Effect of IL-5, glucocorticoid, and Fas ligation on Bcl-2 homologue expression and caspase activation in circulating human eosinophils (original) (raw)
Abstract
IL-5 is a potent eosinophil viability-enhancing factor that has been strongly implicated in the pathogenesis of IgE-mediated inflammation in vivo. Recently published data have suggested that IL-5 (and related cytokines) may act by altering the expression of the anti-apoptotic regulator Bcl-2 or its homologues, but this is controversial. The behaviour of the recently described pro-apoptotic cysteine proteases (caspases) in eosinophils after IL-5 treatment has not been explored. We examined the effect of IL-5 on the expression of four major Bcl-2 homologues, as well as on the expression/activation of key members of the caspase cell death cascade in cultured circulating human eosinophils. The effect of relevant inducers of eosinophil apoptosis (glucocorticoid and Fas ligation) on these regulatory proteins was also examined. We observed baseline expression of the anti-apoptotic Mcl-1 and pro-apoptotic Bax proteins in immunoblots of eosinophil lysates, but not Bcl-x, Bcl-2. IL-5 treatment had the effect of maintaining this basal level of expression over time without altering the balance of Bcl-2 homologues. The (upstream) caspase 8 and (downstream) caspase 3 proenzymes were detected in eosinophils at baseline, and were processed during spontaneous and stimulated eosinophil death. IL-5 completely blocked caspase processing in spontaneous and dexamethasone-induced cell death, and significantly slowed processing during Fas ligation. Our data do not support the theory that IL-5 acts by altering the balance of anti-apoptotic and pro-apoptotic Bcl-2 homologues, but suggest that it may act by regulating activation of the caspase cell death cascade.
Keywords: eosinophil, IL-5, caspase, Bcl-2, Fas
INTRODUCTION
Recent observations that circulating human eosinophils may occasionally synthesize Bcl-2 protein have prompted a number of investigators to examine the regulation of this important anti-apoptotic protein and its homologues in the context of known eosinophil viability-enhancing factors [1–5]. As mature eosinophils do not divide, the regulation of their viability must be a critical determinant of the duration and severity of eosinophilic inflammation. At this point, the current literature is conflicted regarding the effects of IL-5 on Bcl-2, which leave open the question of whether this cytokine truly acts by inducing (or suppressing) specific Bcl-2 homologues. Since eosinophil viability-enhancing factors, particularly IL-5, have been strongly implicated in the pathogenesis of asthma and rhinitis, a clear understanding of their target genes is of major importance.
IL-5 is the prototypic eosinophil viability-enhancing factor, and there is substantial evidence that IL-5 represents a major eosinophil viability-enhancing activity _in vivo_[6–8]. IL-5 is now known to extend eosinophil survival through inhibition of programmed cell death [8–10]. Although certain components of the IL-5 signal transduction pathway have recently been elucidated (reviewed in [11]), the identity of the downstream death effector molecules that IL-5 regulates remains unknown. Potential targets for regulation include members of the Bcl-2 proto-oncogene family as well as members of the apoptotic cysteine protease (caspase) cascade. Bcl-2 was one of the first well-characterized repressors of apoptosis. Its pro-survival activity appears to be modulated by direct interaction with certain of its pro-apoptotic homologues (e.g. bax, bad, and others) such that the balance of these proteins affects whether a cell lives or dies ([12,13], reviewed in [14]) A number of other homologues, including Bcl-xL and Mcl-1, also act as death repressors [15,16].
Caspases are structurally related aspartate-specific cysteine proteases that exist in normal cells as inactive zymogens. They appear to serve as a final common pathway for various apoptotic triggers. More than 10 homologues have now been cloned; the enzymology and behaviour of some of them have been partially characterized in in vitro systems [17]. However, the distribution and regulation of different caspases in mammalian cells is unclear. Caspase activation implies that the zymogen is specifically cleaved to yield the large and small subunits that make up the functional enzyme. Because specific caspases are capable of activating others, it has been proposed that they operate in a cascade, with certain members responsible for upstream (initiator) functions and others performing downstream (effector) functions. Activation of this cascade ultimately results in the cleavage of substrates essential for cell viability [18,19]. Direct activation of the pathway via the ‘upstream’ caspases can be initiated by the engagement of specific cell surface receptors such as Fas [20]. There are few published data regarding the effects of IL-5 on the activation of the caspase cascade.
In light of the controversy in the literature surrounding IL-5 and Bcl-2 expression, we submit the results of our investigation on the effects of IL-5 on Bcl-2 homologue expression, as well as caspase activation in human peripheral blood eosinophils. Relevant inducers of eosinophil apoptosis, including treatment with glucocorticoid and Fas receptor ligation, were also examined in this context [21]. While Fas ligation is believed to result in direct activation of pre-existing caspases, glucocorticoid hormone, after combining with its cytoplasmic receptor, translocates to the nucleus and acts by regulation of gene transcription. Like IL-5, the identity of the pro-apoptotic genes induced (or suppressed) by glucocorticoid hormones during apoptosis remains speculative. The anti-apoptotic homologues Bcl-2, Bcl-x, Mcl-1, and the pro-apoptotic homologue Bax were chosen for study, as they are reasonably well characterized. Caspase 8 (FLICE/Mch5/MACH) and caspase 3 (CPP32/YAMA/apopain) were chosen as representative upstream and downstream markers of cascade activation, respectively [18,20,22,23]. Caspase 8 is considered to be ‘upstream’ as it can be directly activated during Fas ligation by its ability to associate with the cytoplasmic portion of the Fas receptor via the FADD adapter molecule [20]. Furthermore, it is capable of specific cleavage/activation of caspase 3, as well as other caspases [22]. Caspase 3 is considered ‘downstream’, as it has been shown to cleave several substrates critical to cell viability, as well as activate other caspases (amplification) [18,19,24].
