Cytokine expression and synovial pathology in the initiation and spontaneous resolution phases of adjuvant arthritis: Interleukin-17 expression is upregulated in early disease (original) (raw)
Abstract
The aim of this study was to understand the immune processes controlling the initiation and spontaneous resolution of adjuvant arthritis (AA). We investigated synovial T-cell recruitment and mRNA expression of IL-17 and other important disease related cytokines, IFN-γ, IL-2, IL-4, TNF and TGF-β in inguinal lymph node (ILN) and synovial membrane (SM). Arthritis severity was assessed by a numerical rating score and rats were sacrificed every 3–4 days postadjuvant induction. Further assessment involved quantitative radiology and histology of the ankle joints on each day, and the ILN and SM were removed for RNA extraction. Cytokine mRNA expression was measured using RT-PCR and densitometry. Paraffin sections of rat ankle joints were stained for T-cells (CD3) by immunohistochemistry. In the ILN, there was an increase in IL-17, TNF and IFN-γ expression in the early stages of disease, with a secondary sustained increase in IFN-γ expression. In the SM, there was expression of T-cell cytokines in early arthritis (day 13), and prolonged TNF and TGF-β expression, which reflected disease progression. IL-4 mRNA expression increased in the later stages of AA. Synovial T-cell numbers transiently increased at day 6, and remained high from days 13–28. Increased pro-inflammatory cytokine expression, including IL-17, in the ILN reflects the initiating events in the early stage of disease. IL-17 may therefore play an important role in the pathogenesis of AA. The increase in IL-4 (an anti-inflammatory cytokine) in the SM in the later stages of AA suggests that IL-4 is involved in the spontaneous resolution of AA. The initial increase in IFN-γ in the ILN may reflect a pro-inflammatory response, while the prolonged secondary increase may indicate activation of regulatory T-cells.
Keywords: cytokines, adjuvant arthritis, synovium, inguinal lymph node, IL-17
INTRODUCTION
Rheumatoid arthritis (RA) is characterized by chronic inflammation of the joints and progressive destruction of cartilage and bone. Several cytokines have been implicated in the mechanism of synovial cell activation and joint destruction in RA, including TNF, IFN-γ [1] and the newly described IL-17 [2–5]. Although macrophage-derived cytokines are found at much higher levels than T-cell-derived cytokines in the rheumatoid synovium [6], this pattern of cytokine expression has been shown to occur even in T-cell dependent responses such as graft-_versus_-host reaction [7]. Furthermore, although T-cell cytokines have proven difficult to detect in rheumatoid synovium, they have been implicated in the pathogenesis of RA [8] and their role in this disease is intriguing.
Few studies of in vivo cytokine production in models of arthritis have been performed. Although the time course of cytokine expression has been previously investigated in collagen-induced arthritis and antigen-induced arthritis in mice [9, 11], few published studies have investigated cytokine expression in adjuvant arthritis (AA) [12, 13] and changes during the resolution phase have not been reported.
Adjuvant arthritis (AA) is a T-cell dependent disease [14, 15] and is one of the most widely used models of RA in rats. AA has a predictable onset, making it an ideal model to investigate prearthritic induction events; the spontaneous resolution of this model provides an opportunity to study the immunoregulatory events mediating the resolution of inflammatory arthritis. We have therefore utilized the adjuvant model of arthritis in rats to investigate expression of T-cell and macrophage-derived cytokines to elucidate their possible roles in the initiation and resolution phases of inflammatory arthritis.
Although the immunohistochemical changes in the synovial tissue of rats during the course of adjuvant arthritis have previously been examined using the CD5 antibody which stains both T-cells and B-cells [16], the changes in T-cells alone over the entire course of arthritis has not been adequately described.
In the present experiments, we therefore investigated the expression of the important disease-related cytokines, IL-17, IFN-γ, IL-2, IL-4, TNF and TGF-β in inguinal lymph node (ILN) and synovial membrane (SM) over 28 days of adjuvant arthritis to include the prearthritic, established and spontaneous resolution stages of the disease. The changes in cytokine expression were compared to clinical indices of arthritis as well as synovial T-cell recruitment.
MATERIALS AND METHODS
Animals
Male Dark Agouti (DA) rats 4–8-week-old weighing approximately 230 g were obtained from University of Newcastle and housed in cages lined with cellulose bedding and shredded paper in a temperature controlled room (22 ± 1°C) with a 12-h alternating light and dark cycle. Animals were given food (rat chow, Gordon's Speciality Stockfeeds, Yandera, Australia) and water ad libitum for 1–2 weeks prior to, and throughout the experiments. All experiments were approved by the Animal Care and Ethics Committee of the University of New South Wales, Sydney, Australia.
Induction of adjuvant arthritis
Upon arrival, animals were handled 1–2 weeks prior to experiments and every 2–3 days throughout the study. During this time food, fluid intake and body weight were monitored. To induce adjuvant arthritis, rats were anaesthetized with ketamine (50 mg/kg i.p.; Parnell Laboratories, Alexandria, Australia) and xylazine (5 mg/kg i.p.; Troy Laboratories, Smithfield, Australia) and injected with complete Freund's adjuvant (1 mg of heat-killed and dried Mycobacterium butyricum suspension in paraffin oil and mannide monoleate, Difco Laboratories, Detroit, Michigan, USA) intradermally into the tail base. Control rats received similar injections of either 0·9% saline or incomplete Freund's adjuvant (ICFA).
