Src Family Kinases Directly Regulate JIP1 Module Dynamics and Activation (original) (raw)
Abstract
JIP1 is a mammalian scaffold protein that assembles and participates in regulating the dynamics and activation of components of the mixed-lineage kinase-dependent JNK module. Mechanisms governing JIP1-JNK module regulation remain unclear. JIP1 is a multiply phosphorylated protein; for this reason, it was hypothesized that signaling by unidentified protein kinases or phosphatases might determine module function. We find that Src family kinases directly bind and tyrosine phosphorylate JIP1 under basal conditions in several naturally occurring systems and, by doing so, appear to provide a regulated signal that increases the affinity of JIP1 for DLK and maintains the JIP-JNK module in a catalytically inactive state.
Mitogen-activated protein kinase (MAPK) pathways are assembled from a unique combination of protein kinases into distinct protein complexes, or modules (17, 21). The minimal MAPK module uniformly contains a MAPKKK, a MAPKK, and a MAPK. The components of these modules interact via direct protein-protein interactions and/or are tethered to scaffolding proteins. As with other scaffolded protein complexes, assembly of MAPK modules appears to allow segregation of MAPK signaling components into units that are responsive to independent stimuli, that obtain appropriate subcellular targeting, that are insulated from similar modules, and that can regulate functionally distinct substrates (7-9). Identification of the JIP family of JNK scaffolding proteins first established that mammalian cells organize JNK pathways into modules in a fashion similar to that seen in Saccharomyces cerevisiae (32). Four JIP family genes and several splice isoforms have been identified (21). JIP proteins can form homo- and hetero-oligomers and are phosphoproteins. Whereas JIP3 is structurally and functionally divergent from JIP1 and JIP2, each has been demonstrated to associate directly with a mixed-lineage kinase, with MKK7, and with JNK. JIP4 binds MKK3 and MKK6 and appears to participate in a p38mapk pathway (14). It has been proposed that JIP1 and -2 proteins facilitate mixed-lineage kinase-dependent signal transduction to JNK, possibly by aggregating the three components of a JNK module.
Although considerable work has been done to establish the role of JIP1 in regulating JNK signaling (23, 31), less is known about the mechanisms that govern this regulation. We previously demonstrated that the JNK module components DLK and JNK interact with JIP1 in a dynamic manner to regulate the activation of the associated mixed-lineage kinase and ultimately JNK module activity (23, 24). According to previously published results, DLK associates with JIP1 in a monomeric, catalytically inactive state under basal conditions. Upon appropriate stimulation, JNK is recruited to JIP1 and JIP1 is phosphorylated by JNK. Particularly as a result of JNK-dependent phosphorylation on JIP1(T103), DLK dissociates from JIP1, oligomerizes, and becomes catalytically active. While contrary to standard convention, our previous results implied that recruitment of JNK to JIP1 and JNK-dependent phosphorylation of JIP1 is prerequisite to activation of the upstream kinases that are components of a preassembled inactive JIP1 complex. The concept that the JIP1-JNK interaction influences the dynamics or activation state of JIP1 complex components has been supported subsequently in studies by others suggesting, for example, that recruitment of JNK to JIP1 and phosphorylation on T103 regulates JIP1-Akt dynamics and Akt activity (16, 18) or that, in S. cerevisiae, Fus3 catalytic activity is primed by allosteric effects of Ste5 on Fus3 prior to Ste5-Fus3 module activation (2).
While the results of these studies improved our understanding of the dynamics of the JIP1-JNK module, the mechanism by which this module is regulated remained unclear. JIP1 is known to be phosphorylated on multiple serine and threonine residues, and it has been anticipated but not demonstrated that JIP1 might also be phosphorylated on tyrosine residues (5). For this reason, it was hypothesized that inputs to JIP1 from other signaling pathways that are mediated by unidentified protein kinases or phosphatases might play a role in JIP1 phosphorylation and function. Certainly, work in unrelated systems suggested that these JIP1 phosphorylation events might regulate complex assembly, subcellular localization, or activation (26, 28). In work aimed at addressing this hypothesis, we discovered that Src family kinase (SFK) directly binds and tyrosine phosphorylates JIP1 under basal conditions in several naturally occurring systems and by doing so appears to provide a signal that increases the affinity of JIP1 for DLK and maintains the JIP-JNK module in a catalytically inactive state.
MATERIALS AND METHODS
Reagents.
Polyclonal antibodies to JIP1 and DLK were described previously (24). Other antibodies, including anti-FLAG (M2; Sigma), antihemagglutinin (anti-HA) (Sigma), anti-JNK (Santa Cruz Biotechnology, Inc.), antiphosphotyrosine mouse monoclonal antibody (P-Tyr-100; Cell Signaling, catalog no. 9411), and pan-Src, Fyn, c-Src, α-tubulin (Santa Cruz Biotechnology), and Yes (Upstate Biotechnology) antibodies were obtained commercially. Monoclonal JIP1 antibody was obtained from BD Transduction Laboratories (catalog no. 611890). The cell transfection reagents Fugene and Lipofectamine-2000 were purchased from Roche Biochemicals and Invitrogen, respectively. All chemical reagents, including kainic acid and PP2, were obtained commercially from Sigma and Calbiochem, respectively. A phosphoprotein enrichment kit for isolating phosphorylated proteins from cell lysate was purchased from BD Biosciences (catalog no. K1256-1). A JIP1 affinity matrix was prepared by coupling purified His-JIP1 to a cyanogen bromide-activated matrix (Sigma, catalog no. C9142) using the manufacturer's protocol. Recombinant glutathione _S_-transferase (GST)-Fyn fusion proteins, GST-c-Jun-(1-79), and His-JIP1 were prepared and purified from bacterial lysates as described previously (24). Purified active His-Fyn (Upstate Biotechnology) was obtained commercially. Mammalian expression plasmids encoding various SFKs were previously described (30). Fyn−/− mice (129-Fyntm1Sor/J; stock number 002271) were obtained from Jackson Laboratory (Bar Harbor, ME). All animal experiments were approved by the University Committee on the Use and Care of Animals Institutional Review Board at the University of Michigan Medical School.
Preparation of mouse brain lysate.
Brain lysate was prepared based on the procedure described by Kasahara et al. (13) Briefly, mouse brains were homogenized in ice-cold buffer (0.32 M sucrose, 1 mM Tris-HCl [pH 7.4], 0.1 mM EDTA) using a Teflon motor-driven glass homogenizer. The homogenate was centrifuged at 400 × g for 5 min, and the supernatant was centrifuged at 12,000 × g for 20 min. The resulting pellet was solubilized in TNE membrane lysis buffer (50 mM Tris-HCl [pH 7.5], 1% NP-40, 150 mM NaCl, 1 mM Na3VO4, 0.5 mM EDTA, 5 mM NaF, 5 mM Na4P2O7, and a mixture of protease inhibitors) at 4°C for 20 min. These postnuclear supernatants were collected after centrifugation at 20,000 × g for 10 min. Total protein was estimated using a standard Bradford assay. The lysate was subsequently used for affinity chromatography and kinase assays.
JIP1 affinity chromatography.
JIP1-interacting proteins were isolated from mouse brain lysate by using the phosphoprotein enrichment kit from BD Biosciences according to the manufacturer's protocol. Briefly, mouse brain lysate was prepared by homogenization as described above. The resulting tissue pellet was dissolved in extraction buffer (buffer A, phosphoprotein enrichment kit, catalog no. S3406) and subjected to fractionation on a phosphocellulose column (catalog no. S3405). Bound phosphoproteins were eluted with elution buffer (buffer B, catalog no. S3407) and then further fractionated on a His-JIP1 affinity column. Eluted phosphoproteins were incubated with His-JIP beads for 3 h at 4°C and then washed with phosphate-buffered saline (PBS) containing 0.1% Tween 20. Beads were boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and associated proteins were resolved by SDS-PAGE. Gels were stained with Coomassie blue, and stained bands were cut and analyzed by mass spectrometry at the University of Michigan core facility (Michigan Proteome Consortium).
