The proportion of circulating γδ T cells increases after the first week of onset of tularaemia and remains elevated for more than a year (original) (raw)
Abstract
In various human intracellular bacterial diseases, an increase of the proportion of circulating Vγ9Vδ2 T cells has been observed. The prevalence of the finding among infected subjects and the time course of the elevation remain to be investigated. In the present study, comprising blood samples from a large number of cases of ulceroglandular tularaemia, the percentage of Vγ9Vδ2 T cells within the first week of onset of disease (5·3 ± 0·7% (mean±s.e.m.)) did not differ from that of control subjects (5·3 ± 0·8%). Thereafter, percentages increased rapidly and within the interval of 8–40 days mean levels were >20% (P < 0·001). Of 45 individuals sampled within 3 months of onset, 42 showed a percentage of Vγ9Vδ2 T cells of >10%. Significantly increased levels were still recorded at 18 months (13·8 ± 2·4%; P < 0·05) but not at 24 months (10·2 ± 2·1%; _P_ > 0·10). Thus, a consistent increase of circulating Vγ9Vδ2 T cells was demonstrated in tularaemia. The initial delay and the prolonged course of elevation may suggest a role in immunoregulation and/or immunological memory. Furthermore, the percentage of γδ T cells expressing tumour necrosis factor-alpha in response to phorbol myristate acetate was decreased during the first week and up to 40 days after onset, possibly reflecting the modulation of an inflammatory response.
Keywords: tularaemia, γδ T cells, increase
INTRODUCTION
T lymphocytes express either the αβ or the γδ T cell receptor (TCR). The αβ T cells are MHC-restricted and recognize antigen only after generation of peptide–MHC complexes by antigen-presenting cells (APC). Most γδ T cells are not MHC-restricted, and antigen recognition does not require the processing pathways needed to form antigen–peptide complexes [1,2]. In humans, the proportion of peripheral blood T cells expressing the γδ TCR seems to vary among populations, possibly the result of varying exposure to microbial agents that may induce expansion of the cell population. In a healthy Swedish adult population, the percentage of circulating γδ T cells constitutes 4–5% of lymphocytes [3].
The role of γδ T cells in the host response to infectious agents is not completely understood. They are capable of producing cytokines including interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α), crucial mediators of the host defence against intracellular infections. In line with a probable function in these infections, an increase of γδ T cells in peripheral blood has been found in malaria [4–6], toxoplasmosis [7], visceral leishmaniasis [8], tuberculosis [9], Q fever [10], listeriosis [11], brucellosis [12], histoplasmosis [13], and tularaemia [14]. Cells of one single subset of γδ T cells, the Vγ9Vδ2 T cells, seem to account for the increase, their target antigens being small phosphorylated non-peptidic ligands [15–19] or primary alkylamines [20] secreted by bacteria. Although a large proportion of the Vγ9Vδ2 T cells respond in vitro to one and the same antigen, the expansion of the cells is polyclonal by nature [14,21,22]. Increased γδ T cell levels have also been recorded in viral diseases [23–25], but the association with diseases caused by protozoan and intracellular bacterial pathogens is more striking. Clinical studies of the diseases have not however fully elucidated the kinetics of the γδ T cell response. As regards intracellular bacterial diseases, there is virtually no information regarding the interval from onset of disease to start of expansion, or the duration of elevation.
Tularaemia is an acute febrile disease caused by the facultative intracellular Gram-negative bacterium, Francisella tularensis. It is associated with an increase of the proportion of γδ T cells in peripheral blood [14,26]. This may be a result of the production, by F. tularensis, of phosphorylated non-peptidic molecules that stimulate γδ T cells [26].
In the present study, the course of increase and decline of the percentage of γδ T cells was determined by analysing a large number of blood samples from patients with tularaemia. In addition, we assayed the percentage of γδ T cells expressing TNF-α or IFN-γ in response to phorbol myristate acetate (PMA) at various intervals of the disease.
PATIENTS AND METHODS
Patients and volunteers
Outbreaks of ulceroglandular tularaemia occurred in Sweden in August and September of 1995, 1996 and 1998. Blood samples from the patients were obtained through general practitioners at various locations in northern Sweden. The diagnosis was serologically confirmed in all patients by use of ELISA. All patients responded satisfactorily to antibiotic treatment. Repeated blood samples, up to six, were obtained from the patients from 1996 until 1999. In total, 181 samples from 108 patients (2–83 years old, mean age 45·7 ± 18·8 years; 49 females, 59 males) were included. Informed consent was obtained from all patients (or their guardians) included in the study. The study had been approved by the Human Ethical Committee, Umeå University, Umeå.