Experimental protocol
Arthritic rats were sacrificed at days 3, 6, 9, 13, 17, 21, 24 and 28 (n = 7 at each time point). The two nonarthritic control groups, receiving saline or ICFA, were euthanased on day 13 (n = 6 in each group). Day 0 rats did not receive complete Freund's adjuvant (n = 6). Following sacrifice, inguinal lymph nodes from both sides and synovium from the right paw were removed, placed in cryotubes and immediately snap frozen in liquid nitrogen and stored at − 70°C pending RNA extraction.
Assessment of arthritic damage
Disease progression was monitored from the induction of arthritis (day 0) until the day when the last group of rats were sacrificed (day 28). The rats were euthanased using pentobarbital (60 mg i.p.; Lethobarb; Virbac, Sydney, Australia). Three indices of arthritic damage were used; oedema, radiography and histology. Oedema was measured every 3–4 days by plethysmometry (Ugo Basile, Comerio, Italy) in both the left and right ankle. Following sacrifice, the left ankle was removed for quantitative radiographic and histological examination to assess joint damage as described previously [17].
RNA extraction
Samples were processed simultaneously, as previously described [18], at each step of the RT-PCR process to minimize experimental variation. Frozen synovial membrane and ILN tissues were crushed and homogenized in liquid nitrogen on ice. The resulting powder was dissolved in guanidinium isothiocyanate. Total RNA was extracted from samples using the method of Chomczynski and Sacchi [19] and stored at − 70°C in dithiothreitol (DTT; 0·8 µl; 0·1 m; Gibco, Mulgrave, Australia) and 0·1 µl of RNAsin (per 20 µl RNA; Promega, Madison, USA).
CDNA synthesis
To prepare cDNA, RNA (0·13 µg) was added to 1 µl of 5X first strand buffer (75 mm KCl; 50 mm Tris HCl, pH 8·3; 3 mm MgCl; Gibco, Mulgrave, Australia), 2 mm dNTP (10 mm each dATP, dCTP, dTTP and dGTP; Promega, Madison, USA), 0·025 µl of 0·0156 U (0·4 µg) random hexamers (Amersham Pharmacia Biotech, Sydney), 20 U of Moloney Murine Leukaemia Virus (M-MLV) reverse transcriptase (Gibco, Mulgrave, Australia), 0·001 U RNAsin (Promega, Madison, USA) and RNAse free water to a final volume of 5 µl per cDNA. Samples were incubated at 42°C for 60 min. To minimize variability due to experimental processing, cDNA for each tissue (ILN, SM) of all rats was made on the same day, and then stored at − 20°C.
Primers
Sense and antisense oligonucleotide primers used to amplify β-actin, IL-2, IFN-γ, IL-4, TNF and TGF-β had been previously published [20, 22]. IL-17 primers were designed using mRNA sequences from the literature [23] and GenBank. The sequence for the IL-17 primers were as follows:
sense = 5′ − TGGACTCTGAGCCGCATTGA − 3′
antisense = 5′ − GACGCATGGCGGACAATAGA − 3′
Primers were checked for specificity using GenBank and the PCR product was sequenced to confirm identity. An extensive set of preliminary experiments was undertaken to achieve optimal conditions for amplifying mRNA for each of the cytokines.
Polymerase chain reaction (PCR)
RNAse free water (34·75 µl), 5 µl of 10X Reaction Buffer (Promega, Madison, USA) and 0·25 µl (1·25 U) of Taq DNA polymerase (Amersham Pharmacia Biotech, Sydney) per PCR reaction were vortexed and 40 µl was aliquotted per tube. 5′ sense primer (2·5 µl) and 3′ antisense primer (2·5 µl) were added to each tube and overlayed with 50 µl mineral oil (Sigma Chemicals, USA). Samples were heated at 94°C for 1 minute in a thermal cycler (Hybaid Omn-E, Hybaid Ltd, Middlesex, United Kingdom) prior to addition of 5 µl of sample cDNA and cycling. Each cycle involved denaturation at 94°C for 1 minute, annealing at 60°C for 1 minute, and extension at 72°C for 2 min. β-actin primers amplified a 607 bp cDNA fragment and IFN-γ, IL-2, IL-4, TNF, TGF-β and IL-17 primers produced PCR products of 405 bp, 410 bp, 378 bp, 292 bp, 535 bp and 245 bp, respectively. The PCR process was optimized at various cycle numbers with cDNA from synovium and ILN to determine the optimal cycle numbers on the linear portion of the cycle number versus PCR product curve.
Controls
Four hour concanavalin A stimulated ILN cells were used as the positive control because they express high levels of the cytokines. Negative controls contained all components of the reaction mix except cDNA. Each sample was tested for β-actin expression to confirm that the RNA was not degraded prior to testing with other cytokine primers.
Agarose gel electrophoresis
PCR products and molecular weight markers were separated in a 2% agarose gel (ICN, Ohio, USA) using a Tris-borate buffer. Gels were stained with ethidium bromide and photographed with Polaroid 665 film (Polaroid, Hertfordshire, United Kingdom) using UV illumination.
Densitometry
Density of bands on the negative images of the gels were determined using densitometry (GS-700 densitometer, Biorad Laboratories, California, USA) and computer analysis (Molecular Analyst Software, Biorad Laboratories, California, USA), with sample densities normalized for background.
A pilot study was first conducted in the two most severely affected rats (ILN and synovium) at each time point (euthanased at days 0, 3, 6, 9, 13, 17, 21, 24 and 28). RNA was amplified by RT-PCR for each of the cytokines to allow optimization of cycle numbers for the primers in each of the tissues and determination of the most critical days in the time course. From this pilot study, six time points were then selected for the main study; for the ILN, days 0, 6, 9, 13, 17 and 28 were selected, and the synovium, days 0, 9, 13, 17, 24 and 28. Synovial and ILN samples were all amplified in the same PCR reaction, for each tissue, at the optimal cycle number for a specific cytokine and tissue. Synovial and ILN samples were amplified at different cycle numbers, therefore expression of a cytokine in the two tissues cannot be directly compared, however, changes in cytokine expression over time for each tissue are directly comparable.