Bacterial fusion protein construction and expression.
GST-c-Jun(1-79) fusion protein was prepared as described previously (24). GST-JNK3 (preactivated and catalytically competent) used as a control was obtained from Upstate Biotechnology. Hexahistidine-tagged full-length JIP1(1-711) and GST-JIP1 were described previously (24). Tyrosine-phosphorylated GST-JIP1 was produced in Escherichia coli TKB1 (Stratagene) according to the manufacturer's protocol. Purified active His-Fyn (Upstate Biotechnology) was obtained commercially.
In vitro binding assays.
Purified His-Fyn was bound to nickel beads. These beads were incubated for 3 h at 4°C with either purified GST, purified GST-JIP1, or purified GST-JIP1 expressed in TKB1 cells in a volume of 400 μl containing PBS and 0.1% Tween-20. After a washing with PBS containing 0.1% Tween-20, beads were eluted with elution buffer containing imidazole. Eluate was resolved by SDS-PAGE prior to immunoblotting with the indicated panel of antibodies (see Fig. 1). For the overlay assay, purified His-Fyn (0.2 μg per lane), purified GST-Fyn-SH2 and GST-Fyn-SH3 domains, and GST proteins (2 μg per lane) were resolved by SDS-PAGE, transferred to nitrocellulose, and overlaid with GST-JIP1 fusion proteins (2 μg each) for 6 h at 4°C. The associated JIP1 proteins were detected by immunoblotting the membranes with monoclonal JIP1 antibody.
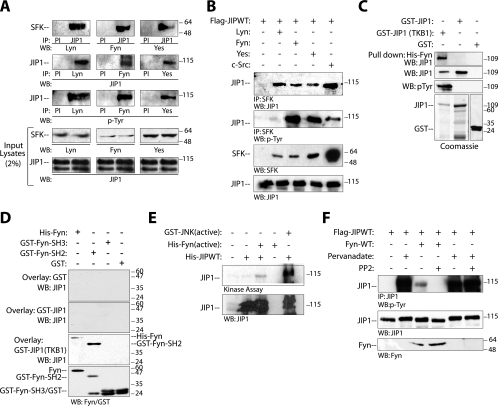
FIG. 1.
JIP1 interacts with SFKs in vivo. (A) Endogenous JIP1 was coimmunoprecipitated with endogenous Lyn, Fyn, or Yes from normal mouse brain lysates. Note that endogenous JIP1 that coimmunoprecipitated with the various SFKs was tyrosine phosphorylated. (B) Flag-JIP1 and the indicated SFKs were coexpressed in COS-7 cells. As in panel A, JIP1 coimmunoprecipitated with the indicated SFK and was tyrosine phosphorylated under these conditions. (C) JIP1 binds directly to Fyn in a tyrosine phosphorylation-dependent fashion. Immobilized His-Fyn was mixed with GST, GST-JIP1 or GST-JIP1 expressed in TKB1 cells. The GST-JIP1 pull-down complex was resolved by SDS-PAGE and immunoblotted using JIP1 antibody. Input of proteins was detected by Coomassie blue staining or immunoblotting. (D) Recombinant His-Fyn, GST-Fyn-SH2, GST-Fyn-SH3, or GST alone was run on SDS-PAGE, transferred to membranes, and overlaid with the indicated GST fusion proteins; bound JIP1 proteins were detected by immunoblotting. (E) Fyn directly phosphorylates JIP1. Purified recombinant His-JIP1 was mixed with either active His-Fyn or active GST-JNK (as a positive control), incubated in a kinase buffer containing [γ-32P]ATP at 30°C for 30 min, and then resolved by SDS-PAGE and autoradiography. The same blot was immunoblotted with JIP1 antibody to ensure that the phosphorylated protein is JIP1. Similar results were obtained in three independent experiments. (F) JIP1 is tyrosine phosphorylated in vivo. Flag-JIP1 was coexpressed with Fyn in COS-7 cells. Where indicated, at 24 h posttransfection cells were pretreated with PP2 (5 μM) or with sodium orthovanadate (50 μM) or both. JIP1 was immunoprecipitated from cell lysates, and immunoprecipitates were analyzed for JIP1 tyrosine phosphorylation by immunoblotting.
Eukaryotic expression constructs.
Construction and characterization of mammalian expression plasmids encoding Flag-DLK, Flag-DLK(K185A), and HA-DLK were described previously (22). Mammalian expression constructs encoding JIP1(Y431F) and JIP1(R160G/P161G/Y431F) were prepared by a previously described method (19) using one long primer (GAGTCGGCCATCGGAGAGGAATTTGAGGAGGCCCCG) in a single cloning step with the templates Flag-JIP1 and Flag-JIP1(R160G/P161G), respectively. Restriction digestion and DNA sequencing were used to validate all constructs.
Cell culture.
Cells of the HN33 line, an immortalized rat hippocampal neuronal cell line, were a gift from Y.-F. Liu (24). Mouse neuroblastoma cell line N2a was obtained from the American Type Culture Collection (ATCC; catalog no. CCL-131). The cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen) and 200 units/ml penicillin and streptomycin (Roche Diagnostics). The transfections were performed using Fugene-6 (Roche Diagnostics) according to the manufacturer's protocols. Where indicated, cells were treated by the addition of various concentrations of kainic acid or reelin (400 μl supernatant from reelin-expressing 293 cells, as described previously [4]). 3T3-L1 cells were grown according to established protocols (15). Where indicated, cells were grown for 2 days postconfluence and treated with MDI (methylisobutylxanthine, dexamethasone, and insulin) for various intervals. The cells were lysed in lysis buffer (50 mM HEPES [pH 7.4], 1% NP-40, 150 mM NaCl, 0.5% deoxycholate, 1 mM Na3VO4, 0.5 mM EDTA, 5 mM NaF, 5 mM Na4P2O7, 10 mM β-glycerophosphate, and a mixture of protease inhibitors). SYF cells (c-Src−/− Yes−/− Fyn−/−) were purchased from the ATCC (CRL-2459) and cultured according to the supplier's instructions. SYF cells were transfected with Lipofectamine-2000 according to manufacturer's protocol.
Immunoprecipitation, immunoblotting, and pull-down assay.
Immunoprecipitation, immunoblotting, and pull-down experiments were performed using the procedures described previously (24).
JNK activation assays.
Cell lysates were prepared 24 h after transfection using 1 ml of TNE membrane lysis buffer (50 mM Tris · HCl [pH 8.0], 1% [wt/vol] Nonidet P-40, 120 mM NaCl, 5 mM EDTA, 0.2 mM sodium vanadate, 50 mM sodium fluoride, 20 mM β-glycerophosphate, and a cocktail of protease inhibitors [Boehringer Mannheim]). Kinase assays for JNK or JIP1 immune complexes were performed as described previously (24). The relative activity from each sample was estimated using ImageQuant software from Molecular Dynamics.
JIP1 peptide array.
JIP1 peptides (10- to 15-mers centered around tyrosine and flanked by alanine) representing the theoretical tyrosine phosphorylation sites (5) were synthesized by Sigma. The peptides were dissolved according to the manufacturer's instructions, and the spot array was generated by spotting these peptides onto polyvinylidene difluoride membranes at high density. Membranes were subjected to an in vitro kinase assay by incubating them with 1 μg of preactivated recombinant Fyn or Yes (both Upstate Biotechnology Inc.) for 4 h at room temperature in kinase reaction buffer (50 mM HEPES [pH 7.5], 2.5 mM MgCl2, 4 mM MnCl2, 0.1 mM sodium orthovanadate) containing 50 μCi of [γ-32P]ATP. The membranes were washed five times with PBS-Tween-20 and autoradiographed.
