Functional TFIIH Is Required for UV-Induced Translocation of CSA to the Nuclear Matrix (original) (raw)
Abstract
Transcription-coupled repair (TCR) efficiently removes a variety of lesions from the transcribed strand of active genes. Mutations in Cockayne syndrome group A and B genes (CSA and CSB) result in defective TCR, but the molecular mechanism of TCR in mammalian cells is not clear. We have found that CSA protein is translocated to the nuclear matrix after UV irradiation and colocalized with the hyperphosphorylated form of RNA polymerase II and that the translocation is dependent on CSB. We developed a cell-free system for the UV-induced translocation of CSA. A cytoskeleton (CSK) buffer-soluble fraction containing CSA and a CSK buffer-insoluble fraction prepared from UV-irradiated CS-A cells were mixed. After incubation, the insoluble fraction was treated with DNase I. CSA protein was detected in the DNase I-insoluble fraction, indicating that it was translocated to the nuclear matrix. In this cell-free system, the translocation was dependent on UV irradiation, CSB function, and TCR-competent CSA. Moreover, the translocation was dependent on functional TFIIH, as well as chromatin structure and transcription elongation. These results suggest that alterations of chromatin at the RNA polymerase II stall site, which depend on CSB and TFIIH at least, are necessary for the UV-induced translocation of CSA to the nuclear matrix.
Nucleotide excision repair (NER) is a versatile DNA repair system that corrects bulky, helix-distorting lesions, including UV-induced cyclobutane pyrimidine dimers and (6-4) photoproducts, as well as chemical-carcinogen-induced lesions (8). There are two pathways in NER: global genome repair (GGR) and transcription-coupled repair (TCR). TCR specifically removes DNA damage on the transcribed strands of transcriptionally active genes (11). GGR occurs throughout the genome, including the nontranscribed strands of active genes. DNA damage is recognized by different modes in the two pathways, and both pathways proceed to the same core NER reactions. GGR requires the XPC-HR23B and DDB complexes for DNA damage recognition in mammalian cells. In TCR, RNA polymerase II stalled at a lesion on the transcribed strand is thought to serve as a damage recognition signal, but the molecular mechanism of TCR remains to be elucidated.
Cockayne syndrome (CS) is a rare autosomal recessive disorder, which shows a diversity of clinical symptoms (2, 8). A comprehensive review of 140 cases of CS (27) indicated that growth failure is a basic clinical feature and generally begins within the first year of life. In addition, individuals with CS develop neurological dysfunctions, such as mental retardation and microcephaly. Associated clinical features are ophthalmologic abnormalities, such as cataracts and optic atrophy; sensorineural hearing loss; and dental caries. Affected individuals also manifest photosensitivity of the skin but have no predisposition to sunlight-induced skin cancer, in contrast to xeroderma pigmentosum (XP) patients (2, 27).
Cells from CS patients are hypersensitive to UV light and show reduced recovery of DNA and RNA synthesis after exposure to UV light. The cellular abnormalities in CS have been attributed to a specific defect in TCR (41, 43). Complementation analysis using the recovery of DNA or RNA synthesis after UV irradiation as a marker has defined two genetic complementation groups in CS: CS-A and CS-B (19, 36). In addition, XP group B (XP-B) patients and certain patients with XP-D or XP-G show features of CS in addition to symptoms of XP (XP-B/CS, XP-D/CS, and XP-G/CS) (2, 8).
The CSA and CSB genes have been cloned (13, 39). The CSA gene encodes a 44-kDa protein with five WD-40 repeats that appears to have the potential to interact with other proteins. It has been shown that the CSA protein interacts with XAB2, CSB, and the p44 subunit of TFIIH (13, 26). We have recently reported that CSA forms a ubiquitin ligase complex containing DDB1, cullin 4A, and Roc1 and that the complex interacts with the COP9 signalosome (10). In addition, this CSA complex binds to the hyperphosphorylated form of RNA polymerase II in the chromatin fraction after UV irradiation. The CSB protein is a member of the SWI2/SNF2 family and has DNA-dependent ATPase and ATP-dependent chromatin-remodeling activities (4, 5, 31). It has been reported that CSB interacts with RNA polymerase II elongation complex in vitro and in vivo (37, 40, 42) and stimulates elongation by RNA polymerase II in vitro (32). However, CSB did not remove a stalled RNA polymerase II elongation complex from the DNA template (31). On the other hand, CSB was found to be involved in transcription driven by RNA polymerases I and III (3, 49).
The nuclear matrix is thought to play an important role in nuclear metabolism. The hyperphosphorylated form of RNA polymerase II is known to associate with the nuclear matrix (24, 28, 47). As for repair, several reports suggest that NER is associated with the nuclear matrix (12, 18, 21). It has been reported that UV-induced repair patches were enriched in the nuclear matrix. Interestingly, this phenomenon was enhanced in TCR-proficient XP-C cells and abolished in TCR-deficient CSB cells (16, 25). These findings indicate that TCR takes place in the nuclear matrix. We have shown that CSA protein is translocated to the nuclear matrix after UV irradiation and that the translocation requires CSB, which is involved in TCR, but not XPC, which acts as a damage sensor in the GGR-specific process, or XPA, which functions in the core NER reactions (15). These results suggested that the UV-induced translocation of CSA is relevant to TCR. In this study, we established a cell-free system for examining the UV-induced translocation of CSA and found that the process is dependent on functional TFIIH, as well as CSB, and on chromatin structure and transcription elongation.
MATERIALS AND METHODS
Cell lines.
The cell lines used in this study were simian virus 40-immortalized human fibroblasts: WI38VA13 (normal), CS3BESV (CS-A), CS1ANSV (CS-B), XP2OSSV (XP-A), XP4PASV (XP-C), XP6BESV (XP-D), XPCS2SV (XP-D/CS), XPCS2BASV (XP-B/CS), TTD1ROSV (XP-D/TTD), and TTD1BRSV (TTD-A). XP6BESV and XPCS2SV cells stably expressing XPD (XPD/XP6BESV and XPD/XPCS2SV) were isolated as described previously (17). The transfectants showed almost the same level of UV resistance as WI38VA13 cells (data not shown). The fibroblasts were cultured in Dulbecco's modified Eagle's medium supplemented with antibiotics and 10% fetal calf serum.
Antibodies.
Antihemagglutinin (anti-HA) antibody (3F10) was purchased from Roche Diagnostics. Anti-green fluorescent protein (anti-GFP) antibody (JL-8) was from Clontech. Peroxidase-conjugated anti-rat immunoglobulin G and Alexa Fluor 488-conjugated anti-rat antibody were from Amersham Biosciences and Molecular Probes, respectively.
Plasmid constructs for CSA expression.
CSA cDNA without a stop codon was amplified by PCR from pBS-CSA (26) with the following primers: 5′-ATTCTCGAGCACCATGCTGGGGTTTTTGTCC-3′ (forward; CSA cDNA flanked by a Kozak sequence and an XhoI site) and 5′-CTGCTCTAGATCCTTCTTCATCACTGCTGC-3′ (reverse; CSA cDNA flanked by an XbaI site). CSA cDNA regions are underlined. The PCR product was cloned into pBluescript (pBS-CSA-C). Sequencing of the plasmid ruled out the presence of PCR-derived mistakes. The XhoI-NotI fragment from the above-described plasmid was cloned in frame and upstream of the sequence encoding a FLAG epitope, followed by an HA epitope in pOZ-C (provided by Y. Nakatani), resulting in pOZ-C-CSA. For the generation of a tagged CSA expression construct, the BglII-AvrII fragment containing the full-length CSA-coding region with the epitope tags from pOZ-C-CSA was ligated between the BamHI and XbaI sites in pcDNA3 (Invitrogen), yielding pcDNA3-C-CSA.
