Oxa1p, an essential component of the N-tail protein export machinery in mitochondria (original) (raw)
Abstract
A number of nuclear encoded inner membrane proteins of mitochondria span the membrane in such a manner that their N termini are located in the intermembrane space. Many of these proteins attain this membrane orientation by undergoing an export step from the matrix across the inner membrane. This export process, which resembles bacterial N-tail export from energetic and topogenic signal requirements, is facilitated by Oxa1p, a protein that has homologues throughout prokaryotes and eukaryotes. Oxa1p, as we have previously shown, is required to export the N and C termini of the mitochondrially encoded pCoxII to the intermembrane space. We demonstrate here that imported nuclear encoded proteins physically interact with Oxa1p and depend on Oxa1p for efficient export of their N termini to the intermembrane space. Furthermore, Oxa1p interacts with nascent polypeptide chains synthesized in mitochondria, including the fully synthesized pCoxII and CoxIII species. Thus, Oxa1p represents a component of a general export machinery of the mitochondrial inner membrane.
Proteins of the inner membrane of mitochondria display a wide range of topological arrangements. These proteins range from being monotopic, spanning the inner membrane once, to those which are polytopic, containing two or more transmembrane segments (1). In addition these proteins differ in their origin of synthesis. The majority of proteins are encoded in the cell nucleus and are imported into the organelle in a posttranslational manner. A few polytopic proteins, all involved in oxidative phosphorylation, are encoded by the mitochondrial genome and are synthesized within the matrix compartment.
How are these various nuclear and mitochondrial encoded proteins sorted to the inner membrane? All of the proteins contain topogenic signals comprised of a hydrophobic core, usually transmembrane segments, which are flanked on both sides by charged amino acid residues. These topogenic signals function to ensure sorting of the protein to the membrane and attainment of the correct topology. We have previously addressed the mechanism of sorting of a subset of inner membrane proteins, which are orientated in the membrane in such a manner that their N termini are exposed to the intermembrane space (1–4). Our results have indicated that these proteins, which include both nuclear as well as mitochondrially encoded proteins, are sorted by way of an export step from the matrix across the inner membrane. The process of export of the N-terminal tails of these proteins bears resemblances to N-terminal tail export in prokaryotes, with regard to both its energetic and topogenic signal characteristics (1–4). In mitochondria and bacteria, this export step is thought to be facilitated by an until now unidentified translocation machinery (4, 5). Export of N-terminal tails and of prokaryotic polytopic proteins has been reported to occur in a Sec-independent but membrane potential-dependent mechanism (5–9). Involvement of a bacterial Sec-type machinery appears to be unlikely as there is no indication, at least in yeast, of the existence of a mitochondrial Sec machinery (10).
Oxa1p is a nuclear encoded mitochondrial protein of the yeast Saccharomyces cerevisiae which is conserved from prokaryotes throughout eukaryotes (11–13). Found in both Gram-positive and Gram-negative bacteria, the function of the prokaryotic homologues of Oxa1p, termed 60-kDa inner membrane protein, has not been identified. Oxa1p spans the inner membrane of mitochondria five times and has an Nout–Cin orientation (4). Although it appears to be involved in the assembly of the cytochrome oxidase complex of respiratory chains in mitochondria, the exact function of Oxa1p was not known until now (11, 12, 14–16). Cells lacking Oxa1p were recently reported to be defective in the export of the precursor of subunit II of cytochrome oxidase (pCoxII) from the mitochondrial matrix, and Oxa1p was suggested to function at an early stage in the biogenesis of CoxII (17). A temperature-sensitive yeast mutant of Oxa1p, termed pet ts1402, was recently isolated and was shown to accumulate pCoxII in vivo when grown at the nonpermissive temperature of 37°C (13). Using this temperature-sensitive yeast mutant, pet ts1402, we have recently shown that Oxa1p plays an essential role in the sorting of pCoxII from the matrix following its synthesis (18). The phenotype of the pet ts1402 mutant can be selectively induced in vitro by briefly exposing mitochondria isolated from this strain grown at permissive temperature to the elevated temperature of 37°C (18). Following induction of the phenotype, newly synthesized pCoxII accumulates in the matrix as it fails to undergo export (18). Oxa1p function is thus essential for the export of the N and C termini of the mitochondrially encoded pCoxII.
A homologue of Oxa1p, ALB3, was reported to be present in chloroplasts (19); the function of this protein can, therefore, not be limited to that of cytochrome oxidase assembly. Rather Oxa1p may act as a component with a more general function in protein sorting and assembly. To test this we analyzed the sorting of a number of nuclear encoded proteins of the inner membrane known to undergo an export step from the mitochondrial matrix following posttranslational import. We demonstrate here that the Oxa1p protein plays a pivotal role in export of N-terminal tails of these proteins from the matrix to the intermembrane space. Thus Oxa1p and its prokaryotic homologues appear to represent essential components of a novel, general N-terminal tail protein export machinery.
