Commensal and pathogenic Escherichia coli use a common pilus adherence factor for epithelial cell colonization (original) (raw)
Abstract
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 is a food-borne pathogen that causes hemorrhagic colitis and the hemolytic uremic syndrome. Colonization of the human gut mucosa and production of potent Shiga toxins are critical virulence traits of EHEC. Although EHEC O157:H7 contains numerous putative pili operons, their role in the colonization of the natural bovine or accidental human hosts remains largely unknown. We have identified in EHEC an adherence factor, herein called E. coli common pilus (ECP), composed of a 21-kDa pilin subunit whose amino acid sequence corresponds to the product of the yagZ (renamed ecpA) gene present in all E. coli genomes sequenced to date. ECP production was demonstrated in 121 (71.6%) of a total of 169 ecpA+ strains representing intestinal and extraintestinal pathogenic as well as normal flora E. coli. High-resolution ultrastructural and immunofluorescence studies demonstrated the presence of abundant peritrichous fibrillar structures emanating from the bacterial surface forming physical bridges between bacteria adhering to cultured epithelial cells. Isogenic ecpA mutants of EHEC O157:H7 or fecal commensal E. coli showed significant reduction in adherence to cultured epithelial cells. Our data suggest that ECP production is a common feature of E. coli colonizing the human gut or other host tissues. ECP is a pilus of EHEC O157:H7 with a potential role in host epithelial cell colonization and may represent a mechanism of adherence of both pathogenic and commensal E. coli.
Keywords: pili, enterohemorrhagic Escherichia coli, normal flora
Bacterial adherence to host tissues is a complex process that, in many cases, involves the participation of several distinct adhesins, all of which may act at the same time or at different stages during infection (1). Many pathogenic bacteria display polymeric adhesive fibers termed “pili” or “fimbriae” that facilitate the initial attachment to epithelial cells and subsequent successful colonization of the host (1). Pili are virulence factors that mediate interbacterial aggregation and biofilm formation, or mediate specific recognition of host-cell receptors (2). It is clear that pili play similar biological roles for commensal bacteria because they also have to colonize specific niches and overcome the host's natural clearing mechanisms. It is thought that commensal and some pathogenic Escherichia coli strains use type I pili or curli to colonize human and animal tissues (3, 4). However, analysis of the genome sequence of E. coli K-12 and some pathogenic E. coli strains shows the presence of multiple putative pili operons (5–8), suggesting that other pili might be produced by pathogenic and normal flora E. coli (NFEC) in the human gut.
Enterohemorrhagic E. coli (EHEC) O157:H7 is a potentially fatal food-borne pathogen that can cause hemorrhagic colitis (9) and the hemolytic uremic syndrome (HUS) (10). Hallmarks of EHEC pathogenicity are the production of intestinal attaching and effacing (AE) lesions (11) and the secretion of potent Shiga toxins, which are responsible for the HUS (10). Most of the EHEC genetic elements responsible for the AE lesions are contained within the pathogenicity island called the “locus of enterocyte effacement” (LEE) (11). Whereas the LEE of enteropathogenic E. coli can confer the ability to cause AE lesions when introduced into a laboratory E. coli K-12 strain, the cloned EHEC LEE cannot (12, 13). This variation is explained by recent reports that show that an EHEC-specific non-LEE effector molecule is required for full development of AE lesions by EHEC (14, 15). EHEC strains are a subset of the Shiga-toxigenic E. coli (STEC) pathogroup. There are STEC strains that do not contain the LEE region and that can cause severe disease in humans, including HUS (16), indicating the existence of other non-LEE virulence factors. Cattle, other farm animals, and wild animals are important reservoirs of STEC strains of different serotypes, including O157:H7 (17). However, the bacteria can only cause disease in neonatal cattle (18).
EHEC strains bind to many cultured cell types (19), and to the intestine of gnotobiotic piglets, newborn rabbits, and neonatal calves (20). Despite efforts to identify putative adhesins, the only factor of EHEC demonstrated to play a role in colonization in vivo is intimin (21, 22). Intimin is an outer-membrane protein encoded on the LEE that mediates close adherence to the enterocyte cell membrane (23) via its own translocated receptor (Tir) (24), integrin (25), or a host cell protein called nucleolin (26). Other less well characterized surface adhesins have been reported (27–31), but their role in in vivo adherence and host colonization remains elusive. The genome of EHEC O157:H7 strains contains 16 loci-encoding genes putatively involved in pili biosynthesis (6, 8, 32); however, what role they play in the pathogenic scheme of these organisms remains largely unknown. In this article, we demonstrate the production of a pilus in EHEC, other E. coli pathogroups, and NFEC isolates that we hypothesize is involved in promoting bacterial adherence and host colonization.
Results
Detection of Unique Pili on EHEC Adhering to Cultured Epithelial Cells.
