Impact of vaccine-induced mucosal high-avidity CD8+CTLs in delay of AIDS viral dissemination from mucosa (original) (raw)
Abstract
Natural HIV transmission occurs through mucosa, but it is debated whether mucosal cytotoxic T lymphocytes (CTLs) can prevent or reduce dissemination from the initial mucosal site to the systemic circulation. Also, the role of CTL avidity in mucosal AIDS viral transmission is unknown. To address these questions, we used delay in acute-phase peak viremia after intrarectal challenge as an indicator of systemic dissemination. We found that a peptide-prime/poxviral boost vaccine inducing high levels of high-avidity mucosal CTLs can have an impact on dissemination of intrarectally administered pathogenic SHIV-ku2 in macaques and that such protection correlates better with mucosal than with systemic CTLs and particularly with levels of high-avidity mucosal CTLs.
Introduction
The gastrointestinal and vaginal mucosae are the primary sites of natural HIV transmission and the former is also a major reservoir for HIV replication.1-4 This mucosa-centric nature of HIV infection provides a strong rationale for development of mucosal HIV vaccines to induce sufficient mucosal response to clear virus from mucosal tissues.5-7 Indeed, we previously demonstrated that intrarectal immunization of macaques with a peptide vaccine was superior to subcutaneous immunization both in the induction of mucosal CD8+cytotoxic T lymphocyte (CTL) responses and in limiting viral titers in the blood and intestine.8,9 In addition, control of viremia in SIV/SHIV/HIV infection correlates with and is dependent on CD8+CTLs in macaques and chimpanzees.10-12 However, it has been debated whether CTLs in the mucosa can eliminate the first round of virally infected cells fast enough to prevent or reduce dissemination of virus to the systemic circulation. Data from several laboratories show that virus remains in the mucosa from 2 to 7 days before spreading to other sites,2,13 suggesting that if CTLs are present at the local site of infection early enough, it may be possible to eradicate the initial nidus of infection before it spreads. However, no data have been available to test this hypothesis, which is important to understand the role of mucosal CTLs in protection and thus in the design of mucosal AIDS vaccines. Here, we use the delay in acute-phase appearance of virus in the systemic circulation after intrarectal inoculation as an indicator of such dissemination from the mucosa to test this hypothesis in rhesus macaques immunized intrarectally with an experimental vaccine.
In murine studies, high-avidity CD8+CTLs are more effective than low-avidity CD8+CTLs in clearance of viral infections.14-17 However, only limited indirect information has been available in primates,18 and the role of CTL avidity has not been studied explicitly in the mucosa. Here, while comparing mucosal vaccine regimens in macaques for protection against mucosal transmission of an AIDS virus, we made the unexpected observation that peptide priming increased mucosal CTL avidity, and further found a correlation between levels of high-avidity mucosal CTLs and delayed appearance of circulating plasma viral particles, which we interpret to reflect dissemination of virus from the initial mucosal site of transmission.
Materials and methods
Macaques and vaccines
Indian rhesus macaques (Macaca mulatta; purchased from Covance and the Caribbean Primate Research Center; Dr. Edmundo N. Kraiselburd, San Juan, Puerto Rico) were matched for genetic origin, source, and comparable age and weight, and maintained in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International (Rockville, MD) and under the approval of the Applied BioScience Laboratories Animal Center Review Committee. All were seronegative for SIV and simian retrovirus 1, 2, and 5. Animals positive for simian T-cell leukemia/lymphotropic virus type 1 (STLV-1+) and herpes B virus were equally distributed among the 4 groups. Mamu-A*01+ major histocompatibility complex (MHC) type was kindly determined by Dr David Watkins (Wisconsin Regional Primate Research Center, Madison, WI) by polymerase chain reaction (PCR) sequence-specific primers and direct sequencing.19 The peptides in Table 1 each contain an HIV envelope helper epitope20 and an SIV Gag,21 Pol,22 Vif,22 or HIV Tat-2 and Tat-3 (this study) CTL epitopes. Recombinant NYVAC-expressing SIVmac239 Gag, pol (5 × 108/immunization) and recombinant NYVAC-expressing HIV-1 IIIB envelope proteins (5 × 108/immunization) were a kind gift of Dr James Tartaglia (Sanofi-Pasteur, Toronto, ON, Canada).23
Table 1.
Combined helper-CTL epitope peptides included in the vaccine
| Sequences | ||
|---|---|---|
| HIV/SIV constructs | T-helper portion | CTL portion |
| PCLUS3-CL10 (HIV-env + SIV-Gag_181) | KQIINMWQEVGKAMYAPPISGQIR | CTPYDINQML |
| PCLUS6.1-CL10 (HIV-env + SIV-Gag_181) | DRVIEVVQGAYRAIRHIPRRIRQGLER | CTPYDINQML |
| PCLUS3-Pol_143 (HIV-env + SIVPol_143) | KQIINMWQEVGKAMYAPPISGQIR | LGPHYTPKIV |
| PCLUS3-Gag_372 (HIV-env + SIVGag_372) | KQIINMWQEVGKAMYAPPISGQIR | LAPVPIPFA |
| PCLUS3-Tat2 (HIV-env + HIV-Tat2) | KQIINMWQEVGKAMYAPPISGQIR | KHPGSQPKTA |
| PCLUS3-Tat3 (HIV-env + HIV-Tat3) | KQIINMWQEVGKAMYAPPISGQIR | VDPRLEPW |
| PCLUS3-Vif (HIV-env + SIV-Vif) | KQIINMWQEVGKAMYAPPISGQIR | QVPSLQYLA |
SHIV-ku2 virus stock
SHIV-ku2 is a chimeric virus containing the HIV-1 IIIB strain (HXBc2) envelope gene and SIVmac239 gag and pol genes and is pathogenic in rhesus macaques.24 The undiluted virus challenge contained approximately 10 animal infectious doses (AID50) per milliliter for intrarectal administration. Because of limited quantities of this titered virus remaining, the dose used in this study was nominally 8 AID50.
