Early IL-12 p70, but not p40, production by splenic macrophages correlates with host resistance to blood-stage Plasmodium chabaudi AS malaria (original) (raw)
Abstract
In this study, we compared synthesis of IL-12, a potent Th1-inducing cytokine, by splenic macrophages recovered from resistant C57Bl/6 (B6) mice, which develop predominantly Th1 responses, and susceptible A/J mice that mount primarily Th2 responses during early Plasmodium chabaudi AS infection. Quantitative analysis of IL-12 p40 and p70 release by ELISA revealed significant differences between resistant B6 and susceptible A/J mice in the synthesis of biologically active IL-12 p70, but not p40, by splenic macrophages during early blood-stage P. chabaudi AS infection. Despite up-regulation in p40 and p35 mRNA levels, spontaneous release of p40 in vitro by splenic macrophages was not significantly increased following infection in either mouse strain. In contrast, spontaneous release of p70 by splenic macrophages was increased in cells from B6 mice and levels were significantly higher compared with A/J mice. Furthermore, compared with infected A/J hosts, splenic macrophages recovered from infected B6 mice produced significantly greater quantities of IL-12 p70, but not p40, in vitro, following stimulation with lipopolysaccharide (LPS) or malaria parasite antigen (PRBC). Moreover, we found significant increases in the percentage of macrophages earlier in the spleens of infected B6 mice that could further contribute to differences in total p70 levels in vivo. Taken together, these data suggest that macrophage IL-12 synthesis may contribute to the polarization of Th responses seen in resistant B6 and susceptible A/J mice during acute blood-stage malaria.
Keywords: IL-12, splenic macrophages, blood-stage malaria
INTRODUCTION
Recent studies indicate that cytokines can profoundly influence the developmental maturation of CD4+ helper T cells towards either a Th1 or Th2 phenotype [1]. Emerging evidence suggests that IL-12 plays a central role in the development of CD4+ T cells towards a Th1 phenotype, whereas IL-4 favours a Th2 phenotype differentiation [2,3]. IL-12 is unique among the cytokines in having a heterodimeric structure consisting of disulphide-linked 35-kD and 45-kD subunits (p35 and p40, respectively). Together, these two subunits form a 70–75-kD protein (p70) that accounts for the main biological actions of IL-12 [4]. The genes for p35 and p40 are located on separate chromosomes and must be co-expressed in the same cell type in order for p70 to be produced [5,6]. IL-12 has potent and pleiotropic effects on natural killer (NK) and T cells, in particular the ability to induce the synthesis of interferon-gamma (IFN-γ) [7]. Protective effects of IFN-γ have been implicated in host resistance to protozoan parasite infections, including blood-stage malaria [8–10].
Resistance or susceptibility of inbred mouse strains to infections with Plasmodium chabaudi AS [11–13], _Leishmania major_[14], or Toxoplasma gondii [10] appear to be critically dependent on the timing and activation of an appropriate Th response. Evidence from our laboratory [11,15] and others [13] have previously shown that, during P. chabaudi AS infection, resistant B6 mice mount early, predominantly Th1 responses, develop moderate levels of primary peak parasitaemia and anaemia, and clear the infection by 4 weeks post-infection. In contrast, susceptible A/J mice mount early, predominantly Th2 responses, develop high levels of primary peak parasitaemia and severe anaemia, and mortality occurs in these hosts by days 10–12 following P. chabaudi AS infection.
Evidence for a crucial role of IL-12 in host defence against lethal blood-stage malaria has come from recent observations from our laboratory that daily treatment with murine rIL-12 for 6 days, beginning on the day of infection, could cure susceptible A/J mice against blood-stage P. chabaudi AS infection [16]. Treatment with rIL-12 protected up to 75% of susceptible A/J hosts, compared with 100% mortality in vehicle-treated controls. Furthermore, this IL-12-induced protection occurred by IFN-γ-, tumour necrosis factor-alpha (TNF-α)- and nitric oxide (NO)-dependent mechanism(s). These data suggest that early IL-12 production might be impaired in P. chabaudi AS-infected A/J mice. IL-12 synthesis has been described in a variety of cell types, including dendritic cells [17], neutrophils [18,19] and microglial cells [20] in the central nervous system (CNS). Monocyte/macrophage(s), however, are generally regarded as the major physiological sources of IL-12 [21].