We found that while IL-5 maintained basal protein expression in cultured eosinophils, there was no detectable effect of IL-5 on the balance of the Bcl-2 homologues studied in cultured peripheral blood eosinophils. However, IL-5 treatment blocked spontaneous and glucocorticoid-induced caspase activation, and interfered with directed caspase activation via Fas ligation.
MATERIALS and METHODS
Isolation of peripheral blood eosinophils
Circulating human eosinophils were obtained from both healthy non-allergic and asymptomatic allergic asthmatic volunteers the method of Hansel _et al._[25]. Briefly, erythrocytes were removed from whole blood by dextran sedimentation (one part 6% dextran (70 000 mol. wt; Sigma, St Louis, MO) in saline to four parts whole blood for 1 h), and the resultant leucocytes centrifuged over Percoll (Pharmacia, Uppsala, Sweden) adjusted to 1·085 g/ml at 1200 g for 20 min. The granulocyte layer was removed, and remaining erythrocytes subjected to hypotonic lysis (30 s in water, on ice). Granulocytes were depleted of neutrophils by incubation with anti-CD16 immunomagnetic beads (Miltenyi Biotech; 25 μl/50 × 106 neutrophils at 4°C for 30 min) and passaged through a magnetic column. The final purity and viability of the eosinophils in the eluate were always > 97% and 98%, respectively.
Cell culture and determination of viability
Eosinophils were cultured in RPMI containing 10% fetal calf serum (FCS) and 1% penicillin/streptomycin (Gibco, Grand Island, NY). Recombinant human cytokines were purchased from R&D Systems (Chicago, IL). Viability was determined by trypan blue exclusion, and apoptosis was assessed in cytospins by morphology and in situ fluorescence labelling of free 3’ DNA ends using the terminal deoxynucleotidyl transferase incorporation of biotinylated deoxyuridine (TUNEL) assay exactly as described in the ApopTag Plus kit (Oncor, Gaithersburg, MD).
Immunoblot analysis
For analysis of protein expression, eosinophils were resuspended at 0·5–1 × 106 cells/ml (depending on the final yield of cells from a particular subject’s blood) and cultured in 24-well plates at 1 ml/well. The cells were harvested at the designated time points, pelleted, and lysed by boiling in 80 μl of SDS-sample buffer and stored at −80°C until use. For immunoblot analysis, 20-μl aliquots of each sample were fractionated over 13% SDS–polyacrylamide minigels. As circulating eosinophils do not divide, and every well was seeded from the same master suspension, equal cell loading within an experiment was assured. Preliminary studies revealed that a 20-μl aliquot typically contained between 10 and 20 μg of total protein depending upon the concentration of the initial cell suspension. The separated proteins were transferred electrophoretically to nitrocellulose membranes, blocked using 10% non-fat dry milk in PBS−0·05% Tween 20, and probed with specific antibody. Primary antibodies were all used at dilutions of 1:300, and included monoclonal Bcl-2 and polyclonal Mcl-1 from PharMingen (San Diego, CA); polyclonal Bcl-x (recognizing both long and short forms) and Bax, and monoclonal caspase 3 from Santa Cruz Biotechnology (Santa Cruz, CA); and monoclonal caspase 8 from Oncogene Research Products (Cambridge, MA). Antibody binding was detected using 1:2500 dilution of anti-rabbit or anti-mouse horseradish peroxidase-linked whole antibody (Amersham Life Science, Arlington Heights, IL) and imaged on film using a chemiluminescent substrate (ECL Western blotting detection reagent; Amersham). Densitometry was performed using a Personal Densitometer and Image Quant software (Molecular Dynamics, Sunnyvale, CA). Lysates of human peripheral blood mononuclear cells (PBMC), Jurkat T cells or the human eosinophil leukaemia cell line AML14.3D10 (kindly supplied by Dr C. Paul, Department of Veterans Affairs Medical Center, Dayton, OH) were used as positive controls.
Statistical analysis
Differences between experimental arms were compared using two-factor anova and the paired _t_-test.