Immunization and challenge of macaques
Each intrarectal peptide vaccine dose contained 0.5 mg of each peptide (total 3.5 mg peptide) mixed with mutant Escherichia coli labile toxin LT(R192G) as mucosal adjuvant (50 μg/immunization)8 and a synergistic combination of cytokines (hGM-CSF, 30 μg/immunization and hIL-12 [Wyeth, Boston, MA], 15 μg/immunization) and 500 μg/immunization of D-type CpG oligodeoxynucleotide (ODN). For intrarectal inoculations, animals were sedated with ketamine hydrochloride (10 mg/kg intramuscularly) and placed within a biosafety cabinet in ventral recumbency with the hindquarters elevated. The tail was elevated dorsally and a 3-mL slip-tip syringe was atraumatically inserted into the rectum. Vaccine or virus was administered and the tail lowered to ensure complete delivery.
Biopsies and isolation of lamina propria lymphocytes
Mesenteric lymph nodes (MLNs) and intestinal tissue were obtained by laparotomy. Para-aortic and para-iliac nodes were not accessible in this procedure. Intestinal tissue was obtained from colonic wedge resection (∼4 cm2) at laparotomy. Lymphocytes were prepared as previously established for macaques tissue.8
CTL, ELISPOT, and tetramer assays
Immune cells were activated overnight in 12-well plates in the presence of 0.02 μM synthetic CTL epitope peptides in CTM. Mamu-A*01 target cells were pulsed with peptide from 10 μM to 10-4 μM for 2 hours during 51Cr labeling, and cultured with effectors at 37°C for 4 hours.8 Specific release was calculated as described.25,26 Soluble tetrameric Mamu-A*01/CM10 complexes conjugated to PE-labeled streptavidin were prepared as described.27 A perforin-release enzyme-linked immunospot (ELISPOT) assay was performed on macaque peripheral blood mononuclear cells (PBMCs) per instructions (Mabtech, Nacka Strand, Sweden), stimulating with 0.1 μM concentration of CL10 peptide for 24 hours.
Determination of viral load
SIVmac251 mRNA in plasma was quantified by nucleic acid sequencebased amplification (NASBA),28 with a lower limit sensitivity of 500 RNA copies/mL.
ELISA for anti-gp160 antibody
Enzyme-linked immunosorbent assay (ELISA) was performed as previously described.29
Software for fitting and statistical analysis
Statistical comparisons were performed using the Mann-Whitney, Wilcoxon, Kruskal-Wallis, and χ2 tests (Statistica 6; StatXact 6, StatSoft, Tulsa, OK), as well as a nonparametric repeated measures ANOVA for plasma viral loads over time,8,30,31 because the data could not be taken as normally distributed.
Results
To examine the role of mucosal CTL avidity in protection against mucosal (intrarectal) AIDS viral transmission, we compared a peptide-based vaccine versus a recombinant vaccinia-based vaccine versus a combination peptide prime-recombinant vaccinia boost vaccine regimen in rhesus macaques (Table 1). The peptide vaccine contained a mixture of epitopes presented by Mamu-A*01, the class I antigen expressed by the macaques selected for study, modified compared with that used previously8 by addition of 2 new peptides from the HIV Tat protein and one from vif. These new Tat epitopes were derived from HIV because the Tat genes of SHIV are derived from HIV not SIV. They were selected by synthesizing 4 peptides predicted to bind to Mamu-A*01 based on a sequence motif32 and then testing the ability of CTLs derived from primates infected with SHIV-Ku2 in our previous study8 to recognize these epitopes presented by MamuA*01. Two of the 4 selected epitopes were recognized. These peptides were added to the peptide mixture (Table 1). We also added a CTL epitope from SIV-vif.22 The peptides were delivered intrarectally with a synergistic combination of molecularly defined adjuvants (GM-CSF, IL-12, and CpG ODN). Previous studies showed synergy of GM-CSF and IL-12 for induction of CTLs both systemically33-35 and mucosally.36 D-type CpG oligonucleotides37 were chosen to mature dendritic cells recruited by the GM-CSF, analogous to CD40L.38
We planned a prime-boost strategy with peptide and recombinant poxviral vector because analogous strategies using DNA priming and viral vector boosting have been shown previously to elicit strong CTL responses,39-42 perhaps due to the ability of the DNA prime to focus the immune response on the target antigens before exposure to the viral vector expressing other antigens. We reasoned that a peptide prime might similarly focus the response on desired epitopes. Recombinant highly attenuated NYVAC SIV/HIV was chosen as a boost because previous studies demonstrated the ability to provide significant protection in the SIV macaque model when given systemically,42 and NYVAC was found immunogenic when given mucosally.43 Even when complete protection is not achieved, a vaccine's impact on the initial nidus of infection may be revealed in a delay or reduction in systemic dissemination reflected by the time of appearance as well as magnitude of acute viremia. Thus, we have focused here on the acute phase of infection and the relationship between vaccine-induced CTL activity and avidity before challenge and the acute viremia kinetics and magnitude shortly after challenge.
The immunization regimens consisted of the following: groups 1 and 2 were immunized intrarectally with the peptide vaccine mixture and the cytokines and adjuvants 5 times at 3-week intervals. Group 1 animals were boosted with 2 additional intrarectal doses of peptide vaccine (with biopsies taken after the second boost), whereas group 2 was boosted with 4 intrarectal doses of recombinant NYVAC vaccinia (delivered in the absence of cytokines and adjuvants); group 3 macaques were given only the adjuvant mixture when groups 1 and 2 received peptide priming and then were immunized only with NYVAC at the same time that group 2 macaques were boosted with NYVAC; finally, group 4 control macaques were given only the cytokines and adjuvants. Two weeks after the last immunization, the macaques were subjected to survival surgery for isolation of cells to be used in CTL assays.
Four weeks following the last administration of vaccine, all macaques were subjected to intrarectal challenge with approximately eight 50% infectious doses (AID50) of SHIV-Ku2 and then monitored to ascertain plasma viral load and immune responses.
CTL responses in mucosal and systemic lymphoid tissue of macaques and effect of peptide priming on CTL magnitude and avidity
We enumerated antigen-specific CD8 CTLs in the colonic lamina propria at the first biopsy and in peripheral blood using a CL10 tetramer-binding assay. All freshly isolated lamina propria lymphocytes and peripheral blood cells in groups 1, 2, and 3 were positive in the tetramer-binding assay (Figure 1A-B), with the most CL10 tetramer-positive CD8 cells in the colons of group 2 macaques. However, CL10 tetramer-positive CD8 cells in blood were more equally distributed between groups 1, 2, and 3, with only slightly more in group 2. All animals in control group 4 were tetramernegative (Figure 1A-B).