In the present study, we have compared IL-12 production by splenic macrophages recovered from resistant B6 and susceptible A/J hosts during acute P. chabaudi AS infection. Our results demonstrate that, during early blood-stage malaria, splenic macrophages obtained from resistant B6 mice produce significantly higher levels of biologically active IL-12 p70 in vitro compared with macrophages from susceptible A/J hosts. Moreover, higher p70 release by splenic macrophages from infected B6 compared with A/J mice correlated with synthesis of greater quantities of IFN-γ by unfractionated spleen cells obtained from these mice and stimulated in vitro with malaria antigen.
MATERIALS AND METHODS
Mice, parasite and experimental infections
Mice, 6–8 weeks old, were age- and sex-matched in all experiments. A/J mice were purchased from Jackson Laboratories (Bar Harbor, ME), and B6 mice were from Charles River Laboratories (St Constant, Quebec, Canada). Plasmodium chabaudi AS was maintained as previously described [22]. Infection was initiated by i.p. injection of 106_P. chabaudi_ AS-infected erythrocytes (PRBC) and the course of infection was monitored by previously described [22] procedures.
Splenic adherent cell preparation
Single cell suspensions of spleen cells and adherent splenic macrophages were prepared as previously described [9,23]. Adherent cells (pooled from two to three mice) were adjusted to a concentration of 2 × 106 macrophages/ml and cultured for 24 h in freshly added medium alone, with 100 ng/ml of Escherichia coli O127:B8 lipopolysaccharide (LPS; Difco Labs, Detroit, MI) or 106/ml of PRBC. In some experiments, adherent cells were cultured in the presence of indicated concentrations of 1.2 μm diameter latex beads (Sigma Chemical Co., St Louis, MO). Cell culture supernatants were removed and assayed for cytokine concentrations by ELISA. Adherent cells were > 95% macrophages based on morphology, phagocytosis of inert latex beads, and non-specific esterase staining. Where indicated, 500-μl aliquots of unfractionated spleen cells (4 × 106/ml), in medium or PRBC (106/ml), were cultured in 24-well plates and 48 h cell-free culture supernatants were assayed for IFN-γ levels by ELISA.
ELISAs
Two-site sandwich ELISAs were performed using previously described procedures for IFN-γ [9] and IL-12 [24]. The detection limits were 100 pg/ml for IFN-γ and IL-12 p70, and 500 pg/ml for IL-12 p40. The p40-specific ELISA detects all forms of IL-12 and does not distinguish between levels of single IL-12 species such as p40 monomers, homodimers or p40-p35 heterodimers. In contrast, the p70-specific ELISA detects only levels of biologically active heterodimer.
Cytokine mRNA determination by reverse transcriptase-polymerase chain reaction
Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed as previously described [25] to detect changes in cytokine mRNA levels. Titrations of input cDNA were performed followed by PCR amplification to ensure that, for the selected number of cycles, a linear relationship existed between input cDNA and PCR product. Both positive and negative controls were included in each assay to ensure efficacy of the reaction and to rule out possible cDNA contamination of reagents. The housekeeping gene G6PDH was simultaneously amplified in each assay to verify that equal amounts of cDNA were added in each PCR reaction. Nucleotide sequences for primers and probes for IL-12 p40 and IL-12 p35 [26], and G6PDH [25] were used as previously published. After hybridization and washing, cytokine mRNA was detected by autoradiography with Kodak Biomax MR film (Rochester, NY). The intensity of bands corresponding to specific cytokines was analysed by high-resolution optical densitometry (SciScan 500; United States Biochemical, Cleveland, OH) and normalized to those of G6PDH.
Statistical analysis
Results are presented as mean ± s.e.m. Statistical significance of differences in means between two groups of mice was determined by Student's _t_-test. Where three or more groups were compared, one-way anova, followed by Dunnett's post-test, was used. Differences in IL-12 p70 levels were analysed by the Mann–Whitney test. P < 0.05 was considered significant.
RESULTS
Ex vivo p40 and p35 mRNA expression and IL-12 protein release by splenic macrophages
As an initial assessment of IL-12 production, we examined whether splenic macrophages from A/J and B6 mice differed in the kinetics of IL-12 p40 and p35 mRNA expression during the first 5 days of P. chabaudi AS infection (Fig. 1). Besides their isolation from unfractionated spleen cells by 2 h adherence to plastic culture dishes, macrophages were not subjected to any further antigenic stimulation in vitro. Thus, it was expected that cytokine mRNA levels and protein secretion by these cells should closely reflect in vivo activation just prior to their isolation from P. chabaudi AS-infected A/J or B6 mice. Splenic macrophages from uninfected controls of either mouse strain were used as controls. We found low and constitutive expression of p40 and p35 mRNA in splenic macrophages from control animals of either mouse strain (Fig. 1). Following P. chabaudi AS infection, the expression of both p40 and p35 mRNA by splenic macrophages was up-regulated in both mouse strains, suggesting an increased capacity for IL-12 protein synthesis by these cells.