RESULTS
The effect of IL-5, glucocorticoid and Fas engagement on survival of human peripheral blood eosinophils in culture
For the purposes of this study and to introduce the basic experimental design, the known viability effects of IL-5, dexamethasone and Fas receptor ligation on human peripheral blood eosinophils are demonstrated. Also, the effect of IL-5 on dexamethasone or Fas-mediated cell death was examined. Eosinophils were purified from the blood of ragweed-allergic asthmatic individuals as well as non-allergic healthy subjects. To avoid (to the extent possible) potential activating effects by exogenous allergen, blood from allergic individuals was obtained out of ragweed season at a time when they were asymptomatic. Cells were resuspended in media and seeded in the presence of IL-5 (1 ng/ml), dexamethasone (1 μm), anti-Fas IgM (250 ng/ml), or media alone. Anti-Fas IgM acts as a Fas receptor agonist, mimicking the effect observed after engagement with natural Fas ligand [26,27]. The concentrations were chosen based on our own titration and numerous published data to induce a maximum anti-apoptotic and pro-apoptotic response. Cells were harvested at t = 0, 24, 48 and 72 h and viability assessed by trypan blue exclusion, and apoptosis by morphology and TUNEL assay. The results are pooled for five sequential subjects (two normal and three asthmatic).
Culture in media alone resulted in gradual cell death that was blocked by addition of IL-5 (Fig. 1a). IL-5 acted by inhibiting apoptosis in cultured eosinophils (Fig. 1b). Glucocorticoid treatment and Fas engagement significantly accelerated the rate of eosinophil death compared with media alone (P < 0·05). Although there was a tendency towards more rapid cell death with anti-Fas compared with dexamethasone, this was not significant. IL-5 treatment completely blocked dexamethasone-induced cell death. Interestingly, the combination of IL-5 and dexamethasone appeared to result in higher viability than IL-5 alone (not significant), a phenomenon that we have observed previously (Zangrilli, unpublished data). IL-5 did not block cell death induced by Fas engagement, but it slowed the rate of cell death significantly (e.g. 54 ± 5·5% versus 9·6 ± 2·3% at 48 h, and 37·6 ± 6·6% versus 0·8 ± 0·4% at 72 h for IL-5 plus anti-Fas versus anti-Fas alone; P < 0·05).
Fig. 1.
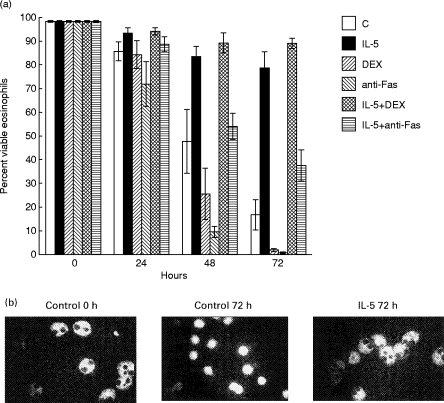
Effect of IL-5, dexamethasone and Fas activation on viability and apoptosis in cultured peripheral blood eosinophils. Cells were cultured in media alone (control), or in the presence of 1 ng/ml IL-5, 1 μm dexamethasone (DEX), or 250 ng/ml anti-Fas IgM. The combinations of IL-5 and DEX, and IL-5 and anti-Fas were also examined. (a) The percent surviving cells over time as determined by trypan exclusion is represented graphically (data are mean ± s.e.m. for three subjects). (b) Cytospins from the beginning (t = 0 h) and end (t = 72 h) of the experiment were stained by the terminal deoxynucleotidyl transferase incorporation of biotinylated deoxyuridine (TUNEL) technique. The control and IL-5 arms for a representative experiment are shown. Apoptotic cells display marked shrinkage and condensation of their cytoplasm and are brightly fluorescent indicating DNA cleavage (e.g. control at 72 h). In contrast, viable eosinophils possess abundant, mildly autofluorescent, granular cytoplasm against which their bilobed nuclei appear black, indicating intact DNA (e.g. control at 0 h, and IL-5-treated at 72 h). The appearance of the DEX and anti-Fas-treated cells was essentially identical to control cells by 72 h (not shown).
Morphologic and biochemical indices of apoptosis occurred first, followed subsequently by loss of viability as detected by trypan blue exclusion (data not shown). As these events are intimately related, and trypan blue exclusion is simpler to perform and quantify for multiple samples than TUNEL assays, viability by trypan exclusion is referred to henceforth for convenience.
Expression of Bcl-2 family members by peripheral blood eosinophils: effect of IL-5 and glucocorticoid
To determine whether the anti-apoptotic effect of IL-5, or the pro-apoptotic effect of glucocorticoid, could be attributed to changes in the expression of Bcl-2 family members, peripheral blood eosinophils were cultured with IL-5 (1 ng/ml), dexamethasone (1 μm), or media control. Anti-Fas was not examined in this arm as current evidence indicates that it acts via direct recruitment and activation of initiator caspase(s) to the Fas receptor, and not by regulating expression of cell death genes. Cell lysates were examined by immunoblot at t = 0 h, t = 24 h, t = 48 h, and t = 72 h for expression of the anti-apoptotic species Bcl-2, Bcl-x, and Mcl-1, and the pro-apoptotic species Bax. Figure 2 demonstrates the ability of the antibodies employed to detect the proteins of interest in control cell lysates (PBMC or the eosinophilic leukaemia cell line AML14.3D10). The complete blots for one subject are shown in Fig. 3a, and are absolutely representative of the two non-asthmatic and three ragweed-allergic subjects studied. We did not detect expression of the anti-apoptotic species Bcl-2 or Bcl-x at baseline in any subject, while a signal corresponding to Mcl-1 protein was detected in all of the subjects. IL-5 did not induce Bcl-2 or Bcl-x protein expression with time, and had the effect of maintaining Mcl-1 at basal levels. The pro-apoptotic species Bax was strongly expressed at baseline in all samples tested; IL-5 had the effect of maintaining Bax at basal levels without evidence of down-regulation. Dexamethasone did not induce Bax, with the signals for both Bax and Mcl-1 declining over time in dying cells (control and dexamethasone), which probably represents gradual proteolysis.