Monoclonal antibodies
Antibodies to the αβ TCR (clone WT31, FITC-conjugated), γδ TCR (11F2, FITC and PE), and the surface markers CD3 (SK7, PerCP), CD19 (4G7, FITC), and CD56 (MY31, PE) were purchased from Becton Dickinson (Sunnyvale, CA). Antibodies to CD56 (clone B-A19, FITC-conjugated), CD3 (UCH-T1, PE), CD19 (FMC63, FITC), αβ TCR (BMA 031, FITC), γδ TCR (5.A6.E9, PE), TCR-Vγ2 (7A5, FITC), and TCR-Vδ2 (I5D, PE) were obtained from Serotec (Oxford, UK). Anti-cytokine antibodies specific for human IFN-γ (4S.B3, PE) and TNF-α (Mab11, PE) and mouse IgG1 PE-conjugated isotype control, TCR Vγ9 (B3, FITC), and TCR Vδ2 (B6, PE) antibodies were purchased from PharMingen (San Diego, CA).
Flow cytometry analysis of lymphocyte subsets
Surface phenotyping of EDTA blood was performed with conjugated MoAbs. Aliquots (50 μl) of blood were incubated with 10 μl of normal mouse serum at a dilution 1:500 (Dako, Glostrup, Denmark) for 15 min at room temperature, and 5 μl of appropriate MoAbs were added, followed by incubation for 25 min at room temperature. Then, 2 ml of FACS-lysing solution (Becton Dickinson) containing 1% paraformaldehyde were added to each tube. After 10 min of incubation, cells were washed twice with Cell-wash solution (Becton Dickinson), resuspended in 500 μl of FACS-flow solution (Becton Dickinson), and stored at 4°C before analysis.
Using a FACSort instrument (Becton Dickinson), 10 000 events per sample were recorded. The data were collected and analysed by use of CellQuest software (Becton Dickinson). Lymphocytes were gated according to their morphological parameters by means of forward scatter and side scatter analysis or for expression of CD3. Results were expressed as the percentage of CD3 cells stained by a given label.
Analysis of cytokine expression by T cells
For enumeration of cytokine-producing cells, a 100-μl blood sample was diluted with 400 μl of RPMI 1640. Cells were stimulated with 1 ng/ml of PMA (Sigma, Madison, WN) and 1 μm of ionomycin (Sigma). Extracellular cytokine transport was blocked by addition of 3 μm of monensin (Sigma), and cells were incubated for 4 h at 37°C. This time period was found optimal when the technique was standardized for demonstration of TNF-α and IFN-γ expression [27]. After stimulation, cells were washed once and stained with conjugated anti-CD3 antibody and anti-TCR αβ or anti-TCR γδ antibodies for 15 min on ice. Then, erythrocytes were lysed by addition of 1 ml FACS-lysing solution (Becton Dickinson) for 10 min, washed once, and fixed for 10 min with ice-cold PBS containing 4% paraformaldehyde and 0·1% saponin (Sigma). Fixed cells were washed once in Cell-wash (Becton Dickinson) containing 0·1% saponin and stained for 15 min on ice with anti-cytokine or isotype control PE-conjugated antibody. Staining was followed by two washes in Cell-wash/saponin and one wash in Cell-wash containing 1% bovine serum albumin (BSA). Finally, cells were resuspended in 500 μl of FACS-flow solution (Becton Dickinson). Thresholds for cytokine signals were assessed after staining of samples with irrelevant isotype-specific antibodies. Results were expressed as the percentage of cytokine-positive cells of the respective subpopulation.
Statistical analysis
For statistical evaluation, Wilcoxon test was used.
RESULTS
Course of elevation of γδ+ T cells
Irrespective of time after onset of tularaemia, percentages of CD3 and CD19 cells of lymphocytes were unaltered. During the first week of disease, there was a transient decrease in the proportion (mean ±s.e.m.) of CD56+ (NK) cells (11·3 ± 1·6% versus 18·8 ± 1·5% in control subjects, P < 0·01). At this time, the percentages of γδ+ T cells (5·3 ± 0·7%) did not differ significantly from those of control subjects (5·3 ± 0·8%) (Fig. 1). In two 2-year-old children, the only patients <4 years old, γδ+ T cells comprised 13·6% and 16·3% of T cells as soon as 1 day after onset of disease. These values were higher than those of any other subject during the first week of disease. The children were not tested repeatedly. In healthy children <4 years old, γδ T cell levels similar to those of the present control subjects have been reported [28].