MATERIALS AND METHODS
Yeast Strains.
Yeast strains used in this study were wild-type D273-10B (ATCC 24657); the temperature-sensitive mutant of the OXA1 gene product, termed pet ts1402, and its isogenic wild-type strain (Sc167) (ref. 13) and a Δ_oxa1_ strain (Mata, leu2, trp1, ura3, ade2, can1, OXA1:HIS3). The wild-type yeast D273-10B was grown on lactate medium at 30°C; the Sc167 and pet ts1402 were grown at 24°C. The Δ_oxa1_ and isogenic wild-type strains were grown on YPGal (2%) supplemented with 0.5% lactate (3). All cells were harvested at an OD578 of about 1.5. Mitochondria were isolated as previously described (4) with the exception that zymolyase treatment was performed at 24°C in the case of the pet ts1402 mutant and its isogenic wild type.
OXA1 Gene Disruption.
The OXA1 disruption strain (Δ_oxa1_) was constructed by inserting a 1.77-kb HIS3 fragment between nucleotides 838 and 872 of the OXA1 gene. This disruption results in a deletion of the C-terminal third of the protein from the beginning of the fourth transmembrane domain (amino acid residue 279 onward). Wild-type yeast W303-1A was transformed with the OXA1:HIS3 linearized DNA fragment, His+ transformants were selected, and the gene disruption was verified by PCR. Western blotting of resulting isolated Δ_oxa1_ mitochondria was performed to confirm that the Oxa1p protein was absent.
Import of Precursor Proteins into Mitochondria.
Precursor proteins were synthesized in the presence of [35S]methionine by coupled transcription/translation in reticulocyte lysate (Promega) as described (2, 3). Import mixtures (100 μl) usually contained 1–3% reticulocyte lysate (vol/vol) in 3% BSA (wt/vol), 500 mM sorbitol, 50 mM Hepes/KOH, 80 mM KCl, 10 mM MgOAc, 2 mM potassium phosphate, 2.5 mM EDTA, and 1 mM MnCl2, pH 7.2. The final concentration of mitochondria in the import reaction was 0.25 mg/ml. Protease treatment (proteinase K, 50 μg/ml) and hypotonic swelling to generate mitoplasts were performed as described (4). Preproteins were imported into wild-type and pet ts1402 mitochondria with similar efficiencies. Following import, the efficiency of swelling was assessed by quantitation of the levels of co-imported radiolabeled preproteins destined for the intermembrane space (precytochrome _b_2, Imp1p-processed mature form) and the matrix-targeted protein [preFeS(1–147)] in the resulting mitochondria and mitoplast samples. Processing of precytochrome _b_2 to its mature size was unaffected in the pet ts1402 mutant (18), thus demonstrating that the activity of the Imp1p protease (20, 21) was normal in these mutant mitochondria.
Cross-Linking and Immunoprecipitation.
Cross-linking analysis of mitochondrial encoded translation products was as follows. In organello translation was performed for 15 min at 25°C in isolated mitochondria in the presence of [35S]methionine. The chemical cross-linkers 1,5-difluoro-2,4-dinitrobenzene (DFDNB) (0.2 mM) or _N_-succinimidyl 3-[2-pyridyldithio]propionate (SPDP) (0.3 mM) were added to one-half of the sample, while the other half was mock-treated with buffer lacking the cross-linker. Samples were further incubated for 15 min at 25°C, and cross-linking and labeling of translation products were stopped by the addition of 100 mM glycine, pH 8, unlabeled methionine (50 mM), and puromycin (50 μg/ml).
Cross-linking of imported radiolabeled pSu9(1–66)pCoxII(1–74)-DHFR was performed following import of the preprotein for 5 min at 25°C into wild-type mitochondria. Cross-linkers (0.2 mM) disuccinimidyl glutarate (DSG) or _m_-maleimidobenzoyl-_N_-hydroxysuccinimide ester (MBS) were added for 30 min at 12°C.
After cross-linking, mitochondria were reisolated, washed, and lysed with 1% Triton X-100, 300 mM KCl, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 10 mM Tris⋅HCl, pH 7.4. Immunoprecipitations of cross-linked adducts were performed, as described (3). Antisera specific for the C-terminal amino acid residues of Oxa1p (DNEKKLQESFKEKR) were used in this study (4).
Miscellaneous.
In vitro labeling of mitochondrial translation products was performed as described previously (3, 22). Following SDS/PAGE (23) and fluorography data were quantified by densitometry (Ultroscan XL, Pharmacia). Protein determination (24), immunoblotting (25), and immunostaining by using the ECL chemiluminescence detection system (Amersham) were performed essentially as previously described (4).