Point mutations identified in CS-A cell lines (CS2SE and CS2IAF) were introduced into pBS-CSA-C using a QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The cDNAs encoding truncated CSA were amplified by PCR with appropriate primers and cloned into pBluescript. DNA sequencing excluded additional mutations introduced elsewhere in the mutant cDNA. The part of the cDNA containing the mutation was isolated by digestion with XhoI-BamHI or BamHI-NotI and exchanged with the corresponding sequences in pcDNA3-C-CSA.
Isolation of stable transfectants.
CS3BESV cells were transfected with the CSA expression constructs using Effectene transfection reagent (QIAGEN) according to the manufacturer's recommendations. Stable transfectants were selected in the presence of G418 (500 μg/ml). By immunoblot analysis using anti-HA antibody, the expression of CSA in each transfectant was compared to that in dtCSA/CS3BESV cells as a control for normal expression levels (15). Isolation of CS3BESV cells stably expressing CSA-GFP has been described previously (35).
Translocation of CSA protein in the cell-free system.
CS3BESV cells were UV irradiated at 20 J/m2 or not irradiated and incubated for 1 h. The cells were harvested by trypsinization, washed with phosphate-buffered saline (PBS), and then extracted in cytoskeleton (CSK)-Triton buffer [10 mM PIPES {piperazine-N,_N_′-bis(2-ethanesulfonic acid)}, pH 6.8, 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 0.5% (vol/vol) Triton X-100, 1 mM dithiothreitol, 1 mM EGTA, and complete protease inhibitor cocktail (Roche)] at 4°C for 10 min. The pellet was separated from soluble proteins by centrifugation at 4,000 × g for 3 min, washed, and resuspended in the same buffer. This suspension was used as the CSK-ppt fraction. CSA-FLAG-HA/CS3BESV cells were treated with the same buffer described above, and the supernatant (CSK-sup fraction) was recovered by centrifugation. The CSK-ppt fraction was incubated with the CSK-sup fraction containing CSA-FLAG-HA at 4°C for 1 h with occasional gentle tapping. After being separated by centrifugation as described above, the pellet was washed twice with the same buffer, treated with DNase I (2.8 units/μl; Takara) at 30°C for 10 min, and then washed three times with the same buffer. The proteins in the remaining pellet (the DNase I-insoluble fraction) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane. CSA in the DNase I- insoluble fraction was detected with anti-HA antibody using enhanced chemiluminescence plus Western blotting detection reagents (Amersham Biosciences).
When the translocation of CSA was measured simultaneously with the synthesis of RNA, the cells were harvested immediately after UV irradiation in the presence of 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole. The CSK-ppt fractions were prepared and resuspended in glycerol storage buffer (50 mM Tris-HCl, pH 8.3, 40% glycerol, 2 mM MgCl2, and 0.1 mM EDTA). The suspension was mixed with an equal volume of 2× reaction buffer (10 mM Tris-HCl, pH 8.0, 5 mM MgCl2, 300 mM KCl, and 5 mM dithiothreitol) containing 1 mM nucleoside triphosphates (NTPs) and purified CSA complex and incubated at 30°C for 1 h.
Survival and recovery of RNA synthesis after UV irradiation.
The survival of the transfectants was assessed based on colony-forming ability. Exponentially growing cells were plated at 5 × 102 to 10 × 102 cells per 100-mm dish and exposed to UV light at various dosages ∼14 h after being plated. The cells were then cultured for 7 to 10 days, fixed with 3% formaldehyde, and stained with 0.1% crystal violet. Colonies were counted using a binocular microscope.
To measure the recovery of RNA synthesis after UV irradiation, cells were seeded in 35-mm dishes at 2 × 105 cells/dish 1 day before being irradiated. The cells were washed with PBS, either irradiated at 10 J/m2 or not irradiated, and incubated in fresh medium. At various times after irradiation, the cells were labeled with 370 kBq/ml of [3H]uridine for 30 min. The labeling was terminated by adding NaN3 at a final concentration of 200 μg/ml to the culture. After being washed with PBS, the cells were lysed with 0.8% SDS at room temperature for 30 min, and then an equal volume of 10% trichloroacetic acid containing 0.1 M sodium pyrophosphate was added to the lysate. Acid-insoluble materials were collected on a Whatman GF/C glass fiber filter, and the radioactivity was measured with a liquid scintillation counter.
Analysis of translocation of CSA protein using in situ visualization and cellular fractionation.
Immunofluorescence microscopy was performed as described previously (15). For the fractionation method, cellular proteins were fractionated as follows. Cells were extracted in CSK-Triton buffer at 4°C for 10 min. The insoluble fractions were separated from soluble proteins (fraction 1) by centrifugation at 4,000 × g for 3 min. The pellet was washed twice with a solution containing 250 mM sucrose and 5 mM MgCl2 (fraction 2) and resuspended with 25 mM Tris-HCl, pH 7.4, 250 mM sucrose, 5 mM MgCl2, and 1 mM phenymethylsulfonyl fluoride. Chromatin was solubilized by digesting DNA with 1 mg/ml of DNase I (Roche Diagnostics; grade II) at 30°C for 1 h. The sample was centrifuged at 4,000 × g for 3 min (fraction 3). The pellet was washed three times with a low-salt buffer (10 mM Tris-HCl, pH 7.4, 0.2 mM MgCl2, 1 mM phenymethylsulfonyl fluoride) (fraction 4), extracted consecutively with the low-salt buffer containing increasingly higher concentrations of NaCl (0.3, 0.5, and 2.0 M) for 15 min, and centrifuged at 18,000 × g for 15 min (fractions 5, 6, and 7, respectively). The high-salt pellet was finally extracted with the low-salt buffer containing 1% (vol/vol) Triton X-100 for 15 min and centrifuged at 18,000 × g for 15 min (fraction 8). The remaining pellet was washed twice with the low-salt buffer and solubilized in SDS-PAGE loading buffer (fraction 9).
RESULTS
Cell-free system for UV-induced translocation of CSA protein to the nuclear matrix.
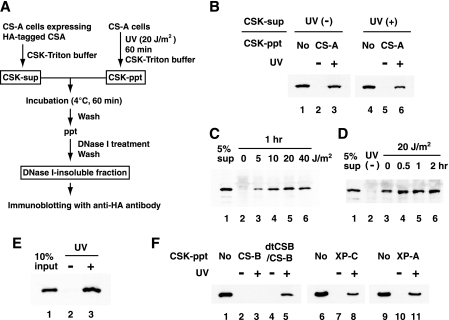
To investigate the mechanism by which UV induces the translocation of the CSA protein to the nuclear matrix, we established a cell-free system for the translocation. The experimental design is summarized in Fig. 1A. CS-A (CS3BESV) cells expressing FLAG and HA epitope-tagged CSA, which are proficient in TCR, were irradiated with UV or not irradiated and incubated for 1 h. CSK-Triton buffer-soluble (CSK-sup) fractions were prepared from these cells. The CSK-sup fractions contained CSA protein (15). CSK-Triton buffer-insoluble (CSK-ppt) fractions were prepared from UV-irradiated or nonirradiated CS-A cells. The CSK-sup and CSK-ppt fractions were incubated at 4°C for 1 h with all combinations, and the insoluble fractions were separated and treated with DNase I. Then, we examined whether the CSA protein was retained in the DNase I-resistant insoluble fraction—that is, the nuclear matrix-equivalent fraction—by immunoblotting it with anti-HA antibody. When the CSK-ppt fractions prepared from nonirradiated cells were used, CSA was not detected in the DNase I-insoluble fractions (Fig. 1B, lanes 2 and 5). On the other hand, when the CSK-ppt fractions derived from UV-irradiated cells were used, CSA was detected in the DNase I-insoluble fractions (lanes 3 and 6). The CSK-ppt fractions prepared from CS-A cells were competent for the CSA translocation, indicating that the CSA protein was not crucial to the CSK-ppt fraction. CSA protein in the CSK-sup fractions prepared from nonirradiated cells was retained in the DNase I-insoluble fractions when the CSK-ppt fraction derived from UV-irradiated cells was used, suggesting that modifications of the CSA protein after UV irradiation are not required for its translocation.
