.M., Oxenius A. Immune responses in the absence of costimulationviruses know the trick. J. Immunol. 1998;161:5791–5794. [PubMed] [Google Scholar]
- Wu Y., Zhou Q., Zheng P., Liu Y. CD28-independent induction of T helper cells and immunoglobulin class switches requires costimulation by the heat-stable antigen. J. Exp. Med. 1998;187:1151–1156. doi: 10.1084/jem.187.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBenedette M.A., Shahinian A., Mak T.W., Watts T.H. Costimulation of CD28− T lymphocytes by 4-1BB ligand. J. Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- Kopf M., Ruedl C., Schmitz N., Gallimore A., Lefrang K., Ecabert B., Odermatt B., Bachmann M.F. OX40-deficient mice are defective in Th cell proliferation, but are competent in generating B cell and CTL responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- Hutloff A., Dittrich A.M., Beier K.C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R.A. ICOS is an inducible T-cell costimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S.K., Whoriskey J.S., Khare S.D., Sarmiento U., Guo J., Horan T., Shih G., Zhang M., Coccia M.A., Kohno T. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F. Evaluation of lymphocytic choriomeningitis virus-specific cytotoxic T cell responses. In: Lefkovits I., editor. Immunology Methods Manual. Academic Press Ltd; New York: 1997. pp. 1921–1933. [Google Scholar]
- Gallimore A., Glithero A., Godkin A., Tissot A.C., Pluckthun A., Elliott T., Hengartner H., Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus–specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I–peptide complexes. J. Exp. Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battegay M., Moskophidis D., Waldner H., Bründler M.A., Fung-Leung W.P., Mak T.W., Hengartner H., Zinkernagel R.M. Impairment and delay of neutralizing antiviral antibody responses by virus specific cytotoxic T cells. J. Immunol. 1993;151:5408–5415. [PubMed] [Google Scholar]
- Finkelman F.D., Shea-Donohue T., Goldhill J., Sullivan C.A., Morris S.C., Madden K.B., Gause W.C., Urban J.F., Jr. Cytokine regulation of host defense against parasitic gastrointestinal nematodeslessons from studies with rodent models. Annu. Rev. Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- Barner M., Mohrs M., Brombacher F., Kopf M. Differences between IL-4R alpha-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr. Biol. 1998;8:669–672. doi: 10.1016/s0960-9822(98)70256-8. [DOI] [PubMed] [Google Scholar]
- Urban J.F., Jr., Noben-Trauth N., Donaldson D.D., Madden K.B., Morris S.C., Collins M., Finkelman F.D. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis . Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- McKenzie G.J., Bancroft A., Grencis R.K., McKenzie A.N. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr. Biol. 1998;8:339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- Coyle A.J., Kohler G., Tsuyuki S., Brombacher F., Kopf M. Eosinophils are not required to induce airway hyperresponsiveness after nematode infection. Eur. J. Immunol. 1998;28:2640–2647. doi: 10.1002/(SICI)1521-4141(199809)28:09<2640::AID-IMMU2640>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Oxenius A., Campbell K.A., Maliszewski C.R., Kishimoto T., Kikutani H., Hengartner H., Zinkernagel R.M., Bachmann M.F. CD40–CD40 ligand interactions are critical in T–B cooperation but not for other anti-viral CD4+ T cell functions. J. Exp. Med. 1996;183:2209–2218. doi: 10.1084/jem.183.5.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D., Lehmann-Grube F. Virus-induced delayed-type hypersensitivity reaction is sequentially mediated by CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA. 1989;86:3291–3295. doi: 10.1073/pnas.86.9.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Kündig T.M. In vivo versus in vitro assays for assessment of T- and B-cell function. Curr. Opin. Immunol. 1994;6:320–326. doi: 10.1016/0952-7915(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Rulifson I.C., Sperling A.I., Fields P.E., Fitch F.W., Bluestone J.A. CD28 costimulation promotes the production of Th2 cytokines. J. Immunol. 1997;158:658–665. [PubMed] [Google Scholar]