RESULTS
Export of N-Terminal Tails of Posttranslationally Imported Proteins Requires a Functional Oxa1p.
A number of nuclear encoded proteins of the inner membrane adopt an orientation whereby their N termini are exposed to the intermembrane space. Many of these proteins, including Oxa1p itself, are directed to mitochondria by N-terminal targeting sequences, which are cleaved by the mitochondrial processing peptidase (MPP) on import into the mitochondrial matrix (1–4). The matured N-terminal tails of these proteins then undergo a membrane potential-dependent export across the inner membrane (2–4).
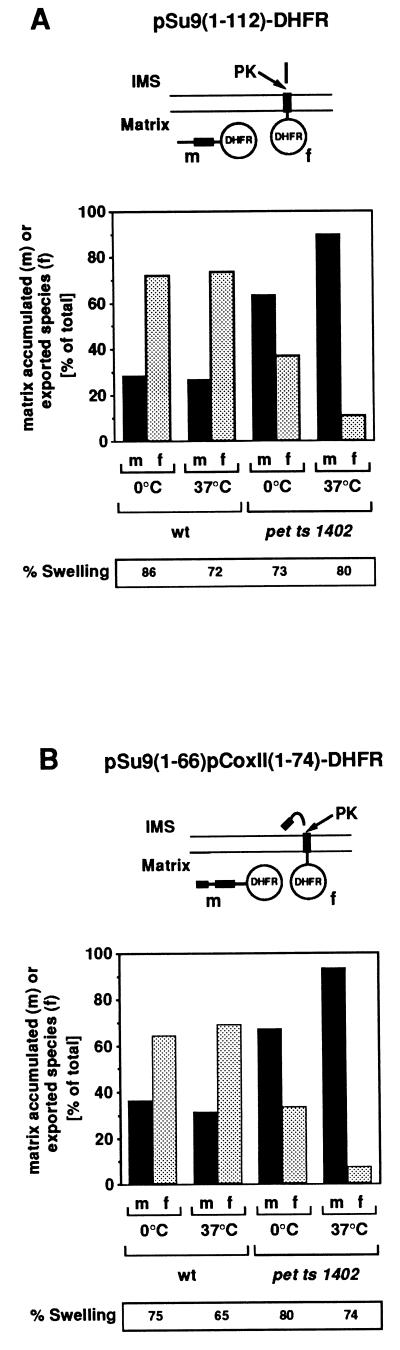
We analyzed the role of Oxa1p in the process of export of these N-terminal tails, following import of the preproteins into isolated pet ts1402 mitochondria. The sorting of two radiolabeled preproteins, pSu9(1–112)-DHFR and pSu9(1–66)pCoxII(1–74)-DHFR, was initially analyzed. These proteins consist of the first transmembrane segment from either subunit 9 of the F1FO-ATP synthase or from pCoxII and of mouse cytosolic dihydrofolate reductase (DHFR) at the C terminus (2, 3). Both proteins were imported into wild-type and pet ts1402 mitochondria with similar efficiencies (data not shown). Attainment of the correct Nout–Cin topology was assessed by disruption of the outer membrane in the presence of protease; this results in the generation of a slightly smaller protease-protected fragment (f) caused by the degradation of the N-terminal tails. In wild-type mitochondria both proteins underwent N-tail export following MPP processing in the matrix (Fig. 1 A and B) as demonstrated (2, 3). In contrast, an inhibition of export of both proteins was observed in the pet ts1402 mitochondria; they remained inaccessible to added protease in mitoplasts. This inhibition of export was almost complete if the pet ts1402 mitochondria had been exposed to the nonpermissive temperature before import (Fig. 1 A and B). We thereby conclude that the function of Oxa1p is required to facilitate the export of the N-terminal tails of both pSu9(1–112)-DHFR and pSu9(1–66)pCoxII(1–74)-DHFR from the matrix across the inner membrane to the intermembrane space.
Figure 1.
Oxa1p is required for the export of N-terminal tails of imported proteins from the mitochondrial matrix. Isolated wild-type (wt) and pet ts1402 mitochondria were preincubated for 10 min in import buffer either at 0°C or at the nonpermissive temperature of 37°C. After cooling on ice, reticulocyte lysate containing radiolabeled precursors pSu9(1–112)DHFR (A) and pSu9(1–66)pCoxII(1–74)DHFR (B) together with NADH (4 mM) were added. Import reactions were performed at 25°C for 30 min. Samples were divided and either mock-treated or subjected to proteinase K (PK) treatment under nonswelling or swelling conditions, as indicated. Samples were analyzed by SDS/PAGE and autoradiography and were quantified by densitometry. Swelling efficiency was determined as described under Materials and Methods and is given as percent mitochondria that had been converted to mitoplasts. m, MPP-processed form of imported species protease inaccessible in the mitoplasts; f, specific fragment of the imported protein generated by the degradation of intermembrane space (IMS)-exposed exported segments of the protein by proteinase K under swelling conditions. Both m and f forms are depicted here and are further described in text.