FIG. 1.
UV-induced translocation of CSA to the nuclear matrix in a cell-free system. (A) Procedures of the cell-free system used for the translocation. (B) UV-dependent translocation of CSA in the cell-free system. CSK-sup fractions were prepared from UV-irradiated or nonirradiated CSA-FLAG-HA/CS3BESV cells. CSK-ppt fractions were prepared from UV-irradiated or nonirradiated CS3BESV cells. The CSK-sup fraction containing HA-tagged CSA was incubated with each CSK-ppt fraction and then treated with DNase I. The CSA retained in the DNase I-insoluble fractions was detected by immunoblotting with anti-HA antibody. Ten percent of the CSK-sup fraction was loaded in lanes 1 and 4 as a control. (C) UV dose-dependent translocation of CSA in the cell-free system. CS3BESV cells were UV irradiated at the indicated doses and incubated for 1 h. The CSK-ppt fractions prepared from those cells were used for the cell-free system. The CSA retained in the DNase I-insoluble fractions is shown in lanes 2 to 6. Five percent of the CSK-sup fraction was loaded in lane 1. (D) Post-UV incubation time-dependent translocation of CSA in the cell-free system. CS3BESV cells were UV irradiated at 20 J/m2 or not irradiated and incubated for the indicated periods. CSK-ppt fractions prepared from those cells were used for the cell-free system. The CSA retained in the DNase I-insoluble fractions is shown in lanes 2 to 6. Five percent of the soluble fraction was loaded in lane 1. (E) A purified CSA complex substitutes for the CSK-sup fraction. The CSA complex (a CSA-DDB1-cullin 4A-Roc1 tetramer) was purified as described previously (10) with some modifications and incubated with CSK-ppt fractions prepared from UV-irradiated or nonirradiated CS3BESV cells. Ten percent of the CSA complex was loaded in lane 1. (F) UV-induced translocation of CSA is dependent on CSB in the cell-free system. CSK-ppt fractions were prepared from UV-irradiated or nonirradiated CS1ANSV (lanes 2 and 3), dtCSB/CS1ANSV (lanes 4 and 5), XP4PASV (lanes 7 and 8), and XP2OSSV (lanes 10 and 11) cells. The CSA retained in the DNase I-insoluble fractions is shown. Ten percent of the CSK-sup fraction was loaded in lanes 1, 6, and 9.
The UV dose-dependent translocation of CSA to the nuclear matrix was then examined. CS3BESV cells were irradiated at 5, 10, 20, and 40 J/m2 and incubated for 1 h. The CSK-ppt fractions prepared from these cells were incubated with the CSK-sup fractions prepared from the CS3BESV cells expressing HA-tagged CSA. The insoluble fractions were separated and then treated with DNase I. As shown in Fig. 1C, CSA was retained in the DNase I-insoluble fraction with 5 J/m2 of UV irradiation. The retention was more pronounced at 10 J/m2 and was saturated at higher doses.
Next, the post-UV incubation time-dependent translocation of CSA to the nuclear matrix was examined. CSK-ppt fractions prepared at various time points after UV irradiation (20 J/m2) were incubated with CSK-sup fractions prepared from the CS3BESV cells expressing HA-tagged CSA (Fig. 1D). CSA was retained in the DNase I-insoluble fractions derived from cells immediately after UV irradiation. More CSA was retained in the DNase I-insoluble fractions from cells incubated for a longer time (0.5 to 2 h) after UV irradiation. These results were consistent with previous results obtained by the conventional cellular-fractionation analysis (15). It took more than 60 min at 4°C for maximum retention of HA-tagged CSA in the DNase I-insoluble fractions (data not shown).
We examined the factor(s) that can substitute for the CSK-sup fraction. Whole-cell extracts (20, 48), extracts fractionated with phosphocellulose (CF-I) (34, 42), and even a purified CSA complex (10) were competent as a CSK-sup fraction for the UV-induced translocation of CSA (Fig. 1E and data not shown).
We have shown that the UV-induced translocation of CSA to the nuclear matrix depends on CSB, not on XPA or XPC (15). We examined whether CSB is required for translocation in this cell-free system as well. When the CSK-ppt fraction prepared from UV-irradiated CS-B (CS1ANSV) cells was used, little CSA was retained in the DNase I-insoluble fractions (Fig. 1F, lane 3). In contrast, a significant amount of CSA was retained in the DNase I-insoluble fractions when the CSK-ppt fractions prepared from UV-irradiated CS1ANSV cells stably expressing double-tagged CSB (dtCSB/CS1ANSV) (42), as well as XP-C and XP-A cells (lanes 5, 8, and 11), were used.
Taken together, the results validated the fact that this cell-free system faithfully reproduces the phenomenon of translocation of CSA to the nuclear matrix in UV-irradiated cells.
CSA in the CSK-ppt fraction is exchangeable with exogenous CSA.
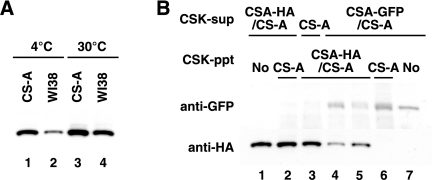
When the CSK-ppt fraction prepared from UV-irradiated normal (WI38VA13) cells was incubated with the CSK-sup fraction containing HA-tagged CSA at 4°C, exogenous CSA was retained in the DNase I-insoluble fraction (Fig. 2A). The retention in the DNase I-insoluble fraction from normal cells was less than that from UV-irradiated CS-A cells (compare lanes 1 and 2). When the CSK-ppt and CSK-sup fractions were incubated at 30°C, the retention was more pronounced. These results suggest that CSA in the CSK-ppt fraction is replaced with exogenous CSA during incubation in this cell-free system. To verify the exchange of CSA, the CSK-ppt fraction prepared from UV-irradiated CSA-HA/CS-A cells and the CSK-sup fraction containing GFP-tagged CSA were used (Fig. 2B). CSA-GFP was competent for translocation in this system (Fig. 2B, lane 6). The amount of CSA-HA retained in the DNase I-insoluble fraction decreased when the CSK-ppt fraction was incubated with the CSK-sup fraction containing CSA-GFP. The decrease of CSA-HA was more pronounced when both fractions were incubated at 30°C (compare lanes 4 and 5 with 2). In contrast, the amount of CSA-GFP retained in the DNase I-insoluble fraction was increased at 30°C (lane 4). These results support the above hypothesis that CSA in the CSK-ppt fraction is exchangeable.
FIG. 2.
CSA in the CSK-ppt fraction is exchangeable with exogenous CSA. (A) CSK-ppt fractions were prepared from UV-irradiated CS3BESV and WI38VA13 cells and incubated with the CSK-sup fraction containing HA-tagged CSA at 4°C or 30°C. (B) CSK-sup fractions were prepared from CS3BESV (lane 3), CSA-FLAG-HA/CS3BESV (lane 2), and CSA-GFP/CS3BESV (lanes 4 to 6) cells. CSK-ppt fractions were prepared from UV-irradiated CS3BESV (lanes 2 and 6) and CSA-FLAG-HA/CS3BESV (lanes 3 to 5) cells. The CSK-sup and CSK-ppt fractions were incubated at 4°C (lanes 2, 3, 5, and 6) or 30°C (lane 4). Five percent of the CSK-sup fraction from CSA-FLAG-HA/CS3BESV and CSA-GFP/CS3BESV cells was loaded in lanes 1 and 7, respectively.
TCR-deficient mutant CSA proteins are not translocated to the nuclear matrix after UV irradiation.
