Expression and functional activity of CXCR-4 and CCR-5 chemokine receptors in human thymocytes (original) (raw)
Abstract
In this paper we addressed the expression of the HIV co-receptors CXCR-4 and CCR-5 in human thymocytes by phenotypic, molecular and functional approaches. Cytofluorimetric analysis disclosed that CXCR-4 was constitutively expressed by freshly isolated thymocytes (˜10 000 molecules/cell in about 30% of thymocytes); the receptor was endowed with functional activity, as it mediated polarization, migration and intracellular Ca2+ increase in response to its ligand, SDF-1. On the contrary, CCR-5 expression in freshly isolated thymocytes was significantly lower (<4000 molecules/cell in less than 5% of the cells), and no functional response to CCR-5 agonists could be documented. Northern blot analysis of freshly isolated thymocytes showed high CXCR-4 mRNA levels, whereas the message for CCR-5 was barely detectable. On the other hand, a modest increase in the expression of CCR-5 was associated with in vitro thymocyte stimulation, and CCR-5 density at the cell surface attained CXCR-4 figures in most cases. None the less, no functional response to CCR-5 agonists could be documented in in vitro stimulated thymocytes. In vitro infection of thymocytes by CAT-expressing recombinant HIV bearing the envelope glycoproteins from different isolates showed that T-tropic strains, which use CXCR-4 as a co-receptor, were more efficient in infecting thymocytes than M-tropic strains, which preferentially use CCR-5. Altogether, these data indicate that expression of the major co-receptors involved in infection by M-tropic HIV strains is very poor in human thymocytes, and would suggest that thymocyte infection by M-tropic HIV strains may be a rare event in vivo.
Keywords: CXCR-4, CCR-5, HIV, thymus
INTRODUCTION
Investigation into human immunodeficiency virus (HIV) infection received a new drive from the discovery of second receptors [1–6], which co-operate with CD4 in HIV–target cell interaction and eventual virus entry. Several members of a large family of chemokine receptors may act as co-receptors; among these CCR-5, whose ligands are MIP-1α, MIP-1β and RANTES [1] and CXCR-4, which binds SDF-1 [7], were best characterized. CCR-5 is thought generally to favour infection by monocytotropic (M-tropic) HIV strains, whereas CXCR-4 expression is generally associated with infection by T-tropic, highly cytopathic strains for reviews, see 8–11]. The importance of these co-receptors in the natural history of HIV infection and acquired immune deficiency syndrome (AIDS) development is currently a major issue, and several studies addressed CCR-5/CXCR-4 expression and cell infectability as a function of different stimuli or cell cycle stages [12–19].
Whether thymus infection by HIV plays a significant role in AIDS pathogenesis is debated; this issue may be of particular importance in the setting of paediatric AIDS infection. In vivo data are scarce; obvious ethical constraints preclude invasive approaches in patients, while post-mortem assessment of thymus histology only reveals the effects of thymic architecture derangement, without providing information on its evolution for a review, see 20]. On the contrary, experiments performed in vitro [21–25] are abundant, and clearly showed infectability of all thymocyte subsets, in particular with more immature phenotypes. Several experimental variables may affect results, however; these include the strain and tropism of the virus employed, the doses utilized for infection, and the experimental procedures adopted for cell activation. Experiments conducted in vivo by employing human thymus/liver-reconstituted severe combined immunodeficiency (SCID-hu) mice [26–29] may mirror more closely what occurs in humans; in this case as well, however, thymus infection and eventual changes depend on the virus strain and doses employed [28,29].
In vitro studies have also shown that the relative expression of CD4 and CCR-5 are a major determinant of the efficiency of viral entry [30], and cells with poor CD4 expression require high HIV co-receptor levels for infection, whereas a few co-receptor molecules are needed when CD4 expression is high. In this paper, we provided a quantitative analysis of the expression of CD4 and CXCR-4/CCR-5 in the major thymus subpopulations as both the percentage of positive cells and the number of surface molecules per cell. In addition, we supplemented phenotypic data by comparing co-receptor mRNA expression, and by investigating the functional thymocyte response to co-receptor natural ligands; finally, we compared the in vitro susceptibility of thymocytes to infection by reporter viruses endowed with different tropism.
MATERIALS AND METHODS
Cell preparation and culture
Informed consent was obtained from the parents of all the patients participating in this study, and institutional human experimentation guidelines were followed. Thymic fragments were obtained from HIV-seronegative children undergoing corrective cardiac surgery (nine males and 11 females, age 0–4 years). The CCR-5 genotype of the patients was determined as described elsewhere [31]; all the donors were homozygous for the wild-type allele of the CCR-5 gene. The tissue was teased with blunt forceps, and the resulting cell suspension was washed twice with RPMI 1640 medium containing 10% fetal calf serum (FCS; Gibco, Grand Island, NY, USA). The cells were counted and either analysed as such, or cultured in vitro as reported [32], alone and in the presence of phytohaemagglutinin (PHA, Difco, Detroit, MI, USA, 1 μg/ml) or recombinant human IL-2 (rIL-2, courtesy of Euro Cetus-Chiron, Milan, Italy, 100 U/ml) or both. After different culture periods the cells were recovered, washed and analysed cytofluorographically. In a set of experiments, to deplete thymus cell suspensions in CD4- and CD8-expressing cells, the thymocyte suspension was incubated with anti-CD4 and anti-CD8 monoclonal antibody (MoAb) conjugated to MACS Colloidal Super-magnetic Microbeads (MACS CD4 and CD8 Microbeads, clones Sk3 and Sk1, Milteny Biotec, Germany), and passed through a depletion column placed in a magnetic separator (Vario-MACS, Milteny Biotec), according to the manufacturer’s instructions. At the end of the separation procedure, the resulting population contained <1% CD4+ and CD8+ cells, and over 70% of the cells did not express CD3. Peripheral blood lymphocytes (PBL) from three thymus donors were purified as reported elsewhere [32].
Cytofluorographic analysis
Freshly isolated or cultured thymocytes were analysed by an EPICS-Elite cytofluorometer (Coulter Electronics, Hialeah, FL, USA) as reported [33]. Three-colour immunofluorescence was carried out with the following MoAbs: anti-CD4 ECD (Coulter), anti-CD8 PE (Becton-Dickinson, San Jose, CA, USA), anti-CCR-5 (clone 2D7, PharMingen, San Diego, CA, USA) or anti-CXCR-4 (clone 12G5, PharMingen), in combination with an anti-mouse FITC MoAb (Dako, Glostrup, Denmark). For evaluation of the number of co-receptor molecules per cell in three- colour immunofluorescence, anti-CXCR-4 FITC (clone 12G5, R&D Systems, Minneapolis, MN, USA) and anti-CCR-5 FITC (clone 45549·111, R&D Systems) were used in place of the non-conjugated anti-co-receptor MoAbs. For the quantification of CD4 density at the cell surface, two-colour immunofluorescence with anti-CD4 FITC and anti-CD8 PE (both from Becton-Dickinson) MoAbs was used. Appropriate isotypic controls were analysed in parallel.