Fig. 2.
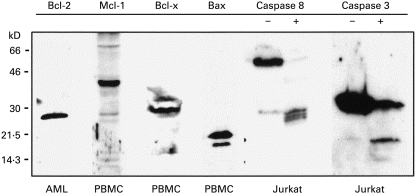
Antibody sensitivities. The ability of the antibodies used in this study to detect the Bcl-2 homologues of interest is demonstrated in lysates of peripheral blood mononuclear cells (PBMC) or AML.3D10 cells (an eosinophil leukaemia cell line). The ability of the caspase 8 and caspase 3 antibodies to detect their respective proenzymes and cleavage products is demonstrated for Jurkat T cells cultured with (+) or without (–) anti-Fas antibody for 24 h. Bcl-2 ran as a single band at 26 kD. Mcl-1 ran around 40 kD, and was often detected as a doublet or with adjacent minor bands. Bcl-xL (long) ran around 29 kD; Bcl-xS (short) was not detected. Bax ran at 21 kD and was usually detected as a doublet. Procaspase-8 was detected around 55 kD (actually a tight doublet of the α and β isoforms) with large subunit cleavage intermediates detected around 43 kD and, more prominently, at 28 kD. Pro-caspase 3 was detected at 32 kD with its large subunit cleavage product running at 17 kD.
Fig. 3.
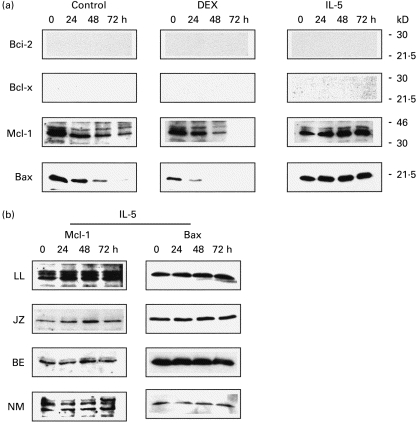
Effect of IL-5 and dexamethasone on Bcl-2 homologue expression in peripheral blood eosinophils. Cells were cultured in the presence of IL-5 (1 ng/ml), dexamethasone (DEX, 1 μm), or media (control) and harvested at the time points shown. Expression of the anti-apoptotic proteins Bcl-2, Mcl-1, and Bcl-x, as well as the pro-apoptotic protein Bax, was examined by immunoblot. (a) The complete results for one asthmatic subject are shown: these blots are completely representative of those obtained in the remaining four subjects, with only Mcl-1 and Bax being detected in eosinophil cell lysates at baseline or during the specific treatment arms. Neither Bcl-2 nor Bcl-x was detected in eosinophils from any subject at baseline or after treatment with IL-5 or DEX. (b) The consistency of these findings is demonstrated in four additional subjects for the IL-5 arm (two asthmatic and two non-asthmatic; letters identify individual subjects).
Figure 3b shows the IL-5 arm from the four additional subjects (two asthmatic and two non-asthmatic) and demonstrates the consistency of Bax and Mcl-1 expression in mature eosinophils, and the effect of IL-5 in maintaining (but not altering) their basal levels over time. Again, induction of Bcl-2 or Bcl-x by IL-5 was not observed in any subject (data not shown). Similar results were obtained using granulocyte-macrophage colony-stimulating factor (GM-CSF) at the same concentration, as well as GM-CSF and IL-5 in combination (data not shown).
Effects of IL-5 on activation of the caspase death cascade in eosinophils
Because IL-5 strongly inhibited eosinophil apoptosis without altering the level of expression of the major Bcl-2 family members examined, we hypothesized that it may act instead by modulating the expression or activation of key members of the caspase cell death cascade. We utilized the upstream initiator, caspase 8, and the downstream effector, caspase 3, as experimental endpoints because their activities are among the best characterized and are likely to span a physiologically relevant portion of the cascade. Figure 2 demonstrates the reactivity of the anti-caspase 8 antibody used towards the proenzyme (around 55 kD) and several of its large subunit cleavage intermediates in cells undergoing apoptosis (typically seen at 43 kD and 28 kD); this antibody recognizes both α and β forms of the proenzyme [28,29]. The caspase 3 antibody detects both the 32-kD proenzyme and the 17-kD (large subunit) cleavage product. Peripheral blood eosinophils were cultured with IL-5 (1 ng/ml), dexamethasone (1 μm), anti-Fas IgM (250 ng/ml) or media alone, or with IL-5 in combination with dexamethasone or anti-Fas. Cells were harvested at t = 0, 24 h, 48 h and 72 h. Four separate experiments were performed (two asthmatic and two non-asthmatic subjects); the representative immunoblots for one subject are shown (Fig. 4a). The mean band densities for pro-caspase 8 and pro-caspase 3 for four subjects were calculated, and the rate of caspase activation is represented as percent of baseline signal (Fig. 4b,c).