RESULTS
JIP1 associates with Src family kinases.
Our previous work suggested that JIP1 is phosphorylated on multiple amino acid residues and that these posttranslational modifications were likely dependent on several protein kinases and phosphatases (5, 24). To begin to investigate this possibility, JIP1-interacting phosphoproteins were identified by affinity chromatography. Phosphoproteins were enriched from mouse brain cortex lysate by phosphocellulose affinity chromatography, and these were subsequently subjected to JIP1 affinity chromatography. Isolated protein bands were analyzed by mass spectrometry. Among the peptides identified was the SFK Lyn, which with other SFKs was chosen for additional investigation. To corroborate that JIP1 can interact with SFKs, JIP1 was immunoprecipitated from mouse brain lysate using JIP1 antibody, and immune complexes were analyzed by immunoblotting for the presence of various SFKs. In these experiments, endogenous JIP1 associated with Lyn, Fyn, and Yes (Fig. 1A). In a reciprocal experiment, immunoprecipitation from mouse brain lysate using Lyn, Fyn, and Yes antibodies followed by immunoblot analysis with JIP1 antibody yielded similar results. Importantly, endogenous JIP1 immunoprecipitated from brain with these SFKs was tyrosine phosphorylated (Fig. 1A). Similar results were also obtained when Lyn, Fyn, c-Src, and Yes were each coexpressed individually with JIP1 in COS-7 cells (Fig. 1B).
JIP1 directly binds to and is directly phosphorylated by Src family kinases.
To examine whether JIP1 and SFKs interact directly, purified bacterial recombinant His-Fyn immobilized on agarose beads was incubated in vitro with GST protein alone, GST-JIP1, or GST-JIP1 that was expressed in TKB1 bacteria in which JIP1 was tyrosine phosphorylated in a nonspecific fashion (Fig. 1C). Pull-down assays showed that JIP1 bound Fyn only when JIP1 was first tyrosine phosphorylated by expression in TKB1 cells. Consistent with this observation, as shown in a protein overlay interaction assay, Fyn bound JIP1 via its SH2 domain and not via its SH3 domain in an interaction that required prior tyrosine phosphorylation of JIP1 (Fig. 1D). To determine whether Fyn can directly phosphorylate JIP1 in vitro, purified His-JIP1 was incubated for 20 min with either activated recombinant His-Fyn or His-JNK (positive control) in a kinase buffer containing radiolabeled ATP (Fig. 1E). Under these conditions, JIP1 became phosphorylated. In additional experiments, JIP1 was expressed in COS-7 cells. When these cells were treated with sodium orthovanadate 30 min prior to cell lysis, JIP1 became tyrosine phosphorylated (Fig. 1F). Orthovanadate-induced JIP1 tyrosine phosphorylation was not blocked by addition of PP2, an inhibitor of SFK activity and possibly other protein kinases, suggesting that JIP1 may be a substrate for additional tyrosine kinases.
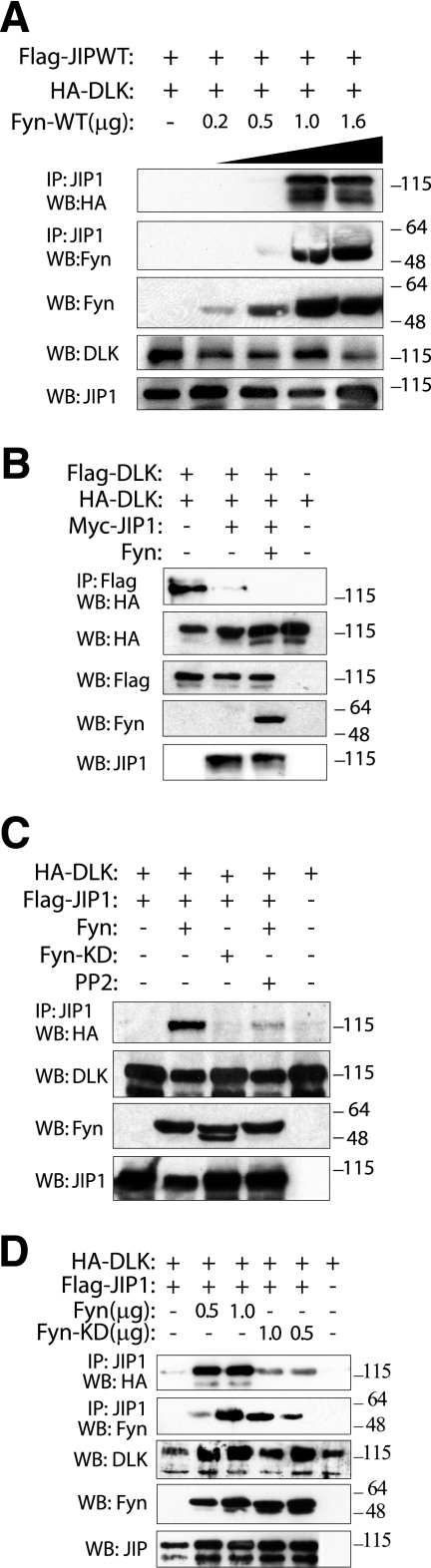
The affinity of JIP1 for DLK increases in the presence of SFK.
Our previous work provided evidence that JIP1 is phosphorylated on multiple serine and threonine residues and suggested that phosphorylation on at least one of these residues was necessary for complex activation (24). Considered with these previous observations, the new results indicating that JIP1 is also tyrosine phosphorylated by SFKs suggested the hypothesis that signaling events culminating in tyrosine phosphorylation of JIP1 residues modulate JIP1 complex dynamics and activation. To begin to test this hypothesis, we examined the influence of Fyn-JIP1 interaction and Fyn-mediated tyrosine phosphorylation of JIP1 on the relationship of JIP1 to other components of the JIP1 module. Flag-JIP1 was coexpressed with HA-DLK, and the interaction between these two proteins was examined in the presence of increasing concentrations of Fyn (Fig. 2A). Here, increased concentration of Fyn resulted in a notable increase in DLK coimmunoprecipitated with JIP1. This observation was extended by examining whether the observed increased binding of JIP1 to DLK in the presence of Fyn was due to increased JIP1-DLK affinity or due to increased oligomerization of DLK while DLK was associated with JIP. Flag-DLK and HA-DLK were coexpressed with Myc-JIP1 in the presence or absence of Fyn (Fig. 2B). Flag-DLK was immunoprecipitated, and immune complexes were analyzed for the presence of HA-DLK. As previously observed, JIP1 inhibited DLK oligomerization (23); the presence of Fyn did not affect this inhibition. Therefore, when this observation is considered with the results noted above, it is concluded that the presence of Fyn results in increased JIP1-DLK binding affinity. This effect appeared to be dependent both on Fyn-JIP1 association and on Fyn kinase catalytic activity. Coexpression of a catalytically inactive mutant of Fyn with JIP1 and DLK did not increase JIP1-DLK binding affinity to the same extent as coexpression with catalytically competent Fyn (Fig. 2C and D). While loss of catalytic activity of Fyn did not affect binding of Fyn to JIP1 (Fig. 2D), inhibition of SFK activity with PP2 attenuated the Fyn-mediated increase in JIP1-DLK binding affinity in the same system (Fig. 2C). Collectively, these results suggest that while DLK-JIP affinity is determined in part by SFK catalytic activity, the physical association of Fyn and JIP1 may also influence JIP1-DLK affinity independent of Fyn catalytic activity.
FIG. 2.