To quantify the number of CD4 and CXCR-4/CCR-5 molecules per cell, the Quantum Simply Cellular® kit (QSC; FCSC, San Juan, Puerto Rico) was employed. QSC is a mixture of four highly uniform microbead populations with varying capabilities to bind mouse monoclonal IgG; this reagent was utilized to construct a calibration curve between fluorescence intensity and the number of antibody-binding sites (ABS). According to manufacturer’s instructions, in parallel to thymocyte staining, 50 μl aliquots of QSC were stained separately for each MoAb used; the mean fluorescence intensities of the samples were converted to ABS through linear regression analysis by the dedicated QuickCal® v2·1 software.
Northern blot analysis
Total RNA was extracted from freshly isolated thymocytes (20 × 106) by the guanidium thiocyanate method, blotted and hybridized as described [34]. Probes were labelled by the Megaprime DNA labelling system (Amersham, Buckinghamshire, UK) with (α-32P)dCTP (specific activity 3000 Ci/mm). Membranes were prehybridized at 42°C in Hybrisol (Oncor, Gaithersburg, MD, USA) and hybridized overnight with 1 × 106 cpm/ml of 32P-labelled probe. Membranes were then washed three times with 2 × SSC (1 × SSC = 0·15 m NaCl, 0·015 m sodium citrate, pH 7·0), 0·1% SDS at room temperature for 10 min, twice with 2 × SSC, 1% SDS at 60°C for 20 min, and finally with 0·1 × SSC for 5 min. Autoradiographs were performed using Kodak XAR-5 films and intensifier screens at –80°C.
Chemokines and polarization assay
SDF-1 was purchased from R&D Systems; MIP-1β was purchased from Peprotech (London, UK). The gp120 glycoprotein of the HIV Ba-L strain (a strictly CCR-5-dependent HIV isolate) was a kind gift of G. Gao (Boston, MA, USA). The polarization of freshly isolated thymocytes in response to chemokines was studied as described [35]. Briefly, thymocytes (1 × 106/ml) were resuspended in RPMI-1% FCS at 37°C in the presence of optimal [36] concentrations of chemokines, zymosan-activated serum (5% final concentration, used as a reference chemoattractant) or medium alone. After 15 min, the cells were fixed with 2·5% glutaraldehyde, washed and scored by phase-contrast microscopy. A cell was considered as polarized when it changed from a spherical shape to a shape characterized by head–tail polarity, typical of migrating cells. In each experiment, at least 200 cells were counted by two independent investigators.
Chemotaxis assay
Chemotaxis assays were carried out in Transwell plates (5 μm pore size, Costar; Corning Science Products, Acton, MA, USA). Freshly isolated or in vitro cultured thymocytes were resuspended in RPMI 1% FCS at 10 × 106/ml. Optimal concentrations of chemoattractants were added to the lower wells of the Transwell system; zymosan-activated serum and plain medium were used as positive and negative controls, respectively. Transwell inserts were placed in site and thymocytes were seeded in the upper well. After incubation for 2 h at 37°C, transmigrated cells were collected in the lower chamber, and counted by phase-contrast microscopy. Four fields of a Burker chamber were counted, and results were expressed as a percentage of migrated cells relative to input cells. In a set of experiments migrated cells were recovered, and their CD4/CD8 phenotype compared to that of freshly isolated cells before chemoattractant addition; the expression of CXCR-4 and CCR-5 was also evaluated cytofluorographically.
Measurement of intracellular Ca2_+_ concentration
Changes in intracellular Ca2+ concentration Ca2+]i were monitored using the fluorescent probe Fura-2 as described previously [36], according to the technique reported by Grynkiewicz et al. [37]. Briefly, thymocytes (10 × 106/ml) were resuspended in RPMI medium and incubated with 1 μm Fura-2 acetoxymethylester (Calbiochem, La Jolla, CA, USA) at 37°C for 20 min. After incubation, the cells were washed and resuspended in HBSS (Biochrom, Berlin, Germany) containing 1·2 mm CaCl2, and kept at room temperature until use. The cells (3–5 × 106/ml) were exposed to different stimuli at 37°C under continuous stirring, and Fura-2 fluorescence was measured in a Perkin-Elmer LS50B spectrophotometer (Perkin-Elmer Instruments, Norwalk, CT, USA). Samples were excited at 340 and 380 nm, and emission at 487 nm was continuously recorded. Calibration was performed as previously described [37].
CAT assay
Recombinant HIV variants encoding chloramphenicol acetyltransferase (CAT) and containing envelope glycoproteins of laboratory-adapted (HXBc2) or primary (ADA and Ba-L) viruses were employed to evaluate the ability of thymocytes to support HIV entry. Recombinant virus was produced in the 293 cell line by the calcium phosphate-mediated cotransfection with 15 μg pHXBH10Δenv CAT and 3 μg pSVIII env plasmids as described previously [38]. The pSP65gptHXBaL molecular clone was kindly provided by Dr M. Reitz (Baltimore, MD, USA). To create the Ba-L envelope expressor plasmid the KpnI(6350)-BamHI(8478) fragment of the pSVIIIenv plasmid was replaced with the equivalent fragment from pSP65gptHXBaL. Freshly isolated thymocytes or thymocytes cultured for 4 days alone and in the presence of rIL-2 or rIL-2 and PHA were infected by overnight incubation at 37°C with the recombinant virions (50 000 cpm/106 cells of reverse transcriptase activity). After washings, the cells were plated in fresh complete medium and cultured at 37°C, 5% CO2 in air; 3 days later, the thymocytes were lysed and CAT activity measured as described previously [38].
Statistical analysis
Data were managed by StatGraphics software (Statgraphics Statistical Graphics System, version 2·6). Wilcoxon’s test was used to compare quantitative variables.