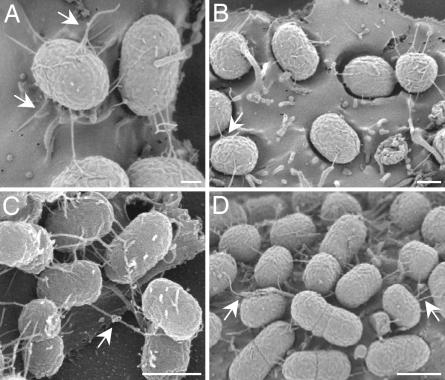
An ultrastructural approach by high-resolution SEM was undertaken to investigate whether any of the 16 loci of EHEC EDL933-encoding putative piliation genes direct the expression of functional pili. Examination of EDL933 adhering to cultured epithelial cells (HEp-2 and HeLa) for a total of 6 h revealed the production of thin (4-nm-wide), flexible fibers resembling pili that extended several micrometers away from the surface of the bacilli (Fig. 1). The peritrichous pili-like structures had a tendency to intertwine and to coil together forming thicker (12-nm-wide) structures that seemed to promote bacteria-to-bacteria interactions. These filamentous bridges were evident on bacteria adhering to the epithelial cell surface as early as 1.5 h after infection (Fig. 1A) and throughout the 6-h duration of the experiment (Fig. 1 B–D). At 3 h after infection, the bacteria formed AE lesions and appeared embedded in concavities formed on the eukaryotic cell surface (Fig. 1B). Transmission EM analysis of the bacteria present in the supernatant of infected cells showed peritrichous long (4-nm-wide) pili (Fig. 2A), which we hypothesized corresponded to those observed by SEM (Fig. 1).
Fig. 1.
SEMs showing production of fibers by adhering EDL933. EDL933 adhering to HEp-2 cells for 1.5 (A), 3 (B), 4.5 (C), and 6 (D) h were visualized by SEM. Note the presence of tethered fibers that create thicker structures, which appear to form physical bridges between bacteria. (Scale bars: 0.1 μm.)
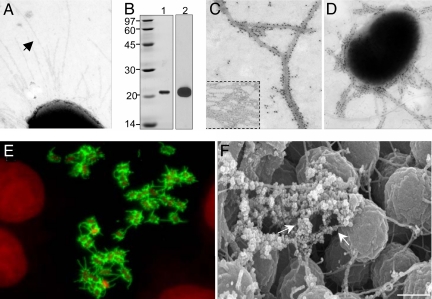
Fig. 2.
Identification of the pili produced by adhering EDL933. (A) Pili (indicated by the arrow) produced by bacteria obtained from the supernatant of infected HEp-2 cells. (B) SDS/PAGE of pili purified; lane 1, Coomasie blue staining showing the pilin subunit with an apparent molecular mass of 21 kDa; lane 2, reactivity of the pilin with anti-ECP antibodies. (C) Immuno-EM of purified ECP (Inset). (D) Immuno-EM of ECP produced by EDL933 recovered from the supernatants of infected HEp-2 cells. (E) IF showing production of ECP (green) by EDL933 adhering to HEp-2 cells. Bacterial and nuclear DNA was stained with propidium iodide (red). (F) Immuno-SEM using anti-ECP antibodies and anti-rabbit IgG conjugated to 30-nm gold particles (arrows). (Scale bar: 0.5 μm.)
Purification and Identification of the Pilin Subunit.
To elucidate the nature of these fibrillar structures, EHEC EDL933 incubated with HEp-2 epithelial cells was collected for isolation of the pili. The major component of these pili was resolved as a 21-kDa protein (Fig. 2B, lane 1) whose size and N terminus amino acid sequence matched that of the predicted protein encoded by the yagZ gene found in the genome of EHEC O157:H7 (6, 8), E. coli K-12 (5), uropathogenic E. coli (7), and meningitis-associated E. coli (33). In light of the apparent wide distribution of yagZ among the E. coli, we propose the generic name “E. coli common pilus” (ECP) for the pilus composed by the subunit protein product of this gene, herein proposed to be renamed “ecpA.”
Sequence analysis of the predicted EcpA subunit revealed that 60% of the protein is hydrophobic (33) and its C terminus does not contain the typical two-cysteine residues present in many pili types (34). Rabbit polyclonal antibodies produced against purified ECP, specifically detected the 21-kDa protein by immunoblotting (IB) (Fig. 2B, lane 2), and decorated the purified ECP filaments by immuno-EM (Fig. 2C). No reactivity was seen with heterologous anti-type-I pili antibody [supporting information (SI) Fig. 5_A_], demonstrating the specificity of the anti-ECP antiserum.
Demonstration of ECP on EHEC O157:H7 Adhering to Host Epithelial Cells.