Fig. 1.
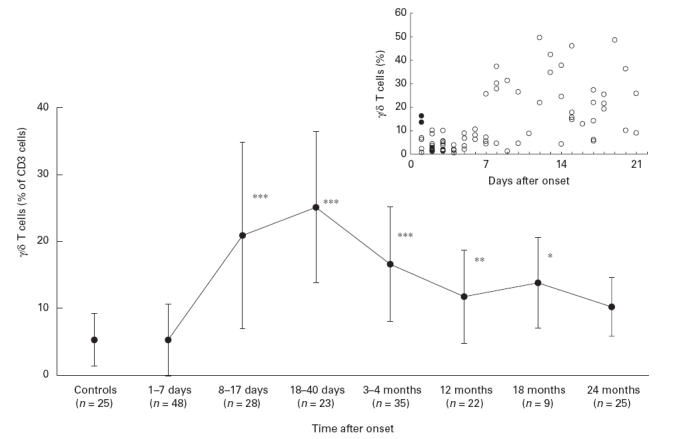
Percentage of γδ+ T cells of CD at various intervals after onset of tularaemia. ***P < 0·001; **P < 0·01; *P < 0·05 in comparison with values of control subjects. n, Number of individuals. Values of individual blood samples obtained during the first 3 weeks after onset are inserted. Samples from two 2-year-old children are shown by filled symbols.
After the first week of disease, percentages of γδ T cells increased rapidly. During the interval of 8–17 days after onset, levels were 20·9 ± 2·7% (P < 0·001), and in the interval of 18–40 days, 25·2 ± 2·4% (_P_ < 0·001). Thereafter the levels declined. A significantly increased mean value was however still recorded at 18 months (13·8 ± 2·4%, _P_ < 0·05), but not at 24 months (10·2 ± 2·1%_, P_ > 0·10). In 10 cases analysed repeatedly (two to six samples per individual), the development of γδ T cell levels was in good agreement with the general trend (data not shown). The increase of γδ T cells was attributed exclusively to cells expressing the Vγ9Vδ2 TCR. In 42/45 individuals sampled within 3 months after onset of disease, a Vγ9Vδ2 T cell percentage of ≥10% was found and one individual showed an approx. three-fold increase.
Concomitantly to the increase of γδ T cells, a decrease in the percentage of αβ T cells was observed (data not shown). The total number of αβ T cells (1·60 ± 0·19 × 106/ml), recorded in eight patients within the interval of 8–30 days of onset of disease, did however not differ significantly (P > 0·10) from values in the first week of disease (2·17 ± 0·26 × 106/ml). Thus, the decline in the percentage of αβ T cells was due to an absolute increase of γδ T cells. No marked increase in expression of the activation markers CD25, CD69 and HLA-DR was observed on peripheral blood lymphocytes of the tularaemia patients (data not shown). All patients responded well to treatment and no complications were observed.
TNF-α and IFN-γ expression of γδ T cells
From a number of patients, selected without regard to the clinical expression or severity of disease, the percentage of γδ T cells expressing TNF-α or IFN-γ was assayed after 4 h of in vitro stimulation with PMA and ionomycin. Within 1–7 days of onset of disease, the mean percentage of TNF-α-expressing γδ T cells (19·8 ± 2·7%) was significantly lower (P < 0·01) than that of control subjects (45·6 ± 10·0%) (Fig. 2). At days 8–17 and 18–40, the mean percentages were still significantly decreased, 10·7 ± 4·8% (_P_ < 0·01) and 15·7 ± 8·0% (_P_ < 0·05), respectively, whereas at 3 months after onset the mean percentage, 25·4 ± 8·4% (_P_ > 0·10), did not differ significantly from that of control subjects. Irrespective of time interval after onset of tularaemia, mean percentages of γδ T cells expressing IFN-γ did not differ significantly from that of control subjects (Fig. 2). Expression of IL-4 was assayed in four samples obtained 4 days after onset of disease, four samples from day 13–18, and five samples obtained at 3 months. In none of the samples did more than a few per cent of γδ T cells stain with IL-4 antibodies, and values did not markedly exceed those obtained by staining with the isotype control. In a previous study, IL-4 was similarly non-detectable in cultures of stimulated Vγ9Vδ2 cells [29].