RESULTS
Cytofluorographic analysis of CXCR-4/CCR-5 expression in freshly isolated thymocytes
We first addressed CXCR-4 and CCR-5 expression within the three major thymocyte subpopulations by three-colour immunofluorescence. All the thymocyte subsets showed high expression of CXCR-4 (Fig. 1a, hatched columns). About 30% of the CD4+CD8– population expressed CXCR-4 (31·6% ± 4·92; range 26·2–37·1). CD4+CD8+ thymocytes showed a percentage expression of CXCR-4 at an intermediate level (26·08% ± 9·63; range 16–39·4) between the two single-positive populations, with no significant differences. The CD4–CD8+ subset showed the lowest CXCR-4 expression (16·7% ± 7·61; range 3–22·8), which was significantly different compared to CD4+CD8– thymocytes (P = 0·021).
Fig. 1.
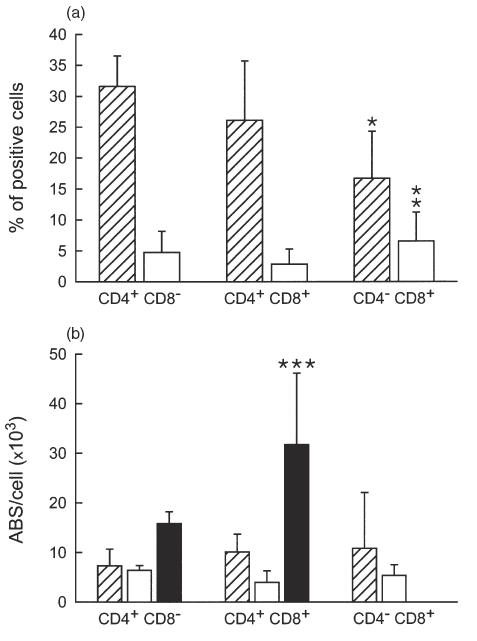
Percentage expression (a) and surface density (b) of CXCR-4 and CCR-5 co-receptors in different thymocyte subsets. (a) The results represent the relative percentage of CD4+CD8–, CD4+CD8+ and CD4–CD8+ cells also expressing CXCR-4 (hatched columns) or CCR-5 (open columns). Mean values of six consecutive experiments were reported; the bars indicate the s.d. (b) CXCR-4 (hatched columns), CCR-5 (open columns) and CD4 (closed columns) antibody-binding sites (ABS)/cell were calculated in CD4+CD8–, CD4+CD8+ and CD4–CD8+ thymocytes. Mean values of six consecutive experiments were reported; the bars indicate the s.d. *P = 0·02 compared to CD4+CD8– thymocytes; **P = 0·02 compared to CD4+CD8+ thymocytes; ***P < 0·01 compared to CD4+CD8– thymocytes.
On the contrary, CCR-5 expression was very low in freshly isolated thymocytes (Fig. 1a, open columns). The percentage of CD4+CD8– cells expressing CCR-5 (mean value 4·78% ± 3·39; range 0·8–9·7) was slightly higher, compared to CD4+CD8+ immature thymocytes (2·85% ± 2·45; range 0·6–6·7); however, this difference was not statistically significant. On the other hand, the CD4–CD8+ subset showed the highest CCR-5 expression values (6·57% ± 4·65; range 1·7–12·7); this value differed significantly, compared to the double-positive population (P = 0·021).
We also quantified the number of available receptors at the cell surface. In general, the density of CXCR-4 expression was higher than that of CCR-5 (Fig. 1b, hatched and open columns, respectively). The gap between CXCR-4 and CCR-5 was particularly evident in CD4+CD8+ and CD4–CD8+ subpopulations, where the number of CCR-5 ABS per cell was approximately one-third, compared to CXCR-4 figures (Fig. 1b). The surface expression of CD4 also was not distributed uniformly among the different subsets; as shown in Fig. 1b (black columns), CD4+CD8+ thymocytes displayed a much higher density of CD4 receptors per cell, compared to the CD4+CD8– subset (31 712 ± 14 433 ABS/cell versus 15 842 ± 2376, respectively; P < 0·01).
CXCR-4 and CCR-5 expression in double-negative and triple-negative thymocytes
To assess co-receptor expression in different stages of thymocyte development, thymocytes were depleted in cells expressing CD4 and CD8 by immunomagnetic separation, and the resulting CD4–CD8– population was analysed by double-fluorescence with anti-CD3 and anti-CXCR-4 or anti-CCR-5 mAbs. As shown in Fig. 2a, CXCR-4 expression was much higher in triple-negative thymocytes (45%) than in CD4–CD8– thymocytes expressing CD3 (11·0%). CCR-5 expression was also (Fig. 2b) more pronounced in triple-negative cells (12%) than in CD3+CD4–CD8– thymocytes (4%). Thus, it is probable that expression of both co-receptors may be regulated developmentally and undergo modulation as thymocytes start rearranging T cell receptor genes; similar data were obtained by Berkowitz et al. [39] in SCID-hu mice.
Fig. 2.
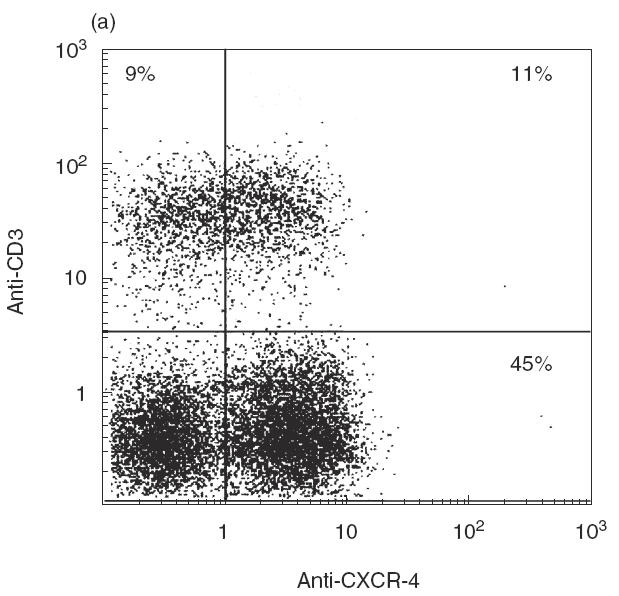
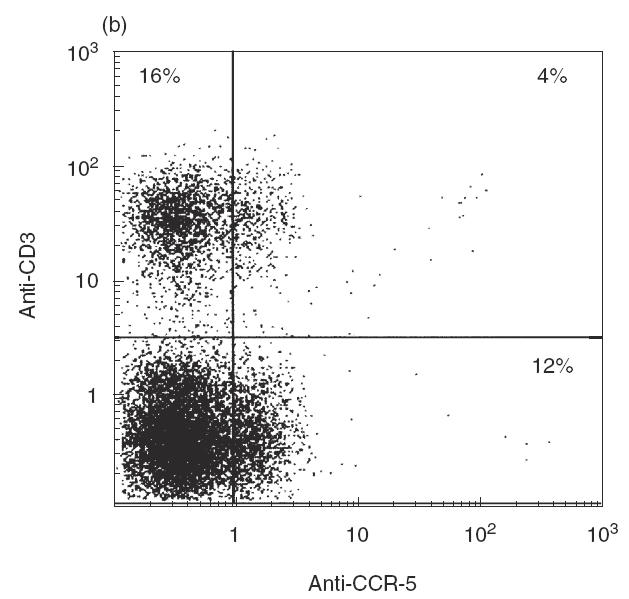
Cytofluorographic analysis of CD3 and CXCR-4/CCR-5 expression in CD4–CD8– (double-negative) thymocytes. Unfractionated thymocytes were depleted in CD4+ and CD8+ cells, and the resulting double-negative population was analysed for CD3 and co-receptor expression: (a) anti-CD3 and anti-CXCR-4; (b) anti-CD3 and anti-CCR-5 by two-colour immunofluorescence. One representative experiment of three consecutive experiments is shown; the numbers indicate the percentage of cells included in each section of the plot.