Binding of DLK to JIP1 but not oligomerization of DLK is increased in the presence of Fyn. (A) COS-7 cells were cotransfected with plasmids encoding Flag-JIP1, HA-DLK, and increasing amounts of Fyn. Cell lysates were immunoprecipitated with anti-JIP1 antibody, separated by SDS-PAGE, and immunoblotted with HA or Fyn antibody. Corresponding lysates were immunoblotted with Fyn, DLK, and JIP1 antibodies to evaluate the expression of these proteins. (B) Plasmids encoding Flag-DLK and HA-DLK were cotransfected with Myc-JIP1 and/or Fyn. Cell lysates were immunoprecipitated with anti-Flag antibody and immunoblotted with anti-HA antibody. These experiments were repeated three times with similar results. (C) Fyn catalytic activity is required for increased affinity of JIP1 for DLK. COS-7 cells were cotransfected with the indicated plasmids. Where noted, cells were treated with PP2 prior to cell lysis. JIP1 was immunoprecipitated from cell lysate, and immune complexes were separated by SDS-PAGE and immunoblotted. (D) Catalytic activity of Fyn is not required for association of Fyn with JIP1. COS-7 cells were cotransfected with plasmids encoding HA-DLK and Flag-JIP1 and with the indicated quantities of either wild-type Fyn or Fyn-KD (kinase-inactive mutant). JIP1 was immunoprecipitated from cell lysates, and immune complexes were separated by SDS-PAGE and immunoblotted with Fyn or HA antibodies.
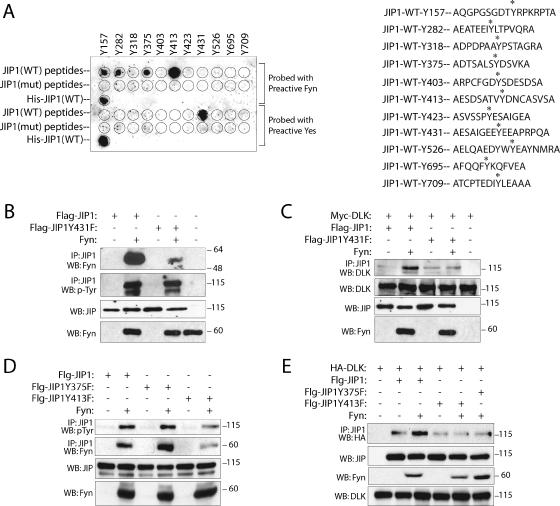
To establish that the observed affect of Fyn on JIP1-DLK affinity was due to a direct affect of Fyn on JIP1, we sought initially to identify a point mutation(s) in JIP1 that would attenuate the interaction of these proteins or would attenuate the SFK-dependent phosphorylation of JIP1. A synthetic oligopeptide-based approach was used to screen for SFK-dependent tyrosine phosphorylation sites in JIP1. Peptides representing theoretical tyrosine-phosphorylated JIP1 residues (5) were synthesized and used to generate a peptide array. As indicated in Fig. 3A, five SFK-dependent tyrosine-phosphorylated residues (Y157, Y282, Y375, Y413, and Y431) were identified in JIP1 using this approach. Examination of the mouse JIP1 primary sequence using Scansite (25) suggested the presence of several putative SH2 binding motifs (including Y413DNC and Y431EEA) that might be necessary for the JIP1-SFK interaction. To investigate the role of SFK-mediated phosphorylation of JIP1 in the activation of the JIP1 module, JIP1(Y375F), JIP1(Y413F), and JIP1(Y431F) mutants were prepared. These mutants and wild-type JIP1 were coexpressed with Fyn in COS-7 cells, and coimmunoprecipitation experiments were performed to examine the relative affinities of JIP1 and JIP1 mutants for Fyn (Fig. 3B and D). In these experiments, Fyn association with JIP1(Y431F) (Fig. 3B) and JIP1(Y413F) (Fig. 3D) but not JIP1(Y375F) (Fig. 3D) was attenuated relative to Fyn association with wild-type JIP1 in control experiments. The ability of Fyn to phosphorylate these mutants was also evaluated (Fig. 3B and D). In repeated experiments, tyrosine phosphorylation by Fyn of JIP1(Y431F) and JIP1(Y375F) was not clearly affected by tyrosine substitution. This observation was not surprising, since it was observed that JIP1 is phosphorylated on multiple tyrosine residues. In contrast, tyrosine phosphorylation of JIP1(Y413F) by Fyn was significantly reduced. Ultimately, the ability of Fyn to alter JIP1 mutant-DLK binding affinity was examined (Fig. 3C and E). Here, while expression of Fyn resulted in increased wild-type JIP1-DLK affinity as noted earlier, expression of Fyn had no effect on DLK binding affinity for any of the three JIP1 mutants examined. In summary, JIP1-SFK interaction and/or SFK-dependent phosphorylation of JIP1 (at Y375, Y413, and Y431) is a necessary determinant of JIP1-DLK affinity. Understanding the mechanism by which these SFK-dependent phosphorylation events determine JIP1-DLK binding affinity will require additional investigation. The specific SFK responsible for phosphorylation of specific JIP1 tyrosine residues will also require additional investigation.
FIG. 3.
SFKs directly phosphorylate JIP1 on tyrosine residues. (A) JIP1 peptides containing theoretical tyrosine phosphorylation sites (5) were used to generate a spot peptide array. As controls, mutant peptides were created in which tyrosine residues were replaced with phenylalanine. Recombinant His-JIP served as an additional control. The array was subjected to an in vitro kinase assay in the presence of recombinant Fyn or Yes. Asterisks indicate the predicted tyrosine residues within JIP1 peptides. (B to E) The indicated plasmids were expressed in COS-7 cells, and lysates were analyzed.
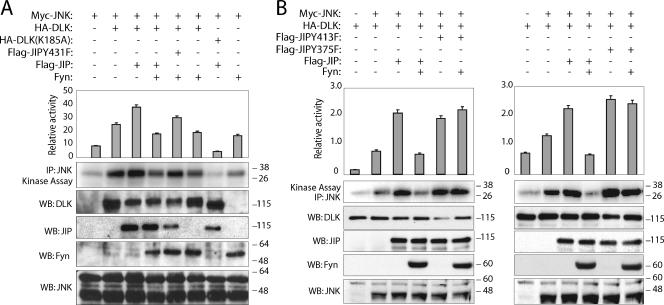
SFK inhibits JIP1-dependent JNK activation.
In previously published studies, we provided experimental evidence for a mechanism by which the DLK-dependent JIP1-JNK module is regulated (24). In this model, under basal conditions, DLK associated with JIP1 is held in a monomeric, unphosphorylated, and catalytically inactive state. Appropriate cellular stimulation results in recruitment of catalytically competent JNK to the JIP1 scaffold. In turn, recruitment of JNK coincides with significantly decreased affinity of JIP1 and DLK. DLK dissociation from JIP1 results in DLK oligomerization, autophosphorylation, activation, and subsequent activation of JNK. Since association of SFK and JIP and SFK-dependent JIP phosphorylation increases JIP-DLK affinity, we hypothesized that SFKs function within the JIP complex to attenuate DLK activation, thereby attenuating the degree to which JIP-JNK module-dependent signaling is propagated. To test this hypothesis, the effect of Fyn on JIP-dependent JNK activation was investigated (Fig. 4). As described previously, addition of overexpressed JIP1 to overexpressed DLK and JNK enhanced DLK-induced JNK activation in COS-7 cells (6). Remarkably, inclusion of overexpressed Fyn in this system resulted in attenuation of JNK activity to a level similar to that observed when JNK and Fyn alone were coexpressed (Fig. 4A, compare lanes 4 and 8). To examine whether the observed effect of Fyn in this experiment was specifically mediated by its influence on JIP1, JIP1 mutants (Y431F, Y375F, and Y413F) were substituted for overexpressed wild-type JIP1. Under these conditions, the ability of Fyn to attenuate JNK activity was attenuated (Fig. 4). These results support the conclusion that SFK interaction with and phosphorylation of JIP1 attenuates DLK-mediated JNK activation by increasing the affinity of JIP1 for DLK and thereby facilitates the effect of JIP1 that maintains DLK in its monomeric, catalytically inactive state.