Fig. 4.
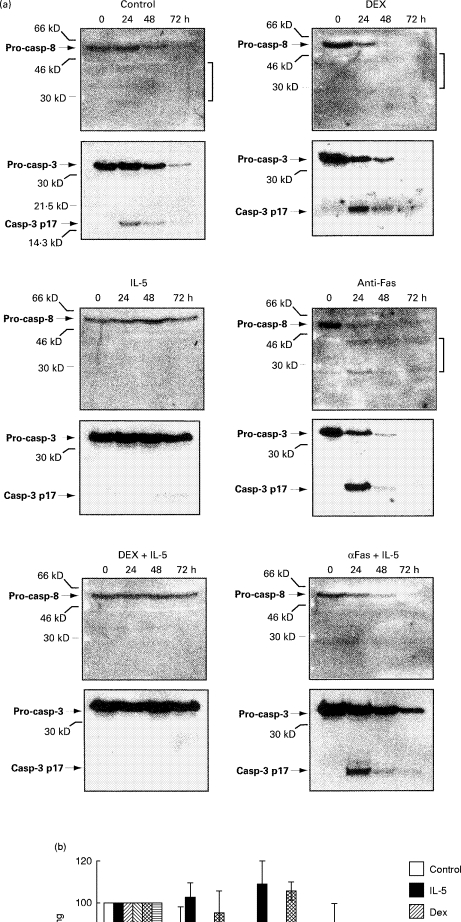
Effects of IL-5, dexamethasone (DEX), or anti-Fas-IgM on expression and activation of caspase 3 and caspase 8 in eosinophils. Peripheral blood eosinophils were cultured in the presence of IL-5 (1 ng/ml), DEX (1 μm), or anti-Fas-IgM (250 ng/ml), or with combinations of IL-5 and DEX or IL-5 and anti-Fas, and harvested at the time points indicated. Immunoblots of the cell lysates were probed first for caspase 8, then stripped and reprobed for caspase 3. The bands representing caspase-8 and 3 are aligned for comparison, according to the experimental time points. (a) Immunoblots for a single subject are shown, and are representative of four separate experiments (two asthmatic and two non-asthmatic subjects). Brackets point to processed intermediates of the caspase 8 large subunit. Pro-casp, Caspase proenzyme; casp-3 p17, the large caspase 3 subunit generated by cleavage of the proenzyme. (b,c) Rate of activation of caspase 8 and caspase 3, respectively, represented as the disappearance of the pro-caspase over time. Band intensities were determined at each time point by densitometry and normalized to baseline (i.e. t = 0:100%). Graphic depicts mean data ± s.e.m. for four subjects.
We observed presence of the caspase 3 and 8 proenzymes in all of the eosinophil samples tested. Activation of the caspase proenzymes was apparent during spontaneous (media alone) and stimulated (dexamethasone or anti-Fas) apoptosis as evidenced by the disappearance of the proenzyme forms over time, with the concomitant appearance of the specific cleavage products. The large subunit intermediates for caspase 8 were present, but tended to be faint during spontaneous and dexamethasone-induced cell death compared with the more vigorous cleavage detected during Fas activation. The rate of disappearance of caspase 8 and caspase 3 proenzymes corresponded closely with the actual rate of cell death (Fig. 1a). Processing of caspase 8 and 3 was obviously more rapid during stimulated cell death compared with media alone, although this did not reach statistical significance, probably due to intersubject variability and a small n (Fig. 4b,c). Of note, IL-5 largely blocked dexamethasone-induced caspase activation. In contrast, Fas-induced caspase activation proceeded in the presence of IL-5, but more slowly. This inhibition was less pronounced for caspase 8 (significant at 72 h only) but marked for caspase 3 (e.g. 66 ± 3·6% versus 8·6 ± 5·7% at 48 h, and 46·7 ± 11% versus 0·8 ± 0·27% at 72 h for IL-5 plus anti-Fas versus anti-Fas alone; P > 0·05).
DISCUSSION
There now exists extensive in vitro and in vivo evidence that the Bcl-2 family of proto-oncogenes is intimately involved in the regulation of programmed cell death. Although their mechanism of action has not been fully elucidated, it appears to depend on the ability of the pro-apoptotic homologues to counter the activity of the anti-apoptotic homologues by direct association [30]. One leading, but evolving, theory regarding the mechanism is that oligomerization of the integral membrane protein Bax is toxic to the cell, possibly by altering mitochondrial membrane permeability. The anti-apoptotic homologues (i.e. Bcl-2, Bcl-x, or Mcl-1) may block this process by associating with Bax so that toxic homodimers are disrupted and apoptosis abrogated [12,31]. Similar results have been observed with various other pro-apoptotic members, which appear to antagonize the mitochondrial protective effect of the pro-survival proteins by direct interaction via their BH-3 domain [14,32]. Thus, the balance of these proteins should be important in determining whether or not a cell will enter the programmed cell death pathway. Accordingly, one might hypothesize that the Bcl-2 homologues are key targets for regulation by growth factor/viability-enhancing factor activities.