Northern blot analysis of freshly isolated thymocytes
In order to confirm phenotypic data, we addressed mRNA expression for CCR-5 and CXCR-4 in freshly isolated thymocytes. CCR-5 message in thymocytes has been reported by PCR analysis [40]; this approach, however, does not permit a quantitative evaluation of the relevant message, which only may be obtained by Northern blotting. In three consecutive experiments using freshly isolated thymocytes from different individuals (Fig. 3), Northern blot analysis of unfractionated thymocytes showed that mRNA levels for CXCR-4 could be evidenced, whereas the message for CCR-5 was barely detectable; this latter was instead clearly evident in freshly isolated PBL (Fig. 3, last lane). Thus, mRNA expression for the two chemokine receptors was in line with data obtained by phenotypic analysis, and confirmed that CCR-5 expression only occurred in a minority of thymocytes.
Fig. 3.
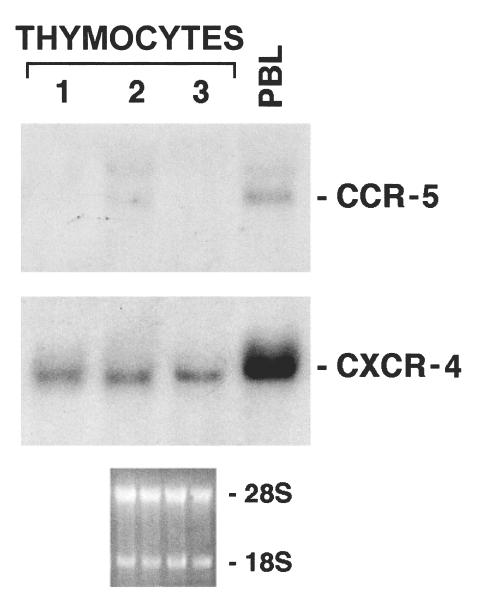
Northern blot analysis of CXCR-4 and CCR-5 mRNA expression in freshly isolated thymocytes. Three thymocyte preparations from different subjects (lanes 1, 2 and 3) were analysed. The CXCR-4 and CCR-5 messages revealed in a representative freshly isolated PBL sample is also shown. Autoradiographs for CCR-5 were obtained after 5-day exposure.
Functional response of freshly isolated thymocytes to CXCR-4 and CCR-5 agonists
The functional response to chemokines of freshly isolated thymocytes was tested initially in a chemotaxis assay. As shown in Fig. 4a, a significant number of cells migrated in response to optimal concentrations of the CXCR-4 agonist SDF-1 (100 and 1000 ng/ml), while no cell migration was observed in response to optimal doses of the CCR-5 agonist MIP-1β (10 and 100 ng/ml), which were able to induce significant migration of purified resting NK cells (not shown in Fig. 4). Phenotypic analysis of the cell population recovered after migration disclosed that the phenotypic composition of the cells responding to SDF-1 was comparable to that of the input population (data not shown); in addition, >80% of the migrated cells expressed CXCR-4. Thus, according to phenotypic data on CXCR-4 expression (Fig. 1), all three major thymocyte subsets migrated in response to the CXCR-4 agonist.
Fig. 4.
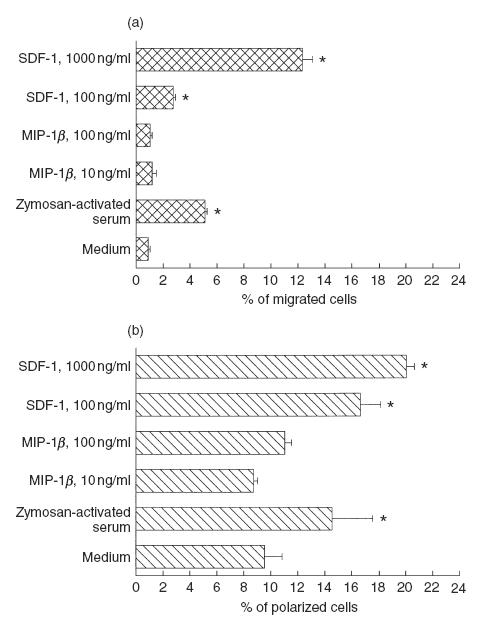
Response of freshly isolated thymocytes to chemokines in chemotactic (a) and polarization (b) assays. (a) The chemotactic assay was carried out in Transwell system with 5 μm pore filter; optimal concentrations of SDF-1 and MIP-1β were added in the lower filter. Unfractionated freshly isolated thymocytes were added in the upper filter and incubated for 2 h. Transmigrated cells were collected from the lower well and counted. (b) Thymocytes were exposed to SDF-1 and MIP-1β for 15 min, and then fixed. Polarized cells were scored by phase contrast microscopy and identified as cells that changed from a spherical shape to a shape characterized by head–tail polarity. In both assays, zymosan-activated serum was used as a reference chemoattractant. Mean values of four consecutive experiments were reported; the bars indicate the s.d., and the asterisks indicate significative responses (P < 0·001), compared to controls (medium alone).
In order to exclude a partial thymocyte unresponsiveness in the classical chemotaxis assay, due to the immaturity of their anchorage apparatus (such as low expression of membrane integrins) we also performed a polarization assay, which measures the change from a spherical shape to a shape characterized by head–tail polarity, typical of migrating cells. In two independent experiments, SDF-1 addition was associated with significant polarization in freshly isolated thymocytes, whereas no effect was observed with MIP-1β (Fig. 4b). The polarization of thymocytes in response to SDF-1 was similar or greater, compared to that obtained with activated serum (as a source of C5a) used as reference chemoattractant.