FIG. 4.
JIP tyrosine mutants attenuate the effect of Fyn on module activation. Myc-JNK was coexpressed with the indicated plasmids in COS-7 cells. JNK kinase catalytic activity was analyzed in kinase buffer containing the substrate GST-c-Jun. The relative activity from each sample was estimated quantitatively. Corresponding lysates were also analyzed for the expression of the indicated proteins. Results are means plus standard errors of the means for five independent experiments.
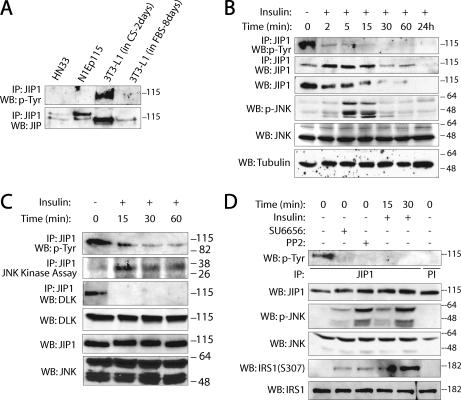
JIP1 is tyrosine phosphorylated in 3T3-L1 preadipocytes in an SFK-dependent fashion.
Models were sought to further establish the physiological significance of SFK-dependent JIP1 tyrosine phosphorylation in regulating JIP1-dependent JNK module activity. Several cell lines, including HN33 (rat hippocampal cells), N1Ep115 (mouse neuronal fibroblasts), N2a (mouse N2a neuroblastoma cells), and 3T3-L1 cells (mouse adipocytes), that express endogenous JIP1 were screened for JIP1 tyrosine phosphorylation under basal cell culture conditions. Under these conditions, tyrosine phosphorylation was observed on JIP1 obtained from 3T3-L1 preadipocytes (Fig. 5A) and JIP1 obtained from N2a cells (see Fig. 7A). Interestingly, in the 3T3-L1 system, tyrosine phosphorylation was not detectable when 3T3-L1 cells were induced to differentiate into adipocytes (after treatment with dexamethasone-methylisobutylxanthine-insulin [DMI] and cultured in DMEM containing 10% fetal bovine serum for 8 days) (Fig. 5A). Upon further investigation, it was observed that JIP1 steady-state abundance declines within minutes following insulin treatment of preadipocytes (Fig. 5A and B). Because it has been established that treatment of 3T3-L1 preadipocytes with insulin results in activation of JNK, we examined whether elements of the model described above would hold in the preadipocyte model system (18). According to our model of JIP1-JNK module dynamics, we hypothesized that stimulation of 3T3-L1 cells with insulin should result in dephosphorylation of JIP1 on tyrosine residues, recruitment of JNK to JIP1, DLK dissociation from JIP1, and activation of JNK. 3T3-L1 cells (2 days postconfluence) were treated with 100 nM insulin for various durations. Insulin stimulation resulted in rapid attenuation of JIP1 tyrosine phosphorylation (Fig. 5B to D). Stimulation with insulin also resulted in dissociation of endogenous DLK from JIP1 and increased JNK activity (Fig. 5C). Maximum JIP1-associated JNK activity peaked after 15 min of stimulation with insulin. Similar results were obtained when DMI was substituted for insulin in this system (data not shown). Tyrosine phosphorylation of JIP1 was also analyzed after pretreatment of 3T3-L1 cells with the SFK inhibitor PP2 or SU6656 (Fig. 5D). Under these conditions, PP2 or SU6656 significantly reduced tyrosine phosphorylation of JIP1 under basal conditions and increased JNK activation (Fig. 5D). Insulin-dependent JNK-mediated phosphorylation of IRS1(S307) was reported by several groups (18). Similarly, in this system, increased phosphorylation of IRS1 at S307 was observed concurrently with JNK activation 15 min after insulin stimulation or when 3T3-L1 cells were treated with PP2 or SU6656 alone (Fig. 5D). Consistent with the experiments described above, these results confirmed a role for SFK catalytic activity in governing JIP1 module dynamics.
FIG. 5.
JIP1 is tyrosine phosphorylated in 3T3-L1 preadipocytes in an SFK-dependent fashion. (A) Survey of JIP1 expression and JIP1 tyrosine phosphorylation in neuronal cell lines (HN33 and NIEp115) or 3T3-L1 cells cultured in either DMEM containing calf serum (CS) for 2 days or in DMEM containing fetal bovine serum (FBS) for 8 days. (B to D) 3T3-L1 cells were grown for 2 days postconfluence in DMEM containing 10% CS and then stimulated with insulin (100 nM) for the indicated times. JIP1 was immunoprecipitated, and immune complexes were separated on SDS-PAGE and immunoblotted with the indicated antibodies. Immunoblot analysis of the corresponding cell lysates was performed to evaluate the presence of indicated proteins using their specific antibodies. In panel C, JIP1 immune complex was also subjected to JNK catalytic assay using GST-c-Jun as the substrate. These experiments were repeated five times with similar results. (D) Tyrosine phosphorylation of JIP1, total JNK activation, and IRS1 phosphorylation were analyzed after pretreatment of 3T3-L1 cells with the SFK inhibitors PP2 (5 μM) and SU6656 (1 μM).
FIG. 7.
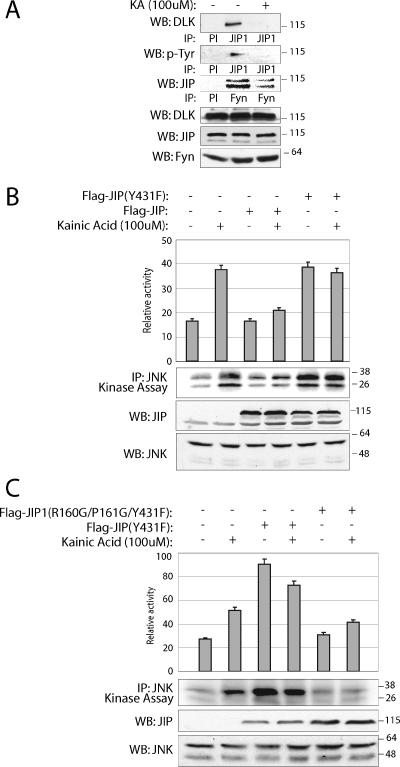
Fyn attenuates JNK module activity by tyrosine phosphorylating JIP1. (A) Extracts were prepared from N2a cells treated with 100 μM kainic acid for 30 min or left untreated. JIP1 complexes were immunoprecipitated using JIP1 antibody and analyzed by immunoblotting with anti-DLK and antiphosphotyrosine antibodies. Immunoprecipitation with preimmune serum (PI) was used as control. (B and C) Mutation of tyrosine 431 in JIP1 induces JNK activation in N2a cells. Flag-JIP1(Y431F) (1 μg) or Flag-JIP1(R160G/P161G/Y431F) was expressed in N2a cells. At 24 h posttransfection, cells were treated with kainic acid for 30 min. Endogenous JNK catalytic activity was assayed in kinase buffer containing [γ-32P]ATP using GST-c-Jun as the substrate. The relative activity from each sample was quantified using a phosphorimager. This experiment was conducted three times with similar results. Results are means plus standard errors of the means.
Stimulation of cultured neuronal cells with either reelin or kainic acid reduces SFK-dependent tyrosine phosphorylation of JIP1 and alters JIP1 module dynamics.