- Corry D.B., Reiner S.L., Linsley P.S., Locksley R.M. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J. Immunol. 1994;153:4142–4148. [PubMed] [Google Scholar]
- King C.L., Xianli J., June C.H., Abe R., Lee K.P. CD28-deficient mice generate an impaired Th2 response to Schistosoma mansoni infection. Eur. J. Immunol. 1996;26:2448–2455. doi: 10.1002/eji.1830261027. [DOI] [PubMed] [Google Scholar]
- Tsuyuki S., Tsuyuki J., Einsle K., Kopf M., Coyle A.J. Costimulation through B7-2 (CD86) is required for the induction of a lung mucosal T helper cell 2 (TH2) immune response and altered airway responsiveness. J. Exp. Med. 1997;185:1671–1679. doi: 10.1084/jem.185.9.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D.J., Walunas T.L., Bluestone J. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Tang A., Judge T.A., Nickoloff B.J., Turka L.A. Suppression of murine allergic contact dermatitis by CTLA4Ig. Tolerance induction of Th2 responses requires additional blockade of CD40-ligand. J. Immunol. 1996;157:117–125. [PubMed] [Google Scholar]
- Schweitzer A.N., Sharpe A.H. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J. Immunol. 1998;161:2762–2771. [PubMed] [Google Scholar]
- Whitmire J.K., Slifka M.K., Grewal I.S., Flavell R.A., Ahmed R. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J. Virol. 1996;70:8375–8381. doi: 10.1128/jvi.70.12.8375-8381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P., Tishon A., Lee S., Xu J., Grewal I.S., Oldstone M.B., Flavell R.A. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J. Exp. Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen A.R., Nansen A., Christensen J.P., Andreasen S.O., Marker O. CD40 ligand is pivotal to efficient control of virus replication in mice infected with lymphocytic choriomeningitis virus. J. Immunol. 1998;161:4583–4590. [PubMed] [Google Scholar]
- Ruedl C., Kopf M., Bachmann M.F. CD8+ T cells mediate CD40-independent maturation of dendritic cells in vivo. J. Exp. Med. 1999;189:1875–1884. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBenedette M.A., Wen T., Bachmann M.F., Ohashi P.S., Barber B.H., Stocking K.L., Peschon J.J., Watts T.H. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J. Immunol. 1999;163:4833–4841. [PubMed] [Google Scholar]
- Chiller J.M., Habicht G.S., Weigle W.O. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science. 1971;171:813–815. doi: 10.1126/science.171.3973.813. [DOI] [PubMed] [Google Scholar]
- Adelstein S., Pritchard B.H., Anderson T.A., Crosbie J., Gammon G., Loblay R.H., Basten A., Goodnow C.C. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991;254:1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- Bennett S.R.M., Carbone F.R., Karamalis F., Flavell R.A., Miller J.F.A.P., Heath W.R. Help for cytotoxic T cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Ridge J.P., DiRosa F., Matzinger P. A conditional dendritic cell can be a temporal bridge between a CD4+ T helper and a T killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Schoenenberger S.P., Toes R.E.M., vanderVoort E.I.H., Offringa R., Melief C.J.M. T cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Oxenius A., Zinkernagel R.M., Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 1998;9:449–457. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- Swallow M.M., Wallin J.J., Sha W.C. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- Bachmann M.F., Wong B.R., Josien R., Steinman R.M., Oxenius A., Choi Y. TRANCE, a TNF family member critical for CD40-independent T helper cell activation. J. Exp. Med. 1999;189:1025–1031. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B.R., Josien R., Lee S.Y., Sauter B., Li H.-L., Steinman R.M., Choi Y. TRANCE, a new TNF family member predominantly expressed in T cells, is a dendritic cell–specific survival factor. J. Exp. Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]