To support further the above data, we finally addressed the effect of co-receptor ligands on Ca2+ influx into freshly isolated thymocytes. As shown in Fig. 5, in the presence of optimal concentrations of MIP-1β no response was observed in terms of Ca2+]i increase. On the contrary, SDF-1 induced a rapid and sharp increase in Ca2+]i (Fig. 5). Comparable data were observed in all the three functional assays when supra-optimal concentrations of MIP-1β up to 300 ng/ml were used, and when thymocytes were stimulated with RANTES, another CCR-5 agonist, at concentrations up to 100 ng/ml (data not shown). In total, these data confirm from a functional point of view the scarce CCR-5 expression in thymocytes observed by phenotypic and molecular approaches.
Fig. 5.
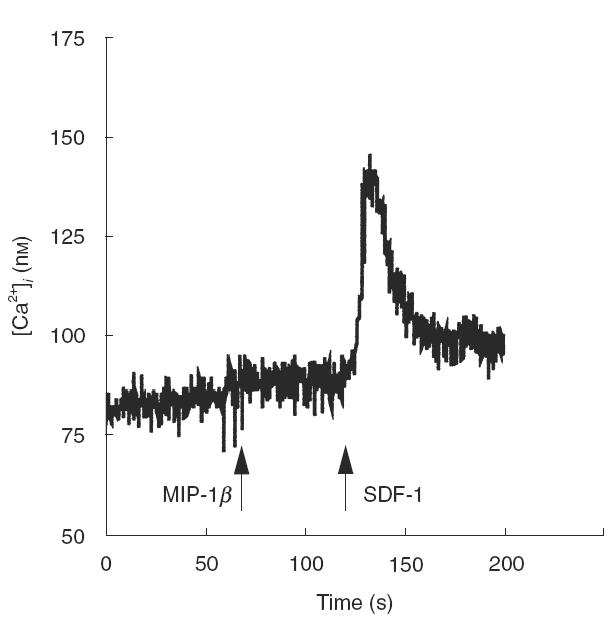
Effect of chemokines on intracellular calcium mobilization. Freshly isolated thymocytes were loaded with Fura-2AM, as detailed in Materials and methods, and exposed to MIP-_1_β (100 ng/ml) or SDF-1 (1000 ng/ml). The flux profile is representative of three consecutive experiments.
In vitro infection of human thymocytes
To determine whether the different expression of HIV co- receptors could correlate with a differential susceptibility to infection with T-tropic and M-tropic viral strains, fresh or cultured human thymocytes were infected in vitro with pseudotyped CAT- expressing recombinant HIV, carrying the envelope glycoproteins of laboratory-adapted T cell-tropic (HXBc2) or M-tropic (ADA and Ba-L) isolates. Since in vitro infection experiments necessarily entail lymphocyte activation, and cell culture and activation may be associated with modulation of membrane molecules [41], the expression and the density of CD4 and HIV co-receptors was monitored in parallel over the entire infection period.
Thymocytes, both freshly isolated and cultured under different conditions, supported viral entry of both M-tropic and T-tropic HIV strains (Fig. 6, left panels). However, CAT activity was consistently lower in the case of the M-tropic virus (open and hatched columns) compared to the T-tropic virus (closed columns). Indeed, the observation that the infectivity of the T-tropic and the M-tropic ADA isolate differed only by about 15–30% was not surprising, in view of the fact that the ADA virus also depends on CCR-3 receptor [2]; this finding suggests strongly that additional co-receptors may be into play. Interestingly, the Ba-L isolate, which is strictly dependent on CCR-5 expression [1], showed the lowest ability to infect thymocytes (Fig. 6). These data are consistent with those already reported by Zaitseva et al. [42], who also reported thymocyte infection by the HIV Ba-L isolate, despite no functional responses to CCR-5 ligands.
Fig. 6.
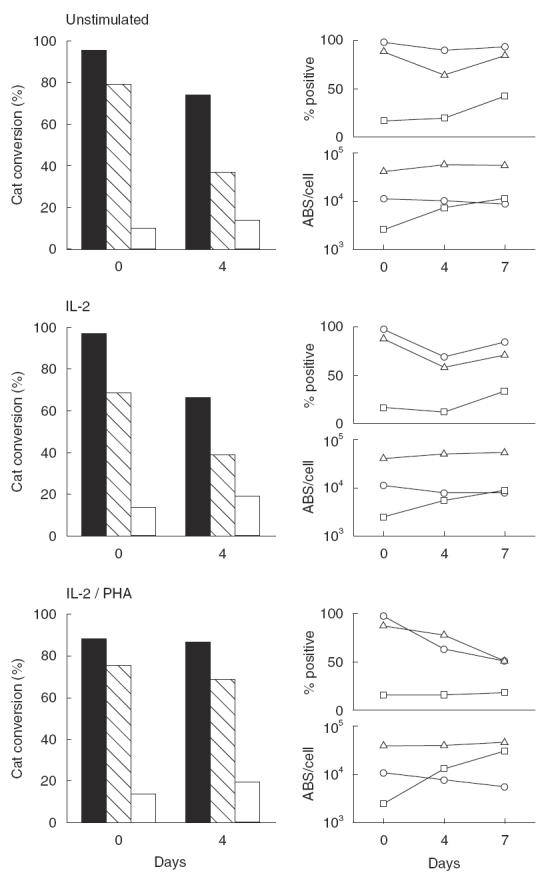
CAT activity in human thymocytes infected with recombinant HIV variants. Left panels: freshly isolated or 4-day cultured thymocytes were infected with recombinant viruses containing envelope glycoproteins of the T-tropic HXBc2 (▪), of the M-tropic ADA ( ), and of the M-tropic Ba-L (□) isolates. The CAT assay was performed as detailed in Materials and methods; data from one of three representative experiments are presented. Right panels: non-infected thymocytes were analysed in parallel for percentage expression and surface density (ABS/cell) of CD4 (Δ), CXCR-4 (○) and CCR-5 (□).
), and of the M-tropic Ba-L (□) isolates. The CAT assay was performed as detailed in Materials and methods; data from one of three representative experiments are presented. Right panels: non-infected thymocytes were analysed in parallel for percentage expression and surface density (ABS/cell) of CD4 (Δ), CXCR-4 (○) and CCR-5 (□).