In these experiments, we examined the expression and activation of key members of the caspase cell death pathway and Bcl-2 homologues in circulating human eosinophils during IL-5 stimulation or during specific apoptotic stressors. Of the three major anti-apoptotic Bcl-2 family members examined, only Mcl-1 protein was consistently detected in both normal and allergic subjects. IL-5 treatment had the effect of maintaining Mcl-1 at basal levels. We found no detectable Bcl-2 or Bcl-x protein expression at baseline or evidence of its induction after 3 days of IL-5 treatment, despite the fact that apoptosis was strongly inhibited. The pro-apoptotic homologue Bax was demonstrated at baseline in all samples. Similar to Mcl-1, IL-5 appeared to maintain Bax protein at its basal levels (and did not suppress it, as one might have hypothesized). For eosinophils, the media control arm of these experiments is analogous to factor withdrawal. In dying control cells, the signal for the two detectable Bcl-2 homologues (Bax and Mcl-1) gradually disappeared over the 3-day time course, an event that was accelerated in the presence of dexamethasone and probably reflects generalized proteolysis in dying cells. Of note, it has been reported that certain anti-apoptotic homologues (including Bcl-2 and Bcl-x) are specifically cleaved during apoptosis by caspases, which theoretically serves to accelerate the process [33,34].
Our results are consistent with studies by Druilhe et al. who observed trace but inconsistent Bcl-2 protein expression by peripheral blood eosinophils, no Bcl-x expression, basal Mcl-1 expression, and no effect of IL-5 on any of these proteins [2]. Similarly, Dibbert et al. failed to observe significant Bcl-2 gene or protein expression in mature eosinophils by reverse transcriptase-polymerase chain reaction (RT-PCR) either at baseline or after IL-5 or GM-CSF stimulation [4]. They did however detect basal Bcl-x gene expression that was maintained over time in the presence of these eosinophil-active cytokines. The Bcl-x protein was also demonstrated, but at apparently low levels as it was visualized only by immunoprecipitation or immunostaining of cells. In contrast, Ochiai et al. presented data suggesting that a dose–response relationship exists between IL-5 and Bcl-2 protein synthesis in mature eosinophils [3]. They did not show the actual immunoblots for review, but provided mean data. More recently, Dewson et al. reported induction of Bcl-2 gene expression by RT-PCR, and Bcl-2 protein expression by in situ cytokine staining using flow cytometry [5]. Interestingly, anti-sense oligonucleotides to Bcl-2 or Bcl-x appeared partially to attenuate the eosinophil viability-enhancing activity of IL-5 [3,4].
Since eosinophil apoptosis was strongly inhibited by IL-5 at the relatively low concentrations used in our experiments without effecting any detectable change from the basal pattern of Bcl-2 homologue expression over time, we conclude that the expression of the major Bcl-2 homologues studied is not directly regulated by IL-5. Rather, the maintenance of the basal levels of these proteins observed after IL-5 treatment is probably non-specific, and simply related to the fact that IL-5 is keeping the cells alive. The positive studies reported above warrant some comment since they conflict with our findings and those of other investigators. As basal Bcl-2 protein expression has been reported in the literature, and seen by us on occasion (Zangrilli, unpublished observations), we feel that it is likely that human eosinophils do express tiny amounts of this apoptotic regulator. Accepted, minimal criteria for ascribing cause and effect for a cytokine activity and specific protein induction would be the demonstration of a quantitative and reproducible dose and time response for the protein of interest. There are several factors regarding the methodology and study design for the positive studies that may be problematic. One is the use of non-quantitative or semiquantitative techniques to analyse gene and protein expression. Also, there was a tendency to compare protein expression between cells cultured in media and those cultured in IL-5. Under such circumstances a significant difference in the level of protein expression in general (Bcl-2 or otherwise) would be expected as apoptosing control cells were compared with viable ones. The anti-sense data observed by others do support a role for Bcl-2 and Bcl-x in the maintenance of eosinophil viability. Again though, this effect may be independent of IL-5 mechanism, and the small attenuation in viability observed with these reagents goes against Bcl-2 or Bcl-x being major downstream effectors of IL-5. Immunoblotting with chemiluminescence detection utilized in our study is a sensitive technique for analysis of protein expression, but it is possible that the proteins of interest are below the level of detection. Then however the significance of tiny changes in protein expression would need to be questioned in light of the current models of Bcl-2 mechanism which are based on direct protein–protein interactions, and where stoichiometry should be important. We have not ruled out the possibility that IL-5 might regulate the expression of an as yet unexamined member of the Bcl-2 family. Nor have we ruled out the possibility that IL-5 regulates activity of Bcl-2 family members rather than their level of expression.