Oxa1p Is Involved in Its Own Biogenesis.
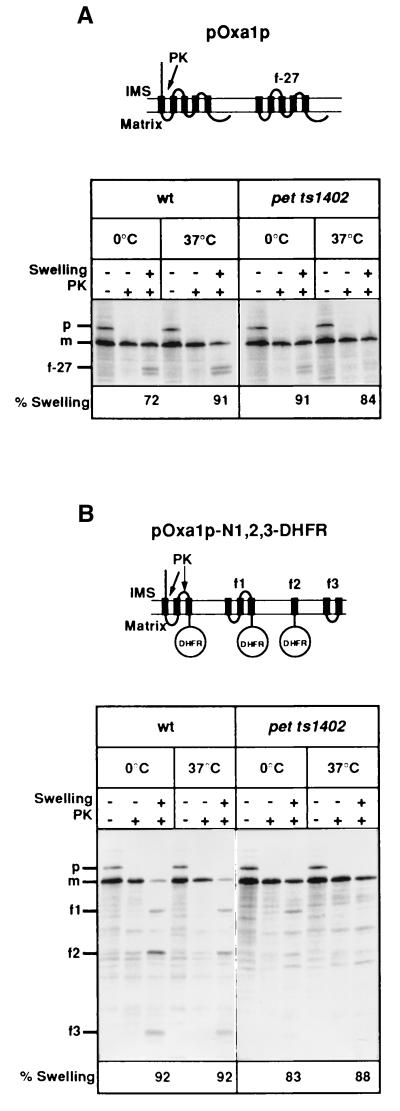
Oxa1p contains an exported N-tail in the intermembrane space (4). We therefore tested whether Oxa1p is required for its own biogenesis by analyzing the sorting of pOxa1p and a pOxa1p-DHFR derivative following their import into isolated pet ts1402 mitochondria. Radiolabeled pOxa1p was efficiently imported and proteolytically matured in both isolated wild-type and pet ts1402 mitochondria (Fig. 2A). Hypotonic swelling of wild-type mitochondria in the presence of proteinase K resulted in the generation of the characteristic 27-kDa fragment (f-27) indicating proteolytic degradation of the correctly exported N terminus of the imported Oxa1p, as described (4). In contrast, Oxa1p imported into pet ts1402 mitochondria remained largely protease-protected in the matrix, demonstrating that N-terminal tail export of Oxa1p was significantly inhibited (Fig. 2A). In addition the sorting of a chimeric protein consisting of the N-terminal region of Oxa1p encompassing the first three transmembrane segments (TM1–TM3) fused to DHFR, pOxa1p-N1,2,3-DHFR (4) was monitored (Fig. 2B). Full-length Oxa1p, after import and attainment of the Nout–Cin orientation, apparently folds into a structure whereby the loop between TM2 and TM3 is no longer accessible to added proteases in mitoplasts (4). This is not the case for the pOxa1p-N1,2,3-DHFR protein, thus enabling us to experimentally follow the insertion of TM2 and TM3 segments, in addition to N-tail export. Wild-type and pet ts1402 mitochondria were exposed to nonpermissive temperature, and radiolabeled pOxa1p-N1,2,3-DHFR was imported. In wild-type mitochondria correct sorting was observed following import (Fig. 2B); this was indicated by the generation of the f1, f2, and f3 fragments, corresponding to the C-terminal fragment containing DHFR and the residual fragment encompassing TM1 and TM2, respectively, as described (4) and as indicated in Fig. 2B. Both of these export events were largely inhibited in the pet ts1402 mitochondria (Fig. 2B).
Figure 2.
Oxa1p is required for its own biogenesis. Isolated wild-type (wt) and pet ts1402 mitochondria were preincubated for 10 min in import buffer either at 0°C or at the nonpermissive temperature of 37°C. Radiolabeled pOxa1p (A) and pOxa1p-N1,2,3-DHFR (B) were imported for 30 min either at 12°C (A) or 25°C (B). Samples were further processed as described in Fig. 1, and resulting autoradiographs are depicted. p, precursor; m, mature form; f-27, f1, f2, and f3 are specific fragments of the imported proteins, which are generated by the degradation of intermembrane space (IMS)-exposed segments of the exported protein by proteinase K (PK) under swelling conditions. Both matrix-accumulated and exported forms of the imported proteins are depicted here and are further described in text.