Co-receptor expression and density over the entire culture period paralleled the infection profile (Fig. 6, right panels). Percentage of CD4 expression declined slowly over time from baseline levels, in particular after 4 days of culture, but the number of ABS/cell was substantially unchanged. Percentage of CXCR-4 expression also did not undergo significant changes, compared to preculture figures, and CXCR-4 density at the cell surface slowly declined under all the culture conditions tested. As far as CCR-5 expression was concerned, although the percentage of CCR-5+ cells increased in most cases over the culture period, it remained constantly lower than CXCR-4 expression (Fig. 6, right panels). On the contrary, the density of CCR-5 expression on the cell surface increased in all the culture conditions tested, and the number of CCR-5 ABS/cell reached CXCR-4 levels in non-stimulated and IL-2-stimulated cultures. Indeed, in the case of IL-2/PHA stimulation, the number of CCR-5 ABS/cell increased strikingly, and was about fivefold higher than that of CXCR-4 ABS by the end of the culture period (Fig. 6, lower right panels).
Effect of in vitro culture on thymocyte functional responses to CXCR-4 and CCR-5 agonists
As in vitro culture was associated with up-regulation of CCR-5 expression, particularly in terms of surface density (Fig. 6, right panels), we also tested the ability of thymocytes cultured under the different conditions to respond to the appropriate ligands. As shown in Table 1, the response of freshly isolated thymocytes to CXCR-4 and CCR-5 agonists was comparable to that shown in Fig. 4, and a strong response to SDF-1 could be demonstrated in thymocytes cultured alone and in the presence of IL-2 or IL-2/PHA (P < 0·01 compared to control); in the case of IL-2/PHA-stimulated thymocytes, although a significant increase in the spontaneous migratory activity was recorded (14·0 ± 0·3 in the presence of plain medium), SDF-1 was still able to evoke a significant migratory response (33·0 ± 3·0, Table 1). On the contrary, no response could be evicenced in any culture condition in the presence of MIP-1β (Table 1). Thus, although CCR-5 is up-regulated following in vitro culture, it is probable that its expression is still too low for the sensitivity limits of our techniques. Finally, we also tested the effect of gp120 glycoprotein from the M-tropic strain Ba-L, that was able to infect freshly isolated thymocytes despite poor CCR-5 expression, on the migratory capacity of thymocytes. As shown in Table 1, the Ba-L envelope glycoprotein did not cause any functional response up to doses of 10 μg/ml; no response was also observed when the effect of gp120 was evaluated on Ca2+ influx into the cells (data not shown).
Table 1.
Migratory response of freshly isolated and in vitro culturedthymocyes to CCR-5 and CXCR-4 agonists a
| In vitro cultured | ||||
|---|---|---|---|---|
| Freshly isolated | Unstimulated | IL-2 | PHA + IL-2 | |
| Control | 1·2 ± 0·5b | 0·6 ± 0·2 | 0·7 ± 0·1 | 14·0 ± 0·3 |
| SDF-1 | 5·8 ± 0·7* | 1·7 ± 0·1* | 4·1 ± 0·3* | 33·0 ± 3·0* |
| MIP-1β | 1·2 ± 0·6 | 0·7 ± 0·2 | 0·7 ± 0·1 | 12·8 ± 0·6 |
| Ba-L gp120 | ||||
| 0·1 μg/ml | 1·1 ± 0·5 | 0·4 ± 0·1 | 0·5 ± 0·1 | 14·6 ± 0·9 |
| 1 μg/ml | 1·8 ± 0·7 | 0·5 ± 0·2 | 0·5 ± 0·3 | 13·2 ± 0·2 |
| 10 μg/ml | 1·3 ± 0·5 | 0·2 ± 0·1 | 0·9 ± 0·2 | 13·9 ± 1·0 |
DISCUSSION
By different phenotypic and functional approaches, we showed that freshly isolated thymocytes have significant expression of CXCR-4, whereas CCR-5 expression is very low; for the first time, the difference in the expression of the two co-receptors was quantified as both the percentage of cells expressing the relevant molecules and their density at the cell surface. Expression of CCR-5 was up-regulated, although with some variability, following in vitro cell culture and activation; these findings confirm the profile of co-receptor expression observed in peripheral blood lymphocytes following in vitro activation [41]. Moreover, the expression of co-receptors correlated with thymocyte susceptibility to viral infection with HIV strains endowed with different tropism and co-receptor usage.
Our findings confirm and extend previously published observations [42,43], and may contribute to resolve apparently contradictory data. Dairaghi et al. [40] reported expression of CCR-5 in thymocytes; this evidence was based on non-quantitative approaches, however, such as demonstration of CCR-5 mRNA by RT-PCR analysis, MIP-1β binding to thymocytes, signal transduction events and thymocyte migration in response to co-receptor ligands. These data are in contrast with those by other workers [42,43], who showed by both phenotypic and functional analyses poor or absent CCR-5 expression on thymocytes; nonetheless, these authors also did not provide a quantitative evaluation of CCR-5 expression. In our study, we found poor CCR-5 expression in freshly isolated thymocytes (˜4000 ABS/cell in less than 5% of freshly isolated cells), whereas the expression of the CXCR-4 co-receptor was much higher (˜10 000 ABS/cell in about 30% of all the thymic subpopulations). This gap was confirmed by Northern blot analysis of co-receptor mRNA, and by the different behaviour observed in functional responses to co-receptor agonists. In this regard, our findings confirm and extend data by Zaitseva et al. [42] and Taylor et al. [43]; by using three different assays, we showed that exposure of thymocytes to a CXCR-4 agonist such as SDF-1, but not to a CCR-5 agonist such as MIP-1β, induced functional responses in freshly isolated thymocytes. Other CCR-5 agonists, such as MIP-1α and RANTES, were also inactive (data not shown).