Next, we sought to confirm the identity of the structures produced by EHEC adhering to cultured epithelial cells (Fig. 1) and also of the pili seen on bacteria recovered from the supernatants by immuno-EM, immunofluorescence (IF), and immuno-SEM. Bacteria recovered from the supernatants of infected cells displayed ECP that were decorated by anti-ECP antibodies (Fig. 2D). Bacteria adhering to HEp-2 cells produced abundant ECP, which were seen by IF as a specific fluorescent fibrillar pattern associated with the bacteria (Fig. 2E). Lastly, using immuno-SEM we demonstrated that the fibers tethering the adhering bacteria are ECP (Fig. 2F). The fibers were not detected with anti-type-I-pili antibody and anti-curli antibodies, used as negative controls (Fig. 5 B and C). Attempts to inhibit adherence of EHEC by using anti-ECP antibodies were unsuccessful (data not shown). The bases for this result are unknown, although we cannot rule out the possibility that our antibodies have no access for those ECP epitopes or regions involved in adherence.
Expression of ecpA and Environmental Regulation.
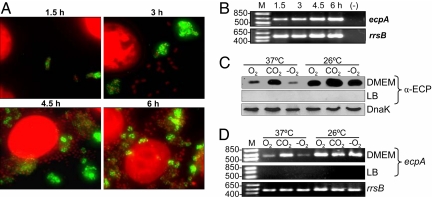
Consistent with the ultrastructural (Fig. 1) and kinetics studies of ECP production followed by IF (Fig. 3A), RT-PCR experiments showed that ecpA expression occurs in bacteria interacting with cultured epithelial cells between 1.5 and 6 h after infection, showing an increase of 1.34-fold over time (Fig. 3B). Production of ECP is apparently driven from a putative operon comprised of six genes, among which four are predicted to encode proteins with homology to proteins involved in pili bioassembly (SI Table 2).
Fig. 3.
Kinetics and environmental regulation of ecpA expression. (A) Time-dependent production of ECP by adhering bacteria demonstrated by IF. (B) Expression of ecpA mRNA analyzed during the course of cell infection (1.5–6 h). Influence of temperature and oxygen tension in environmental regulation of ECP demonstrated by IB (C) and RT-PCR (D). DnaK and amplification of 16S RNA (rrsB) were used as loading controls; RNA from uninfected cells was also used as a negative control (−). M, mass standards.
Expression of bacterial pili is under the influence of environmental cues and, sometimes, host factors (35). Previously, it was shown that meningitis-associated E. coli strains, but not any other E. coli pathogroups, were able to assemble the EcpA protein (formerly YagZ) into pili named “Mat” (meningitis-associated and temperature-regulated pilus) only after growth at 20°C in LB broth (33). EHEC is indeed able to assemble the EcpA protein into pili upon infection of cultured epithelial cells at host temperature, a phenotype that is biologically significant from the host–pathogen interaction standpoint. We sought to determine whether eukaryotic cells triggered ECP production and what environmental cues might be signals to activate the ecp operon. Adherence experiments performed with formalin-killed HEp-2 cells or with cells separated from the bacteria by a 0.2-μm filter showed similar levels of production of ECP (SI Table 3), suggesting that a host-cell product or direct contact of the bacteria with host cells are not required for induction.
We then compared ecpA transcription and production of ECP after growth of EHEC in LB versus DMEM at 26°C versus 37°C and with aeration versus 5% CO2 atmosphere versus anaerobiosis. No ECP was observed in LB medium in any of the conditions examined (SI Table 3 and Fig. 3 C and D). In general, bacterial growth in DMEM at 26°C yielded higher levels of expression of the pili compared with growth at 37°C; this property could be relevant during the life of the bacteria outside the bovine or human hosts. The presence of 5% CO2 was generally a stimulator of ECP production at either temperature (SI Table 3 and Fig. 3 C and D). The level of ecpA transcription found under the conditions examined was in line with the phenotypic data. Thus, like many other pili types, production of ECP is subjected to regulation by environmental cues such as temperature, oxygen tension, and growth media. The molecular mechanisms that modulate ECP expression are unknown and need to be investigated.
Distribution of ecpA Among E. coli Strains.
To assess the distribution of ecpA among E. coli strains from different sources, we performed a PCR-based ecpA survey in a collection of 176 strains representing NFEC and the major E. coli pathogroups (EHEC, enteropathogenic, enterotoxigenic, enteroaggregative, enteroinvasive, rabbit pathogenic, avian pathogenic, and uropathogenic). Using primers G84 and G85 (SI Table 4), which derive from the 5′- and 3′-ends of ecpA, we found that this gene was present in 169 (96%) of these strains (data not shown), supporting the notion that this locus is highly common among intestinal and extraintestinal E. coli strains. We investigated whether the remaining 4% of the strains lacked ecpA or possessed genetic differences in the ecp operon that would account for their negativity with the primers used. We performed a multiplex PCR by using primers (SI Table 4) for internal sequences of ecpR, ecpA, ecpB, and ecpC, and found that these genes were absent in all cases (SI Fig. 6), indicating that the remaining 4% of the strains actually lack the ecp operon.