Fig. 2.
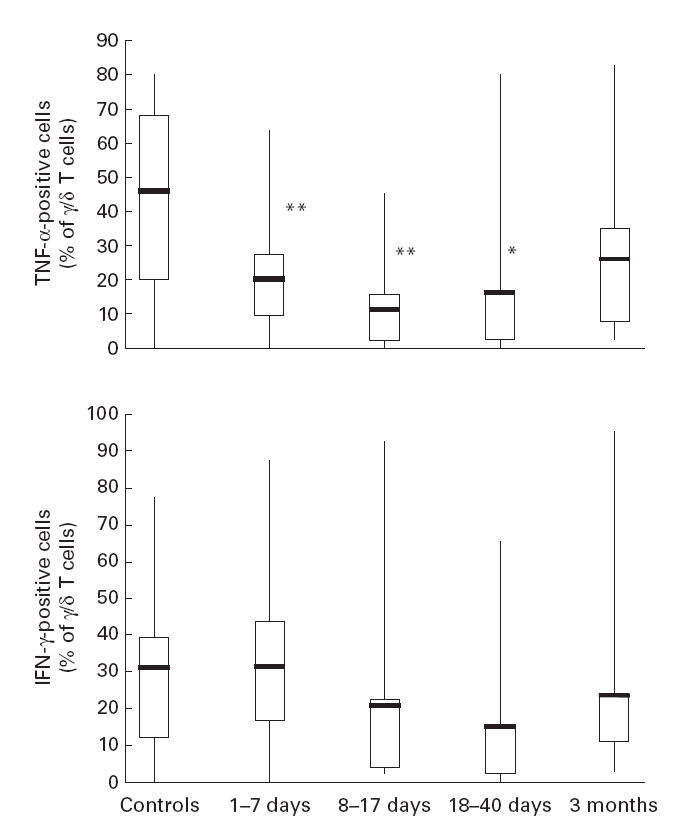
Frequency of cytokine-producing γδ+ T cells at various intervals after onset of tularaemia. Line through box shows median, with quartiles at either end. The vertical lines indicate maximum and minimum values. **P < 0·01; *P < 0·05 in comparison with values of control subjects. n, Number of individuals.
DISCUSSION
After onset of tularaemia, 1 week passed before an increase of the percentage of Vγ9Vδ2 T cells was discerned. In other intracellular infections, Vγ9Vδ2 T cell levels have not been analysed at brief intervals within the acute phase and it therefore remains to be shown whether such a delay may be a general feature of these diseases. In fact, it would be in line with in vitro data indicating that Vγ9Vδ2 T cells need IL-2 for proliferation [30,31], a support provided primarily by stimulated CD4+ T cells. Notably, a recall CD4 T cell response to protein antigens of F. tularensis is not usually demonstrable in circulation before the second week of disease [32,33].
Most, but not all, blood samples analysed within the first week of onset of tularaemia had a normal percentage of γδ T cells. The exceptions were two 2-year-old children, both displaying a pronounced increase of Vγ9Vδ2 T cells as soon as 1 day after onset of disease. Besides the possibility that in children some days may lapse before illness is recognized, we have no plausible explanation for the observation. In any case, it lends no support to the hypothesis suggesting that γδ T cells, due to their need for IL-2 support from αβ T cells, would be less prone to respond to a primary infection early in life, when an αβ T cell memory to various cross-reactive T cell epitopes has not yet developed [34].
The peak of percentage of Vγ9Vδ2 T cells was extended over several weeks and normalization did not occur until more than a year after recovery. Both the magnitude and duration of the response were at least as prominent as in intracellular infections previously studied. In acute Plasmodium falciparum or P. vivax malaria, γδ T cells increased gradually during the first 2 months after infection, peaking during the second month [6]. In another study of malaria, the peak occurred at an interval similar to that of the present tularaemia patients and normalization occurred within 6 months [35]. As regards intracellular bacteria, there are fragmentary data indicating the presence of an increased percentage of Vγ9Vδ2 T cells several months after onset of symptoms [12]. Increased levels have been observed also in healthy individuals exposed to intracellular parasites by living in highly endemic regions or working with tuberculosis patients [3,36].