In vitro thymocyte activation was associated with up- regulation of CCR-5 expression; in this regard, it is noteworthy that the density of expression of CCR-5 at the cell surface increased beyond CXCR-4 density levels when thymocytes were cultured in the presence of PHA and IL-2, a usual culture condition for in vitro HIV infection experiments [44]. Thus, although percentage expression of CCR-5 did not attain the figures of CXCR-4+ thymocytes, this activation-associated increase in CCR-5 density may explain why, despite poor basal CCR-5 expression, thymocytes are susceptible to in vitro infection by both T-tropic and M-tropic HIV strains. In this regard, however, an apparent paradox seemed to emerge from our data. By phenotypic and molecular approaches we could not document a sizeable CCR-5 expression on thymocytes; in addition, functional responses to CCR-5 agonists could not be detected in freshly isolated and in vitro stimulated thymocytes, despite the fact that in vitro culture was associated with up-regulation of co-receptor expression. Yet, in vitro infection by an M-tropic, R5 strain could be easily obtained, although at lower efficiency compared to a T-tropic strain. The observation that the envelope glycoprotein of the Ba-L strain used for infection did not signal into freshly isolated and cultured thymocytes (Table 1), which fully confirms data published by Zaitseva et al. [42], does not necessarily imply that CCR-5 signalling is not required for thymocyte infection; other workers [45] showed a strict correlation between the ability of HIV or SIV envelopes to signal through CCR-5 and the ability to infect in vitro cultured monocytes. This apparent discrepancy may be due to the different cell populations studied, as well as to the different technical approaches used, each endowed with its own sensitivity. Thus, while we and others [42,43] did not succeed in demonstrating functional consequences of CCR5–ligand interaction, we cannot exclude that a very low co-receptor expression, inadequate to mediate measurable biological effects in our functional readouts, could be sufficient to allow virus entry and cell infection.
In addition, the complex interplay of CD4 and the different, often multiple co-receptors involved in HIV–target cells interaction may be of paramount importance. As demonstrated by Platt et al. [30], CD4 and CCR-5 interact in a concentration-dependent manner to promote HIV infection. In Hela- CD4 clones, low traces of CCR-5 (between 700 and 2000 molecules/cell) were sufficient for maximal susceptibility to HIV infection in the presence of large amounts of CD4 (400 000 molecules/cell), whereas ˜10 000 CCR-5 ABS/cell were needed in the presence of relatively few CD4 molecules (10 000/cell) [30]. Thus, it is possible that the low number of CCR-5 receptors at the cell surface is compensated in immature thymocytes by the high level of CD4 expression, and CD4+CD8+ cells undergo preferential in vitro infection, compared to other subsets [21,22,25]. In this regard, it would be intriguing to speculate that the much higher density of CD4 at the cell surface observed ex vivo in CD4+CD8+ thymocytes, compared to the more mature, CD4+ single positive subset (Fig. 1b) could account for the reported [21,22,25] greater susceptibility of this immature CD4+CD8+ population to M- and T-tropic HIV strains, compared to more mature subsets. In any case, our findings suggest that in vitro studies on the differential susceptibility of thymocyte subpopulations to HIV strains of any tropism may need restatement, since infection experiments usually require cell activation, and this latter translates into a perturbation of the expression of both co-receptors.
In general, our data could also cast some doubt on the possibility that thymus infection by the more frequent M-tropic strains that use CCR-5 as co-receptor could be a common event in vivo. In fact, although thymocytes are susceptible to in vitro infection with both M- and T-tropic HIV isolates [20], in SCID-hu mice thymus infection and involution were mainly apparent when T-tropic, highly cytopathic strains were employed for infection [20,26,27,29]. It is probable that the low density of CCR-5 expression in a minority of thymocytes could be inadequate for efficient in vivo infection by M-tropic strains, and the dynamics of HIV receptor and co-receptor expression on thymocyte surface following in vitro activation could explain why a relatively good target in vitro may long escape in vivo infection. In fact, Reynes et al. [46] demonstrated that the density of CCR-5 expression in peripheral blood lymphocytes of seropositive patients was directly correlated to plasmaviraemia levels. Based on the high constitutive expression of CXCR-4 by thymocytes, it is conceivable that a phenotypic switch from an M-tropic to a T- or dual-tropic HIV strain may translate into virus spread to the thymic tissue, and hence precursor infection and destruction; indeed, a preferential tropism for T cell lines was documented in primary viral isolates from thymus of an HIV-infected patient [47]. The pattern of co-receptor expression in thymus may also account for the observation that a shift towards CXCR-4 usage is associated with accelerated disease progression [9,10,48,49]. In this regard, however, in view of the promiscuity of co-receptor usage by HIV, which may involve other members of the cytokine receptor family such a CCR-2b, CCR3 and CCR8 [2,4,50], a complete profile of co-receptor expression by thymic cells ex vivo is required.
Acknowledgments
We are indebted to G. Gao (Boston, MA, USA) for the kind supply of Ba-L gp120 glycoprotein. We would like to thank Dr Simone Minardi for performing the intracellular calcium influx experiments. The help of Ms Patricia Segato in the preparation of this manuscript is gratefully acknowledged. We are also grateful to P. Gallo for artwork and C. Tognon for secretarial assistance. These studies were supported in part by grants from the Istituto Superiore di Sanità (AIDS Project); MURST 60%; Associazione Italiana Ricerca sul Cancro (AIRC) and Fondazione Italiana Ricerca sul Cancro (FIRC). V. R. is an AIRC fellow.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder CC, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Choe H, Farzan M, Sun Y, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–48. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 3.Deng H, Liu R, Ellmeier W, et al. Identification of a major co- receptor for primary isolates of HIV-1. Nature. 1996;381:661–6. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 4.Doranz BJ, Rucker J, Yi Y, et al. A dual-tropic primary HIV-1 isolate that uses Fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–58. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 5.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 7.Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 8.Moore JP, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–62. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 9.Dittmar MT, McKnight A, Simmons G, Clapham PR, Weiss RA, Simmonds P. HIV-1 tropism and co-receptor use. Nature. 1997;385:495–6. doi: 10.1038/385495a0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Huang Y, He T, Cao Y, Ho DD. HIV-1 subtype and second-receptor use. Nature. 1996;383:768. doi: 10.1038/383768a0. [DOI] [PubMed] [Google Scholar]