To further investigate synovial IL-17 expression, another experiment was conducted, in which adjuvant arthritis was induced in a group of 16 rats (n = 8 in each group). Rats were sacrificed at days 0 and 13 and the synovium was removed and snap frozen for RNA extraction.
Cell culture
To determine whether TNF and IFN-γ protein could be detected in tissue culture supernatants, synovium and inguinal lymph nodes from arthritic rats were cultured and protein measured by bioassay or ELISA (see below). A sheep antimouse polyclonal antibody was kindly donated by Dr Gisa Tiegs (University of Erlangen-Nurnberg, Germany).
Synovial culture
Synovial membrane was dissected from rat ankle joints and cultured as previously described [24].
Inguinal lymph node (ILN) culture
Inguinal lymph nodes were dissected from DA rats on days 0, 6 and 17 postinduction of AA. A single cell suspension was prepared and cells were washed with RPMI (LPS free) and cultured in RPMI (Gibco, Mulgrave, Australia) supplemented with 2 mm l-glutamine (Trace, Castle Hill, Australia) and 10% (v/v) heat inactivated fetal calf serum (Trace). 1 × 106 cells/ml were incubated at 37°C in an atmosphere of 5% CO2 in 96 well plates (Trace). Cell supernatants were collected after 48 h for protein analysis by bioassay and ELISA.
WEHI 164 bioassay (MTS Cytotoxicity Assay)
Biologically active TNF was detected in the WEHI 164 murine fibrosarcoma cytotoxicity assay as described previously [25]. The WEHI cell line was obtained from Dr Robert Miller (Peptech, North Ryde, Sydney, Australia). The MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2H-tetrazolium, inner salt) cytotoxicity assay was used to measure percentage viable cells. In brief, 50 µl WEHI-164 medium [RPMI 1640 tissue culture medium (Gibco, Mulgrave, Australia), 50 IU/ml Penicillin/Streptomycin (Trace), 2 mm L-Glutamine (ICN), 10 mm HEPES buffer (Trace) and 10% FCS (heat inactivated) (Trace)] was added to each well of a 96 well plate. A standard curve of recombinant rat TNF (Peprotech, Australia) ranging from 0 to 2500 pg/ml or 50 µl of cell culture supernatants were added in triplicate on the same plate. WEHI-164 cells were then added to all wells (50 µl; 2 × 104 cells/well). Actinomycin D (0·625 µg) (Sigma Chemicals, Sydney, Australia) was finally added to all wells. Plates were incubated at 37°C in a 5% CO2 humidified incubator for 20 h. MTS/PMS (30 µl phenazine methosulphate) solution (3 ml MTS + 150 µl PMS (Promega, Madison, USA) was added to each well and incubated for a further 1–4 h. OD at 490 nm (ref 630 nm) was read using an ELISA plate reader (Multiskan MS, Labsystems, Helsinki, Finland). The lower limit of sensitivity of the bioassay was 5 pg/ml.
IFN-γ ELISA
Secreted rat IFN-γ protein from inguinal lymph node cell cultures was measured using a commercial rat IFN-γ immunoassay kit (Endogen, MA, USA). The lower limit of sensitivity of the assay was 2 pg/ml.
Immunohistochemistry for T-cells
Paraffin sections were cut from prefixed ankle blocks at 4 μm on a rotary microtome, set on Superfrost Plus Gold slides (Menzel, Germany) and dried at 56°C. Slides were baked at 80°C for 30 min in an oven prior to de-waxing in xylene and ethanol, and washing with Tris buffered saline (TBS; 0·025 m Tris base, 0·025 m Tris HCl and 0·85% NaCl, pH 7·6; Sigma Chemicals, USA). Endogenous peroxidase was blocked with 3% hydrogen peroxide in methanol for 5 mins followed by TBS wash. Slides were autoclaved (121°C for 20 min). They were then rinsed with distilled water and then TBS, blocked with skim milk (2%, 15 mins), dipped in distilled water and then incubated with polyclonal rabbit antihuman CD3 (Dako, Carpinteria, CA, USA) 1 : 200 for 1 h at 37°C. Negative controls were incubated with rabbit serum (Gibco, Mulgrave, Australia) at the same protein concentration. Slides were washed and then incubated with goat antirabbit-biotinylated secondary antibody (Dako, Carpinteria, CA, USA; 1 : 300) for 30 mins. After further washes with TBS, Avidin Biotin Complex (ABC kit; Vector Laboratories, Carpinteria, CA, USA) was applied for 30 mins. After washing, sections were developed in 0·01% DAB (3,3′-diaminobenzidine tetrahydrochloride; Sigma Chemicals, USA; with 10 µl of hydrogen peroxide, 30%) for 5 mins and rinsed in TBS. Sections were counterstained in Harris' haematoxylin for 10 s, washed in water and dipped in Scott's blue for 5 s. Slides were then washed in water, dehydrated in alcohol and xylene and mounted using DPX (BDH Laboratory Supply, Poole, England).
Quantification of CD3 cells
Stained cells were counted in high-power fields (x 400) using a 0·02-mm2 graticule. Up to 10 fields were counted for all samples (coefficient of variation = 15%) by two independent observers who were unaware of the treatment regimen. Some sections from nonarthritic rats had less than 10 fields counted (mean = 6 fields). The number of cells was expressed per mm2 of joint tissue.