The export from the matrix of the N-terminal tails of the proteins described above requires the presence of a membrane potential (2–4). Measurement of the membrane potential in the pet ts1402 mitochondria showed that although it was slightly reduced in comparison with that of wild-type mitochondria, it was still sufficient to support the export events (18). On the basis of these observations, we conclude therefore that the function of Oxa1p is essential for the export of a diverse range of N-terminal tails of mitochondrial proteins, both nuclear and mitochondrially encoded; hence its function is not limited to the sorting of pCoxII.
Oxa1p Interacts with Nascent Polypeptide Chains Synthesized in Mitochondria.
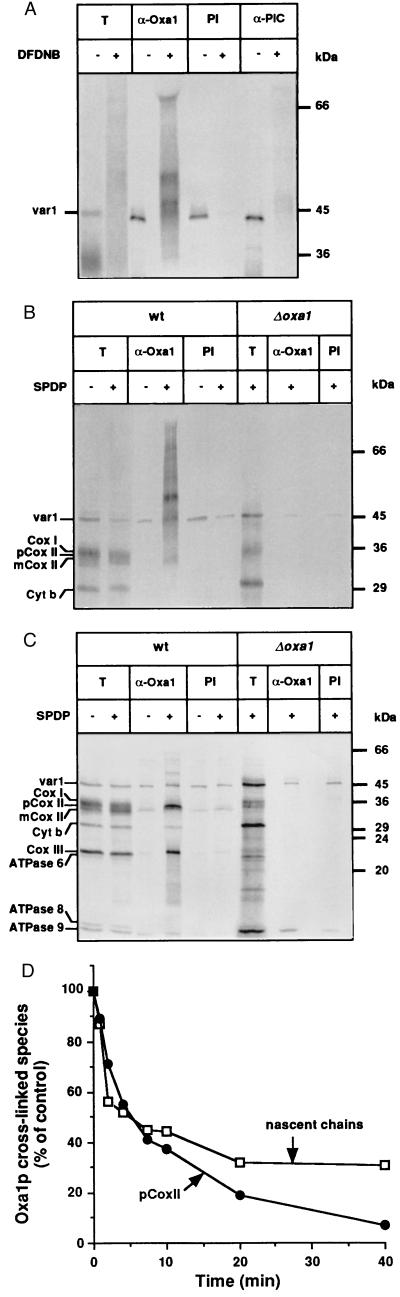
Translationally active ribosomes can be observed in close proximity to the mitochondrial inner membrane in vivo. Thus the synthesis of membrane proteins encoded within the mitochondria has been proposed to occur concomitantly with their translocation across the inner membrane (26, 27). Association of these nascent chains during their synthesis with Oxa1p was analyzed by chemical cross-linking experiments initially by using the cross-linker DFDNB (Fig. 3A). DFDNB was added during the incubation of isolated wild-type mitochondria for labeling of translation products. Cross-linked adducts with apparent molecular masses between 36 and 80 kDa were immunoprecipitated by using antiserum specific for Oxa1p. No cross-linked products were precipitated with preimmune serum or with antibodies against other hydrophobic integral inner membrane proteins such as the phosphate carrier (Fig. 3A) or the ADP/ATP carrier (data not shown). A similar cross-linking analysis with the reductant-cleavable cross-linker SPDP was performed with mitochondria from a yeast strain in which the OXA1 gene was disrupted (Δ_oxa1_) and with mitochondria from the corresponding wild type (Fig. 3B). Cross-linked adducts were immunoprecipitated with Oxa1p-specific antiserum in the wild-type mitochondria but were notably absent in the Δ_oxa1_ mitochondria (Fig. 3B). The immunoprecipitated cross-linked products containing Oxa1p could be cleaved by the addition of 2-mercaptoethanol; in addition to nascent chains, the full-length forms of pCoxII and subunit III of the cytochrome oxidase complex (CoxIII) were found in association with Oxa1p (Fig. 3C).
Figure 3.