- 11.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 co-receptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 12.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Paxton WA, Wolinsky SM, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nature Med. 1996;2:1240–3. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 14.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–62. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 15.Eugen Olsen J, Iversen AK, Garred P, et al. Heterozygosity for a deletion in the CKR-5 gene leads to prolonged AIDS-free survival and slower CD4 T-cell decline in a cohort of HIV-seropositive individuals. AIDS. 1997;11:305–10. doi: 10.1097/00002030-199703110-00007. [DOI] [PubMed] [Google Scholar]
- 16.Michael NL, Chang G, Louie LG, et al. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nature Med. 1997;3:338–40. doi: 10.1038/nm0397-338. [DOI] [PubMed] [Google Scholar]
- 17.Wu L, Paxton WA, Kassam N, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–91. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hori T, Sakaida H, Sato A, et al. Detection and delineation of CXCR-4 (fusin) as an entry and fusion cofactor for T cell-tropic HIV-1 by three different monoclonal antibodies. J Immunol. 1998;160:180–8. [PubMed] [Google Scholar]
- 19.Hesselgesser J, Liang M, Hoxie J, et al. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J Immunol. 1998;160:877–83. [PubMed] [Google Scholar]
- 20.McCune JM. Thymic function in HIV-1 disease. Semin Immunol. 1997;9:397–404. doi: 10.1006/smim.1997.0098. [DOI] [PubMed] [Google Scholar]
- 21.De Rossi A, Calabro ML, Panozzo M, et al. In vitro studies of HIV-1 infection in thymic lymphocytes: a putative role of the thymus in AIDS pathogenesis. AIDS Res Hum Retroviruses. 1990;6:287–98. doi: 10.1089/aid.1990.6.287. [DOI] [PubMed] [Google Scholar]
- 22.Schnittman SM, Denning SM, Greenhouse JJ, et al. Evidence for susceptibility of intrathymic T-cell precursors and their progeny carrying T-cell antigen receptor phenotypes TCR alpha beta + and TCR gamma delta + to human immunodeficiency virus infection: a mechanism for CD4+ (T4) lymphocyte depletion. Proc Natl Acad Sci USA. 1990;87:7727–31. doi: 10.1073/pnas.87.19.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tremblay M, Numazaki K, Goldman H, Wainberg MA. Infection of human thymic lymphocytes by HIV-1. J Acquir Immune Defic Syndr. 1990;3:356–60. [PubMed] [Google Scholar]
- 24.Hays EF, Uittenbogaart CH, Brewer JC, Vollger LW, Zack JA. In vitro studies of HIV-1 expression in thymocytes from infants and children. AIDS. 1992;6:265–72. doi: 10.1097/00002030-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Uittenbogaart CH, Anisman DJ, Jamieson BD, et al. Differential tropism of HIV-1 isolates for distinct thymocyte subsets in vitro. AIDS. 1996;10:F9–16. doi: 10.1097/00002030-199606001-00001. [DOI] [PubMed] [Google Scholar]
- 26.Stanley SK, McCune JM, Kaneshima H, et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–63. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneshima H, Su L, Bonyhadi ML, Connor RI, Ho DD, McCune JM. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J Virol. 1994;68:8188–92. doi: 10.1128/jvi.68.12.8188-8192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grandadam M, Cesbron JY, Candotti D, et al. Dose-dependent systemic human immunodeficiency virus infection of SCID-hu mice after intraperitoneal virus injection. Res Virol. 1995;146:101–12. doi: 10.1016/0923-2516(96)81079-x. [DOI] [PubMed] [Google Scholar]
- 29.Kollmann TR, Kim A, Pettoello Mantovani M, et al. Divergent effects of chronic HIV-1 infection on human thymocyte maturation in SCID-hu mice. J Immunol. 1995;154:907–21. [PubMed] [Google Scholar]
- 30.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–64. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamarchi R, Indraccolo S, Minuzzo S, et al. Frequency of a CCR-5 mutated allele (Δ32) among Italian healthy donors and individuals at risk of parenteral HIV infection. AIDS Res Hum Retroviruses. 1999;4:337–44. doi: 10.1089/088922299311303. [DOI] [PubMed] [Google Scholar]
- 32.Coppola V, Veronesi A, Indraccolo S, et al. Lymphoproliferative disease in human peripheral blood mononuclear cell-injected SCID mice. IV. Differential activation of human Th1 and Th2 lymphocytes and influence of the atopic status on lymphoma development. J Immunol. 1998;160:2514–22. [PubMed] [Google Scholar]
- 33.Amadori A, Zamarchi R, De Silvestro G, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nature Med. 1995;1:1279–83. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- 34.Sozzani S, Ghezzi S, Iannolo G, et al. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med. 1998;187:439–44. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson PC, Liew FY. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995;181:1255–9. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sozzani S, Zhou D, Locati M, et al. Receptors and transduction pathways for monocyte chemotactic protein-2 and monocyte chemotactic protein-3. Similarities and differences with MCP-1. J Immunol. 1994;152:3615–22. [PubMed] [Google Scholar]
- 37.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- 38.Parolin C, Borsetti A, Choe H, et al. Use of murine CXCR-4 as a second receptor by some T-cell-tropic human immunodeficiency viruses. J Virol. 1998;72:1652–6. doi: 10.1128/jvi.72.2.1652-1656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berkowitz RD, Beckerman KP, Schall TJ, McCune JM. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J Immunol. 1998;161:3702–10. [PubMed] [Google Scholar]
- 40.Dairaghi DJ, Franz Bacon K, Callas E, et al. Macrophage inflammatory protein-1beta induces migration and activation of human thymocytes. Blood. 1998;91:2905–13. [PubMed] [Google Scholar]
- 41.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV co-receptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaitseva MB, Lee S, Rabin RL, et al. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–13. [PubMed] [Google Scholar]
- 43.Taylor JR, Kimbrell KC, Scoggins R, Delaney M, Wu L, Camerini D. Expression and function of chemokine receptors on human thymus: implications for infection by human immunodeficiency virus type 1. J Virol. 2001;75:8752–60. doi: 10.1128/JVI.75.18.8752-8760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rübsamen-Waigmann H, Von Briesen H, Holmes H, et al. Standard conditions of virus isolation reveal biological variability of HIV type 1 in different regions of the world. AIDS Res Hum Retroviruses. 1994;10:1401–8. doi: 10.1089/aid.1994.10.1401. [DOI] [PubMed] [Google Scholar]
- 45.Arthos J, Rubbert A, Rabin RL, et al. CCR5 signal transduction in macrophages by human immunodeficiency virus and simian immunodeficiency virus envelopes. J Virol. 2000;74:6418–24. doi: 10.1128/jvi.74.14.6418-6424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynes J, Portales P, Segondy M, et al. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with HIV-1. J Infect Dis. 2000;181:927–32. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- 47.Calabrò ML, Zanotto C, Calderazzo F, et al. HIV-1 infection of the thymus: evidence for a cytopathic and thymotropic viral variant in vivo. AIDS Res Hum Retroviruses. 1995;11:11–9. doi: 10.1089/aid.1995.11.11. [DOI] [PubMed] [Google Scholar]
- 48.Scarlatti G, Tresoldi E, Bjorndal A, et al. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nature Med. 1997;3:1259–65. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 49.Glushakova S, Grivel JC, Fitzgerald W, Sylwester A, Zimmerberg J, Margolis LB. Evidence for the HIV-1 phenotype switch as a causal factor in acquired immunodeficiency. Nature Med. 1998;4:346–9. doi: 10.1038/nm0398-346. [DOI] [PubMed] [Google Scholar]
- 50.Horuk R, Hesselgesser J, Zhou Y, et al. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273:386–91. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]