Production of ECP by EHEC and non-EHEC E. coli.
We investigated the production of ECP by flow cytometry in 169 ecpA+ clinical and natural E. coli isolates grown statically overnight at 26°C in DMEM. This collection included 43 EHEC strains belonging to different serotypes (O157:H7 and non-O157:H7), among which 38 were LEE+ and 5 were LEE−. ECP production was demonstrated in 35 of the 38 LEE+ and 2 of the 5 LEE− EHEC (Table 1). The level of production of ECP varied among the strains tested, as determined by IB and IF (SI Fig. 7). Furthermore, 84 (66%) of the 126 non-EHEC E. coli strains tested produced ECP, albeit to different levels (Table 1). Representative strains of this collection showed ECP when adhering to HEp-2 cells (SI Fig. 7_B_). We speculate that the remaining ECP− E. coli might produce ECP in vivo or under other in vitro growth conditions. These data suggest that most pathogenic and nonpathogenic E. coli strains are able to produce ECP.
Table 1.
Production of ECP by ecpA + E. coli strains
| N | ECP+ | % | |
|---|---|---|---|
| EHEC O157:H7 | 20 | 19 | 95 |
| EHEC non-O157:H7* | 23† | 18† | 78 |
| EPEC | 10 | 7 | 70 |
| ETEC | 12 | 5 | 42 |
| EAEC | 20 | 19 | 95 |
| EIEC | 3 | 1 | 33 |
| UPEC | 10 | 9 | 90 |
| APEC | 10 | 7 | 70 |
| REPEC | 1 | 0 | 0 |
| NFEC | 60 | 36 | 60 |
| Total | 169 | 121 | 71.6 |
Antibody Reactivity of Human and Bovine Sera to ECP.
The presence of antibodies against a particular antigen is a biological marker of the production of that antigen in a host. The presence of anti-ECP antibodies in three pools of sera (five normal human sera, five sera from HUS patients, and five sera from bovines) was investigated. Regardless of the origin of the sera, IgG reactivity against EcpA was seen by IB (data not shown). These results suggest that circulating anti-ECP antibodies are already present in healthy humans and bovines, which correlates with our observations that NFEC are also able to produce ECP.
ecpA Mutants of EHEC and NFEC Are Deficient in Adherence to Epithelial Cells.
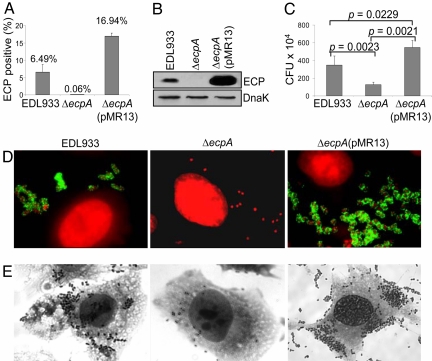
To provide genetic evidence of the involvement of ECP in bacterial adherence, the ecpA gene of EHEC EDL933 was targeted for mutagenesis. The resulting EDL933Δ_ecpA_ strain lacked ECP production (Fig. 4A, B, and D) and was significantly impaired in adherence compared with the wild-type strain (P = 0.0023) or the ecpA mutant transcomplemented with plasmid pMR13 containing ecpA (Fig. 4 C and E). EDL933Δ_ecpA_(pMR13) produced abundant ECP, and this observation correlated well with the level of adherence seen for this strain (Fig. 4). To our knowledge, this is the first report of a mutation in an EHEC O157:H7 pilus gene that significantly affects adherence to human epithelial cells.
Fig. 4.
EHEC EDL933 ecpA mutant is deficient in adherence to epithelial cells. Demonstration of production of ECP by EHEC strains by flow cytometry (A) and IB (B). (C) Quantification of adhering bacteria. The results shown represent the average of three separate experiments. (D) IF showing ECP (green) on bacteria (red). (E) Giemsa staining showing reduction of adherence in the ecpA mutant.
Next, we investigated the role of ECP for NFEC adherence by mutating ecpA in a HEp-2 cell-adherent E. coli isolate (Leo21) obtained from the stool of a healthy child. The resulting NFEC Leo21Δ_ecpA_ lacked ECP production (data not shown) and, consequently, became significantly deficient in adherence (≈94% reduction, P = 0.0008) in comparison to the parental strain (SI Fig. 8). In support of this observation, similar results were obtained with a second NFEC (Leo6) ecpA mutant (data not shown). In all, our data point to a significant role for ECP in cell adherence and perhaps in colonization of the host gut mucosa by EHEC and nonpathogenic E. coli.