Oxa1p interacts cotranslationally with mitochondrially encoded proteins. (A) Oxa1p is in direct contact with nascent chains of mitochondrial translation products. In organello translation was performed for 15 min at 25°C in wild-type mitochondria in the presence of [35S]methionine (3). Samples were divided and incubated with the chemical cross-linker DFDNB (0.2 mM) or were mock-treated with buffer lacking the cross-linker for 15 min at 25°C. Cross-linking and labeling were stopped by the addition of glycine, unlabeled methionine, and puromycin. Mitochondria were reisolated, washed, and lysed. Solubilized cross-linked products were immunoprecipitated with either preimmune serum (PI) or antiserum specific for Oxa1p (α-Oxa1) (4) or the phosphate carrier (α-PiC). T, 10% of the total solubilized material used for immunoprecipitation. (B and C) Oxa1p interacts not only with nascent chains but also with fully synthesized translation products. In organello translation and cross-linking of mitochondrial translation products with the cleavable cross-linker SPDP (0.3 mM) were performed in mitochondria isolated from Δ_oxa1_ and its corresponding wild type (wt), as described above. Immunoprecipitation of cross-linked products was performed with preimmune serum (PI) or Oxa1p-specific antiserum (α-Oxa1). Samples were divided and analyzed by SDS/PAGE with sample buffer without 2-mercaptoethanol (B) or containing 2-mercaptoethanol (C). T, 2% and 10% of total solubilized material from the wild-type and Δ_oxa1_ mitochondria, respectively, which were used for immunoprecipitation reactions. (D) Nascent chains and pCoxII interact with Oxa1p in a transient manner. In organello translation was performed for 15 min at 25°C in wild-type mitochondria in the presence of [35S]methionine, after which an excess of unlabeled methionine (10 mM) was added, and samples were incubated further at 25°C for the times indicated. Cross-linking with SPDP was carried out as described above. Immunoprecipitation of cross-linked products was performed with Oxa1p-specific antiserum, and samples were analyzed by SDS/PAGE in the absence of 2-mercaptoethanol (for quantitation of nascent chains) or in the presence of 2-mercaptoethanol (for quantitation of pCoxII). The amounts of nascent chains and pCoxII that could be cross-linked with Oxa1p were quantified by phosphorimaging; they are expressed for both species as a percent of their amount associated with Oxa1p at the beginning of the chase period.
The finding that specifically the pCoxII species can be cross-linked to Oxa1p is highly significant when one considers that the bulk of the CoxII species accumulated during translation is in fact the mCoxII form. The pCoxII, which represents truly a minor fraction (<5% of total CoxII) in this case, is selectively associated with Oxa1p. Thus once correctly sorted and matured by Imp1p, mCoxII is no longer in association with Oxa1p. This observation supports the idea that Oxa1p interacts transiently with the pCoxII species as it undergoes export, rather than as an assembly factor mediating the subsequent assembly of the exported mature CoxII species.
In further support of an export function for Oxa1p, we have performed a pulse–chase cross-linking experiment. Mitochondrial translation was monitored in the presence of [35S]methionine for a short pulse period, after which excess unlabeled methionine was added to prevent further incorporation of radiolabeled methionine (“chase”). The interaction of newly synthesized proteins with Oxa1p was monitored during the chase period by the addition of cross-linking (SPDP) after various time periods of incubation (Fig. 3D). The interaction of nascent chains and pCoxII with Oxa1p was transient in nature as shown by the decrease of their Oxa1p cross-links during the chase period (Fig. 3D).
Oxa1p Interacts with Posttranslationally Imported Protein, pSu9(1–66)pCoxII(1–74)-DHFR.
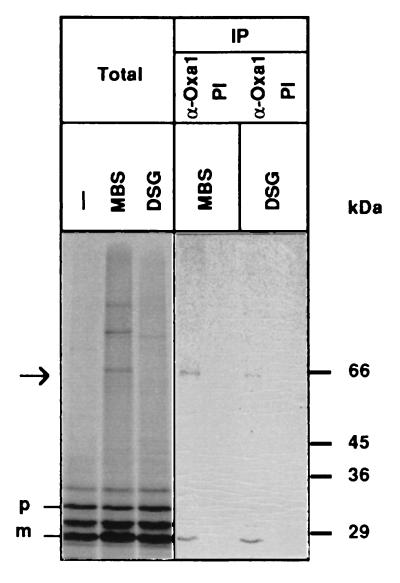
We tested whether Oxa1p physically interacted with a posttranslationally imported protein that undergoes N-terminal tail export. Radiolabeled pSu9(1–66)pCoxII(1–74)-DHFR was imported into isolated mitochondria, and an interaction with Oxa1p was analyzed by using the chemical cross-linkers DSG or MBS. A cross-linked adduct of 65 kDa was observed with both cross-linkers and could be immunoprecipitated with Oxa1p-specific antiserum (Fig. 4). These results demonstrate that the imported pSu9(1–66)pCoxII(1–74)-DHFR could be found in physical association with Oxa1p during its sorting pathway.
Figure 4.
Imported pSu9(1–66)pCoxII(1–74)DHFR interacts with Oxa1p. Radiolabeled pSu9(1–66)pCoxII(1–74)DHFR was imported into wild-type mitochondria for 5 min at 25°C in the absence of NADH. Following cooling on ice, NADH (2 mM) and 0.2 mM cross-linker (MBS or DSG as indicated) were added, and the sample was incubated for another 30 min at 12°C. A cross-linked product of 65 kDa is obtained (indicated by the arrow), which was immunoprecipitated (IP) with antiserum specific for Oxa1p (α-Oxa1) but not with preimmune serum (PI). Total, 10% of solubilized material used for the immunoprecipitation; p, pSu9(1–66)pCoxII(1–74)DHFR; m, MPP-processed species.