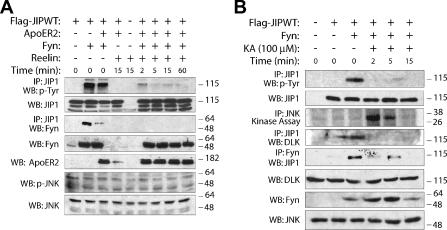
Next we sought to determine whether the proposed model of stimulation-induced attenuation of JIP1 tyrosine phosphorylation could be generalized to other systems. Therefore, two additional systems were examined that might provide further evidence for the proposed model of SFK-dependent JIP1 module dynamics. According to previously published work, JIP1 interacts with apolipoprotein E receptor 2 (ApoER2) that is localized to a lipid microdomain that contains Fyn (29). ApoER2 interacts with its ligand, reelin (29, 31). We speculated that reelin-activated ApoER2 might stimulate Fyn-dependent JIP1 module dynamics because JIP1 and Fyn might colocalize in this system. To examine this possibility, the neuronal cell line HN33 was cotransfected with Flag-JIP1, ApoER2, and Fyn. After 24 h, cells were stimulated with reelin for various durations (Fig. 6A). Stimulation of cells with reelin resulted in rapid dissociation of Fyn from JIP1 and attenuation of JIP1 tyrosine phosphorylation. As reported previously (29), JNK activation was not induced in this system (Fig. 6A). An additional model system was also examined. We previously showed that treatment of HN33 cells with kainic acid results in recruitment of JNK to JIP1, dissociation of DLK from JIP1, and JNK activation (24). To extend these observations in the context of the work described herein, HN33 cells were cotransfected with Flag-JIP1 and Fyn and then treated with 100 μM kainic acid. Treatment of cells with kainic acid resulted in rapid attenuation of JIP1 tyrosine phosphorylation (Fig. 6B). This coincided with increased JNK activity and decreased affinity of endogenous DLK with JIP1 (Fig. 6B) as previously demonstrated (24).
FIG. 6.
Stimulation of neuronal cells with either reelin or kainic acid decreases Fyn-dependent JIP1 tyrosine phosphorylation. (A) HN33 neuronal cells were cotransfected with plasmids encoding Flag-JIP1 (1 μg), ApoER2 (0.5 μg), and Fyn (0.2 μg). At 24 h posttransfection, cells were treated with reelin for the indicated times. JIP1 was immunoprecipitated from the cell lysate, and the immune complexes were analyzed for JIP1 tyrosine phosphorylation and Fyn binding. (B) HN33 cells were cotransfected with Flag-JIP1 (1 μg) and Fyn (0.2 μg). At 24 h posttransfection, cells were treated with 100 μM kainic acid for the indicated times. Tyrosine phosphorylation of JIP1 was analyzed by immunoprecipitating JIP1 from cell lysates and immunoblotting with antiphosphotyrosine antibody. JNK catalytic activity was assayed by immunoprecipitating total JNK from lysate and incubating the immune complex with kinase buffer containing [γ-32P]ATP and GST-c-Jun.
A similar experiment was carried out in the N2a neuronal cell line, because we sought to conduct this experiment in a model system that did not require expression of exogenous proteins. N2a cells express endogenous JIP1 and DLK at steady-state concentrations greater than those observed in HN33 cells. (Fig. 7A). N2a cells were stimulated with kainic acid, and the interactions of endogenous JIP1 with DLK and JIP1 tyrosine phosphorylation were evaluated. The association of JIP1 with DLK and Fyn and the tyrosine phosphorylation of JIP1 were readily observed under basal conditions; however, these were significantly reduced upon stimulation of N2a cells with kainic acid (Fig. 7A). Using this model, we also sought to reconfirm our hypothesis that SFK-dependent JIP1 phosphorylation maintains the complex in an inactive state. It was anticipated that in this model, where endogenous Fyn, JIP1, DLK, and JNK coexist, interference with SFK-dependent JIP1 tyrosine phosphorylation should result in increased JNK activity when the pathway is otherwise not stimulated. Wild-type JIP1 and JIP1(Y431F) (acting as an interfering mutant) were overexpressed in N2a cells. In results similar to those reported by others (8), overexpression of wild-type JIP1 suppressed kainic acid-induced JNK activity. In contrast, simple overexpression of JIP1(Y431F) increased JNK activity to a level equivalent to that achieved following stimulation of cells with kainic acid (Fig. 7B). The observed effect on JNK activity of mutating JIP1(Y431) was not a result of a change in the affinity of JIP1 and JNK (data not shown). To establish that JIP1(Y431F) was mediating this effect on JNK activity via its effect on a pool of JNK associated with JIP1, a compound JIP1 mutant was constructed. We previously demonstrated that mutation in the JNK binding domain of JIP1 [JIP1(R160G/P161G)] prevents JNK binding to JIP1 (23). Therefore, a JIP1(R160G/P161G/Y431F) compound mutant was constructed and expressed in N2a cells. As shown in Fig. 7C, while expression of JIP1(Y431F) in N2a cells increased basal JNK activity, transfection of JIP1(R160G/P161G/Y431F) abolished this effect.
Deletion of Fyn in mice affects JIP-JNK dynamics.
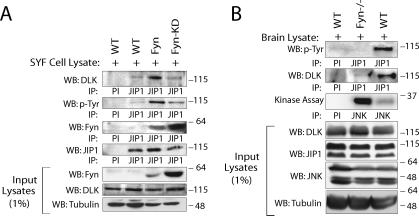
Collectively, these results provide evidence from three cell culture model systems to support the contention that appropriate cell stimulation results in decreased SFK-dependent JIP1 phosphorylation, resulting in turn in disinhibition of JIP1-JNK module activity, and that this is a common mechanism employed by JIP-dependent signaling modules. In a final set of confirmatory experiments, the proposed mechanism was examined in model systems that were genetically deficient of Fyn or of Fyn, c-Src, and Yes. In the first experiment, SYF (cSrc−/− Fyn−/− Yes−/−) cells were rescued by re-expressing Fyn or catalytically inactive Fyn to examine whether Fyn catalytic activity was sufficient to alter JIP-DLK affinity and modulate JNK activity; the effect of expression of Yes or c-Src was not examined in this system. As shown in Fig. 8A, expression of Fyn in SYF cells resulted in significantly enhanced interaction between JIP1 and DLK compared to transfection of cells with either catalytically inactive Fyn or vector control. As observed in earlier experiments (Fig. 2D), binding of Fyn to JIP1 was not dependent on the catalytic activity of Fyn (Fig. 8A). In a second experiment, JIP1 was immunoprecipitated from brain lysate obtained from Fyn-null mice or wild-type littermates, and these immune complexes were analyzed for JIP1 tyrosine phosphorylation, the association of DLK, and JNK activity. As shown in Fig. 8B, tyrosine phosphorylation of JIP1 was significantly reduced in the brain lysates of Fyn-null mice. Moreover, as predicted from the results shown above and consistent with the hypothesis that Fyn-dependent phosphorylation of JIP1 maintains the JIP1-DLK-JNK module in a basal inactive state, the affinity of DLK for JIP1 in Fyn-null mice was attenuated, and basal JNK activity in brains of Fyn-null mice was increased relative to that in brains of wild-type littermates. Collectively, these results provide confirmatory genetic evidence for the role of Fyn and Fyn-dependent tyrosine phosphorylation of JIP1 in the regulation of JIP1-dependent JNK module activation. We cannot entirely exclude a role for other SFKs in this mechanism, since additional results reported above suggest that other SFKs might participate to some extent in this mechanism.
FIG. 8.