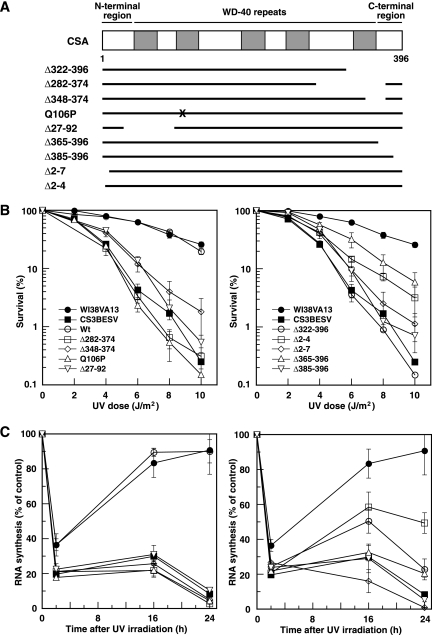
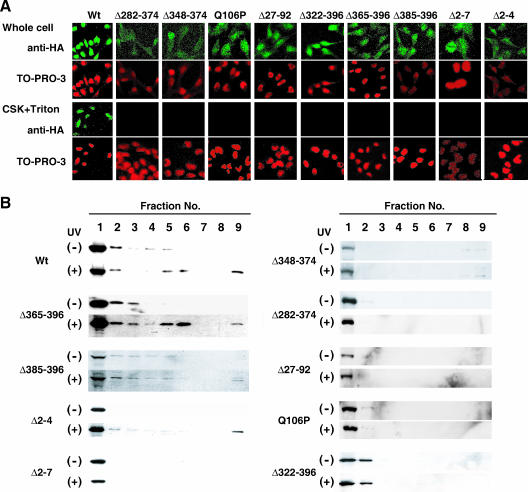
To investigate whether the UV-induced translocation of CSA to the nuclear matrix is relevant to TCR reactions, various types of cDNA constructs that express mutant CSA proteins containing FLAG and HA epitope tags at the C terminus were constructed and transfected into CS-A (CS3BESV) cells. The UV-induced translocation of CSA to the nuclear matrix, survival, and the recovery of RNA synthesis after UV irradiation were examined in these stable transfectants. The mutant CSA proteins examined in this study are summarized in Fig. 3A. Δ322-396, Δ282-374, Δ348-374, and Q106P are mutant forms of CSA derived from CS patients. The CSA patient CS2IAF is homozygous for the Tyr 322-to-stop mutation (Δ322-396) (13, 22). Two deletions removing either exon 11 (Δ348-374) or exons 10 and 11 (Δ282-374) were identified in CSA cDNA isolated from CS-A patients CS5BR and CS6BR and are presumably derived from a homozygous mutation at a splice donor site (13). An amino acid substitution at Gln 106 to Pro (Q106P) was identified in the CS-A patient CS2SE (29). The transfectants expressing each of these mutant CSAs were for convenience designated Δ322-396 cells, Δ282-374 cells, Δ348-374 cells, and Q106P cells, respectively. In addition, we established transfectants expressing mutant CSA with an in-frame deletion of exons 2 and 3 (Δ27-92 cells), with a truncated N terminus (Δ2-4 and Δ2-7 cells), and with a truncated C terminus (Δ365-396 and Δ385-396 cells). All the WD-40 repeat motifs are preserved in these terminally truncated mutants. We chose the transfectants that expressed a normal amount of CSA protein.
FIG. 3.
Survival and recovery of RNA synthesis after UV irradiation in the mutant CSA transfectants. (A) Schematic representation of CSA mutants. The WD-40 repeats are indicated by gray boxes. Each mutant CSA was tagged at the C terminus with FLAG and HA epitopes and expressed in CS3BESV cells. The X shows the location of the amino acid substitution Q106P. (B) Colony-forming abilities of the UV-irradiated CS3BESV cells expressing mutant CSA with an internal deletion, point mutation (left), or terminal deletion (right) were measured. The points are averages for at least three independent experiments, and the vertical bars indicate standard errors. (C) RNA synthesis was measured at various time points after UV irradiation as the relative incorporation of [3H]uridine in cells irradiated with 10 J/m2 of UV compared with that in nonirradiated cells. The points are averages for at least three independent experiments, and the vertical bars indicate standard errors. Symbols in panel C are as defined in panel B. Wt, wild type.
First, the colony-forming abilities of the UV-irradiated transfectants were measured (Fig. 3B). The transfectant expressing wild-type CSA exhibited almost the same level of UV resistance as normal (WI38VA13) cells. Δ322-396 and Δ282-374 cells were very sensitive to UV irradiation, as were the parental CS3BESV cells. Q106P cells were also extremely sensitive to UV irradiation (29). Δ348-374, Δ27-92, Δ2-7, and Δ385-396 cells were slightly more resistant than the parental CS3BESV cells. Δ2-4 and Δ365-396 cells showed less UV hypersensitivity than Δ348-374, Δ27-92, Δ2-7, and Δ385-396 cells. Thus, all the transfectants expressing mutant CSA showed more or less UV hypersensitivity.
Next, the recovery of RNA synthesis after UV irradiation, which is an index of TCR, was measured. RNA synthesis in WI38VA13 and CS3BESV cells at 2 h after UV irradiation was about 35% and 20% of that in nonirradiated cells, respectively (Fig. 3C). The RNA synthesis in WI38VA13 cells and the transfectant expressing wild-type CSA at 16 and 24 h after UV irradiation had recovered to about 90% of the level in nonirradiated cells, but in the parental CS3BESV cells, no such recovery was observed. None of the transfectants expressing mutant CSA showed a recovery of RNA synthesis at 16 and 24 h after UV irradiation. Thus, all the CSA mutants were deficient in TCR.
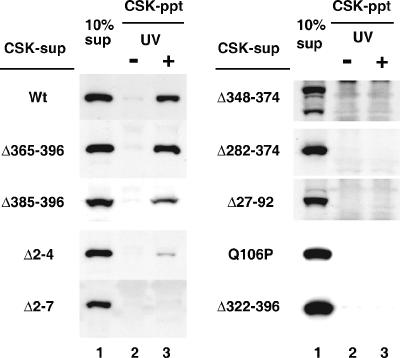
We then examined whether the mutant CSA proteins were translocated to the nuclear matrix after UV irradiation by using the cell-free method. CSK-sup fractions were prepared from CS3BESV cells expressing various types of mutant CSA (Fig. 4). When these fractions were incubated with the CSK-ppt fractions from nonirradiated CS3BESV cells, no mutant CSA proteins were retained in the DNase I-insoluble fractions. When the CSK-sup fractions prepared from Δ365-396 and Δ385-396 cells, as well as wild-type cells, were incubated with the CSK-ppt fractions derived from UV-irradiated CS3BESV cells, CSA proteins were detected in the DNase I-insoluble fractions. When the CSK-sup fraction was prepared from Δ2-4 cells, a small amount of CSA was translocated to the DNase I-insoluble fraction. However, no CSA protein was retained in the DNase I-insoluble fractions when the CSK-sup fractions derived from Δ2-7, Δ348-374, Δ282-374, Δ27-92, Q106P, and Δ322-396 cells were used.
FIG. 4.
Translocation of mutant CSA to the nuclear matrix in the cell-free system. CSK-sup fractions prepared from CS3BESV cells expressing HA-tagged mutant CSA were incubated with CSK-ppt fractions prepared from UV-irradiated or nonirradiated CS3BESV cells (lanes 2 and 3) and then treated with DNase I. The CSA retained in the DNase I-insoluble fraction was detected by immunoblotting with anti-HA antibody. Ten percent of the CSK-sup fraction was loaded in lane 1. Wt, wild type.