Data treatment
Inflammation
Data are presented as mean ± standard error of the mean (SEM). Raw scores for both left and right paw volumes were normalized as percentage change from day 0. The three indices of arthritic damage: paw swelling, radiography and histology were expressed as a percentage of day 17 values (when all arthritis parameters peaked; defined as 100%) and summed to obtain a pooled severity index (PSI).
Differences between means were calculated using one-way anova. If a significant difference was found (P < 0·05), posthoc analysis was performed on planned comparisons using Fisher's LSD multiple comparison tests (Number Cruncher Statistical System, NCSS, Kaysville, Utah). For ordinal data, such as the radiology and histology scoring, the non parametric test, Newman-Keuls multiple comparison posthoc test, was used. Pearson correlation coefficient was used to evaluate the correlation between histology, radiology, paw volume, T-cell numbers and cytokine mRNA expression. _T_-tests were used to compare means for the IFN-γ ELISA data.
PCR products
PCR product values were expressed as a percentage of the density of positive control samples amplified simultaneously in the same PCR reaction.
WEHI 164 bioassay
Results were calculated by averaging the replicates. Wells containing cells only were considered as 100% viable. The percentage viability of experimental wells was calculated: OD490 experimental wells/OD490 100% viable × 100. The standard curve of percentage viability versus rTNF concentration was used to obtain TNF levels in cell culture supernatants.
RESULTS
Development of adjuvant arthritis
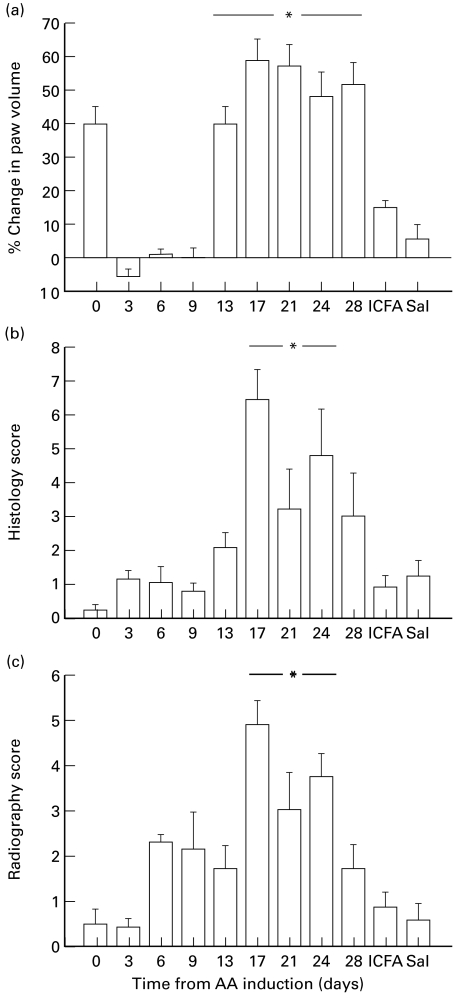
All rats treated with Freund's complete adjuvant developed disease. None of the control rats, which received 0·9% saline or ICFA, developed any disease. All arthritic animals groomed themselves well and maintained their original bodyweight throughout the progress of the disease (day 0 versus day 28: 188 ± 6 g versus 201 ± 11 g, P > 0·05). In arthritic rats, paw volume significantly increased at day 13, with the maximum increase in paw volume occurring between days 17–21 (P < 0·05). Radiology and histology scores followed a similar pattern, with scores increasing significantly from day 17–24 (P < 0·05; Fig. 1).
Fig. 1.
Percentage change in (a) paw volume, (b) histology scores and (c) radiography scores of rats during the time course of adjuvant arthritis, and in the saline (Sal) and Incomplete Freund's Adjuvant (ICFA) control groups. * represents a significant increase from day 0, P < 0·05 (n = 56, 7, 7, 7, 35, 28, 21, 14 and 7 on days 0, 3, 6, 9, 13, 17, 21, 24 and 28, respectively).
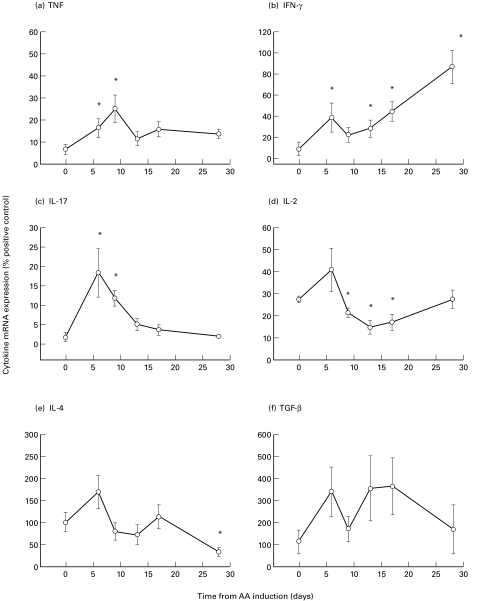
Cytokine mRNA expression in the inguinal lymph nodes of rats with adjuvant arthritis
IL-17 mRNA expression was significantly increased at days 6 and 9 (P < 0·05; Fig. 2c). TNF mRNA expression also significantly increased at days 6 and 9 (_P_ < 0·05; Fig. 2a). IFN-γ mRNA expression followed a biphasic pattern, with an initial significant increase at day 6, followed by a sustained increase from day 13 through 28 (_P_ < 0·05; Fig. 2b). In contrast, IL-2 mRNA expression significantly decreased from days 9–17 (_P_ < 0·05; Fig. 2d). However, IL-4 mRNA expression did not change until a significant decrease was observed at day 28 (_P_ < 0·05; Fig. 2e). TGF-β mRNA expression did not significantly change over the time course of the disease (_P_ > 0·05; Fig. 2f).