In intracellular infections previously subjected to analysis of γδ T cells, i.e. malaria, brucellosis, tuberculosis, and Q fever, persistent infection or latency is a well-recognized trait, implying that continued exposure to the infective agent cannot readily be excluded. In tularaemia, latency does not occur. On the contrary, the bacteria are completely eradicated and only a few cases of reinfection have been documented [37]. In line with this, all patients in our study responded well to treatment. Due to the sporadic occurrence of F. tularensis in the environment, re-exposure is also unlikely. As far as tularaemia is concerned, it can be concluded that the increase of the γδ T cell proportion is maintained long after recovery and the complete eradication of the causative agent.
The role of Vγ9Vδ2 T cells in intracellular infections is unknown. The increase in blood after the acute phase of tularaemia, when symptoms tended to recede, and the persistent elevation long after recovery, may suggest a regulatory function and/or a role within the T cell memory. Even though protective immunity to F. tularensis seems to depend strictly on the development of a pronounced αβ T cell response to various bacterial proteins [38–40], Vγ9Vδ2 T cells might nonetheless have a contributory role.
By intracellular staining of cytokines, a significant decrease in the percentage of TNF-α-positive cells was found in the present patients already within the first week of disease. This might possibly reflect a down-regulation or modulation of an otherwise harmful inflammatory response in acute-phase disease. In vitro studies of human γδ T cells suggest that these cells are subjected to a fine-tuned combination of stimulation and inhibition by interaction with different membrane receptors [41–44]. The decrease in percent of TNF-α-producing γδ T cells endured for at least 6 weeks, a finding which may contribute to explaining why an expansion of the cell population was not associated with clinical signs of inflammation.
In conclusion, a delay of approx. 1 week before the appearance of increased percentages of Vγ9Vδ2 T cells in peripheral blood after onset of ulceroglandular tularaemia was demonstrated. Thereafter, almost all patients showed increased percentages. In spite of rapid eradication of the bacteria and clinical recovery, levels of Vy9Vδ2 T cells remained significantly increased as long as 18 months after recovery. A decrease in the percentage of TNF-α-expressing γδ cells, apparent from the first week after onset and during the next month, was possibly the result of modulation to mitigate pathogenic effects.
Acknowledgments
We thank Drs Pekka Ruuska and Lennart Berglund for providing the patient samples. Financial support was obtained from the Swedish Medical Research Council (project no. 9485), the Medical faculty of Umeå University, and the County Council of Västerbotten.
REFERENCES
- 1.Morita CT, Beckman EM, Bukowski JF, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 2.Davis MM, Chien Y. Issues concerning the nature of antigen recognition by αβ and γδ T-cell receptors. Immunol Today. 1995;16:316–8. doi: 10.1016/0167-5699(95)80143-x. [DOI] [PubMed] [Google Scholar]
- 3.Esin S, Shigematsu M, Nagai S, et al. Different percentages of peripheral blood γδ+ T cells in healthy individuals from different areas of the world. Scand J Immunol. 1996;43:593–6. doi: 10.1046/j.1365-3083.1996.d01-79.x. [DOI] [PubMed] [Google Scholar]
- 4.Perera MK, Carter R, Goonewardene R, et al. Transient increase in circulating γδ T cells during Plasmodium vivax malarial paroxysms. J Exp Med. 1994;179:311–5. doi: 10.1084/jem.179.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho M, Webster HK, Tongtawe P, et al. Increased γδ T cells in acute Plasmodium falciparum malaria. Immunol Letters. 1990;25:139–41. doi: 10.1016/0165-2478(90)90105-y. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz E, Shapiro R, Shina S, et al. Delayed expansion of Vδ2+ and Vδ1+γδ T cells after acute Plasmodium falciparum and Plasmodium vivax malaria. J Allergy Clin Immunol. 1996;97:1387–92. doi: 10.1016/s0091-6749(96)70208-7. [DOI] [PubMed] [Google Scholar]