Figure 1.
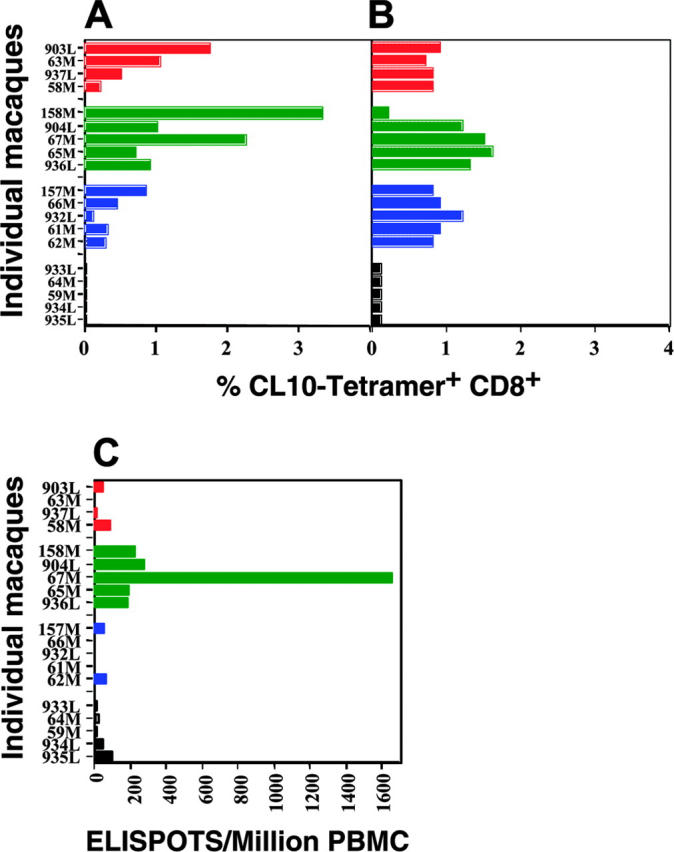
Mucosal prime-boost SHIV vaccine strategy induces CL-10–specific CD8 CTLs in mucosal and systemic lymphoid tissues. (A) Number of CL-10–specific tetramer-positive cells in colonic lamina propria. (B) Number of CL-10–specific tetramer-positive cells in peripheral blood. (C) Number of CL-10–specific cells secreting perforin in peripheral blood by ELISPOT assay. Individual data points for macaques in group 1 are color-coded as red; group 2, green; group 3, blue; and group 4, black.
We also asked whether mucosal immunization with peptide and recombinant vaccine induced antigen-specific CD8 CTLs systemically (in peripheral blood) that expressed perforin on stimulation. By ELISPOT assay, we studied the number CL10-specific perforin-positive CD8+CTLs in the blood after intrarectal immunization in all 4 groups (Figure 1C). A significant number of perforin-producing CD8+ CTLs appeared only in group 2 (prime-boost; Figure 1C), not in groups 1, 3, and 4 (Figure 1C), suggesting that this regimen induced more functionally active T cells.
To understand the role of vaccine-induced CTL avidity in protection, we studied mucosal CTL avidity after the various immunizations. We examined CTL avidity in the MLNs, where sufficient cells were available. It was impossible to study CTL avidity in colonic tissue because of the limited number of cells recoverable from the colon wedges.
CTL activity in MLNs obtained 2 weeks after completion of the immunization schedule (week 34) (Figure 2A) was measured after just overnight stimulation to avoid skewing the CTL repertoire or avidity by culturing for a week.44 Most group 2 macaques made a substantial CTL response to all SIV and HIV epitopes and most group 1 macaques had somewhat lower, but still considerable CTL responses to all epitopes (except the HIV-Tat3 epitope). In contrast, most group 3 macaques registered somewhat weaker CTL responses only against the 2 gag CTL epitopes, as neither Tat nor vif is expressed by the recombinant NYVAC. The ability of the NYVAC boost to increase the response to HIV Tat or SIV vif in group 2 may represent a bystander effect, for example, through the induction of T-cell help. Finally, the group 4 unimmunized macaques manifested virtually no responses to any of the epitopes.
Figure 2.
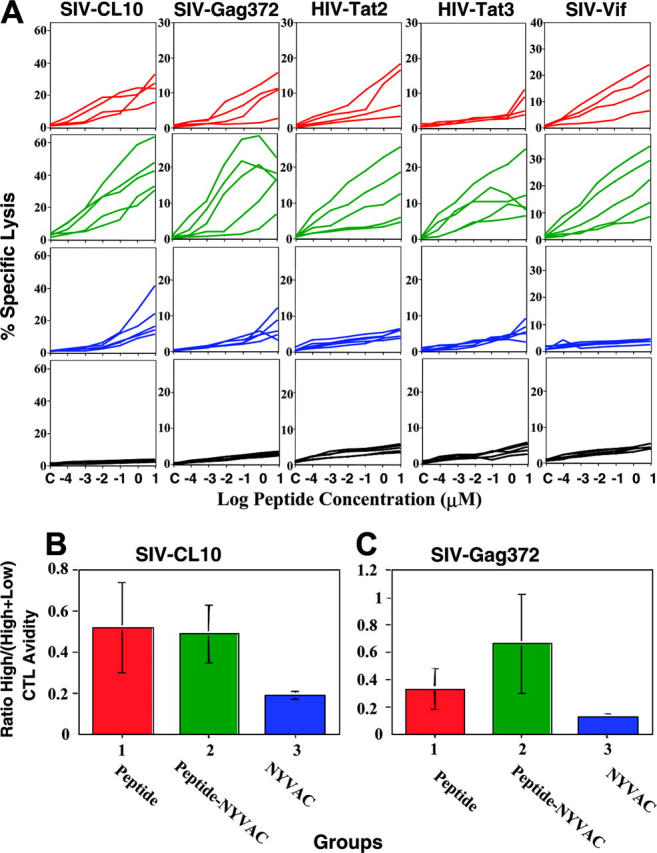
Mucosal prime-boost SHIV vaccine strategy induces high avidity and high magnitude of CD8+ CTL responses in mucosal and systemic lymphoid tissues. (A) CTL activity as a function of peptide concentration for SIV Gag CL10, Gag 372, Pol 143, Vif, HIV Tat 2, Tat 3, as indicated, in MLNs after mucosal immunizations with HIV/SIV peptide vaccines with GM-CSF, rhIL-12, CpG ODN, and LT(R192G) (group 1), recombinant NYVAC (group 3), HIV/SIV peptide prime and recombinant NYVAC boost (group 2), or GM-CSF, rhIL-12, CpG ODN, and LT(R192G) alone without a specific antigen (group 4). Mamu-A*01+ target cells were pulsed with different concentration of peptides from 10 μM to 10-4 μM, as indicated, and lysis by MLN cells stimulated just overnight (to avoid skewing the repertoire) was studied by 51Cr-release assay at 100:1 effector-target ratio. (B-C) High-/low-avidity CTL ratio. (B) CL-10 peptide, (C) Gag372 peptide. Ratio between percent specific lysis against 0.001 μM peptide (high-avidity CTLs) to percent specific lysis against 10 μM peptide (total low- and high-avidity CTLs).