Fig. 1.
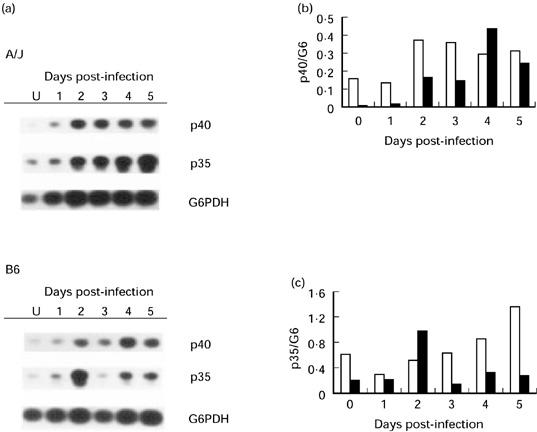
Ex vivo IL-12 p40 and p35 mRNA levels in splenic macrophages (pooled from two to three mice) recovered from uninfected or Plasmodium chabaudi AS-infected A/J and B6 hosts during the first 5 days of infection. Cytokine mRNA levels were determined by reverse transcriptase-polymerase chain reaction (RT-PCR) followed by agarose gel electrophoresis, Southern detection and autoradiography (a). The density of bands corresponding to p40 (b) or p35 (c) mRNA was normalized to those of the housekeeping gene G6PDH (G6). □, A/J; ▪, B6. A representative result of three experiments is shown.
Splenic macrophages from uninfected controls or malaria-infected resistant B6 or susceptible A/J mice at day 4 were selected for a quantitative analysis and comparison of IL-12 p40 and p70 protein secretion in vitro. Substantial increases in IL-12 protein levels in splenic macrophage culture supernatants from infected mice of either strain were not detectable at days earlier than day 4 post-infection (data not shown). Splenic macrophages from uninfected controls of both mouse strains spontaneously secreted basal levels of p40 protein (Fig. 2a). Despite increases in mRNA expression by splenic macrophages for IL-12 p40 following P. chabaudi AS infection, there were no significant increases in the spontaneous secretion of p40 protein in supernatants of cells from infected compared with uninfected controls of either strain. In contrast, spontaneous p70 production was undetectable in cells from uninfected controls of both mouse strains (Fig. 2b). Importantly, p70 levels were significantly greater in supernatants of splenic macrophages from infected B6 compared with A/J mice (P < 0.05, Fig. 2b).
Fig. 2.
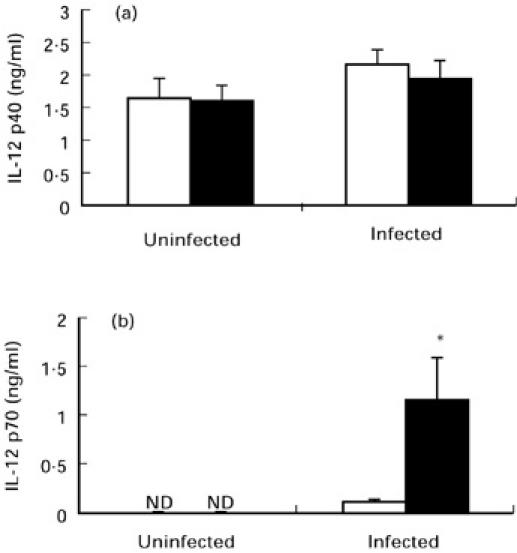
Spontaneous release of IL-12 p40 (a) and p70 (b) by splenic macrophages recovered from uninfected controls or day 4 Plasmodium chabaudi AS-infected A/J (□) and B6 (▪) mice. Cells were cultured in the presence of medium alone for 24 h. IL-12 p40 and p70 levels in culture supernatants were determined by ELISAs. Data are pooled from two to three replicate experiments and are presented as mean ± s.e.m. (n = 8–15). Statistically significant differences: *P < 0.05 versus infected A/J mice. ND, Not detectable.