Fig. 2.
Cytokine mRNA expression of (a) TNF, (b) IFN-γ, (c) IL-17, (d) IL-2, (e) IL-4 and (f) TGF-β in the inguinal lymph nodes of rats with adjuvant arthritis (AA). * represents a significant difference from day 0, P < 0·05 (n = 5–7 at each time point).
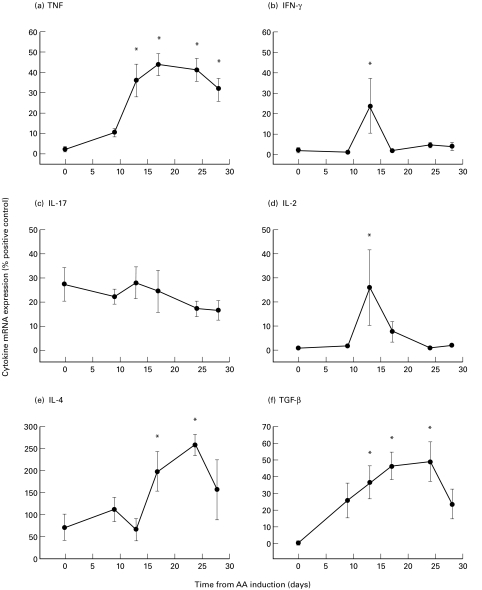
Cytokine mRNA expression in the synovium of rats with adjuvant arthritis
In the synovium, TNF mRNA expression significantly increased at day 13 and remained high until day 28 (P < 0·05; Fig. 3a). IFN-γ and IL-2 mRNA expression significantly increased in the synovium at day 13 (_P_ < 0·05; Fig. 3b,d). IL-4 mRNA expression increased significantly at days 17 and 24 in the synovium (_P_ < 0·05; Fig. 3e). TGF-β mRNA expression significantly increased from days 13–24 (_P_ < 0·05; Fig. 3f). In the initial study, IL-17 mRNA expression did not change throughout the time course (_P_ > 0·05; Fig. 3c), but additional experiments demonstrated that there was a significant increase in IL-17 mRNA expression at day 13 compared with day 0 (% positive control: day 0 versus day 13: 22 ± 6 versus 37 ± 5, P < 0·05).
Fig. 3.
Cytokine mRNA expression of (a) TNF, (b) IFN-γ, (c) IL-17, (d) IL-2, (e) IL-4 and (f) TGF-β in the synovium of rats with adjuvant arthritis (AA). * represents a significant difference from day 0, P < 0·05 (n = 5–7 at each time point).
IFN-γ protein
Inguinal lymph node cultures at day 0 and 6 of AA produced 42 ± 12 and 59 ± 13 pg/ml IFN-γ, respectively (P > 0·05).
TNF protein
Synovial membrane cultures at day 17 of AA produced between 5 and 30 pg/ml of biologically active TNF. A sheep antimouse polyclonal antibody completely blocked the TNF produced by the synovial cultures.
Inguinal lymph node cultures at day 17 of AA produced between 50 and 150 pg/ml biologically active TNF. A sheep antimouse polyclonal antibody did not completely block the TNF produced by these cultures suggesting lymphotoxin was also produced.
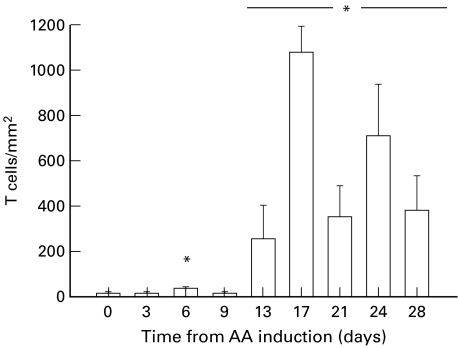
T-cell numbers in the joints of rats with adjuvant arthritis
The number of T-cells in the joints of rats with AA showed a transient, but significant increase at day 6, with a sustained increase from days 13–28, compared with day 0 (P < 0·05; Fig. 4).
Fig. 4.
T cell numbers in the joints of rats over the time course of adjuvant arthritis (AA). * represents a significant difference from day 0, P < 0·05 (n = 6–7 at each time point).
Correlations between cytokine expression and radiology, histology and paw volume
In the synovium, there was a significant correlation between TNF mRNA expression and radiology, histology and paw volume scores (_r_2 = 0·39, 0·66 and 0·65, respectively, P < 0·05). There was also a significant correlation between TGF-β mRNA expression and radiology, histology and paw volume scores (_r_2 = 0·59, 0·53 and 0·46, respectively, P < 0·05). In addition, there was a significant correlation between IL-4 mRNA expression and radiology and histology scores (_r_2 = 0·43 and 0·40, respectively, P < 0·05).
Similarly, there was a significant correlation between synovial T-cell numbers and radiology, histology and paw volume scores (_r_2 = 0·51, 0·8 and 0·6, respectively, P < 0·05). Furthermore, there was a significant correlation between synovial T-cell numbers and expression of TNF, IL-4 and TGF-β (_r_2 = 0·67, 0·39 and 0·39, respectively, P < 0·05).