Endogenous JIP1 module dynamics are altered after genetic deletion of Fyn in cell culture and in vivo. (A) SYF cells deficient in Src, Fyn, and Yes were transfected with plasmids expressing Fyn or a catalytically inactive Fyn (Fyn-KD) or with a vector control. JIP1 was immunoprecipitated from these cell lysates and examined. (B) JIP1 tyrosine phosphorylation and association of JIP1 with DLK is reduced in the brains of Fyn-deficient mice. JIP1 was immunoprecipitated from brain lysate of Fyn−/− or wild-type littermates using JIP1 antibody or preimmune control serum (PI). Immunoprecipitates were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with DLK or antiphosphotyrosine antibodies. Endogenous JNK kinase catalytic activity was assayed as before. This experiment used two mice from each group and was repeated three times with similar results.
DISCUSSION
Rather than simply serving as static backbones upon which MAPK signaling complexes are assembled, accumulating evidence has shown that scaffold proteins play a central role in regulating MAPK module function by regulating the dynamic interrelationship of associated protein complex components. Importantly, phosphorylation and/or dephosphorylation events on these scaffold proteins have emerged as primary mechanisms by which the relationship between scaffold protein and complex components and therefore complex-dependent signaling are governed (17). Collectively, our present observations and those previously published by us and others suggest a model of JIP1-JNK module regulation in which JIP1 serves as a focal point for module regulation by integrating inhibitory tyrosine phosphorylation-mediated signals from Src family kinases and permissive serine/threonine phosphorylation-mediated signals from JNK. In this model, under basal conditions, DLK is maintained in a high-affinity interaction with JIP1; this DLK-JIP1 interaction requires that JIP1 both interact with and be tyrosine phosphorylated by an SFK in a constitutive manner. Under these basal conditions, DLK is held in a monomeric, catalytically inactive state in which it is unable to activate MKK7 and ultimately JNK (22, 23). Upon appropriate stimulation, the interaction between JNK and JIP1 is altered such that JIP1 is serine and threonine phosphorylated by catalytically competent JNK (2, 5, 24). Particularly as a result of JIP1 T103 phosphorylation by JNK, DLK is dissociated from JIP1, oligomerizes, and becomes catalytically active (24). Activated DLK phosphorylates and activates MKK7, which subsequently phosphorylates JNK. This model implies the existence of a positive feedback mechanism for JNK activation that moves the module in a switch-like manner from a low to high rate of catalysis (24).
Several observations support the conclusion that under basal conditions, JIP1 interaction with and tyrosine phosphorylation by SFKs maintain the JIP1 module in an inactive state; most important among these are our observations that inhibition of endogenous SFK activity in naturally occurring modules or that targeted deletion of Fyn in mouse brain results in decreased JIP1 tyrosine phosphorylation, attenuated DLK-JIP1 affinity, and increased JNK catalytic activity. Further, it is remarkable that simple overexpression of the JIP1(Y431F) mutant in which SFK-dependent tyrosine phosphorylation is prevented results in activation of endogenous JNK in a JIP-dependent fashion. Presumably, this dominant gain-of-function effect of JIP1(Y431F) results from competition of mutant JIP1 with endogenous JIP1 for assembly of components of the JIP1-JNK module in a fashion that promotes JNK activation. In part, this may be due to an effect on JIP1-DLK affinity; however, the possibility that the SFK-JIP1 interaction also influences the functional interaction of JIP and JNK or JIP with additional regulatory proteins cannot be excluded.
Our previously published results showed that stimulus-induced JNK recruitment to JIP1 precedes JNK-dependent Ser/Thr phosphorylation of JIP1, a priming event that is required for subsequent activation of the JIP1-dependent JNK module (23, 24). We have hypothesized that an initiating JIP1 Ser/Thr phosphorylation event and/or an event that results in JIP tyrosine dephosphorylation results in JIP conformational changes that increase the affinity of JIP for JNK, resulting in recruitment of catalytically competent JNK to the module and then in module activation after JNK-mediated phosphorylation at T103. Recently published work by Bhattacharyya et al. suggests a potentially relevant mechanism by which changes in phosphorylation on JIP could result in a subsequent priming JNK-dependent JIP1 phosphorylation event (2). These investigators demonstrated that the yeast scaffold Ste5 activates by an allosteric mechanism the mitogen-activated protein kinase Fus3, inducing autophosphorylation of Fus3 on only one of two residues in its activation loop. By a feedback mechanism, this partial autoactivation of Fus3 is sufficient to promote phosphorylation on specific residues on the yeast module scaffold. A similar mechanism might be employed in the partial activation of mammalian JNK, which then primes full activation of the module via a positive feedback mechanism.
The intracellular signaling mechanisms by which proximal signals are transduced to the JIP module remain unstudied. It is intriguing that following cellular stimulation, JIP1 is rapidly tyrosine dephosphorylated in each of the model systems investigated. Collectively, these results suggest the possibility that JIP1 module activation is achieved through a rapid switch between a basal state in which JIP1 is tyrosine phosphorylated and an activated state in which JIP1 becomes Ser/Thr phosphorylated by JNK. JIP1 tyrosine dephosphorylation might be influenced by a change in the activation state of associated SFKs. However, the manner in which JIP tyrosine dephosphorylation is influenced by the activation state of associated SFKs requires study, especially since insulin, reelin, and kainic acid stimulation induce rather than attenuate total cellular SFK catalytic activity (3, 20, 27). Alternative hypothetical mechanisms by which JIP module activation is initiated are worthy of investigation. For example, one might speculate that the rapid dephosphorylation of endogenous JIP1 observed in response to cellular stimulation requires the induction of a JIP tyrosine phosphatase activity that subsequently alters JIP conformation and primes JIP module activation. In addition, it is possible that initiating Ser/Thr phosphorylation on JIP1 by several kinases, including JNK, could result in attenuation of the affinity of JIP1 for SFK, decreased JIP tyrosine phosphorylation, and subsequent module activation. Indeed, in data not shown, overexpression of catalytically competent JNK relative to JIP1 and SFK results in attenuated JIP1-SFK interaction in addition to previously reported decreased JIP1-DLK interaction and module activation.
Activation of the JIP1-dependent JNK module via the mechanism described above can be stimulated in a variety of cell types by several extracellular stimuli, including insulin, reelin, and kainic acid. These results suggest that this mechanism of JIP1 module regulation is employed commonly by JIP modules in diverse naturally occurring signaling pathways. Despite the common mechanism employed, assembly of distinct JIP modules creates units that are targeted to specific subcellular compartments, that are insulated from similar modules, and that can regulate functionally distinct substrates; as a result, these modules provide for signaling specificity in response to unique stimuli. Given the large number of mixed-lineage kinases that have the potential to interact with multiple JIP scaffold proteins, it is likely that many unique JIP complexes that subserve distinct cellular functions will be identified. The specific functions of DLK and its associated JIP module are just beginning to be identified (10). Therefore, it is noteworthy that our results place DLK in an endogenous insulin-sensitive pathway. JNK1 is thought to serve as an intermediary in a negative-feedback loop that attenuates the cellular response to insulin-stimulation (1). Indeed, JNK1 activity is induced in muscle and adipose tissue of obese mice, and deletion of JNK1 or JIP1 in obese mice results in decreased insulin resistance relative to controls (11, 12). Collectively, these observations suggest the hypothesis that a specific DLK-JNK1-JIP1 module participates in insulin-stimulated signal transduction.
Acknowledgments
This work was supported by the NIDDK (R01 to L.B.H.) and by an O'Brien Renal Center Grant from the NIH.
Footnotes
▿
Published ahead of print on 22 January 2007.