IL-12 p40 and p70 release in response to stimulation with various microbial products
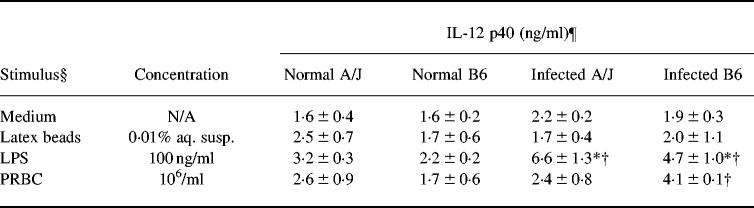
Next, we analysed the capacity of splenic macrophages to produce IL-12 p40 and p70 following stimulation in vitro with microbial antigens. As previously reported, LPS is a potent stimulus for monocyte/macrophage IL-12 p40 release in vitro [27], and systemic IL-12 p40 production in murine hosts [28]. Differences in the potency of different microbial products to induce IL-12 p40 release by splenic macrophages were revealed by the comparison of stimulated versus unstimulated release in medium alone (Table 1). In cells from infected mice of either strain, LPS-induced p40 release was significantly higher than production in medium alone (P < 0.01). In cells from infected B6, but not A/J, mice, PRBC-induced p40 release was also significantly greater than production in medium alone (P < 0.01). In contrast, small inert latex beads (1.2 μm diameter) were ineffective in inducing greater release of p40 over production in medium alone, consistent with studies by other investigators [27,29].
Table 1.
Effect of stimulation with microbial products on IL-12 p40 production by splenic macrophages‡
In contrast to spontaneous production, LPS-induced p40 levels were significantly higher in splenic macrophage culture supernatants from infected versus uninfected mice of both strains (P < 0.01, Table 1). However, LPS-induced p40 protein levels were not significantly different in culture supernatants of splenic macrophages obtained from infected B6 compared with A/J mice. Similar results were obtained when a range of doses of LPS, from 25 to 1000 ng/ml, was used (data not shown). As seen with LPS, PRBC-induced p40 levels in culture supernatants of splenic macrophages were not significantly different between the two mouse strains (Table 1).
In contrast to p40, LPS-induced p70 production by splenic macrophages in vitro was significantly greater in infected B6 compared with A/J mice (P < 0.01), whereas similar production was seen in cells from uninfected controls of either mouse strain (Fig. 3a). In addition, in B6, but not A/J mice, LPS-induced p70 production was significantly greater in splenic macrophages from infected versus uninfected controls (P < 0.05). Secretion of p70 in response to PRBC was also significantly higher in supernatants of splenic macrophages from infected versus uninfected B6 mice (P < 0.01) or compared with cells from infected A/J (P < 0.01) hosts (Fig. 3b). Taken together, these data suggest that, following P. chabaudi AS infection, splenic macrophages from B6 compared with cells from A/J mice produce greater quantities of IL-12 p70, but not p40, both spontaneously and in response to stimulation with LPS or PRBC. We also analysed the levels of IFN-γ in 48 h culture supernatants of spleen cells obtained from uninfected or infected mice of both strains. Consistent with previous studies [11,30], and in correlation with higher IL-12 p70 release by splenic macrophages from infected B6 mice, malaria parasite antigen (PRBC)-induced IFN-γ synthesis was significantly greater (P < 0.05) in spleen cells recovered from day 4 P. chabaudi AS-infected B6 compared with A/J mice (Fig. 3c).
Fig. 3.