- 7.Scalise F, Gerli R, Castellucci G, et al. Lymphocytes bearing the γδ T-cell receptor in acute toxoplasmosis. Immunology. 1992;76:668–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Raziuddin S, Telmasani AW, el-Hag el-Awad M, et al. γδ T cells and the immune response in visceral leishmaniasis. Eur J Immunol. 1992;22:1143–8. doi: 10.1002/eji.1830220506. [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Kojiro N, Ikeda T, et al. Increased proportions of peripheral blood γδ T cells in patients with pulmonary tuberculosis. Chest. 1992;102:195–7. doi: 10.1378/chest.102.1.195. [DOI] [PubMed] [Google Scholar]
- 10.Schneider T, Jahn HU, Liesenfeld O, et al. The number and proportion of Vγ9Vδ2 T cells rise significantly in the peripheral blood of patients after the onset of acute Coxiella burnetii infection. Clin Infect Dis. 1997;24:261–4. doi: 10.1093/clinids/24.2.261. [DOI] [PubMed] [Google Scholar]
- 11.Jouen-Beades F, Paris E, Dieulois C, et al. In vivo and in vitro activation and expansion of γδ T cells during Listeria monocytogenes infection in humans. Infect Immun. 1997;65:4267–72. doi: 10.1128/iai.65.10.4267-4272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertotto A, Gerli R, Spinozzi F, et al. Lymphocytes bearing the γδ T cell receptor in acute Brucella melitensis infection. Eur J Immunol. 1993;23:1177–80. doi: 10.1002/eji.1830230531. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann PF, Sawyer T, Donabedian H. Novel abnormality in subpopulations of circulating lymphocytes. T γδ and CD2−, 3+, 4−, 8− lymphocytes in histoplasmosis-associated immunodeficiency. Int Arch Allergy Appl Immunol. 1989;90:213–8. doi: 10.1159/000235027. [DOI] [PubMed] [Google Scholar]
- 14.Sumida T, Maeda T, Takahashi H, et al. Predominant expansion of Vγ9Vδ2 T cells in a tularemia patient. Infect Immun. 1992;60:2554–8. doi: 10.1128/iai.60.6.2554-2558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Constant P, Davodeau F, Peyrat MA, et al. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 16.Burk MR, Mori L, De Libero G. Human Vγ9Vδ2 T cells are stimulated in a cross-reactive fashion by a variety of phosphorylated metabolites. Eur J Immunol. 1995;25:2052–8. doi: 10.1002/eji.1830250737. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer K, Schoel B, Gulle H, et al. Primary responses of human T cells to mycobacteria: a frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990;20:1175–9. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka Y, Morita CT, Tanaka Y, et al. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y, Sano S, Nieves E, et al. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci USA. 1994;91:8175–9. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bukowski JF, Morita CT, Brenner MB. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 21.Ho M, Tongtawe P, Kriangkum J, et al. Polyclonal expansion of peripheral γδ T cells in human Plasmodium falciparum malaria. Infect Immun. 1994;62:855–62. doi: 10.1128/iai.62.3.855-862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boullier S, Poquet Y, Halary F, et al. Phosphoantigen activation induces surface translocation of intracellular CD94/NKG2A class I receptor on CD94− peripheral Vγ9Vδ2 T cells but not on CD94− thymic or mature gammadelta T cell clones. Eur J Immunol. 1998;28:3399–410. doi: 10.1002/(SICI)1521-4141(199811)28:11<3399::AID-IMMU3399>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Dechanet J, Merville P, Berge F, et al. Major expansion of γδ T lymphocytes following cytomegalovirus infection in kidney allograft recipients. J Infect Dis. 1999;179:1–8. doi: 10.1086/314568. [DOI] [PubMed] [Google Scholar]
- 24.Poccia F, Boullier S, Lecoeur H, et al. Peripheral Vγ9Vδ2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J Immunol. 1996;157:449–61. [PubMed] [Google Scholar]
- 25.De Paoli P, Gennari D, Martelli P, et al. γδ T cell receptor-bearing lymphocytes during Epstein–Barr virus infection. J Infect Dis. 1990;161:1013–6. doi: 10.1093/infdis/161.5.1013. [DOI] [PubMed] [Google Scholar]
- 26.Poquet Y, Kroca M, Halary F, et al. Expansion of Vγ9Vδ2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect Immun. 1998;66:2107–14. doi: 10.1128/iai.66.5.2107-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascher B, Schlenke P, Seyfarth M. Expression and kinetics of cytokines determined by intracellular staining using flow cytometry. J Immunol Methods. 1999;223:115–21. doi: 10.1016/s0022-1759(98)00200-2. [DOI] [PubMed] [Google Scholar]