To examine avidity, these CTL responses were evaluated by plotting specific lysis versus epitope concentration. We observed that the shape of the dose-response curves was more convex in the groups receiving the peptide vaccine compared with those receiving NYVAC alone, indicating a higher proportion of high-avidity CTLs that could kill targets at low peptide concentrations. We calculated the ratio between the percent specific lysis against a low (0.001 μM) epitope concentration (reflecting high-avidity CTLs) and the percent specific lysis against a high (10 μM) concentration (reflecting both high- and low-avidity CTLs) for the CTLs to the SIV-CL10 or SIV-Gag372 epitopes. We reasoned that higher values of this ratio (close to 1) indicate skewing of the CTL population toward high-avidity cells, whereas lower values of this ratio indicate skewing toward low-avidity cells. Despite the higher magnitude of the group 2 CTL response versus the group 1 CTL response (Figure 2B), both groups had avidity ratios that were not statistically different from each other for CTLs against the SIV-CL10 epitope and both groups had substantially higher ratios than group 3 CTLs (P < .05); similarly, although the avidity ratio of group 2 was higher than that of group 1 for CTLs against the SIV-Gag372 epitope, the ratios of both of these groups was higher than that of group 3 (P < .05; Figure 2C). Thus, both regimens including peptides, cytokines, and CpG ODNs led to similar avidity profiles, whereas mucosal NYVAC immunization alone led to CTL responses lower in both magnitude and avidity. Thus, the peptide/cytokine/CpG immunization improved CD8+CTL avidity and therefore quality, whereas boosting with recombinant vaccinia vector increased the magnitude of the CD8+CTL responses, so that the combination gave the highest high-avidity response.
Peak viremia delay during acute infection after intrarectal SHIV-Ku2 challenge
As noted, macaques in each group were subjected to intrarectal challenge with SHIV-Ku2. However, 3 macaques in group 4, the control group, did not become infected as determined by 3 independent criteria: the NASBA assay for viral RNA in the peripheral blood, PCR assay for the detection of proviral DNA in cells, and ELISA for the detection of anti-HIV gp160 on day 14 after viral challenge. In addition, 2 macaques in group 2 did not become viremic although they were clearly infected as determined by detection of proviral DNA in cells and anti-HIV gp160 on day 14 after viral challenge. It is possible that this lack of viremia was due to protection from productive infection by the immunization; however, we cannot exclude the alternative possibility that the virus dose administered to these macaques was lower than expected due to deterioration in storage. In any case, in the following analysis we considered only those macaques that became viremic.
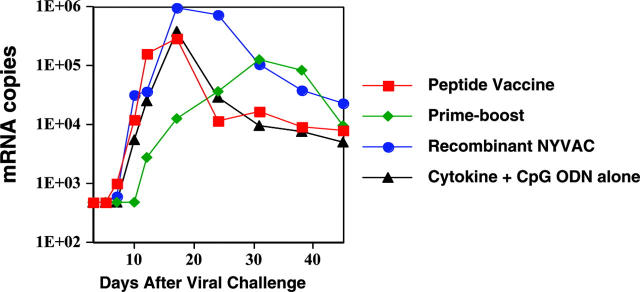
The various immunization regimens resulted in several notable differences in the patterns of viremia exhibited in the 4 groups. First, group 2 macaques exhibited a delay in the peak of viremia of approximately 2.5 weeks compared with group 1 and 3 macaques given peptide or NYVAC alone (from day 19 or 21 to day 38), or indeed compared with unimmunized group 4 macaques (peak day 20; Figure 3, P = .006). This delay was also accompanied by a reduction in the magnitude of the peak viral load, which did not quite attain statistical significance (P > .05, P < .10). These data indicate that peptide priming followed by NYVAC boosting led not only to the greatest high-avidity mucosal CTL response but also to the greatest delay in acute viremia, most likely reflecting a retardation of the dissemination of virus from the mucosa.
Figure 3.
Mucosal prime-boost SHIV vaccine induces delay in viremia after intrarectal infection of rhesus macaques. Viral load is expressed as viral RNA copies per milliliter plasma (by NASBA assay) versus time after challenge. By the exact Wilcoxon-Mann-Whitney test (StatXact 6), the prime-boost group 2 peak viral titers (tmax) were significantly delayed (P = .006) compared with those of groups 1 and 3, whereas groups 1 and 3 were not significantly different from control group 4. The day (mean ± SE) of tmax peaks for groups 1, 2, 3, and 4 were 18.8 ± 1.7 (median, 17), 38.0 ± 4.04 (median, 38), 21.2 ± 1.72 (median, 24), and 20.5 ± 3.50 (median, 20.5), respectively.
Relation of mucosal versus systemic CTL responses prior to viral challenge to acute viremia after viral challenge
To understand the role of vaccine-induced mucosal versus systemic immune responses in protection, we studied the correlation between log of viral load in blood on day 17 after challenge and the number of CL10-specific tetramer-positive CD8+CTLs in the colonic lamina propria and in peripheral blood prior to SHIV challenge for all infected animals that were vaccinated (Figure 4A-B). We found a strong inverse correlation (r = -0.84; P < .001) between numbers of tetramer-positive cells in the colon and the level of viremia on day 17 (Figure 4A). These data suggested the importance of local mucosal CD8+CTLs in the colon for protection against dissemination of virus from the mucosa after mucosal challenge with pathogenic SHIV virus. In contrast, we found no correlation (r = .007, P = .77) between numbers of tetramer-positive cells in peripheral blood and the level of viremia on day 17 (Figure 4B). Also, we found a very weak and barely significant inverse correlation (r = -0.4, P = .049) between the number of CD8 cells in peripheral blood releasing perforin in response to the CL10 peptide and the log viral load in the blood on day 17 (Figure 4C). These results suggest that mucosal CTLs play a more important role than systemic ones in preventing virus from disseminating from the initial mucosal site of infection.