We confirmed the above-mentioned results by using previously described methods (15). First, we performed immunofluorescence staining (Fig. 5A). When the transfectants were fixed 2 h after UV irradiation by using paraformaldehyde without pretreatment with CSK-Triton buffer, all the mutant CSA proteins, as well as the wild-type CSA protein, were detected in the nuclei, and some mutant CSA proteins (Δ282-374, Δ348-374, Q106P, and Δ365-396) were also detected in the cytoplasm. On the other hand, wild-type CSA protein was retained in the nuclei even after the pretreatment with CSK-Triton buffer, as described previously (15), but none of the mutant CSA proteins were. Neither the wild-type nor any of the mutant CSA proteins was retained in the nuclei when the cells were not irradiated with UV (data not shown). Next, the translocation of the mutant CSA proteins to the nuclear matrix was monitored by immunoblotting of fractionated cellular proteins (Fig. 5B). In the nonirradiated cells, CSA was mainly detected in the fractions extracted with CSK-Triton buffer (fractions 1 and 2). As described previously (15), and consistent with the results of the immunofluorescence analysis, wild-type CSA was detected in the nuclear-matrix fraction (fraction 9) after UV irradiation. Three truncated CSA proteins, Δ365-396, Δ385-396, and Δ2-4, were also translocated to the nuclear-matrix fraction after UV irradiation, although the amounts translocated to the nuclear matrix appeared to be less than that of wild-type CSA protein. The other mutant CSA proteins were not translocated to the nuclear-matrix fraction after UV irradiation. The difference between the results by immunofluorescence staining and immunoblotting could be caused by the difference in the sensitivities of these two detection methods.
FIG. 5.
UV-induced translocation of CSA in the transfectants expressing various types of mutant CSA. (A) Immunofluorescent staining of CSA in the transfectants expressing various types of mutant CSA. CS3BESV cells expressing HA-tagged mutant CSA were irradiated with 20 J/m2 of UV, incubated for 2 h, and fixed (upper row; Whole cell) or else pretreated with CSK-Triton buffer before fixation (lower row; CSK+Triton). CSA was detected with anti-HA antibody and visualized with Alexa Fluor 488-conjugated anti-rat antibody (green). DNA was stained by TO-PRO-3 (red). Wt, wild type. (B) Cellular-fractionation analysis. CS3BESV cells expressing HA-tagged mutant CSA were irradiated with 20 J/m2 of UV or not irradiated and incubated for 2 h. Fractionation of cellular proteins was performed as described in Materials and Methods. Proteins from each fraction with equivalent cell numbers were loaded for SDS-PAGE and analyzed by immunoblotting them with anti-HA antibody. The lane numbers correspond to the fraction numbers. Fraction 9 is the nuclear-matrix fraction. Note that Δ365-396, Δ385-396, and Δ2-4, as well as wild-type CSA, were translocated to some extent to the nuclear matrix while other mutant CSAs were not.
These results indicate that the cell-free system faithfully reproduces the phenomenon in UV-irradiated cells and that the WD-40 repeat motifs and some N-terminal regions of CSA are required for the translocation, which is well correlated with TCR activity.
Involvement of TFIIH in the UV-induced translocation of CSA.
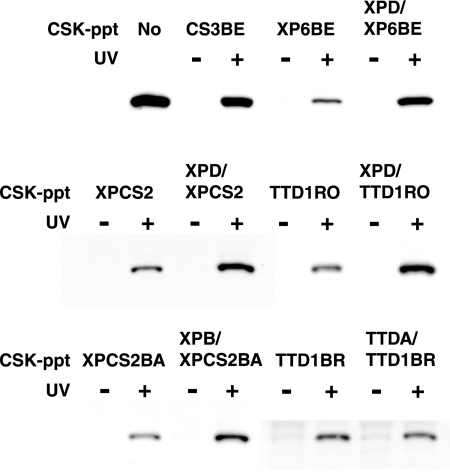
TFIIH consists of 10 subunits and is involved in TCR, as well as GGR and transcription. Hereditary mutations in the subunits, XPB, XPD, and TTDA, are associated with three UV-sensitive disorders: XP, XP combined with CS, and trichothiodystrophy (TTD) (2, 9). We examined whether TFIIH participates in the CSA translocation by using the cell-free system. We prepared CSK-ppt fractions from various cell lines mutated in XPD, XPB, or TTDA and tested their competence for the UV-induced CSA translocation (Fig. 6). When CSK-ppt fractions were prepared from UV-irradiated XPD- or XPB-deficient cells (XP6BE [XP-D], XPCS2 [XP-D/CS], TTD1RO [XP-D/TTD], and XPCS2BA [XP-B/CS]), a small amount of CSA was retained in the DNase I-insoluble fractions, while a significant amount of CSA was retained when the CSK-ppt fraction from XPD- or XPB-corrected cells (XPD/XP6BE, XPD/XPCS2, XPD/TTD1RO [46], and XPB/XPCS2BA [14], respectively) was used. On the other hand, there were no significant differences in the retention of CSA between TTDA-deficient (TTD1BR) and corrected (TTDA/TTD1BR [9]) cells. It was reported that the steady-state level of TFIIH in TTD1BR cells was reduced but the main enzymatic activities of TFIIH in TTD1BR cells were globally intact (45). These results suggest that functional TFIIH is involved in the UV-induced translocation of CSA to the nuclear matrix and that the CSA translocation is not correlated with phenotypes caused by mutations in the subunits of TFIIH.
FIG. 6.
Involvement of TFIIH in the UV-induced translocation of CSA to the nuclear matrix. CSK-ppt fractions prepared from various cells with mutations in XPD, XPB, or TTDA and their corrected cells were tested for competence for the CSA translocation. The CSK-sup fraction prepared from CSA-FLAG-HA cells was incubated with the CSK-ppt fraction from a UV-irradiated or nonirradiated cell line and then treated with DNase I. The CSA retained in the DNase I-insoluble fractions was detected by immunoblotting with anti-HA antibody.
Requirements for chromatin structure and transcription for UV-induced translocation.
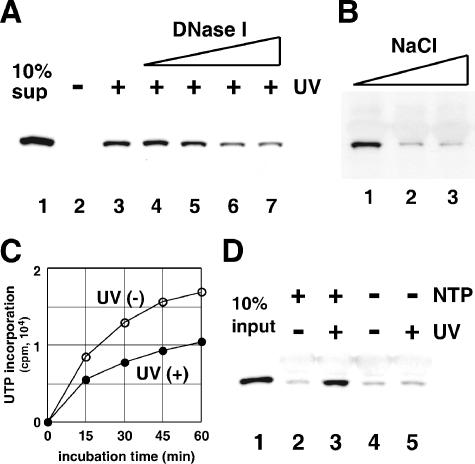
Our results indicated that the CSK-ppt fractions are crucial for the UV-induced translocation of CSA protein to the nuclear matrix. Therefore, we examined whether some pretreatments of the CSK-ppt fractions affect their activities to translocate the CSA protein in the DNase I-insoluble fraction. As shown in Fig. 7A and B, pretreatment with DNase I or high concentrations of salt (more than 300 mM) decreased the UV-induced translocation of CSA to the nuclear-matrix fraction, suggesting that appropriate chromatin structure in the CSK-ppt fraction is necessary for the UV-induced translocation.
FIG. 7.