DISCUSSION
In this study we have described the changes in cytokine mRNA in the synovium and inguinal lymph nodes of rats with adjuvant arthritis up to 28 days post‐induction, including the onset, established and later remission phases, in the context of oedematous, histological and radiological changes. There have been no previous studies investigating changes in cytokine mRNA expression beyond day 20 of adjuvant arthritis. We have shown for the first time that IL-17 mRNA is expressed in the initial immune activation within the inguinal lymph node. In addition, we have described cytokine changes that may mediate the spontaneous resolution of adjuvant arthritis later in the disease process.
The expression of pro-inflammatory cytokines (TNF, IFN-γ, IL-2 and IL-17) was increased in the inguinal lymph nodes in the early stages of adjuvant arthritis, reflecting the immunological events within these lymph nodes which drain the adjuvant injection site [26–28]. If the inguinal lymph nodes are removed before the fifth day after injection of adjuvant, arthritis does not develop [28]. TGF-β expression also increased at this stage, but no increases were seen in IL-4 expression. The expression of these cytokines was transient, with the exception of IFN-γ, which showed a second sustained increase later in the disease. In other models, a rapid increase in TNF expression in the inguinal lymph nodes has also been observed [11]. Studies in collagen-induced arthritis in rats have shown an increase in IFN-γ expression in the inguinal lymph nodes in established arthritis [11], consistent with our findings, although earlier time points (e.g. prearthritis, days 3–6) were not investigated. Previous studies in collagen-induced arthritis in mice have not detected IL-4 expression in the inguinal lymph nodes [11]. The presence of TNF and IFN-γ protein and their patterns of release in both synovial and inguinal lymph node culture supernatants supports the mRNA expression data which showed mRNA for TNF and IFN-γ in the synovium and inguinal lymph nodes.
In our study, increased expression of cytokines in the synovial membrane was observed only at the time of arthritis onset with transient increases in IFN-γ and IL-2, consistent with other studies [13], and in a second experiment, IL-17, at day 13 only. In contrast, TNF and TGF-β expression was increased for a prolonged period, only decreasing during the resolution phase. IL-4 mRNA expression became significantly elevated during the later stages of the disease. TNF expression followed a similar pattern to the clinical expression of disease as measured by paw volume. In murine antigen-induced arthritis and collagen-induced arthritis, TNF expression [10] and production [9] was maximal at the time of peak paw inflammation, consistent with our findings. Furthermore, an increase in TNF protein levels in whole joint extracts between days 18–20 in rats with adjuvant arthritis has been demonstrated by others [29–30] using a TNF bioassay, ELISA's and immunohistochemistry. The increase in IFN-γ expression in the joint at day 13 was consistent with studies in collagen-induced arthritis, which have shown an increase in IFN-γ in the joint early in arthritis development [9]. Similarly, studies in antigen-induced arthritis have demonstrated an increase in IFN-γ expression in the joints in the acute phase of the disease [10]. Despite the lack of IL-4 in the joints of mice with collagen-induced arthritis [9], IL-4 was strongly detected in the joints in the acute phase of antigen-induced arthritis, around days 1–2 after induction. In our study, synovial IL-4 expression increased later in the disease, at days 17 and 21. This increase in expression of synovial IL-4, an anti-inflammatory cytokine, may be important in the spontaneous resolution of adjuvant arthritis. Similarly, studies in experimental autoimmune encephalomyelitis (EAE) have shown that natural recovery of the disease is associated with upregulation of IL-4 in the central nervous system [31].
The recruitment of T-cells to the joints of rats with adjuvant arthritis observed in this study was similar to that reported in collagen-induced arthritis [32] and adjuvant arthritis [16], with T-cell numbers peaking at the time of maximum paw inflammation. A small increase in T-cell numbers was noted in the joint at day 6, similar to Holmdahl's study in collagen-induced arthritis [32]. This transient increase in T-cell numbers may play an important role in the initiation of adjuvant arthritis. The later increase in synovial T-cell numbers in rats with adjuvant arthritis follows a similar pattern to mast cells and macrophages, which we have previously found to significantly increase from day 13–28 [33]. In the present study, synovial T-cell numbers were strongly correlated with radiography changes, suggesting that T-cells may be associated with joint damage in adjuvant arthritis. In support of this finding, activated T-cells have recently been shown to produce osteoprotegerin ligand, a major mediator of bone loss [34], providing a novel paradigm for T-cells as regulators of bone damage in inflammatory arthritis.
IL-17 is a newly described T-cell cytokine secreted by CD4 + T-cells [2, 35] and is spontaneously produced by RA synovium [36]. It has been shown to induce the release of IL-6, IL-8, G-CSF and PGE2 from synovial fibroblasts, to activate the transcription factor κB (NF-κB) and to enhance the surface expression of ICAM-1 [2,35]. IL-17 also stimulates the secretion of inflammatory cytokines IL-1β and TNF by macrophages [37]. It increases inflammatory infiltration of the synovium and cartilage degradation when injected into the knee joint of mice [3].
In our study, IL-17 expression demonstrated a similar time course to TNF in the inguinal lymph nodes, consistent with its pro-inflammatory effects. IL-17 mRNA expression was found at low levels in normal joint tissue and there was a significant increase in IL-17 expression in the synovium at day 13, compared with day 0. This increase occurred at the same time as IFN-γ and IL-2, suggesting it was part of a synovial T-cell activation process in the early phase of adjuvant arthritis. This is the first report of IL-17 expression in experimental adjuvant arthritis. In addition to its pro-inflammatory properties, IL-17 shows synergy with TNF for bone resorption [4] and has been shown to stimulate osteoclast differentiation [38]. Therefore, IL-17 has properties which could contribute to both pro-inflammatory immune mechanisms and to cartilage and bone damage. The role of IL-17 in the initiation of adjuvant arthritis and in the joint damage in adjuvant arthritis will require further studies using IL-17 blocking strategies.