DISCUSSION
The biogenesis of a group of mitochondrial inner membrane proteins involves export of their N-terminal tails from the matrix. This process resembles bacterial N-tail export with regard to energetic requirements and adherence to the positive-inside rule (1–4). In bacteria the export of N-terminal tails has been described to occur in a Sec-independent mechanism. In the search for a machinery of N-terminal tail export in mitochondria, we have investigated the role of Oxa1p in the sorting of a number of nuclear and mitochondrially encoded inner membrane proteins.
Oxa1p is a integral protein of the inner membrane of mitochondria. Disruption of the OXA1 gene results in gross defects in the assembly of the cytochrome oxidase complex (11, 12). This assembly defect reflects a dependence on Oxa1p for the export of at least one of the subunits of this complex, the mitochondrially encoded pCoxII (17, 18). On the basis of the evidence presented here, we conclude the function of Oxa1p is not limited to mediating the export of pCoxII. Rather, Oxa1p also plays a pivotal role in mediating the membrane potential-dependent export of N-terminal tails of nuclear encoded proteins from the matrix to the intermembrane space.
We demonstrate here that a variety of nuclear encoded inner membrane proteins, which are initially imported into the mitochondrial matrix, physically interact with Oxa1p and depend on Oxa1p for efficient export of their N termini to the intermembrane space. Furthermore, Oxa1p interacts with nascent polypeptide chains synthesized in mitochondria including the fully synthesized pCoxII and CoxIII species. The interaction of mitochondrially synthesized proteins with Oxa1p is transient, as one may expect from nascent chains undergoing export at the site of an Oxa1p export machinery. Once exported though to a Nout–Cout topology, mCoxII is no longer found in association with Oxa1p. Our preliminary data suggest that the interaction with Oxa1p occurs early during their synthesis as nascent chains, rather than after the completion of their synthesis as fully synthesized polypeptide chains (K.H., unpublished data). Thus cross-linking of Oxa1p to fully synthesized pCoxII and CoxIII observed here was a result of their cross-linking as early nascent chains to Oxa1p followed by their subsequent completion of synthesis to full-length species. This observation would be consistent with the notion that these mitochondrially encoded inner membrane proteins initiate insertion into the inner membrane at the site of the Oxa1p export machinery during their synthesis rather than in a posttranslational manner.
Export of both the N- and C-terminal tails of CoxII requires the function of Oxa1p as the accumulated pCoxII was completely located in the matrix (18). The possibility that the export of the N terminus must precede that of the C terminus, however, cannot be excluded; the inhibition of export of the C-terminal domain of CoxII in the pet ts1402 mitochondria could therefore be an indirect effect. The function of Oxa1p in mitochondria may not be limited to the export of N-terminal tails; rather, Oxa1p appears to be involved in the insertion of pairs of transmembrane segments into the inner membrane leading to the export of hydrophilic segments between them. The following observations support this. First, export of the hydrophilic loop between the second and third transmembrane segment of pOxa1p (by using the chimeric pOxa1p-N1,2,3-DHFR as a tester protein) was inhibited in the pet ts1402 mitochondria. Second, the newly synthesized, mitochondrially encoded CoxIII protein could also be cross-linked to Oxa1p, yet it is a protein with an Nin–Cin topology. CoxIII spans the inner membrane six times exposing three hydrophilic loops of approximately 5, 60, and 20 amino acid residues to the intermembrane space. A function of Oxa1p in mediating the export of these hydrophilic domains is currently being tested.
In conclusion, Oxa1p represents a component of a novel, general export machinery in the mitochondrial inner membrane. Consistent with this function, determination of the native molecular mass of Oxa1p demonstrated it to be a constituent of an oligomeric complex (K.H., unpublished results). Whether this complex is composed entirely of Oxa1p subunits or represents a heterooligomer is presently under investigation. Homologues of Oxa1p are present in chloroplasts and in both Gram-positive and Gram-negative bacteria (11–13, 18); the function of these homologues was unknown up until now. On the basis of the observations made here, we propose they perform a similar function as Oxa1p in mitochondria and mediate the Sec-independent export of, at least, hydrophilic N-terminal tails across the thylakoid membrane of chloroplasts and across the plasma membrane of bacteria.
Acknowledgments
We thank Steffi Glocker and Sandra Weinzierl for excellent technical assistance. This work was supported by the Sonderforschungsbereich 184 (Teilprojekt B2), the Münchener Medizinische Wochenschrift (to R.A.S.), and a predoctoral fellowship from the Boehringer Ingelheim Fonds (to K.H.).