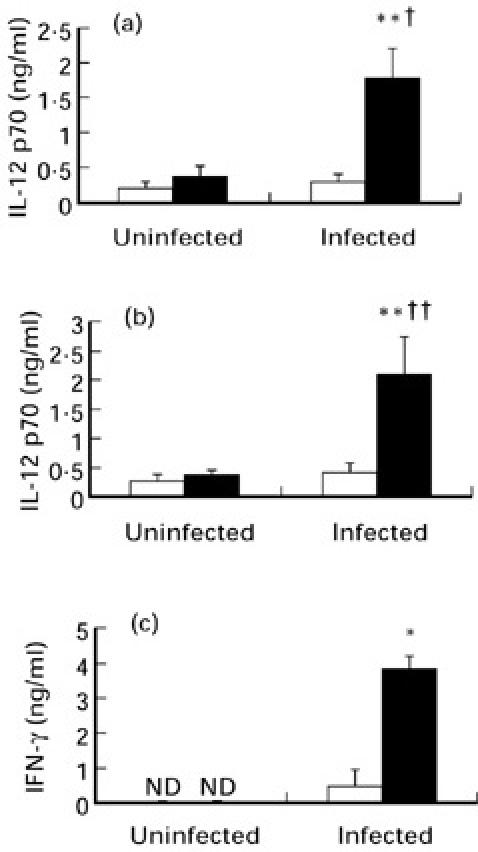
Stimulated release of IL-12 p70 in vitro by splenic macrophages recovered from uninfected controls or day 4 Plasmodium chabaudi AS-infected A/J (□) and B6 mice (▪). Cells were cultured for 24 h in the presence of 100 ng/ml of lipopolysaccharide (LPS) (a) or 106/ml of P. chabaudi AS-infected erythrocytes (PRBC) (b). IFN-γ production by unfractionated splenocytes following 48 h culture in the presence of 106/ml of PRBC (c). IL-12 p40, p70 or IFN-γ levels in culture supernatants were determined by ELISAs. Data are pooled from three replicate experiments and are presented as mean ± s.e.m. (n = 4–8). Statistically significant differences: *P < 0.05; **P < 0.01 versus infected A/J mice; †P < 0.05 and ††P < 0.01 versus uninfected B6 mice. ND, Not detectable.
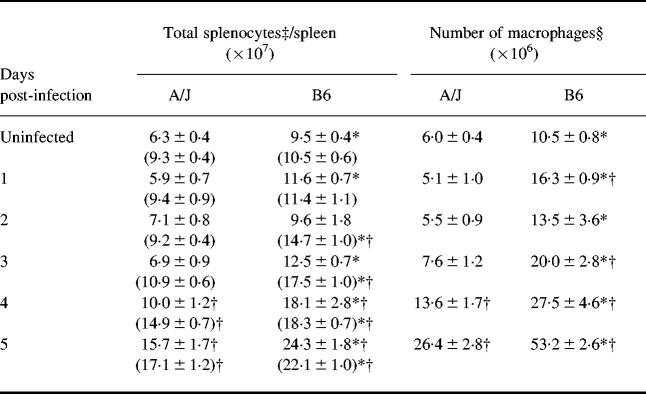
Kinetics of increases in spleen cellularity in vivo
In the results presented above, comparisons between resistant B6 and susceptible A/J hosts for IL-12 p40 and p70 synthesis by splenic macrophages were done on a per cell basis. However, following P. chabaudi AS infection, rapid and dramatic changes occurred in spleen cellularity of both mouse strains. These changes could impact significantly on total levels of IL-12 produced by splenic macrophages in vivo. In both mouse strains, spleen cell numbers were significantly higher on days 4 (P < 0.05 and P < 0.01 for A/J and B6, respectively) and 5 (P < 0.01 for both strains) post-infection compared with uninfected controls on day 0 (Table 2). However, spleen cell numbers were significantly higher in uninfected B6 compared with A/J mice (P < 0.05), and during the first 5 days of P. chabaudi AS infection, with the exception of day 2. Unlike the total splenocyte numbers, the percentage of macrophages in the spleen was not significantly different between A/J and B6 mice before P. chabaudi AS infection. Following infection, nonetheless, the percentage of splenic macrophages was significantly higher in B6 mice on days 2–3 (P < 0.001) and 4–5 (P < 0.01). Thus, resistant B6 mice show earlier increases in the percentage of macrophages in the spleen. As a result of these differences between the strains, in terms of total spleen cell numbers and percentage of macrophages, the absolute number of splenic macrophages was significantly higher in B6 mice on all days examined.
Table 2.
Changes in percentage and number of macrophages in the spleen during Plasmodium chabaudi AS infection
DISCUSSION
The development of adaptive host responses to blood-stage malaria has been shown to be dependent on the timely activation of both cellular [15,22,23,31] and humoral [32,33] immune mechanism(s). It is thought that cell-mediated mechanism(s) can result in the activation of cells of the mononuclear phagocyte lineage that are capable of destroying _Plasmodia_-infected erythrocytes [34]. In vivo depletion of monocyte/macrophages by treatment with silica- or liposome-encapsulated muramyl dipeptide-glycerol dipalmitate demonstrated that these cells play a critical role in the elimination of P. chabaudi AS infection [34]. The role of activated monocyte/macrophages in host resistance against blood-stage malaria may be mediated in part by the release of cytokines, most notably TNF-α, as well as reactive nitrogen and oxygen species. Our laboratory recently demonstrated that an early Th1-associated increase in TNF-α mRNA expression in the spleen correlates with resistance of B6 mice against P. chabaudi AS malaria [30]. Moreover, IL-12-induced protection of susceptible A/J mice was shown to occur by a mechanism involving IFN-γ, TNF-α and NO [16].