Discussion
It is well established that EHEC adheres to and colonizes the intestinal tracts of humans and farm animals and that, in vitro, it attaches to a variety of epithelial cell lines (19, 20). There are 16 putative piliation operons present in the genome of EHEC O157:H7 (6, 8), but what role pili play in the colonization of the human or bovine gastrointestinal tracts remains to be elucidated. Here, we found that EHEC expresses ecpA (yagZ), a highly conserved gene present in the genomes of E. coli K-12 (5) and strains with pathogenic attributes (6–8), and assembles the encoded protein EcpA into pili structures. In addition, we found that 96% of a collection of intestinal (NFEC, enteropathogenic E. coli, enterotoxigenic E. coli, enteroaggregative E. coli, EHEC, enteroinvasive, and rabbit pathogenic E. coli) and extraintestinal (avian pathogenic and uropathogenic) E. coli strains contain ecpA. Further analysis by multiplex PCR of the _ecpA_− strains revealed that the remaining 4% of strains lacks the ecp operon. The importance of the biological role of ECP may expand beyond E. coli because homologs of ecpA are found in the genomes of Shigella boydii, Aeromonas hydrophila, and Yersinia mollaretii (data not shown).
To judge the biological significance of ECP in E. coli adherence, we chose EHEC EDL933 (O157:H7) as a model of study over other pathogroups because of its clinical importance and because this organism does not routinely produce pili. We began this study by investigating the production of pili on EHEC adhering to human cultured epithelial cells by high-resolution SEM. Compelling data demonstrate that EHEC strains produce a pilus structure composed of a 21-kDa pilin subunit whose amino acid sequence corresponded to that of EcpA. Ultrastructural EM and IF studies suggested that ECP contribute to EHEC adherence by mediating direct binding of the bacteria to the cell membrane through recognition of specific host cell receptors or by forming physical bridges between adhering bacteria. Our results provokingly suggest that the production of ECP may be necessary for stabilizing the adhering bacteria to the host cell membrane favoring tissue colonization.
Expression of bacterial virulence factors is generally regulated by complex molecular mechanisms that respond to host and environmental signals (35). The presence of host cells was not required for ECP production as initially thought, eliminating the hypothesis that eukaryotic cells or a soluble product were triggering signals. In contrast to meningitis-associated E. coli (33), EHEC produced ECP after growth in DMEM at 26°C or 37°C, but not in LB; suggesting that subtle differences in the mechanisms regulating ECP production may exist between different strains. Production of ECP at temperatures <37°C might have important implications during the life of the organism outside their bovine or human hosts, allowing for their persistence in the environment and contamination of produce. It is also possible that ECP mediate biofilm formation in some E. coli categories. The presence of 5% CO2, but not anaerobiosis, was favorable for ECP production. That ECP are produced at 37°C under low oxygen tension is an indication that they might be produced by EHEC in the intestine.
The expression of the 16 putative pili operons present in EHEC O157:H7 was previously investigated through transcriptional analysis (32). Except for genes encoding curli (csgA), a _fimA_-like gene, and ybgD and yehD (putative pilin genes), the expression of the remaining 12 pilin subunit genes, including ecpA, was not demonstrated under the conditions examined (32). Likewise, genome-wide-scale efforts using transposon or signature-tagged mutagenesis have not evidenced a role for ECP in adherence to Caco-2 cells or in colonization of the bovine gastrointestinal tract (36, 37). These apparently contradictory results may be attributed to differences in the experimental assays and conditions used.
To provide genetic evidence of the role for ECP in EHEC cell adherence, an isogenic ecpA mutant of EDL933 was constructed. This mutant was substantially reduced in adherence to HEp-2 cells in comparison with the parental strain. The residual adherence observed in the ECP mutant was likely due to intimin. It is still an open question as to how commensal E. coli strains adhere to the gut mucosa. The finding that two NFEC strains that were mutated in ecpA were also significantly reduced in their adherence to HEp-2 cells further supports a role for ECP in EHEC and NFEC adherence. We attempted to block EHEC adherence by using anti-ECP antibodies, but the results were unsuccessful. Although the reason for this result is unknown, it is possible that the anti-ECP serum lacks antibodies directed to epitopes involved in cell adherence.