Figure 4.
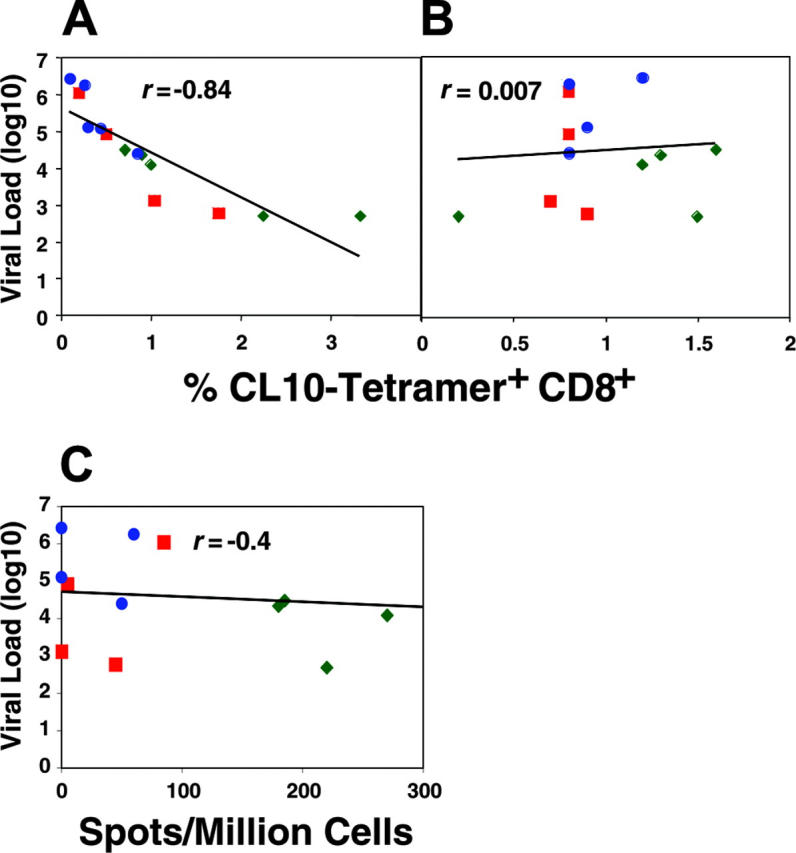
The viral load during the early stages of infection inversely correlated with number of CL-10–specific tetramer-positive CD8+CTLs in colonic lamina propria before SHIV challenge, but not in blood. (A) Correlation between log viral load in blood on day 17 and number of CL-10–specific tetramer-positive CD8+CTLs in colonic lamina propria before SHIV challenge. (B) Correlation between log viral load in blood on day 17 and number of CL-10–specific tetramer-positive CD8+CTLs in peripheral blood before SHIV challenge. (C) Correlation between CL-10–specific perforin-positive CD8+ T cells in peripheral blood and log viral load in blood on day 17. Animal (67M) in group 2 with more than 1600 spots/million cells was included in determining the correlation but is not shown on this scale.
To study the relation between the avidity of the mucosal CTLs after immunization but before viral challenge and the acute plasma viral load after viral challenge, we constructed correlation plots of CTL avidity before challenge (derived from SIV-CL10 CTL data in MLNs harvested after completion of immunization) versus viral load at 2 different time points after viral challenge. In this analysis, cytotoxicity values obtained at different epitope concentrations for each macaque in every group (Figure 2A) were plotted against the viral load for that macaque. Because the values at the different epitope concentrations were a measure of CTL avidity (high avidity represented by cytotoxicity at low epitope concentrations and vice versa), these plots allowed us to estimate the correlation coefficient values at different levels of CTL avidity for the entire study group.
By this analysis, at day 17 after viral challenge when the viral loads were generally near their peak, there was a strong inverse correlation (correlation coefficient > 0.6) for both high- and high- plus low-avidity CTLs, but the correlation was better for the higher avidity CTLs (Figure 5A). In contrast, a similar analysis at day 31 showed a strong correlation only with high-avidity CTLs (data not shown). Consistent with their generally higher CTL response and lower viral load, the group 2 animals clustered in the high-avidity, low viral load portion of the plot, contributing to the correlation observed. In contrast, the group 3 animals clustered mostly at the opposite end of the curve. However, the correlation among all the animals makes the point that regardless of the immunization regimen, those animals that responded to the vaccine with a stronger, higher avidity CTL response were more protected.
Figure 5.