ABBREVIATIONS
DFDNB
1,5-difluoro-2,4-dinitrobenzene
SPDP
_N_-succinimidyl 3-[2-pyridyldithio]propionate
DSG
disuccinimidyl glutarate
MBS
_m_-maleimidobenzoyl-_N_-hydroxysuccinimide ester
MPP
mitochondrial processing peptidase
DHFR
dihydrofolate reductase
References
- 1.Stuart R A, Neupert W. Trends Biochem Sci. 1996;21:261–267. [PubMed] [Google Scholar]
- 2.Rojo E E, Stuart R A, Neupert W. EMBO J. 1995;14:3445–3451. doi: 10.1002/j.1460-2075.1995.tb07350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrmann J M, Koll H, Cook R A, Neupert W, Stuart R A. J Biol Chem. 1995;270:27079–27086. doi: 10.1074/jbc.270.45.27079. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann J M, Neupert W, Stuart R A. EMBO J. 1997;16:2217–2226. doi: 10.1093/emboj/16.9.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalbey R E, Kuhn A, von Heijne G. Trends Cell Biol. 1995;5:380–383. doi: 10.1016/s0962-8924(00)89079-0. [DOI] [PubMed] [Google Scholar]
- 6.Andersson H, von Heijne G. EMBO J. 1993;12:683–691. doi: 10.1002/j.1460-2075.1993.tb05702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao G, Cheng S, Whitley P, von Heijne G, Kuhn A, Dalbey R E. J Biol Chem. 1994;269:26898–26903. [PubMed] [Google Scholar]
- 8.Cao G, Dalbey R E. EMBO J. 1994;13:4662–4669. doi: 10.1002/j.1460-2075.1994.tb06789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitley P, Gafvelin G, von Heijne G. Proc Natl Acad Sci USA. 1995;270:29831–29835. doi: 10.1074/jbc.270.50.29831. [DOI] [PubMed] [Google Scholar]
- 10.Glick B S, von Heijne G. Protein Sci. 1996;5:1–2. doi: 10.1002/pro.5560051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnefoy N, Chalvet F, Hamel P, Slonimski P P, Dujardin G. J Mol Biol. 1994;239:201–212. doi: 10.1006/jmbi.1994.1363. [DOI] [PubMed] [Google Scholar]
- 12.Bonnefoy N, Kermorgant M, Groudinsky O, Minet M, Slonimski P P, Dujardin G. Proc Natl Acad Sci USA. 1994;91:11978–11982. doi: 10.1073/pnas.91.25.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer M, Behrens M, Esser K, Michaelis G, Pratje E. Mol Gen Genet. 1994;245:272–278. doi: 10.1007/BF00290106. [DOI] [PubMed] [Google Scholar]
- 14.Kermorgant M, Bonnefoy N, Dujardin G. Curr Genet. 1997;31:302–307. doi: 10.1007/s002940050209. [DOI] [PubMed] [Google Scholar]
- 15.Meyer W, Bauer M, Pratje E. Curr Genet. 1997;31:401–407. doi: 10.1007/s002940050222. [DOI] [PubMed] [Google Scholar]
- 16.Altamura N, Capitanio N, Bonnefoy N, Papa S, Dujardin G. FEBS Lett. 1996;382:111–115. doi: 10.1016/0014-5793(96)00165-2. [DOI] [PubMed] [Google Scholar]
- 17.He S, Fox T D. Mol Biol Cell. 1997;8:1449–1460. doi: 10.1091/mbc.8.8.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hell K, Herrmann J M, Pratje E, Neupert W, Stuart R A. FEBS Lett. 1997;418:367–370. doi: 10.1016/s0014-5793(97)01412-9. [DOI] [PubMed] [Google Scholar]
- 19.Sundberg E, Slagter J G, Fridborg I, Cleary S P, Robinson C, Coupland G. Plant Cell. 1997;9:717–730. doi: 10.1105/tpc.9.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behrens M, Michaelis G, Pratje E. Mol Gen Genet. 1991;228:167–176. doi: 10.1007/BF00282462. [DOI] [PubMed] [Google Scholar]
- 21.Nunnari J, Fox T D, Walter P. Science. 1993;262:1997–2004. doi: 10.1126/science.8266095. [DOI] [PubMed] [Google Scholar]
- 22.McKee E E, Poyton R O. J Biol Chem. 1984;259:9320–9331. [PubMed] [Google Scholar]
- 23.Laemmli U K. Nature (London) 1979;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;79:267–271. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vignais P V, Huet J, André J. FEBS Lett. 1969;3:177–181. doi: 10.1016/0014-5793(69)80128-6. [DOI] [PubMed] [Google Scholar]
- 27.Watson K. J Cell Biol. 1972;55:721–726. doi: 10.1083/jcb.55.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]