To determine whether production of ECP was a generalized phenomenon among the E. coli, we surveyed a collection of 169 ecpA+ intestinal and extraintestinal E. coli strains. Production of ECP was demonstrated in 71.6% of the E. coli tested. Among STEC strains (including LEE+ and LEE−), 86% produced ECP. This finding suggests that production of ECP is a common property of STEC strains and perhaps a biological marker of their ability to colonize the intestinal epithelium. Notably, within the enteroaggregative E. coli group, 95% of the strains studied produced ECP. This observation is particularly significant given that only a minority of enteroaggregative E. coli strains produce any of the three AAF/I-III fimbriae reported in this diarrheagenic E. coli group (11). Also of note is our finding that 9 of 10 (90%) uropathogenic E. coli strains produced ECP, which suggested that, in addition to type I and Pap pili, ECP may contribute to the adherence properties of this pathogroup. It is possible that the ECP− E. coli strains found may have genetic alterations or, simply, may produce ECP under other experimental conditions. This idea is supported by the results showing that not all ecpA+ strains produced ECP under the conditions tested in this study. The remarkable high percentage of E. coli strains producing ECP is an indication that the pili must play a significant biological role in the host–bacteria interplay. The presence of anti-ECP IgG in sera from healthy individuals and HUS patients may reflect the ability of NFEC and EHEC to produce ECP in the intestine. It is unlikely that the bacteria would expend a significant amount of energy in producing pili that play no biological function. These observations have extensive implications regarding pathogenesis of disease caused by the major diarrheagenic E. coli pathogroups and their evolution.
This article represents a reproducible demonstration of pilus production by EHEC O157:H7 and shows that a mutation in a pilus gene of EHEC O157:H7 results in a substantial decrease in epithelial cell adherence. If ECP-mediated events are critical for EHEC to establish a successful intestinal infection, then it is tempting to speculate that pathogenic E. coli strains use ECP to mimic commensal E. coli and provide themselves with an ecological advantage for host colonization and evasion of the immune system. This study supports our standing hypothesis that ECP is a common E. coli attribute that was inherited and conserved during the evolution of intestinal and extraintestinal E. coli, providing a widespread mechanism of host colonization of different hosts and host tissues.
Materials and Methods
Bacterial Strains and Plasmids.
E. coli strains and plasmids are described in SI Table 5. E. coli reference and diarrheagenic E. coli collections were kindly donated by Howard Ochman (University of Arizona). Bacterial strains were propagated overnight in LB broth or DMEM (Invitrogen, Carlsbad, CA) at 26°C or 37°C, statically, with aeration, under 5% CO2 atmosphere or anaerobiosis. Anaerobiosis was achieved by using the GasPak EZ anaerobe gas-generating pouch system (Beckton Dickinson, Franklin Lakes, NJ). For testing ECP production, bacterial cultures were normalized by spectrometry. Antibiotics were added, when necessary, at concentrations of 100 μg/ml (ampicillin) or 50 μg/ml (kanamycin). Arabinose (Sigma, St. Louis, MO) was used at a 100 mM concentration.
Interaction with Eukaryotic Cells.
HEp-2 and HeLa epithelial cells (ATCC CCL-23 and CCL-2, respectively) were used in adherence assays carried out from 0 to 6 h of infection as described (38). The results obtained on ECP production by EHEC strains were identical for both cell lines. Adherent bacteria were quantified by plating out 10-fold serial dilutions on LB agar. Replica samples were used for IF and Giemsa staining. Inhibition of adherence was performed by incubation of EDL933 and cultured cells with 1:10 and 1:100 dilutions of anti-ECP. Statistical analysis was performed by using Student's t test.
Ultrastructural Studies.
The presence of pili on bacterial cells was visualized by EM in a Phillips CM12 electron microscope at 80 kV as described (38, 39). Immuno-EM was performed with anti-EHEC ECP antibodies and anti-rabbit IgG conjugated to 10- or 30-nm gold particles as described (38). Initial studies used rabbit anti-MatB antibodies (kindly provided by Timo K. Korhonen, University of Helsinki, Helsinki, Finland) (33). Glass coverslips containing fixed mammalian cells with adhering bacteria were prepared for SEM as described (38) and visualized by using a Hitachi S-4500 scanning electron microscope (Hitachi, Tokyo, Japan).
Pili Purification.
Tissue culture bottles containing monolayers of HEp-2 cells were infected with EHEC EDL933 for 6 h at 37°C under 5% CO2 atmosphere. The bacteria were collected and the pili were purified as described (38). The pili were denatured with HCl (40) and resolved by SDS/PAGE (41). A 21-kDa protein was excised and subjected to Edman degradation (Stanford University, Stanford, CA). For IB, bacteria were normalized to equal amounts and HCl-treated before SDS/PAGE and then reacted with primary anti-ECP-antibodies, followed by the secondary peroxidase conjugate (Sigma, St. Louis, MO). The substrate was a chemoluminescent reagent (GE Healthcare, Chalfont St. Giles, U.K.). Anti-DnaK (Stressgen Bioreagents, Victoria, BC, Canada) was used as control for the amount of protein loaded into the gel.
Human and Animal Sera.
Sera from HUS patients were a kind gift of Phillip Tarr (Washington University School of Medicine, St. Louis, MO) to J.B.K. Normal human and bovine sera were obtained from the collection of J.B.K. These sera were tested for reactivity against ECP by IB.
Flow Cytometry.