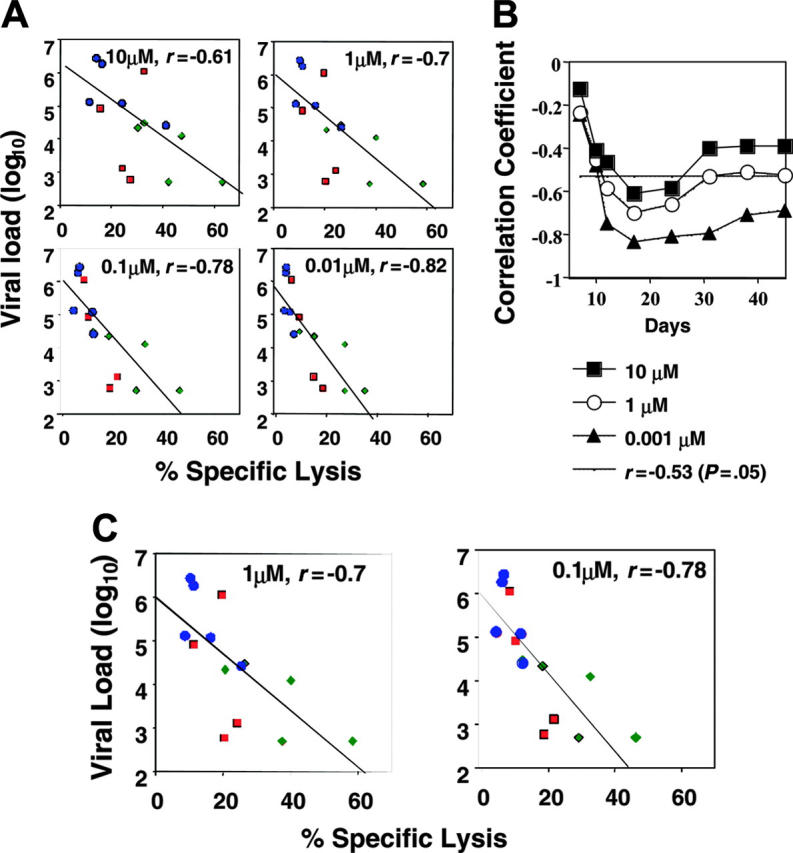
The viral load during the early stages of infection inversely correlated with the level of high-avidity SHIV-specific CD8+CTLs in the mucosal lymphoid tissues but not in blood. (A) Dynamics of the relationships between log viral load in blood on day 17 and avidity of the CL10-specific CTLs (against target cells pulsed with 10 μM, 1 μM, 0.1 μM, and 0.01 μM peptide) in MLNs before SHIV challenge. Individual data points for macaques in group 1 are color-coded as red squares; group 2, green diamonds; and group 3, blue circles. Spearman correlation coefficient and its P value were calculated. (B) Dynamics of correlation between high-avidity (0.001 μM), intermediate-avidity (1 μM), or low-avidity (10 μM) CL10-specific responses and log plasma viral load over time. The dynamics of high-avidity (0.001 μM) CTLs were significantly different from those of the CTLs measured at 1 and 10 μM peptide (P < .001). All correlation coefficients were negative (inverse correlation), but those with absolute value < 0.5 were not significant (above the horizontal line). (C) Correlation between high- and intermediate-avidity (0.1 μM and 1 μM) Gag372-specific responses before viral challenge and log of plasma viral load on day 17.
In a longitudinal analysis we plotted correlation coefficients from this type of analysis for a series of time points after challenge covering the acute phase of infection for cytotoxicity values obtained at each of 3 epitope concentrations (Figure 5B). A significant inverse correlation between high-avidity CTL response (0.001 μM) and viral load was seen through the first 45 days after challenge, whereas a significant inverse correlation was seen with the total (high- plus low-avidity) CTL (10 μM) response only during the first few weeks after challenge (Figure 5B). (The negative values of r below the dashed line in Figure 3D at approximately -0.5 were statistically significant.) Thus, in the first 7 weeks after challenge, CTL avidity correlated with viral load and the higher the avidity the better the inverse correlation. This early period reflects, in part, the time in which viral load kinetics were delayed in some animals, suggesting a relationship between high-avidity CTLs and the delay in peak viral load in group 2.
To examine whether these correlations could be generalized beyond the Gag CL10 immunodominant epitope, we studied the correlation of CTL response and avidity before challenge specific for another (non–immunodominant) CTL epitope, Gag372, in MLNs versus plasma viral load at day 17 after viral challenge. Consistent with the CL10 correlation data, we found very strong inverse correlations between CTL avidity after immunization and viral load (Figure 5C). The correlation was again stronger for the response measured at 0.1 μM, corresponding to the higher avidity population for this subdominant epitope.
Discussion
It is now clear that the role of mucosal tissues in HIV infection extends beyond its well-known function as an initial site of HIV entry and transmission. Recent studies establish that the gastrointestinal mucosa also serves as a reservoir of HIV infection and persistence.1,3,4 Thus, a primary goal of an HIV vaccine is to establish immunity in the mucosal tissues that prevents the virus from establishing the mucosa as a site of continued replication and dissemination to other tissues.8,9,45-47
Previous studies from our laboratory as well as from other laboratories focusing on responses of the gastrointestinal mucosa and intrarectal viral challenge provide support of this thesis.5,8,48-50 Thus, in studies in mice infected with a surrogate virus, a recombinant vaccinia virus expressing HIV gp160, we have shown that mucosal CTLs are essential for resistance to mucosal transmission of the surrogate organism.5 Later, in studies in primates infected with SHIV, we demonstrated that intrarectal immunization of rhesus macaques with a peptide vaccine was more effective than systemic immunization with the same vaccine in reducing the set point plasma viral load, due to reduction of the mucosal viral reservoir seeding the bloodstream.8 Thus, although a systemically administered AIDS vaccine might also contribute to mucosal immune responses (both CD8 CTLs and antibody) and protection,51-53 these results strongly suggested that mucosal immunization more effectively reduces the mucosal reservoir.
Correspondingly, female rhesus macaques chronically infected with SIV after intravaginal inoculation of SIV developed SIV-specific CD8+intraepithelial lymphocytes mostly located in the vaginal tissue.54 Such intravaginal infection may be initially localized to the mucosa because SIV is found only in endocervical tissue during the first 2 to 7 days of infection.2,13 These studies raise the question whether early CTL responses in mucosal tissues may be able to limit HIV infection to the mucosal area, at least for a period of time, and eradicate the infection before it becomes systemic.
In the current study, we tested this hypothesis that mucosal CTLs of sufficiently high avidity and quantity can actually affect dissemination from the initial mucosal nidus of infection. The inverse correlations with viral load were stronger for CTL responses in the colon and MLNs than for those in peripheral blood, and particularly strong for high-avidity mucosal CTLs. Animals in group 2 showed a significant delay in the time of peak of viremia (and a decrease in the magnitude of the peak just missing statistical significance) compared with that observed in the other experimental groups, correlating with their higher avidity and magnitude CTL response. This delayed onset of acute infection is based only on animals that became viremic and is primarily a comparison with the groups immunized with other regimens, so it is a robust finding despite the failure of some control group 4 macaques to become infected. Indeed, group 2 also initially contained 2 macaques with definite evidence of having been infected that never expressed virus in the blood, whereas other macaques that never expressed virus in the blood had no evidence of ever being infected. Thus, although prevention of mucosal transmission of virus was not complete in group 2, there was considerable evidence that movement of virus away from the initial site of infection was being resisted.