Chromatin structure and transcription elongation are crucial to the UV-induced translocation of CSA. (A) Pretreatment of the CSK-ppt fraction with DNase I decreased translocation. CSK-ppt fractions were prepared from UV-irradiated CS3BESV cells, treated with various concentrations of DNase I (lanes 3 to 7; 0, 0.7, 1.4, 2.8, and 5.6 units/ml) at 30°C for 15 min, and then incubated with the CSK-sup fraction containing CSA. The CSK-ppt fraction prepared from nonirradiated CS3BESV cells was used in lane 2. Ten percent of the CSK-sup fraction was loaded in lane 1. (B) Pretreatment of the cell extracts with high-salt buffer decreased the translocation of CSA. UV-irradiated CS3BESV cells were extracted with CSK-Triton buffer containing 100 (lane 1), 300 (lane 2), or 500 mM (lane 3) NaCl and washed with CSK-Triton buffer containing 100 mM NaCl. These insoluble fractions were used as the CSK-ppt fractions for the CSA translocation in the cell-free system. (C) The CSK-ppt fractions were prepared immediately after UV irradiation or nonirradiation and incubated at 30°C for various periods in reaction buffer containing NTPs. RNA synthesis was evaluated by measuring the incorporation of [α-32P]UTP into the acid-insoluble materials. (D) CSK-ppt fractions were prepared immediately after UV irradiation or nonirradiation and incubated with purified CSA complex in the presence (lanes 1 and 2) or absence (lanes 3 and 4) of NTPs at 30°C for 1 h. Ten percent of the CSA complex was loaded in lane 1.
The CSK-ppt fractions, even if prepared from UV-irradiated cells, were competent for RNA synthesis (transcription elongation) in the presence of NTPs (Fig. 7C). On the other hand, RNA synthesis did not occur in the absence of NTPs (data not shown). We examined whether active transcription in the CSK-ppt fraction is required for translocation. CSK-ppt fractions were prepared immediately after UV irradiation and incubated with purified CSA complex with or without NTPs (Fig. 7D). CSA was retained in the DNase I-insoluble fraction in the presence of NTPs, but not in their absence. These results indicate that the transcription elongation by RNA polymerase II is required for the UV-induced translocation of CSA to the nuclear matrix.
DISCUSSION
We developed a cell-free system for studying the UV-induced translocation of CSA to the nuclear matrix. After CSK-sup fractions containing the CSA protein were incubated with CSK-ppt fractions derived from UV-irradiated CSA cells, the CSA protein in the CSK-sup fractions was translocated to the DNase I-insoluble fractions. The translocation was dependent on UV irradiation and CSB in the CSK-ppt fractions. Thus, the cell-free system faithfully reproduced the phenomenon in terms of translocation of CSA to the nuclear matrix in UV-irradiated cells. It made no difference whether the CSK-sup fractions were derived from UV-irradiated or nonirradiated cells. In addition, the purified CSA complex could substitute for the CSK-sup fraction. These results suggest that no modifications of CSA upon UV irradiation in the CSK-sup fractions are necessary for the translocation of CSA.
We have shown that CSA forms a ubiquitin ligase complex with DDB1, cullin 4A, Roc 1, and the COP9 signalosome (10). However, our cell-free system with a purified CSA complex does not support a ubiquitination reaction. In the absence of ATP, the translocation was detected (Fig. 1E). These results suggest that the ubiquitin ligase activity of the CSA complex is not involved in the translocation.
In order to identify which regions in CSA are required for the UV-induced translocation to the nuclear matrix, we established CS-A (CS3BESV) cells stably expressing various types of mutant CSA (Fig. 3A). The UV-induced translocation to the nuclear matrix was examined with both the cell-free system and conventional fractionation and immunofluorescence methods. As shown in Fig. 3 to 5, the CSA mutants derived from CS-A patients (Δ282-374, Δ348-374, Δ322-396, and Q106P) were not translocated to the nuclear matrix. Moreover, the transfectants expressing these mutants showed UV hypersensitivity and were deficient in the recovery of RNA synthesis after UV irradiation (Fig. 3B and C). CSA protein has five WD-40 repeat motifs and is thought to form a β-propeller structure. All the mutants derived from CS-A patients used here lacked the last WD-40 repeat motif or had a substitution of amino acid residues in the second motif. It is conceivable that these mutants could not form the proper structure and so were deficient in the UV-induced translocation to the nuclear matrix and TCR function. In addition, the CSA mutant Δ27-92 lacks the first WD-40 repeat motif and the phenotypes of Δ27-92 cells were similar to those of the CS-A patients' cells, as expected. On the other hand, the four CSA mutants with terminal deletions (Δ2-4, Δ2-7, Δ365-396, and Δ385-396) retained all the WD-40 repeat motifs and are assumed to form a β-propeller structure. In fact, the Δ2-4, Δ365-396, and Δ385-396 CSA mutants were translocated to the nuclear matrix to some extent, and these mutant cells showed partial resistance to UV, although the Δ2-7 cells were deficient in UV-induced translocation. These results suggest that the WD-40 repeat motifs and some N-terminal regions are indispensable for the UV-induced translocation of CSA and TCR while some C-terminal regions are dispensable for the translocation to the nuclear matrix but required for full TCR activity.
The C-terminal region (amino acid residues 365 to 396) of CSA is highly conserved and has clusters of acidic amino acid residues. Δ365-396 lacks the entire C-terminal region, while Δ385-396 retains half of it. However, Δ365-396 cells were less UV-sensitive than Δ385-396 cells. These results suggest that incomplete deletion of the C-terminal region has negative effects on the viability of cells after UV irradiation, although there were no significant differences in the recovery of RNA synthesis after UV irradiation between Δ365-396 and Δ385-396 cells.
We showed, using a cell-free system, that TFIIH, as well as CSB, is involved in the UV-induced translocation of CSA. In NER, XPB and XPD helicases in TFIIH cooperate in the unwinding of duplex DNA around lesions to allow the recruitment of the NER factors XPA, RPA, XPG, and XPF-ERCC1 to the damaged site. In this study, we tested three cell lines with mutations in the XPD gene. All the mutations found in the cell lines were shown to impair XPD helicase activity (7). Impairment of TFIIH function in the XPB-deficient cell line has also been reported (44). In contrast, functional TFIIH was shown to exist in the TTDA-deficient cell line, although the steady-state level of TFIIH was reduced (45). This could be a reason why the UV-induced translocation of CSA was not impaired in the TTDA-deficient cell line. We do not yet know the molecular mechanism of TFIIH function in the CSA translocation process. It has been reported that TFIIH interacts with the RNA polymerase II-CSB-DNA-RNA complex (38) and remodels the stalled RNA polymerase II in a manner dependent on the hydrolysis of ATP (30). These results suggest that TFIIH is involved in the UV-induced translocation of CSA to the nuclear matrix by remodeling the stalled RNA polymerase II in collaboration with CSB.
We showed that the CSK-ppt fraction was competent for RNA synthesis even if it was prepared from UV-irradiated cells and that the UV-induced translocation of CSA to the DNase I-insoluble fraction was dependent on RNA synthesis in the CSK-ppt fraction. It has been shown that the synthesis of RNA by RNA polymerase II is completely blocked at UV-damaged sites (6, 23, 33). These results suggest that the blockage of elongating RNA polymerase II is necessary for the translocation of CSA.
We also showed that the pretreatment of the CSK-ppt fraction with either DNase I or a high-salt buffer reduced the UV-induced translocation of CSA, indicating that the disruption of the chromatin structure resulted in the decrease in translocation and, therefore, that alteration of the chromatin structure at the RNA polymerase II stall sites is a prerequisite for the UV-induced translocation of CSA. It has been reported that CSB interacts with RNA polymerase II engaged in ternary complexes containing DNA and RNA in vitro (30, 37) and that the interaction between CSB and the transcription elongation machinery is stabilized by the presence of DNA damage in vivo (40). It has also been reported that CSB wraps DNA around its surface, dependent on ATP binding (1), and has ATP-dependent chromatin-remodeling activity in vitro (5). Taken together, these results suggest that CSB and TFIIH cooperate to alter the chromatin structure at the DNA-damaged site where the elongating RNA polymerase II is stalled so that CSA can be recruited to the RNA polymerase II stall site in the nuclear matrix.
Acknowledgments
We thank W. Vermeulen for providing XPCS2BA, TTD1RO, and TTD1BR cells and their corrected cells and Y. Nakatsu and I. Kuraoka for helpful suggestions.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), by the Solution Oriented Research for Science and Technology program of the Japan Science and Technology Agency, and also by the Takeda Science Foundation.