Flow cytometry was used to detect the production of ECP by all E. coli strains studied, as described (42). Briefly, bacteria grown overnight in DMEM were incubated with anti-ECP antibodies (1:1,000) followed by goat anti-rabbit IgG Alexa fluor conjugate (Invitrogen). The Alexa Fluor fluorescence emission was collected through a 30-nm band pass filter centered at 530 nm in which 50,000 events were measured. Bacteria were labeled with propidium iodide (Sigma) and detected through a 42-nm band pass centered at 585 nm. These experiments were repeated three times in triplicate. The samples were analyzed in a Becton Dickinson FACScan.
Mutagenesis, Multiplex PCR, and RT-PCR.
Detailed methods and protocols for the generation of nonpolar mutants, plasmid construction, multiplex PCR and RT-PCR can be found in the SI Text.
Supplementary Material
Supporting Information
Acknowledgments
We thank Diana R. Hernandez and Fabiola Avelino for technical assistance; Dr. Timo K. Korhonen for the kind gift of anti-MatB antibodies; Dr. Iruka Okeke for critical discussions; Dr. Harry L. Mobley (University of Michigan, Ann Arbor, MI) for uropathogenic E. coli CTF073; Drs. Melha Mellata (Arizona State University, Phoenix, AZ) and Roy Curtiss for avian pathogenic E. coli strains; Dr. Elizabeth Hartland (Monash University, Melbourne, Australia) for the rabbit pathogenic E. coli strain; and Dr. Howard Ochman for the ECOR and DEC strains. This study was supported by National Institutes of Health Grants AI66012 (to J.A.G.), DK58957 (to J.B.K.), and AI21657 (to J.B.K.); Dirección General de Asuntos del Personal Acadèmico Grant IN201703-3; Consejo Nacional de Ciencia y Technología 42918Q; and Howard Hughes Medical Institute Grant 75301-565101 (to J.L.P.). J.A.G. thanks the Arizona Hispanic Center of Excellence.
Abbreviations
AE
attaching and effacing
EHEC
enterohemorrhagic E. coli
ECP
E. coli common pilus
HUS
hemolytic uremic syndrome
IB
immunoblotting
IF
immunofluorescence
NFEC
normal flora E. coli
STEC
Shiga-toxigenic E. coli.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ofek I, Hasty DL, Doyle RJ. Bacterial Adhesion. Washington, DC: Am Soc Microbiol; 2002. pp. 63–96. [Google Scholar]
- 2.Jonson A-B, Normak S, Rhen M. In: Concepts in Bacterial Virulence. Russell W, Herwarld H, editors. Vol 12. Basel: Karger; 2005. pp. 67–89. [Google Scholar]
- 3.Schilling JD, Mulvey MA, Hultgren SJ. J Infect Dis. 2001;183(Suppl 1):S36–S40. doi: 10.1086/318855. [DOI] [PubMed] [Google Scholar]
- 4.Olsén A, Jonsson A, Normark S. Nature. 1989;338:652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- 5.Blattner FR, Plunkett G, III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, et al. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 6.Perna NT, Plunkett G, III, Burland V, Mau B, Glasner JD, Rose DJ, Mayhew GF, Evans PS, Gregor J, Kirkpatrick HA, et al. Nature. 2001;409:529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- 7.Welch RA, Burland V, Plunkett G, III, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, et al. Proc Natl Acad Sci USA. 2002;99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, Yokoyama K, Han CG, Ohtsubo E, Nakayama K, Murata T, et al. DNA Res. 2001;8:11–22. doi: 10.1093/dnares/8.1.11. [DOI] [PubMed] [Google Scholar]
- 9.Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, Hebert RJ, Olcott ES, Johnson LM, Hargrett NT, et al. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 10.Karmali MA, Petric M, Lim C, Fleming PC, Steele BT. Lancet. 1983;2:1299–1300. doi: 10.1016/s0140-6736(83)91167-4. [DOI] [PubMed] [Google Scholar]
- 11.Kaper JB, Nataro JP, Mobley HL. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 12.Elliott SJ, Yu J, Kaper JB. Infect Immun. 1999;67:4260–4263. doi: 10.1128/iai.67.8.4260-4263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDaniel TK, Kaper JB. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 14.Campellone KG, Robbins D, Leong JM. Dev Cell. 2004;7:217–228. doi: 10.1016/j.devcel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Garmendia J, Phillips AD, Carlier MF, Chong Y, Schuller S, Marches O, Dahan S, Oswald E, Shaw RK, Knutton S, et al. Cell Microbiol. 2004;6:1167–1183. doi: 10.1111/j.1462-5822.2004.00459.x. [DOI] [PubMed] [Google Scholar]