A major finding was that both the intrarectally administered peptide vaccine regimen and the combined peptide/NYVAC regimen induced a higher CTL response in the colon and MLNs, more than in the peripheral blood, as well as a higher avidity mucosal CTL response than did the intrarectal NYVAC regimen alone. This result may relate to the effect of the adjuvant cocktail on dendritic cells presenting the peptide antigen. Thus, our laboratory has previously shown that the augmentation of T- cell costimulation (signal 2) could compensate for stimulation with low level of antigen (signal 1) and induce high-avidity CTLs.55 Furthermore, we found that the delivery of antigen as well as IL-15 by a single vaccine vector also augments induction of high-avidity CTLs by promoting avidity maturation over time.56 These findings suggest that in the current study the repeated use of GM-CSF, IL-12, and D-type CpG ODNs as adjuvant with peptide augmented both costimulation and IL-15 production by the dendritic cells presenting the peptide and thus contributed to both of these mechanisms of induction of high-level, high-avidity CTLs.
One possible reason for the greater ability of high-avidity CTLs to clear viral infection is suggested by our previous studies that such CTLs kill recently infected cells expressing low levels of viral antigen.16 Thus, in the mucosa of the macaques under study here, high-affinity CTLs were potentially better able to recognize infected cells expressing low levels of SHIV antigens and eliminate these cells before much viral progeny were made. The correlations with avidity extend the evidence for greater efficacy of high-avidity CTLs against viral infection from mice14-16 to nonhuman primates, and from systemic immunity to mucosal immunity.
In conclusion, the time to peak viremia may be an important measure of control of the primary local mucosal infection, and the data therefore emphasize that both the level of the mucosal CTL response (its quantity) as well as its avidity (quality) determine the effectiveness of a mucosal vaccine. Furthermore, the strong inverse correlation between colonic CTL and high-avidity CTL activity in the MLNs before challenge and the plasma viremia early after challenge (ie, the delay in the acute phase of infection) supports the conclusions that it is indeed possible for mucosal CTLs to have an impact on the initial mucosal nidus of infection before it disseminates and that high-avidity mucosal CTLs are likely to be important for protection against mucosal transmission of AIDS viruses in primates.
Acknowledgments
We thank Dr David I. Watkins, Wisconsin Regional Primate Research Center, for Mamu-A*01 typing of macaques; Dr John Parrish, Martin Spano, and the veterinary and animal care staff at the Advanced BioScience Laboratories for their assistance in carrying out these experiments. We also thank Drs Jack R. Bennink, Jeffrey D. Lifson, and Gene Shearer for critical reading of the manuscript and helpful suggestions. We thank Drs John H. Eldridge and John Ryan, Wyeth Research, for recombinant human IL-12.
Prepublished online as Blood First Edition Paper, December 22, 2005; DOI 10.1182/blood-2005-11-4374.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280: 427-431. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z-Q, Schuler T, Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286: 1353-1357. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200: 749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200: 761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belyakov IM, Ahlers JD, Brandwein BY, et al. The importance of local mucosal HIV-specific CD8+ cytotoxic T lymphocytes for resistance to mucosal-viral transmission in mice and enhancement of resistance by local administration of IL-12. J Clin Invest. 1998;102: 2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belyakov IM, Derby MA, Ahlers JD, et al. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci U S A. 1998;95: 1709-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belyakov IM, Ahlers JD, Berzofsky JA. Mucosal AIDS vaccines: current status and future directions. Expert Rev Vaccines. 2004;3(suppl): 65-73. [DOI] [PubMed] [Google Scholar]
- 8.Belyakov IM, Hel Z, Kelsall B, et al. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001;7: 1320-1326. [DOI] [PubMed] [Google Scholar]
- 9.Belyakov IM, Berzofsky JA. Immunobiology of mucosal HIV infection and the basis for development of a new generation of mucosal AIDS vaccines. Immunity. 2004;20: 247-253. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283: 857-860. [DOI] [PubMed] [Google Scholar]
- 11.Jin X, Bauer DE, Tuttleton SE, et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189: 991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro BA, Homsy J, Lennette E, Murthy KK, Eichberg JW, Levy JA. HIV-1 expression in chimpanzees can be activated by CD8+ cell depletion or CMV infection. Clin Immunol Immunopathol. 1992;65: 227-233. [DOI] [PubMed] [Google Scholar]
- 13.Spira AI, Marx PA, Patterson BK, et al. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183: 215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high or low avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci U S A. 1996;93: 4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallimore A, Dumrese T, Hengartner H, Zinkernagel RM, Rammensee HG. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187: 1647-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derby MA, Alexander-Miller MA, Tse R, Berzofsky JA. High avidity CTL exploit 2 complementary mechanisms to provide better protection against viral infection than low avidity CTL. J Immunol. 2001;166: 1690-1697. [DOI] [PubMed] [Google Scholar]
- 17.Snyder JT, Alexander-Miller MA, Berzofsky JA, Belyakov IM. Molecular mechanisms and biological significance of CTL avidity. Curr HIV Res. 2003;1: 287-294. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor DH, Allen TM, Vogel TU, et al. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat Med. 2002;8: 493-499. [DOI] [PubMed] [Google Scholar]
- 19.Knapp LA, Lehmann E, Piekarczyk MS, Urvater JA, Watkins DI. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens. 1997;50: 657-661. [DOI] [PubMed] [Google Scholar]
- 20.Berzofsky JA, Pendleton CD, Clerici M, et al. Construction of peptides encompassing multideterminant clusters of HIV envelope to induce in vitro T-cell responses in mice and humans of multiple MHC types. J Clin Invest. 1991;88: 876-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuroda MJ, Schmitz JE, Barouch DH, et al. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J Exp Med. 1998;187: 1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen TM, Mothe BR, Sidney J, et al. CD8(+) lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule mamu-A*01: implications for vaccine design and testing. J Virol. 2000;75: 738-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tartaglia J, Perkus ME, Taylor J, et al. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992;188: 217-232. [DOI] [PubMed] [Google Scholar]