In the present study, we report significant differences between resistant B6 and susceptible A/J mice in splenic macrophage synthesis of biologically active IL-12 p70 during early blood-stage P. chabaudi AS infection. Compared with infected A/J hosts, splenic macrophages recovered from infected B6 mice produced significantly greater quantities of IL-12 p70 in vitro, both spontaneously and in response to LPS or malaria parasite stimulation. The greater release of p70 by splenic macrophages in infected B6 mice correlates with the development of early, predominantly Th1 responses and resistance of this host to this infection. In contrast, reduced p70 synthesis by splenic macrophages from infected A/J mice is consistent with the development of early, primarily Th2 responses and susceptibility to blood-stage malaria.
Since p70, but not p40, production by splenic macrophages was significantly different between the two mouse strains following P. chabaudi AS infection, our results suggest differences between the regulation of IL-12 p70 versus p40 secretion by splenic macrophages from the two mouse strains. This conclusion was suggested by the fact that whereas basal secretion of p40 could readily be detected in cells from uninfected mice of either strain, p70 production was undetectable. Although p40 mRNA levels were higher in splenic macrophages from uninfected A/J compared with B6 mice, spontaneous secretion of p40 protein was comparable in both strains. Moreover, following infection, substantial increases in p40 mRNA levels occurred in splenic macrophages from both mouse strains, yet these changes in p40 mRNA expression resulted in no significant increases in spontaneous release of p40 protein in vitro. Thus, we found no proportional relationship between the level of p40 mRNA expression and the spontaneous release of p40 protein in vitro by splenic macrophages of either strain. The expression of p35 mRNA was constitutive in cells from uninfected mice of either strain. Following infection, p35 mRNA expression was up-regulated in cells from both strains, with a substantial peak in cells from B6 but not A/J mice on day 2. This early peak in p35 mRNA expression in splenic macrophages from B6 mice might be in part responsible for the higher spontaneous release of p70 by these cells on day 4. Alternatively, P. chabaudi may induce the release of factors that suppress p70 release by splenic macrophages in A/J but not B6 mice. Further studies are necessary to elucidate these potential mechanisms that regulate p40 and p70 release.
The suggestion that p70 secretion might be much more tightly regulated than p40 release is supported by earlier studies showing a tendency for p40 to be produced in large excess over p70 levels [4,18,35]. The essential difference in IL-12 release by splenic macrophages from susceptible A/J compared with resistant B6 mice appears to be reduced p70 synthesis in the former hosts in the presence of unimpaired p40 release. The capacity for similar production of p40 protein by splenic macrophages recovered from the two mouse strains acutely infected with P. chabaudi AS was demonstrated when cells were cultured in medium alone, or in the presence of LPS or PRBC. These results are in agreement with our recent findings on the differential production of IL-12 p70 versus p40 in vivo between the two mouse strains during early blood-stage malaria [24]. We found significantly greater levels of p70 in sera from infected B6 compared with A/J mice, whereas total serum p40 levels were comparable between the two mouse strains. Production of limited quantities of p70 in P. chabaudi AS-infected A/J mice, despite normal p40 release, raises the interesting possibility that most of the IL-12 p40 synthesized might be present as p40 monomers and homodimers, forms of IL-12 shown to be capable of antagonizing the activities of heterodimeric p70 [36,37]. Thus, reduced p70 synthesis in A/J mice may further be compromised by the inhibitory actions of p40 monomers and homodimers.
In earlier studies addressing the role of IL-12 production during parasitic infections with L. major [38], Schistosoma mansoni [39], T. gondii [10], and Listeria monocytogenes [40], as well as in graft-_versus_-host disease [25], p40 mRNA and protein production were used as markers of biologically active IL-12 p70 release. However, in recent years the availability of reagents for specifically quantifying p70 levels has revealed important differences between p40 and p70 release. For example, it was recently demonstrated that Salmonella dublin LPS stimulation induced substantial production of p40 by macrophages, while p70 release was minimal [41]. In the present study, we analysed production of both p40 and p70 by splenic macrophages, and to our knowledge this is the first study to demonstrate significant differences between resistant and susceptible mouse strains in splenic macrophage IL-12 p70 release during early blood-stage malaria.