REFERENCES
- 1.Aguirre, V., T. Uchida, L. Yenush, R. Davis, and M. F. White. 2000. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem. 275**:**9047-9054. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya, R. P., A. Remenyi, M. C. Good, C. J. Bashor, A. M. Falick, and W. A. Lim. 2006. The Ste5 scaffold allosterically modulates signaling output of the yeast mating pathway. Science 311**:**822-826. [DOI] [PubMed] [Google Scholar]
- 3.Bock, H. H., and J. Herz. 2003. Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 13**:**18-26. [DOI] [PubMed] [Google Scholar]
- 4.Brandes, C., L. Kahr, W. Stockinger, T. Hiesberger, W. J. Schneider, and J. Nimpf. 2001. Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not alpha 2-macroglobulin. J. Biol. Chem. 276**:**22160-22169. [DOI] [PubMed] [Google Scholar]
- 5.D'Ambrosio, C., S. Arena, G. Fulcoli, M. H. Scheinfeld, D. Zhou, L. D'Adamio, and A. Scaloni. 2006. Hyperphosphorylation of JNK-interacting protein 1, a protein associated with Alzheimer disease. Mol. Cell Proteomics 5**:**97-113. [DOI] [PubMed] [Google Scholar]
- 6.Dickens, M., J. S. Rogers, J. Cavanagh, A. Raitano, Z. Xia, J. R. Halpern, M. E. Greenberg, C. L. Sawyers, and R. J. Davis. 1997. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277**:**693-696. [DOI] [PubMed] [Google Scholar]
- 7.Garrington, T. P., and G. L. Johnson. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11**:**211-218. [DOI] [PubMed] [Google Scholar]
- 8.Harding, T. C., L. Xue, A. Bienemann, D. Haywood, M. Dickens, A. M. Tolkovsky, and J. B. Uney. 2001. Inhibition of JNK by overexpression of the JNK binding domain of JIP-1 prevents apoptosis in sympathetic neurons. J. Biol. Chem. 276**:**4531-4534. [DOI] [PubMed] [Google Scholar]
- 9.Hiesberger, T., M. Trommsdorff, B. W. Howell, A. Goffinet, M. C. Mumby, J. A. Cooper, and J. Herz. 1999. Direct binding of reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates Tau phosphorylation. Neuron 24**:**481-489. [DOI] [PubMed] [Google Scholar]
- 10.Hirai, S., F. Cui de, T. Miyata, M. Ogawa, H. Kiyonari, Y. Suda, S. Aizawa, Y. Banba, and S. Ohno. 2006. The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J. Neurosci. 26**:**11992-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirosumi, J., G. Tuncman, L. Chang, C. Z. Gorgun, K. T. Uysal, K. Maeda, M. Karin, and G. S. Hotamisligil. 2002. A central role for JNK in obesity and insulin resistance. Nature 420**:**333-336. [DOI] [PubMed] [Google Scholar]
- 12.Jaeschke, A., M. P. Czech, and R. J. Davis. 2004. An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Genes Dev. 18**:**1976-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasahara, K., Y. Watanabe, T. Yamamoto, and Y. Sanai. 1997. Association of Src family tyrosine kinase Lyn with ganglioside GD3 in rat brain. Possible regulation of Lyn by glycosphingolipid in caveolae-like domains. J. Biol. Chem. 272**:**29947-29953. [DOI] [PubMed] [Google Scholar]
- 14.Kelkar, N., C. L. Standen, and R. J. Davis. 2005. Role of the JIP4 scaffold protein in the regulation of mitogen-activated protein kinase signaling pathways. Mol. Cell. Biol. 25**:**2733-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennell, J. A., and O. A. MacDougald. 2005. Wnt signaling inhibits adipogenesis through beta-catenin-dependent and -independent mechanisms. J. Biol. Chem. 280**:**24004-24010. [DOI] [PubMed] [Google Scholar]
- 16.Kim, A. H., H. Yano, H. Cho, D. Meyer, B. Monks, B. Margolis, M. J. Birnbaum, and M. V. Chao. 2002. Akt1 regulates a JNK scaffold during excitotoxic apoptosis. Neuron 35**:**697-709. [DOI] [PubMed] [Google Scholar]
- 17.Kolch, W. 2005. Coordinating Erk/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 6**:**827-837. [DOI] [PubMed] [Google Scholar]
- 18.Lee, Y. H., J. Giraud, R. J. Davis, and M. F. White. 2003. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J. Biol. Chem. 278**:**2896-2902. [DOI] [PubMed] [Google Scholar]
- 19.Makarova, O., E. Kamberov, and B. Margolis. 2000. Generation of deletion and point mutations with one primer in a single cloning step. BioTechniques 29**:**970-972. [DOI] [PubMed] [Google Scholar]
- 20.Mastick, C. C., and A. R. Saltiel. 1997. Insulin-stimulated tyrosine phosphorylation of caveolin is specific for the differentiated adipocyte phenotype in 3T3-L1 cells. J. Biol. Chem. 272**:**20706-20714. [DOI] [PubMed] [Google Scholar]
- 21.Morrison, D. K., and R. J. Davis. 2003. Regulation of map kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 19**:**91-118. [DOI] [PubMed] [Google Scholar]
- 22.Nihalani, D., S. Merritt, and L. B. Holzman. 2000. Identification of structural and functional domains in mixed lineage kinase dual leucine zipper-bearing kinase required for complex formation and stress-activated protein kinase activation. J. Biol. Chem. 275**:**7273-7279. [DOI] [PubMed] [Google Scholar]
- 23.Nihalani, D., D. Meyer, S. Pajni, and L. B. Holzman. 2001. Mixed lineage kinase-dependent JNK activation is governed by interactions of scaffold protein JIP with MAPK module components. EMBO J. 20**:**3447-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nihalani, D., H. N. Wong, and L. B. Holzman. 2003. Recruitment of JNK to JIP1 and JNK-dependent JIP1 phosphorylation regulates JNK module dynamics and activation. J. Biol. Chem. 278**:**28694-28702. [DOI] [PubMed] [Google Scholar]
- 25.Obenauer, J. C., L. C. Cantley, and M. B. Yaffe. 2003. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 31**:**3635-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ory, S., M. Zhou, T. P. Conrads, T. D. Veenstra, and D. K. Morrison. 2003. Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites. Current Biology 13**:**1356-1364. [DOI] [PubMed] [Google Scholar]
- 27.Salter, M. W., and L. V. Kalia. 2004. Src kinases: a hub for NMDA receptor regulation. Nat. Rev. Neurosci. 5**:**317-328. [DOI] [PubMed] [Google Scholar]
- 28.Song, J. J., and Y. J. Lee. 2005. Dissociation of Akt1 from its negative regulator JIP1 is mediated through the ASK1-MEK-JNK signal transduction pathway during metabolic oxidative stress: a negative feedback loop. J. Cell Biol. 170**:**61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stockinger, W., C. Brandes, D. Fasching, M. Hermann, M. Gotthardt, J. Herz, W. J. Schneider, and J. Nimpf. 2000. The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J. Biol. Chem. 275**:**25625-25632. [DOI] [PubMed] [Google Scholar]
- 30.Verma, R., B. Wharram, I. Kovari, R. Kunkel, D. Nihalani, K. K. Wary, R. C. Wiggins, P. Killen, and L. B. Holzman. 2003. Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J. Biol. Chem. 278**:**20716-20723. [DOI] [PubMed] [Google Scholar]
- 31.Whitmarsh, A. J., C.-Y. Kuan, N. J. Kennedy, N. Kelkar, T. F. Haydar, J. P. Mordes, M. Appel, A. A. Rossini, S. N. Jones, R. A. Flavell, P. Rakic, and R. J. Davis. 2001. Requirement of the JIP1 scaffold protein for stress-induced JNK activation. Genes Dev. 15**:**2421-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasuda, J., A. J. Whitmarsh, J. Cavanagh, M. Sharma, and R. J. Davis. 1999. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell. Biol. 19**:**7245-7254. [DOI] [PMC free article] [PubMed] [Google Scholar]