Footnotes
▿
Published ahead of print on 22 January 2007.
REFERENCES
- 1.Beerens, N., J. H. J. Hoeijmakers, R. Kanaar, W. Vermeulen, and C. Wyman. 2005. The CSB protein actively wraps DNA. J. Biol. Chem. 280**:**4722-4729. [DOI] [PubMed] [Google Scholar]
- 2.Bootsma, D., J. E. Cleaver, K. H. Kraemer, and J. H. J. Hoeijmakers. 1998. Xeroderma pigmentosum and Cockayne syndrome, p. 245-274. In C. R. Scriver, A. L. Beaudet, W. S. Sly, and D. Valle (ed.), The metabolic basis of inherited disease. McGraw-Hill, New York, NY.
- 3.Bradsher, J., J. Auriol, L. Proietti de Santis, S. Iben, J.-L. Vonesch, I. Grummt, and J.-M. Egly. 2002. CSB is a component of RNA pol I transcription. Mol. Cell 10**:**819-829. [DOI] [PubMed] [Google Scholar]
- 4.Citterio, E., S. Rademakers, G. T. J. van der Horst, A. J. van Gool, J. H. J. Hoeijmakers, and W. Vermeulen. 1998. Biochemical and biological characterization of wild-type and ATPase-deficient Cockayne syndrome B repair protein. J. Biol. Chem. 273**:**11844-11851. [DOI] [PubMed] [Google Scholar]
- 5.Citterio, E., V. van den Boom, G. Schnitzler, R. Kanaar, E. Bonte, R. E. Kingston, J. H. J. Hoeijmakers, and W. Vermeulen. 2000. ATP-dependent chromatin remodeling by the Cockayne syndrome B DNA repair-transcription-coupling factor. Mol. Cell. Biol. 20**:**7643-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donahue, B. A., S. Yin, J.-S. Taylor, D. Reines, and P. C. Hanawalt. 1994. Transcript cleavage by RNA polymerase II arrested by a cyclobutane pyrimidine dimer in the DNA template. Proc. Natl. Acad. Sci. USA 91**:**8502-8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubaele, S., L. Proietti De Santis, R. J. Bienstock, A. Keriel, M. Stefanini, B. Van Houten, and J.-M. Egly. 2003. Basal transcription defect discriminates between xeroderma pigmentosum and trichothiodystrophy in XPD patients. Mol. Cell 11**:**1635-1646. [DOI] [PubMed] [Google Scholar]
- 8.Friedberg, E. C., G. C. Walker, W. Siede, R. D. Wood, R. A. Schultz, and T. Ellenberger. 2005. DNA repair and mutagenesis. ASM Press, Washington, DC.
- 9.Giglia-Mari, G., F. Coin, J. A. Ranish, D. Hoogstraten, A. Theil, N. Wijgers, N. G. J. Jaspers, A. Raams, M. Argentini, P. J. van der Spek, E. Botta, M. Stefanini, J.-M. Egly, R. Aebersold, J. H. J. Hoeijmakers, and W. Vermeulen. 2004. A new, tenth subunit of TFIIH is responsible for the DNA repair syndrome trichothiodystrophy group A. Nat. Genet. 36**:**714-719. [DOI] [PubMed] [Google Scholar]
- 10.Groisman, R., J. Polanowska, I. Kuraoka, J. Sawada, M. Saijo, A. F. Kisselev, R. Drapkin, K. Tanaka, and Y. Nakatani. 2003. Ubiquitin ligase activity in the DDB2 and CSA complexes, which are linked to xeroderma pigmentosum and Cockayne syndrome respectively, is differentially regulated by the COP9 signalosome in response to UV irradiation. Cell 113**:**357-367. [DOI] [PubMed] [Google Scholar]
- 11.Hanawalt, P., and G. Spivak. 1999. Transcription-coupled DNA repair. Which lesions? Which diseases? p. 169-179. In M. Dizdaroglu, and A. E. Karakaya (ed.), Advances in DNA damage and repair. Kluwer Academic Publishers, New York, NY.
- 12.Harless, J., and R. R. Hewitt. 1987. Intranuclear localization of UV-induced DNA repair in human VA13 cells. Mutat. Res. 183**:**177-184. [DOI] [PubMed] [Google Scholar]
- 13.Henning, K. A., L. Li, N. Iyer, L. D. McDaniel, M. S. Reagan, R. Legerski, R. A. Schultz, M. Stefanini, A. R. Lehmann, L. V. Mayne, and E. C. Friedberg. 1995. The Cockayne syndrome group A gene encodes a WD repeat protein that interacts with CSB protein and a subunit of RNA polymerase II TFIIH. Cell 82**:**555-564. [DOI] [PubMed] [Google Scholar]
- 14.Hoogstraten, D., A. L. Nigg, H. Heath, L. H. F. Mullenders, R. van Driel, J. H. J. Hoeijmakers, W. Vermeulen, and A. B. Houtsmuller. 2002. Rapid switching of TFIIH between RNA polymerase I and II transcription and DNA repair in vivo. Mol. Cell 10**:**1163-1174. [DOI] [PubMed] [Google Scholar]
- 15.Kamiuchi, S., M. Saijo, E. Citterio, M. de Jager, J. H. J. Hoeijmakers, and K. Tanaka. 2002. Translocation of Cockayne syndrome group A protein to the nuclear matrix: possible relevance to transcription-coupled DNA repair. Proc. Natl. Acad. Sci. USA 99**:**201-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karmakar, P., and A. T. Natarajan. 2000. Characteristics of UV-induced repair patches relative to the nuclear skeleton in human fibroblasts. Mutagenesis 15**:**115-120. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi, T., I. Kuraoka, M. Saijo, Y. Nakatsu, A. Tanaka, Y. Someda, S. Fukuro, and K. Tanaka. 1997. Mutations in the XPD gene leading to xeroderma pigmentosum symptoms. Hum. Mutat. 9**:**322-331. [DOI] [PubMed] [Google Scholar]
- 18.Koehler, D. R., and P. C. Hanawalt. 1996. Recruitment of damaged DNA to the nuclear matrix in hamster cells following ultraviolet irradiation. Nucleic Acids Res. 24**:**2877-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehman, A. R. 1982. Three complementation groups in Cockayne syndrome. Mutat. Res. 106**:**347-356. [DOI] [PubMed] [Google Scholar]
- 20.Manley, J. L., A. Fire, M. Samuels, and P. A. Sharp. 1983. In vitro transcription: whole-cell extract. Methods Enzymol. 101**:**568-582. [DOI] [PubMed] [Google Scholar]
- 21.McCready, S. J., and P. R. Cook. 1984. Lesions induced in DNA by ultraviolet light are repaired at the nuclear cage. J. Cell Sci. 70**:**189-196. [DOI] [PubMed] [Google Scholar]
- 22.McDaniel, L. D., R. Legerski, A. R. Lehman, E. C. Friedberg, and R. A. Schultz. 1997. Confirmation of homozygosity for a single nucleotide substitution mutation in a Cockayne syndrome patient using monoallelic mutation analysis in somatic cell hybrids. Hum. Mutat. 10**:**317-321. [DOI] [PubMed] [Google Scholar]
- 23.Mei Kwei, J. S., I. Kuraoka, K. Horibata, M. Ubukata, E. Kobatake, S. Iwai, H. Handa, and K. Tanaka. 2004. Blockage of RNA polymerase II at a cyclobutane pyrimidine dimer and 6-4 photoproduct. Biochem. Biophys. Res. Commun. 320**:**1133-1138. [DOI] [PubMed] [Google Scholar]