Comparisons between A/J and B6 mice for IL-12 production by splenic macrophages were done on a per cell basis. The percentage of macrophages in unfractionated spleen cells was not significantly different in uninfected controls of either strain, even though B6 mice had higher numbers of splenocytes per spleen. Following P. chabaudi AS infection, the percentage of macrophages in the spleen was significantly higher in B6 compared with A/J mice on days 2–5, in agreement with our previous observation of a higher percentage of Mac-1+ cells in the spleens of B6 mice on day 6 post-infection [42]. A more severe depletion of marginal metallophilic macrophages in A/J versus B6 mice may be in part responsible for these differences [43]. The recruitment of higher numbers of macrophages to the spleens of B6 mice early in infection could further augment the differences between the strains in splenic macrophage-derived IL-12 levels in vivo.
Taken together, the results of the present study suggest an important role of macrophage-derived IL-12 in host resistance to blood-stage P. chabaudi AS malaria. In addition to aiding in the destruction of _Plasmodia_-infected erythrocytes, splenic macrophages, through IL-12 production, may be crucial in shaping the development of an appropriate Th phenotype during early blood-stage malaria. In turn, which Th phenotype is activated has vital consequences for host resistance or susceptibility to this infection.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Mifong Tam in setting up IL-12 ELISAs and Krikor Kichian for help with RT-PCR set up. This work was supported by NIH Grant (AI 35955) and MRC Grants (MT 12638 and MT 14663). H.S. is a recipient of a MD/PhD studentship from MRC.
REFERENCES
- 1.Chehimi J, Trinchieri G. Interleukin-12: a bridge between innate resistance and adaptive immunity with a role in infection and acquired immunodeficiency. J Clin Immunol. 1994;14:149–61. doi: 10.1007/BF01533364. [DOI] [PubMed] [Google Scholar]
- 2.Manetti R, Parronchi P, Giudizi MG, Piccinni M-P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper Type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rude E, Germann T. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-γ and is inhibited by transforming growth factor-β. Eur J Immunol. 1994;24:793–8. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 4.Schoenhaut DS, Chua AO, Wolitzky AG, et al. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–40. [PubMed] [Google Scholar]
- 5.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–81. [PubMed] [Google Scholar]
- 6.Gubler U, Chua AO, Schoenhaut DS, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–7. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson MM, Tam MF, Nowotarski M. Role of interferon-γ and tumor necrosis factor in host resistance to Plasmodium chabaudi AS. Immunol Letters. 1990;25:115–21. doi: 10.1016/0165-2478(90)90101-u. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson MM, Tam MF, Belosevic M, van der Meide PH, Podoba JE. Role of endogenous gamma interferon in host response to infection with blood-stage Plasmodium chabaudi AS. Infect Immun. 1990;58:3225–32. doi: 10.1128/iai.58.10.3225-3232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–43. [PubMed] [Google Scholar]
- 11.Stevenson MM, Tam M-F. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin Exp Immunol. 1993;92:77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor-Robinson AW, Phillips SR, Severn A, Moncada S, Liew FY. The role of Th1 and Th2 cells in a rodent malaria infection. Science. 1993;260:1931–4. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 13.Langhorne J, Gillard S, Simon B, Slade S, Eichmann K. Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int Immunol. 1989;1:416–24. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- 14.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–9. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yap GS, Jacobs P, Stevenson MM. Th cell regulation of host resistance to blood-stage Plasmodium chabaudi AS. Res Immunol. 1994;145:419–22. doi: 10.1016/s0923-2494(94)80171-1. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson MM, Tam MF, Wolf SF, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–56. [PubMed] [Google Scholar]
- 17.Heufler C, Koch F, Stanzl U, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper 1 cells. Eur J Immunol. 1996;26:659–68. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 18.Cassatella MA, Meda L, Gasperini S, D'andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 19.Romani L, Mencacci A, Cenci E, et al. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–56. [PubMed] [Google Scholar]