We next examined the anti-apoptotic effects of IL-5 in the context of caspase expression and activation in eosinophils. Data derived from in vitro engineered systems, where the individual components can be controlled, implicate caspase 8 and 3 in a physiological cell death cascade in which activated caspase 8 processes caspase 3 to its active form [22,35]. Yet extensive in vivo validation is lacking, and caspase expression and behaviour in eosinophils undergoing apoptosis have not been explored. We were able to demonstrate basal expression of caspase 3 and caspase 8 in mature eosinophils, and activation during apoptosis. Activation rather than non-specific proteolysis is supported based on the detection of known cleavage products for both caspases. There was a tendency for caspase 8 to be processed faster than the caspase 3 initially, tending to support its presumed apical role in the cascade, but this was not significant. Rather unexpectedly, the rate of caspase 8 and 3 processing was not significantly different between direct Fas activation and dexamethasone-induced apoptosis. This, plus the fact that caspase 8 was activated even during spontaneous apoptosis, suggests that there is feedback from downstream caspases. The mechanism behind this kind of non-receptor-mediated caspase activation during spontaneous or glucocorticoid-induced apoptosis in eosinophils is unknown, but may relate to the recently described phenomenon of cytochrome c release from mitochondria with resultant activation of caspase 9 [36].
IL-5 treatment strongly inhibited spontaneous and glucocorticoid-mediated eosinophil death, and prevented caspase 8 and 3 processing in these cells. One interpretation of these data would be that IL-5 exerts its anti-apoptotic effect primarily through inhibition of caspase activation, although the specificity of this effect is unclear. However, we find the effects of IL-5 on direct activation of the caspase cascade via Fas ligation interesting, and at least supportive of the notion that IL-5 may act by modulating this pathway. Specifically, IL-5 significantly slowed the rate of caspase processing and cell death during direct receptor-mediated caspase activation even with the supra-maximal concentrations of anti-Fas antibody used in these experiments. This effect was marked for caspase 3, and less strong for caspase 8, suggesting that the antagonism may occur at a level upstream of caspase 3 and downstream of caspase 8. The mechanism by which IL-5 might exert this effect must remain speculative at present, as our understanding of regulatory checkpoints in mammalian caspase activation is still in its infancy. Certainly a major checkpoint for any cascade would be during its initiation, and for caspases this equates to the formation of the ‘apoptosome’ during receptor-mediated death (i.e. the death receptor, associated adapter molecules such as FADD, and the initiator caspases), or possibly the formation of the recently described caspase 9/APAF complex. It will be interesting to see if IL-5 affects the assembly of these complexes. Alternatively, IL-5 could affect an amplification arm of the cascade.
In conclusion, we observed that circulating human eosinophils express high levels of the anti-apoptotic species Mcl-1 and the pro-apoptotic species Bax at baseline, confirming the findings of other investigators. IL-5 had the effect of maintaining basal expression of these species, but not altering their levels. Unlike some investigators we did not detect constitutive or IL-5-stimulated Bcl-2 or Bcl-x expression. These data lessen enthusiasm for the theory that IL-5 acts by altering expression of Bcl-2 family members. The expression and processing of the key initiator and effector caspases 8 and 3 in eosinophils support the existence of a caspase death cascade in these cells. IL-5 significantly antagonizes spontaneous and stimulated caspase processing and subsequent apoptosis, suggesting that IL-5 may exert its major anti-apoptotic effect at the level of caspase activation. As IL-5 expression in the asthmatic airway is well documented, and Fas ligand expression in the airway was recently described [37,38], these findings are likely to have clinical significance. Further research is this area is warranted.
Acknowledgments
We are grateful to Steven Galati for his excellent technical support on this project. J.Z. is a recipient of an ALA research grant and a Mentored Clinical Scientist Development Award (NIH K08HL03663). This work was also supported by NIH grants AI24509-08A2 and AI/HL 40976-1.