Although IFN-γ is considered to be an archetypal Type I pro-inflammatory cytokine, it has been shown to have biphasic effects in adjuvant arthritis and collagen-induced arthritis [39–41]. Clear evidence from anti-IFN-γ monoclonal antibody treatment studies in adjuvant arthritis suggest that IFN-γ may enhance the pro-inflammatory immune response during the induction phase of experimental arthritis, but that blocking IFN-γ function later in the disease leads to an exacerbation of adjuvant arthritis. This suggests that IFN-γ may have an immunoregulatory role later in the course of AA, as has been demonstrated in other animal models such as EAE.
In EAE, there is an initial expansion of Type I CD4 cells and a late expansion of Type II cells. It has been shown that the spontaneous remission of EAE is due to the development of regulatory T-cells, of the CD4 and CD8 subtype [42], which use IFN-γ dependent mechanisms to disable disease initiating CD4 T-cells. Similar mechanisms have been shown in collagen-induced arthritis [41]. The mechanism of spontaneous remission in adjuvant arthritis has not been fully explored, but our data suggest that it could involve regulatory T-cells acting through IFN-γ dependent mechanisms [40, 41]. The initial increase in IFN-γ in the inguinal lymph node may be a pro-inflammatory response which when blocked by anti-IFN-γ monoclonal antibody results in disease suppression. In contrast, the prolonged increase later in the disease may reflect regulatory T-cell activity, which when blocked with anti-IFN-γ monoclonal antibody results in prolonged disease activity.
In summary, we have described the time course of cytokine expression and characterized the radiology, histology and T-cell recruitment to the joint over 28 days of adjuvant arthritis. The early increase in IL-17 expression in the inguinal lymph node and T-cell recruitment to the joint may play an important role in the initiation of adjuvant arthritis. The increase in IL-4 in the synovium in the later stages of disease may be important in the spontaneous resolution of adjuvant arthritis, and the secondary increase in IFN-γ in the ILN may reflect activation of regulatory T-cells.
Acknowledgments
This study was funded by grants from the National Health and Medical Research Council of Australia (970851 to JSW), ARC and Glaxo Wellcome (to KB).
The authors thank Dr Caroline Scott for her excellent technical assistance with the study and Angelina Enno for her help with the immunohistochemistry.
REFERENCES
- 1.Arend WP, Dayer JM. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–60. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- 2.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudler J, Busso N, Peclat V, Lotz M. & So, A. In vivo effects of murine recombinant interleukin-17 on synovial joint in mice. Arthritis Rheum. 1997;40:S273. (Abstract) [Google Scholar]
- 4.van Bezooijen RL, Farih-Sips HCM, Papapoulos SE, Löwik CWGM. Interleukin-17: a new bone acting cytokine in vitro. J Bone Miner Res. 1999;14:1513–21. doi: 10.1359/jbmr.1999.14.9.1513. [DOI] [PubMed] [Google Scholar]
- 5.Chabaud M, Durand JM, Buchs N, et al. Human interleukin-17. A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–70. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 7.Troutt AB, Kelso A. Enumeration of lymphokine mRNA-containing cells in vitro in a murine graft-versus-host reaction using PCR. Proc Natl Acad Sci USA. 1992;89:5276–80. doi: 10.1073/pnas.89.12.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panayi GS, Lanchbury JS, Kingsley GH. The importance of the T-cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992;35:729–35. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- 9.Mussener A, Litton MJ, Lindroos E, Klareskog L. Cytokine production in synovial tissue of mice with collagen-induced arthritis (CIA) Clin Exp Immunol. 1997;107:485–93. doi: 10.1046/j.1365-2249.1997.3181214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hersmann GHW, Kriegsmann J, Simon J, Huttich C, Brauer R. Expression of cell adhesion molecules and cytokines in murine antigen-induced arthritis. Cell Adhes Commun. 1998;6:69–82. doi: 10.3109/15419069809069761. [DOI] [PubMed] [Google Scholar]
- 11.Mussener A, Klareskog L, Lorentzen JC, Kleinau S. TNF-α dominates cytokine mRNA expression in lymphoid tissues of rats developing collagen-induced and oil-induced arthritis. Scand J Immunol. 1995;42:128–34. doi: 10.1111/j.1365-3083.1995.tb03635.x. [DOI] [PubMed] [Google Scholar]
- 12.Connolly AR, Cleland LG. Cytokine mRNA expression in adjuvant arthritis. Arthritis Rheum. 1997;39:S79. (Abstract) [Google Scholar]
- 13.Schmidt-Weber CB, Pohlers D, Buchner E, et al. Cytokine gene activation in synovial membrane, regional lymph nodes, and spleen during the course of rat adjuvant arthritis. Cell Immunol. 1999;195:53–65. doi: 10.1006/cimm.1999.1509. [DOI] [PubMed] [Google Scholar]
- 14.Pelegri C, Morante MP, Castellote C, Franch A, Castell M. Treatment with an anti-CD4 monoclonal antibody strongly ameliorates established rat adjuvant arthritis. Clin Exp Immunol. 1996;103:273–8. doi: 10.1046/j.1365-2249.1996.d01-624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billingham MEJ, Hicks C, Carney S. Monoclonal antibodies and arthritis. Agents Actions. 1990;29:77–87. doi: 10.1007/BF01964727. [DOI] [PubMed] [Google Scholar]
- 16.Pelegri C, Franch A, Castellote C, Castell M. Immunohistochemical changes in synvoial tissue during the course of adjuvant arthritis. J Rheumatol. 1995;22:124–32. [PubMed] [Google Scholar]
- 17.Binder W, Scott C, Walker JS. Involvement of substance P in the anti-inflammatory effects of the peripherally selective κ-opioid asimadoline and the NK1 antagonist GR205171. Eur J Neurosci. 1999;11:2065–72. doi: 10.1046/j.1460-9568.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirkham B, Portek I, Lee CS, et al. Intraarticular variability of synovial membrane histology, immunohistology, and cytokine mRNA expression in patients with rheumatoid arthritis. J Rheumatol. 1999;26:777–84. [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 20.Farges O, Morris PJ, Dallman MJ. Spontaneous acceptance of rat liver allografts is associated with an early downregulation of intragraft interleukin-4 messenger RNA expression. Hepatology. 1995;21:767–75. [PubMed] [Google Scholar]
- 21.McKnight AJ, Barclay AN, Mason DW. Molecular cloning of rat interleukin 4 cDNA and analysis of the cytokine repertoire of subsets of CD4+ T cells. Eur J Immunol. 1991;21:1187–94. doi: 10.1002/eji.1830210514. [DOI] [PubMed] [Google Scholar]
- 22.Connolly AR. Cytokine gene expression in a rat model of polyarthritis. PhD Thesis University of Adelaide. 1998.