- 16.Paton JC, Paton AW. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caprioli A, Morabito S, Brugere H, Oswald E. Vet Res. 2005;36:289–311. doi: 10.1051/vetres:2005002. [DOI] [PubMed] [Google Scholar]
- 18.Dean-Nystrom EA, Bosworth BT, Moon HW. Adv Exp Med Biol. 1997;412:47–51. doi: 10.1007/978-1-4899-1828-4_5. [DOI] [PubMed] [Google Scholar]
- 19.Tarr PI, Bilge SS. In: Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Kaper JB, O'Brien AD, editors. Washington, DC: Am Soc Microbiol; 1998. pp. 157–162. [Google Scholar]
- 20.Moxley RA, Francis DH. In: Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Kaper JB, O'Brien AD, editors. Washington, DC: Am Soc Microbiol; 1998. pp. 249–260. [Google Scholar]
- 21.McKee ML, Melton-Celsa AR, Moxley RA, Francis DH, O'Brien AD. Infect Immun. 1995;63:3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnenberg MS, Tzipori S, McKee ML, O'Brien AD, Alroy J, Kaper JB. J Clin Invest. 1993;92:1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jerse AE, Yu J, Tall BD, Kaper JB. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 25.Frankel G, Lider O, Hershkoviz R, Mould AP, Kachalsky SG, Candy DC, Cahalon L, Humphries MJ, Dougan G. J Biol Chem. 1996;271:20359–20364. doi: 10.1074/jbc.271.34.20359. [DOI] [PubMed] [Google Scholar]
- 26.Sinclair JF, O'Brien AD. J Biol Chem. 2002;277:2876–2885. doi: 10.1074/jbc.M110230200. [DOI] [PubMed] [Google Scholar]
- 27.Low AS, Dziva F, Torres AG, Martinez JL, Rosser T, Naylor S, Spears K, Holden N, Mahajan A, Findlay J, et al. Infect Immun. 2006;74:2233–2244. doi: 10.1128/IAI.74.4.2233-2244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholls L, Grant TH, Robins-Browne RM. Mol Microbiol. 2000;35:275–288. doi: 10.1046/j.1365-2958.2000.01690.x. [DOI] [PubMed] [Google Scholar]
- 29.Paton AW, Srimanote P, Woodrow MC, Paton JC. Infect Immun. 2001;69:6999–7009. doi: 10.1128/IAI.69.11.6999-7009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarr PI, Bilge SS, Vary JC, Jr, Jelacic S, Habeeb RL, Ward TR, Baylor MR, Besser TE. Infect Immun. 2000;68:1400–1407. doi: 10.1128/iai.68.3.1400-1407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres AG, Giron JA, Perna NT, Burland V, Blattner FR, Avelino-Flores F, Kaper JB. Infect Immun. 2002;70:5416–5427. doi: 10.1128/IAI.70.10.5416-5427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Low AS, Holden N, Rosser T, Roe AJ, Constantinidou C, Hobman JL, Smith DG, Low JC, Gally DL. Environ Microbiol. 2006;8:1033–1047. doi: 10.1111/j.1462-2920.2006.00995.x. [DOI] [PubMed] [Google Scholar]
- 33.Pouttu R, Westerlund-Wikstrom B, Lang H, Alsti K, Virkola R, Saarela U, Siitonen A, Kalkkinen N, Korhonen TK. J Bacteriol. 2001;183:4727–4736. doi: 10.1128/JB.183.16.4727-4736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simons BL, Rathman P, Malij CR, Oudega B, de Graaf FK. FEMS Microbiol Lett. 1990;55:107–112. doi: 10.1016/0378-1097(90)90177-r. [DOI] [PubMed] [Google Scholar]
- 35.Edwards RA, Puente JL. Trends Microbiol. 1998;6:282–287. doi: 10.1016/s0966-842x(98)01288-8. [DOI] [PubMed] [Google Scholar]
- 36.Dziva F, van Diemen PM, Stevens MP, Smith AJ, Wallis TS. Microbiology. 2004;150:3631–3645. doi: 10.1099/mic.0.27448-0. [DOI] [PubMed] [Google Scholar]
- 37.Tatsuno I, Kimura H, Okutani A, Kanamaru K, Abe H, Nagai S, Makino K, Shinagawa H, Yoshida M, Sato K, et al. Infect Immun. 2000;68:5943–5952. doi: 10.1128/iai.68.10.5943-5952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girón JA, Torres AG, Freer E, Kaper JB. Mol Microbiol. 2002;44:361–379. doi: 10.1046/j.1365-2958.2002.02899.x. [DOI] [PubMed] [Google Scholar]
- 39.Girón JA, Ho AS, Schoolnik GK. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- 40.McMichael JC, Ou JT. J Bacteriol. 1979;138:969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 42.Humphries AD, Raffatellu M, Winter S, Weening EH, Kingsley RA, Droleskey R, Zhang S, Figueiredo J, Khare S, Nunes J, et al. Mol Microbiol. 2003;48:1357–1376. doi: 10.1046/j.1365-2958.2003.03507.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information