REFERENCES
- 1.Zangrilli JG, Robertson NM, Alnemri E, et al. Expression of ICE/CED-3 related cysteine proteases and Bcl-2 in human eosinophils. Am J Respir Crit Care Med. 1996;153:A56. (Abstr.) [Google Scholar]
- 2.Druilhe A, Arock M, Le Goff L, Pretolani M. Human eosinophils express Bcl-2 family proteins: modulation of Mcl-1 expression by IFN-gamma. Am J Respir Cell Mol Biol. 1998;18:315–22. doi: 10.1165/ajrcmb.18.3.3019. [DOI] [PubMed] [Google Scholar]
- 3.Ochiai K, Kagami M, Matsumura R, Tomioka H. IL-5 but not interferon-gamma (IFN-gamma) inhibits eosinophil apoptosis by up-regulation of bcl-2 expression. Clin Exp Immunol. 1997;107:198–204. doi: 10.1046/j.1365-2249.1997.d01-884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dibbert B, Daigle I, Braun D, et al. Role of Bcl-xL in delayed eosinophil-apoptosis mediated by granulocyte-macrophage colony-stimulating factor and interleukin-5. Blood. 1999;92:778–83. [PubMed] [Google Scholar]
- 5.Dewson G, Walsh GM, Wardlaw AJ. Expression of Bcl-2 and its homologues in human eosinophils. Am J Respir Cell Mol Biol. 1999;20:720–8. doi: 10.1165/ajrcmb.20.4.3453. [DOI] [PubMed] [Google Scholar]
- 6.Egan RW, Umland SP, Cuss FM, Chapman RW. Biology of interleukin-5 and its relevance to allergic disease. Allergy. 1996;51:71–81. doi: 10.1111/j.1398-9995.1996.tb04561.x. [DOI] [PubMed] [Google Scholar]
- 7.Ohnishi T, Kita H, Weiler D, et al. IL-5 is the predominant eosinophil-active cytokine in the antigen-induced pulmonary late-phase reaction. Am Rev Respir Dis. 1993;147:901–7. doi: 10.1164/ajrccm/147.4.901. [DOI] [PubMed] [Google Scholar]
- 8.Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–10. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi Y, Suda T, Ohta S, Tominaga K, Miura Y, Kasahara T. Analysis of the survival of mature human eosinophils: interleukin-5 prevents apoptosis in mature human eosinophils. Blood. 1991;78:2542–7. [PubMed] [Google Scholar]
- 10.Her E, Frazer J, Austen KF, Owen WF. Eosinophil hematopoietins antagonize the programmed cell death of eosinophils. J Clin Invest. 1991;88:1982–7. doi: 10.1172/JCI115524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousefi S, Blaser K, Simon H-U. Activation of signaling pathways and prevention of apoptosis by cytokines in eosinophils. Int Arch Allergy Immunol. 1998;112:9–12. doi: 10.1159/000237424. [DOI] [PubMed] [Google Scholar]
- 12.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 13.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–91. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 14.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 15.Boise LH, Gonzalez-Garcia M, Postema CE, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 16.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig R. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci USA. 1993;90:3516–20. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997;64:33–42. doi: 10.1002/(sici)1097-4644(199701)64:1<33::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Tewari M, Quan LT, O’Rourke K, et al. Yama/CPP32β, a mammalian homolog of CED-3, is a crmA-inhibitable protease that cleaves the death substrate Poly (ADP-ribose) polymerase. Cell. 1995;81:801–9. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–84. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 20.Muzio M, Chinnaiyan AM, Kischkel FC, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–27. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Schleimer RP, Saito H, Iikura Y, Bochner BS. Induction of apoptosis in human eosinophils by anti-Fas antibody treatment in vivo. Blood. 1995;86:1437–43. [PubMed] [Google Scholar]
- 22.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri ES. Molecular ordering of the Fas-apoptotic pathway: the Fas/APo-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–91. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes-Alnemri T, Litwack G, Alnemri E. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein CED-3 and mammalian interleukin-1-beta-converting enzyme. J Biol Chem. 1994;269:30761–4. [PubMed] [Google Scholar]
- 24.Srinivasula SM, Fernandes-Alnemri T, Zangrilli J, et al. The Ced-3/IL-1β converting enzyme-like homologue Mch6 and the lamin cleaving enzyme Mch2α are substrates for the apoptotic mediator CPP32. J Biol Chem. 1996;271:27099–106. doi: 10.1074/jbc.271.43.27099. [DOI] [PubMed] [Google Scholar]
- 25.Hansel TT, De Vries IJ, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991;145:105–10. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 26.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–56. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas x can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 28.Fearnhead HO, Rodriguez J, Govek EE, Guo W, Kobayashi R, Hannon G, Lazebnik YA. Oncogene-dependent apoptosis is mediated by caspase-9. Proc Natl Acad Sci USA. 1998;95:13664–9. doi: 10.1073/pnas.95.23.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scaffidi C, Medema JP, Krammer PH, Peter ME. FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. J Biol Chem. 1997;272:26953–8. doi: 10.1074/jbc.272.43.26953. [DOI] [PubMed] [Google Scholar]
- 30.Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson KB, Korsemyer SJ. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA. 1995;92:7834–8. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonsson B, Conti F, Ciavatta AM, et al. Inhibition of Bax channel forming activity by Bcl-2. Science. 1997;277:370–2. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 32.Sattler M, Liang H, Nettesheim D, et al. Structure of Bcl-x sub L-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–6. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 33.Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–8. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 34.Clem RJ, Cheng EH, Karp CL, et al. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci USA. 1998;95:554–9. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang JJ, Schaber MD, Srinivasula SM, Alnemri ES, Litwack G, Hall DJ, Bjornsti M-A. Cascades of mammalian caspase activation in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:3189–98. doi: 10.1074/jbc.274.5.3189. [DOI] [PubMed] [Google Scholar]
- 36.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 37.Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, Rabe KF, White SR. Expression of fas (CD95) and fast (CD95L) in human airway epithelium. Am J Respir Cell Mol Biol. 1998;19:537–42. doi: 10.1165/ajrcmb.19.4.3100. [DOI] [PubMed] [Google Scholar]
- 38.Druile A, Wallaert B, Tsicopoulos A, Lapa e Silva J-R, Tillie-Leblond I, Tonnel A-B, Pretolani M. Apoptosis, proliferation, and expression of Bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol. 1999;19:747–57. doi: 10.1165/ajrcmb.19.5.3166. [DOI] [PubMed] [Google Scholar]