- 24.Joag SV, Li Z, Foresman L, et al. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pigtailed macaques. J Virol. 1996;70: 3189-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belyakov IM, Wyatt LS, Ahlers JD, et al. Induction of mucosal CTL response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing HIV 89.6 envelope protein. J Virol. 1998;72: 8264-8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belyakov IM, Moss B, Strober W, Berzofsky JA. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc Natl Acad Sci U S A. 1999;96: 4512-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman JD, Moss PAH, Goulder PJR, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274: 94-96. [PubMed] [Google Scholar]
- 28.Romano JW, Shurtliff RN, Dobratz E, et al. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J Virol Methods. 2000;86: 61-70. [DOI] [PubMed] [Google Scholar]
- 29.Pal R, Venzon D, Letvin NL, et al. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol. 2002;76: 292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuznetsov VA, Stepanov VS, Berzofsky JA, Belyakov IM. Assessment of the relative therapeutic effects of vaccines on virus load and immune responses in small groups at several time points: An efficacy of mucosal and subcutaneous polypeptide vaccines in rhesus macaques exposed to SHIV. J Clin Virol. 2004: S69-S82. [DOI] [PubMed]
- 31.Belyakov IM, Earl P, Dzutsev A, et al. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci U S A. 2003;100: 9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen TM, Sidney J, Del Guercio MF, et al. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J Immunol. 1998;160: 6062-6071. [PubMed] [Google Scholar]
- 33.Ahlers JD, Dunlop N, Alling DW, Nara PL, Berzofsky JA. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: GM-CSF and TNFa synergize with IL-12 to enhance induction of CTL. J Immunol. 1997;158: 3947-3958. [PubMed] [Google Scholar]
- 34.O'Neill E, Martinez I, Villinger F, et al. Protection by SIV VLP DNA prime/protein boost following mucosal SIV challenge is markedly enhanced by IL-12/GM-CSF co-administration. J Med Primatol. 2002;31: 217-227. [DOI] [PubMed] [Google Scholar]
- 35.Ahlers JD, Belyakov IM, Berzofsky JA. Cytokine, chemokine and costimulatory molecule modulation to enhance efficacy of HIV vaccines. Curr Mol Med. 2003;3: 285-301. [DOI] [PubMed] [Google Scholar]
- 36.Belyakov IM, Ahlers JD, Clements JD, Strober W, Berzofsky JA. Interplay of cytokines and adjuvants in the regulation of mucosal and systemic HIV-specific cytotoxic T lymphocytes. J Immunol. 2000;165: 6454-6462. [DOI] [PubMed] [Google Scholar]
- 37.Verthelyi D, Kenney RT, Seder RA, Gam AA, Friedag B, Klinman DM. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J Immunol. 2002;168: 1659-1663. [DOI] [PubMed] [Google Scholar]
- 38.Ahlers JD, Belyakov IM, Terabe M, et al. A push-pull approach to maximize vaccine efficacy: abrogating suppression with an IL-13 inhibitor while augmenting help with GM-CSF and CD40L. Proc Natl Acad Sci U S A. 2002;99: 13020-13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanke T, Blanchard TJ, Schneider J, et al. Enhancement of MHC class I-restricted peptide-specific T cell induction by a DNA prime/MVA boost vaccination regime. Vaccine. 1998;16: 439-445. [DOI] [PubMed] [Google Scholar]
- 40.Amara RR, Villinger F, Altman JD, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292: 69-74. [DOI] [PubMed] [Google Scholar]
- 41.Shiver JW, Fu TM, Chen L, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415: 331-335. [DOI] [PubMed] [Google Scholar]
- 42.Hel Z, Nacsa J, Tryniszewska E, et al. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8++ T cell responses. J Immunol. 2002;169: 4778-4787. [DOI] [PubMed] [Google Scholar]
- 43.Stevceva L, Alvarez X, Lackner AA, et al. Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIV(gpe) recombinant vaccine result in gag-specific CD8(+) T-cell responses in mucosal tissues of macaques. J Virol. 2002;76: 11659-11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belyakov IM, Wang J, Koka R, et al. Activating CTL precursors to reveal CTL function without skewing the repertoire by in vitro expansion. Eur J Immunol. 2001;31: 3557-3566. [DOI] [PubMed] [Google Scholar]
- 45.Berzofsky JA, Ahlers JD, Derby MA, Pendleton CD, Arichi T, Belyakov IM. Approaches to improve engineered vaccines for human immunodeficiency virus (HIV) and other viruses that cause chronic infections. Immunol Rev. 1999;170: 151-172. [DOI] [PubMed] [Google Scholar]
- 46.Berzofsky JA, Ahlers J, Janik J, et al. Progress on new vaccine strategies against chronic viral infections. J Clin Invest. 2004;114: 450-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev Immunol. 2001;1: 209-219. [DOI] [PubMed] [Google Scholar]
- 48.Belyakov IM, Hammond SA, Ahlers JD, Glenn GM, Berzofsky JA. Transcutaneous immunization induces mucosal CTL and protective immunity by migration of primed skin dendritic cells. J Clin Invest. 2004;113: 998-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehner T, Bergmeier L, Wang Y, Tao L, Mitchell E. A rational basis for mucosal vaccination against HIV infection. Immunol Rev. 1999;170: 183-196. [DOI] [PubMed] [Google Scholar]
- 50.Murphey-Corb M, Wilson LA, Trichel AM, et al. Selective induction of protective MHC class I restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J Immunol. 1999;162: 540-549. [PubMed] [Google Scholar]
- 51.McMichael A, Hanke T. The quest for an AIDS vaccine: is the CD8+ T-cell approach feasible? Nat Rev Immunol. 2002;2: 283-291. [DOI] [PubMed] [Google Scholar]
- 52.Calarota SA, Weiner DB. Present status of human HIV vaccine development. AIDS. 2003;17(suppl 4): S73-S84. [DOI] [PubMed] [Google Scholar]
- 53.Johnson RP, Lifson JD, Czajak SC, et al. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J Virol. 1999;73: 4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lohman BL, Miller CJ, McChesney MB. Antiviral cytotoxic T lymphocytes in vaginal mucosa of simian immunodeficiency virus-infected rhesus macaques. J Immunol. 1995;155: 5855-5860. [PMC free article] [PubMed] [Google Scholar]
- 55.Oh S, Hodge JW, Ahlers JD, Burke DS, Schlom J, Berzofsky JA. Selective induction of high avidity CTL by altering the balance of signals from antigen presenting cells. J Immunol. 2003;170: 2523-2530. [DOI] [PubMed] [Google Scholar]
- 56.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15R α-mediated avidity maturation of memory CD8+T cells. Proc Natl Acad Sci U S A. 2004;101: 15154-15159. [DOI] [PMC free article] [PubMed] [Google Scholar]