- 28.De Weerd W, Twilhaar WN, Kimpen JL. T cell subset analysis in peripheral blood of children with RSV bronchiolitis. Scand J Infect Dis. 1998;30:77–80. doi: 10.1080/003655498750002349. [DOI] [PubMed] [Google Scholar]
- 29.Subauste CS, Chung JY, Do D, et al. Preferential activation and expansion of human peripheral blood gamma delta T cells in response to Toxoplasma gondii in vitro and their cytokine production and cytotoxic activity against T. gondii-infected cells. J Clin Invest. 1995;96:610–9. doi: 10.1172/JCI118076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kjeldsen-Kragh J, Quayle AJ, Skalhegg BS, et al. Selective activation of resting human γδ T lymphocytes by interleukin-2. Eur J Immunol. 1993;23:2092–9. doi: 10.1002/eji.1830230908. [DOI] [PubMed] [Google Scholar]
- 31.Elloso MM, van der Heyde HC, Troutt A, et al. Human γδ T cell subset-proliferative response to malarial antigen in vitro depends on CD4+ T cells or cytokines that signal through components of the IL-2R. J Immunol. 1996;157:2096–102. [PubMed] [Google Scholar]
- 32.Tärnvik A, Sandström G, Löfgren S. Time of lymphocyte response after onset of tularemia and after tularemia vaccination. J Clin Microbiol. 1979;10:854–60. doi: 10.1128/jcm.10.6.854-860.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syrjala H, Herva E, Ilonen J, et al. A whole-blood lymphocyte stimulation test for the diagnosis of human tularemia. J Infect Dis. 1984;150:912–5. doi: 10.1093/infdis/150.6.912. [DOI] [PubMed] [Google Scholar]
- 34.Riley EM. Is T-cell priming required for initiation of pathology in malaria infections? Immunol Today. 1999;20:228–33. doi: 10.1016/s0167-5699(99)01456-5. [DOI] [PubMed] [Google Scholar]
- 35.Roussilhon C, Agrapart M, Guglielmi P, et al. Human TcR γδ+ lymphocyte response on primary exposure to Plasmodium falciparum. Clin Exp Immunol. 1994;95:91–97. doi: 10.1111/j.1365-2249.1994.tb06020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueta C, Tsuyuguchi I, Kawasumi H, et al. Increase of γδ T cells in hospital workers who are in close contact with tuberculosis patients. Infect Immun. 1994;62:5434–41. doi: 10.1128/iai.62.12.5434-5441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke DS. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977;135:55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- 38.Ericsson M, Sandström G, Sjöstedt A, et al. Persistence of cell-mediated immunity and decline of humoral immunity to the intracellular bacterium Francisella tularensis 25 years after natural infection. J Infect Dis. 1994;170:110–4. doi: 10.1093/infdis/170.1.110. [DOI] [PubMed] [Google Scholar]
- 39.Sjöstedt A, Sandström G, Tärnvik A. Several membrane polypeptides of the live vaccine strain Francisella tularensis LVS stimulate T cells from naturally infected individuals. J Clin Microbiol. 1990;28:43–48. doi: 10.1128/jcm.28.1.43-48.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surcel HM. Diversity of Francisella tularensis antigens recognized by human T lymphocytes. Infect Immun. 1990;58:2664–8. doi: 10.1128/iai.58.8.2664-2668.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poccia F, Cipriani B, Vendetti S, et al. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive Vγ9Vδ2 T lymphocytes. J Immunol. 1997;159:6009–17. [PubMed] [Google Scholar]
- 42.Carretero M, Cantoni C, Bellon T, et al. The CD94 and NKG2-A C-type lectins covalently assemble to form a natural killer cell inhibitory receptor for HLA class I molecules. Eur J Immunol. 1997;27:563–7. doi: 10.1002/eji.1830270230. [DOI] [PubMed] [Google Scholar]
- 43.Lazetic S, Chang C, Houchins JP, et al. Human natural killer cell receptors involved in MHC class I recognition are disulfide-linked heterodimers of CD94 and NKG2 subunits. J Immunol. 1996;157:4741–5. [PubMed] [Google Scholar]
- 44.Lopez-Botet M, Perez-Villar JJ, Carretero M, et al. Structure and function of the CD94 C-type lectin receptor complex involved in recognition of HLA class I molecules. Immunol Rev. 1997;155:165–74. doi: 10.1111/j.1600-065x.1997.tb00949.x. [DOI] [PubMed] [Google Scholar]