- 23.Rouvier E, Luciani M, Mattei M, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell bearing AU-rich messenger RNA instability sequences, and homologous to a Herpesvirus Saimiri gene. J Immunol. 1993;150:5445–56. [PubMed] [Google Scholar]
- 24.Butler DM, Maini RN, Feldmann M, Brennan FM. Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures. Comparison of monoclonal anti TNF-α antibody with the interleukin-1 receptor antagonist. Eur Cytokine Netw. 1995;6:225–30. [PubMed] [Google Scholar]
- 25.Espevik T, Nissen-Meyer J. A hightly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 26.Koga T, Vande Sande B, Yeaton R, Pearson CM. Reevaluation of inguinal lymph node injection for production of adjuvant arthritis in the rat. Int Arch Allergy Immunol. 1976;51:359–67. doi: 10.1159/000231609. [DOI] [PubMed] [Google Scholar]
- 27.Spargo LDJ, Hawkes JS, Cleland LG, Mayrhofer G. Recruitment of lymphoblasts derived from peripheral and intestinal lymph to synovium and other tissues in normal rats and rats with adjuvant arthritis. J Immunol. 1996;157:5198–207. [PubMed] [Google Scholar]
- 28.Newbould BB. Role of lymph nodes in adjuvant-induced arthritis in rats. Ann Rheum Dis. 1964;23:392–6. doi: 10.1136/ard.23.5.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith-Oliver T, Noel LS, Stimpson SS, Yarnall DP, Connolly KM. Elevated levels of TNF in the joints of adjuvant arthritic rats. Cytokine. 1993;5:298–304. doi: 10.1016/1043-4666(93)90060-i. [DOI] [PubMed] [Google Scholar]
- 30.Szekanecz Z, Halloran MM, Volin MV, et al. Temporal expression of inflammatory cytokines and chemokines in rat adjuvant arthritis. Arthritis Rheum. 2000;43:1266–77. doi: 10.1002/1529-0131(200006)43:6<1266::AID-ANR9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 31.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–64. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmdahl R, Rubin K, Klareskog L, Dencker L, Gustafson G, Larsson E. Appearance of different lymphoid cells in synovial tissue and in peripheral blood during the course of collagen II-induced arthritis in rats. Scand J Immunol. 1985;21:197–204. doi: 10.1111/j.1365-3083.1985.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 33.Walker J, Wilson J, Binder W, et al. 9th World Congress on Pain. Vienna, Austria: Immunopharmacology of opioids in inflammatory arthritis; p. 20. August 22–27(Abstract) [Google Scholar]
- 34.Kong Y, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 35.Yao Z, Painter SL, Fansow W, Ulrich D, Macduff B, Spriggs M. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–6. [PubMed] [Google Scholar]
- 36.Fouilhoux A, Chabaud M, Fossiez F. & Miossec, P. Production of IL-17 and its regulation in rheumatoid synovium. Arthritis Rheum. 1997;40:S272. (Abstract) [Google Scholar]
- 37.Jovanovic D, DiBattista J, Martel-Pelletier J, et al. IL-17 stimulates the secretion of proinflammatory cytokines by human macrophages. Arthritis Rheum. 1997;40:S272. (Abstract) [Google Scholar]
- 38.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiesenberg I, Van Der Meide PH, Schellekens H, Alkan SS. Suppression and augmentation of rat adjuvant arthritis with monoclonal anti-interferon-gamma antibody. Clin Exp Immunol. 1989;78:245–9. [PMC free article] [PubMed] [Google Scholar]
- 40.Jacob CO, Holoshitz J, Van der Meide P, Strober S, McDevitt HO. Heterogeneous effects of IFN-γ in adjuvant arthritis. J Immunol. 1989;142:1500–5. [PubMed] [Google Scholar]
- 41.Boissier M, Chiocchia G, Bessis N, et al. Biphasic effect of interferon-γ in murine collagen-induced arthritis. Eur J Immunol. 1995;25:1184–90. doi: 10.1002/eji.1830250508. [DOI] [PubMed] [Google Scholar]
- 42.Kumar V, Sercarz E. Induction or protection from experimental autoimmune encephalomyelitis depends on the cytokine secretion profile of TCR peptide-specific regulatory CD4 T cells. J Immunol. 1998;161:6585–91. [PubMed] [Google Scholar]