- 24.Mortillaro, M. J., B. J. Blencowe, X. Wei, H. Nakayasu, L. Du, S. L. Warren, P. A. Sharp, and R. Berezney. 1996. A hyperphosphorylated form of the large subunit of RNA polymerase II is associated with splicing complexes and the nuclear matrix. Proc. Natl. Acad. Sci. USA 93**:**8253-8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullenders, L. H., A. C. van Kesteren van Leeuwen, A. A. van Zeeland, and A. T. Natarajan. 1988. Nuclear matrix associated DNA is preferentially repaired in normal human fibroblasts exposed to a low dose of ultraviolet light but not in Cockayne's syndrome fibroblasts. Nucleic Acids Res. 16**:**10607-10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakatsu, Y., H. Asahina, E. Citterio, S. Rademakers, W. Vermeulen, S. Kamiuchi, J.-P. Yeo, M.-C. Khaw, M. Saijo, N. Kodo, T. Matsuda, J. H. J. Hoeijmakers, and K. Tanaka. 2000. XAB2, a novel tetratricopeptide repeat protein, involved in transcription-coupled DNA repair and transcription. J. Biol. Chem. 275**:**34931-34937. [DOI] [PubMed] [Google Scholar]
- 27.Nance, M. A., and S. A. Berry. 1992. Cockayne syndrome: review of 140 cases. Am. J. Med. Genet. 42**:**68-84. [DOI] [PubMed] [Google Scholar]
- 28.Patturajan, M., X. Wei, R. Berezney, and J. L. Corden. 1998. A nuclear matrix protein interacts with the phosphorylated C-terminal domain of RNA polymerase II. Mol. Cell. Biol. 18**:**2406-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren, Y., M. Saijo, Y. Nakatsu, H. Nakai, M. Yamaizumi, and K. Tanaka. 2003. Three novel mutations responsible for Cockayne syndrome group A. Genes Genet. Syst. 78**:**93-102. [DOI] [PubMed] [Google Scholar]
- 30.Sarker, A. H., S. E. Tsutakawa, S. Kostek, C. Ng, D. S. Shin, M. Peris, E. Campeau, J. A. Tainer, E. Nogales, and P. K. Cooper. 2005. Recognition of RNA polymerase II and transcription bubbles by XPG, CSB, and TFIIH: Insights for transcription-coupled repair and Cockayne syndrome. Mol. Cell 20**:**187-198. [DOI] [PubMed] [Google Scholar]
- 31.Selby, C. P., and A. Sancar. 1997. Human transcription-repair coupling factor CSB/ERCC6 is a DNA-stimulated ATPase but is not a helicase and does not disrupt the ternary transcription complex of stalled RNA polymerase II. J. Biol. Chem. 272**:**1885-1890. [DOI] [PubMed] [Google Scholar]
- 32.Selby, C. P., and A. Sancar. 1997. Cockayne syndrome group B protein enhances elongation by RNA polymerase II. Proc. Natl. Acad. Sci. USA 94**:**11205-11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selby, C. P., R. Drapkin, D. Reinberg, and A. Sancar. 1997. RNA polymerase II stalled at a thymine dimer: footprint and effect on excision repair. Nucleic Acids Res. 25**:**787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shivji, M. K. K., M. K. Kenny, and R. D. Wood. 1992. Proliferating cell nuclear antigen is required for DNA excision repair. Cell 69**:**367-374. [DOI] [PubMed] [Google Scholar]
- 35.Solovjeva, L., M. Svetlova, L. Sasina, K. Tanaka, M. Saijo, I. Nazarov, M. Bradbury, and N. Tomilin. 2005. High mobility of Flap endonuclease 1 and DNA polymerase n associated with replication foci in mammalian S-phase nucleus. Mol. Biol. Cell 16**:**2518-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka, K., K. Kawai, Y. Kumahara, M. Ikenaga, and Y. Okada. 1981. Genetic complementation groups in Cockayne syndrome. Somatic Cell Genet. 7**:**445-455. [DOI] [PubMed] [Google Scholar]
- 37.Tantin, D., A. Kansal, and M. Carey. 1997. Recruitment of putative transcription-repair coupling factor CSB/ERCC6 to RNA polymerase II elongation complexes. Mol. Cell. Biol. 17**:**6803-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tantin, D. 1998. RNA polymerase II elongation complexes containing the Cockayne syndrome group B protein interact with a molecular complex containing the transcription factor IIH components xeroderma pigmentosum B and p62. J. Biol. Chem. 273**:**27794-27799. [DOI] [PubMed] [Google Scholar]
- 39.Troelstra, C., A. van Gool, J. de Wit, W. Vermeulen, D. Bootsma, and J. H. Hoeijmakers. 1992. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell 71**:**939-953. [DOI] [PubMed] [Google Scholar]
- 40.van den Boom, V., E. Citterio, D. Hoogstraten, A. Zotter, J.-M. Egly, W. A. van Cappellen, J. H. J. Hoeijmakers, A. B. Houtsmuller, and W. Vermeulen. 2004. DNA damage stabilizes interaction of CSB with the transcription elongation machinery. J. Cell Biol. 166**:**27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Hoffen, A., A. T. Natarajan, L. V. Mayne, A. A. van Zeeland, L. H. Mullenders, and J. Venema. 1993. Deficient repair of the transcribed strand of active genes in Cockayne's syndrome cells. Nucleic Acids Res. 21**:**5890-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Gool, A. J., E. Citterio, S. Rademakers, R. van Os, W. Vermeulen, A. Constantinou, J.-M. Egly, D. Bootsma, and J. H. J. Hoeijmakers. 1997. The Cockayne syndrome B protein, involved in transcription-coupled DNA repair, resides in an RNA polymerase II-containing complex. EMBO J. 16**:**5955-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venema, J., L. H. Mullenders, A. T. Natarajan, A. A. van Zeeland, and L. V. Mayne. 1990. The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc. Natl. Acad. Sci. USA 87**:**4707-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vermeulen, W., A. J. van Vuuren, M. Chipoulet, L. Schaeffer, E. Appeldoorn, G. Weeda, N. G. J. Jaspers, A. Priestley, C. F. Arlett, A. R. Lehmann, M. Stefanini, M. Mezzina, A. Sarasin, D. Bootsma, J.-M. Egly, and J. H. J. Hoeijmakers. 1994. Three unusual repair deficiencies associated with transcription factor BTF2 (TFIIH): Evidence for the existence of a transcription syndrome. Cold Spring Harbor Quant. Symp. Biol. 59**:**317-329. [DOI] [PubMed] [Google Scholar]
- 45.Vermeulen, W., E. Bergmann, J. Auriol, S. Rademakers, P. Frit, E. Appeldoorn, J. H. J. Hoeijmakers, and J.-M. Egly. 2000. Sublimiting concentration of TFIIH transcription/DNA repair factor causes TTD-A trichothiodystrophy disorder. Nat. Genet. 26**:**307-313. [DOI] [PubMed] [Google Scholar]
- 46.Vermeulen, W., S. Rademakers, N. G. J. Jaspers, E. Appeldoorn, A. Raams, B. Klein, W. J. Kleijer, L. K. Hansen, and J. H. J. Hoeijmakers. 2001. A temperature-sensitive disorder in basal transcription and DNA repair in humans. Nat. Genet. 27**:**299-303. [DOI] [PubMed] [Google Scholar]
- 47.Wei, X., S. Somanathan, J. Samarabandu, and R. Berezney. 1999. Three-dimensional visualization of transcription sites and their association with splicing factor-rich nuclear speckles. J. Cell Biol. 146**:**543-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood, R. D., P. Robins, and T. Lindahl. 1988. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell 53**:**97-106. [DOI] [PubMed] [Google Scholar]
- 49.Yu, A., H.-Y. Fan, D. Liao, A. D. Bailey, and A. M. Weiner. 2000. Activation of p53 or loss of the Cockayne syndrome group B repair protein causes metaphase fragility of human U1, U2, and 5S genes. Mol. Cell 5**:**801-810. [DOI] [PubMed] [Google Scholar]