- 20.Aloisi F, Penna G, Cerase J, Menendez Iglesias B, Adorini L. IL-12 production by central nervous system microglia is inhibited by astrocytes. J Immunol. 1997;159:1604–12. [PubMed] [Google Scholar]
- 21.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Ann Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 22.Podoba JE, Stevenson MM. CD4+ and CD8+ T lymphocytes both contribute to acquired immunity to blood-stage Plasmodium chabaudi AS. Infect Immun. 1991;59:51–58. doi: 10.1128/iai.59.1.51-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson MM, Huang DY, Podoba JE, Notowotarski ME. Macrophage activation during Plasmodium chabaudi AS infection in resistant C57BL/6 and susceptible A/J mice. Infect Immun. 1992;60:1193–201. doi: 10.1128/iai.60.3.1193-1201.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sam H, Stevenson MM. In vivo IL-12 production and IL-12 receptors β1 and β2 mRNA expression in the spleen are differentially up-regulated in resistant B6 and susceptible A/J mice during early blood-stage Plasmodium chabaudi AS malaria. J Immunol. 1999;162:1582–9. [PubMed] [Google Scholar]
- 25.Kichian K, Nestel FP, Kim D, Ponka P, Lapp WS. IL-12 p40 messenger RNA expression in target organs during acute graft-versus-host disease: possible involvement of IFN-γ. J Immunol. 1996;157:2851–6. [PubMed] [Google Scholar]
- 26.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–9. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skeen MJ, Miller MA, Shinnick TM, Ziegler HK. Regulation of murine macrophage IL-12 production. Activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J Immunol. 1996;156:1196–206. [PubMed] [Google Scholar]
- 28.Heinzel FP, Rerko RM, Ling P, Hakimi J, Schoenhaut DS. Interleukin 12 is produced in vivo during endotoxemia and stimulates synthesis of gamma interferon. Infect Immun. 1994;62:4244–9. doi: 10.1128/iai.62.10.4244-4249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladel CH, Szalay G, Riedel D, Kaufmann SH. Interleukin-12 secretion by Mycobacterium tuberculosis-infected macrophages. Infect Immun. 1997;65:1936–8. doi: 10.1128/iai.65.5.1936-1938.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs P, Radzioch D, Stevenson MM. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–41. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langhorne J, Simon-Haarhaus B, Meding SJ. The role of CD4+ T cells in the protective immune response to Plasmodium chabaudi in vivo. Immunol Letters. 1990;25:101–7. doi: 10.1016/0165-2478(90)90099-c. [DOI] [PubMed] [Google Scholar]
- 32.von der Weid T, Honarvar N, Langhorne J. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J Immunol. 1996;156:2510–6. [PubMed] [Google Scholar]
- 33.Yap GS, Stevenson MM. Differential requirements for an intact spleen in induction and expression of B-cell-dependent immunity to Plasmodium chabaudi AS. Infect Immun. 1994;62:4219–25. doi: 10.1128/iai.62.10.4219-4225.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevenson MM, Ghadirian E, Phillips NC, Rae D, Podoba JE. Role of mononuclear phagocytes in elimination of Plasmodium chabaudi AS infection. Parasite Immunol. 1989;11:529–44. doi: 10.1111/j.1365-3024.1989.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 35.Stern AS, Podlaski FJ, Hulmes JD, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87:6808–12. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattner F, Fischer S, Gukes S, Jin S, Kaulen H, Schmitt E, Rude E, Germann T. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur J Immunol. 1993;23:2202–8. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 37.Heinzel FP, Hujer AM, Ahmed FN, Rerko RM. In vivo production and function of IL-12 p40 homodimers. J Immunol. 1997;158:4381–8. [PubMed] [Google Scholar]
- 38.Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994;179:447–56. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–61. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W, Kurlander RJ. Analysis of the interrelationship between IL-12, TNF-α, and IFN-γ production during murine listeriosis. Cell Immunol. 1995;163:260–7. doi: 10.1006/cimm.1995.1125. [DOI] [PubMed] [Google Scholar]
- 41.Bost KL, Clements JD. Intracellular Salmonella dublin induces substantial secretion of the 40-kilodalton subunit of interleukin-12 (IL-12) but minimal secretion of IL-12 as a 70-kilodalton protein in murine macrophages. Infect Immun. 1997;65:3186–92. doi: 10.1128/iai.65.8.3186-3192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohan K, Moulin P, Stevenson MM. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J Immunol. 1997;159:4990–8. [PubMed] [Google Scholar]
- 43.Stevenson MM, Kraal G. Histological changes in the spleen and liver of C57BL/6 and A/J mice during Plasmodium chabaudi AS infection. Exp Mol Pathol. 1989;51:80–95. doi: 10.1016/0014-4800(89)90009-9. [DOI] [PubMed] [Google Scholar]