Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism (original) (raw)
Abstract
Primary cilia are sensory organelles that translate extracellular chemical and mechanical cues into cellular responses. Bone is an exquisitely mechanosensitive organ, and its homeostasis depends on the ability of bone cells to sense and respond to mechanical stimuli. One such stimulus is dynamic fluid flow, which triggers biochemical and transcriptional changes in bone cells by an unknown mechanism. Here we report that bone cells possess primary cilia that project from the cell surface and deflect during fluid flow and that these primary cilia are required for osteogenic and bone resorptive responses to dynamic fluid flow. We also show that, unlike in kidney cells, primary cilia in bone translate fluid flow into cellular responses in bone cells independently of Ca2+ flux and stretch-activated ion channels. These results suggest that primary cilia might regulate homeostasis in diverse tissues by allowing mechanical signals to alter cellular activity via tissue-specific pathways. Our identification of a mechanism for mechanotransduction in bone could lead to therapeutic approaches for combating bone loss due to osteoporosis and disuse.
Keywords: intracellular signaling, mechanotransduction, osteoblast, osteocyte, fluid flow
Mammalian cells are sensitive to their environments and can detect both chemical and mechanical signals (1, 2). In tissues such as bone, cartilage, endothelium, and kidney, mechanical signals play important roles in proliferation (3, 4), differentiation (4, 5), and pathology (6–8). However, in many of these tissues the cellular mechanosensors are unknown.
Bone alters its morphology and density in response to external loads. Lack of mechanical stimulation has been linked to bone loss in osteoporosis, a highly prevalent and debilitating disease of aging (9). Models of bone tissue predict that during loading, fluid flows through the compartments (lacunae) that house osteocytes within mineralized bone and through the channels (canaliculae) that connect lacunae to each other and to bone-forming osteoblasts at the bone surface (10, 11). Experiments in cultured bone cells have shown that dynamic fluid flow stimulates osteogenic, and inhibits bone resorptive, responses (12–14). However, it remains unknown how bone cells translate loading-induced signals such as dynamic fluid flow into biochemical and transcriptional responses.
Primary cilia are solitary, immotile, microtubule-based organelles that grow from the centrosome and project from the cell surface in many vertebrate tissues (15), including bone (16, 17). Their construction requires intraflagellar transport (IFT), a coordinated process involving a system of motor proteins and adaptors (18). Primary cilia possess receptors for signaling molecules such as somatostatin and PDGF (19, 20) and have been implicated in Sonic hedgehog signaling (21) and the establishment of left–right asymmetry (22). Primary cilia also function as flow sensors in kidney tubule epithelial cells. When mechanically stimulated, cultured kidney cell primary cilia induce extracellular Ca2+-dependent intracellular Ca2+ release (23, 24), leading to activation of the STAT transcription factor pathway (7). This Ca2+ response is lost in cells bearing mutations in the IFT component polaris (23, 25) or after removal of primary cilia by treatment with the drug chloral hydrate (26). In kidney cells, cilium-mediated Ca2+ entry requires polycystin 2, a stretch-activated Ca2+ channel that localizes to the primary cilium (27). Mutations in polycystin 2, or mutations that result in abnormal cilia, cause polycystic kidney disease in humans and mice (28–30).
The extension of the primary cilium from the cell surface, and a high concentration of receptors in the ciliary membrane, along with its putative role in sensing fluid flow in the kidney, make it a promising candidate for flow-sensing in bone (31). Although there is evidence that primary cilia play a role in bone development and patterning (17, 32), to date there is no direct evidence that primary cilia play a mechanosensory role in bone cells. In this study, we show that bone cells possess primary cilia with physical characteristics consistent with a flow-sensing function. We then show that primary cilia are required for bone-specific cellular responses to fluid flow. Finally, we show that, unlike in kidney cells, flow-induced Ca2+ flux in bone cells is independent of primary cilia and calcium influx.
Results
Primary Cilia Extend from the Surface of Bone Cells and Deflect During Fluid Flow.
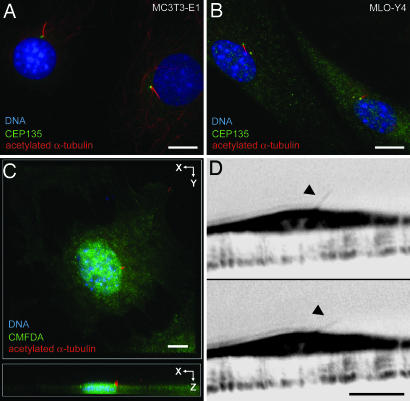
To investigate whether primary cilia might play a role in mechanotransduction in bone, we sought to determine whether primary cilia extend from bone cells into the extracellular environment and, if so, whether bone cell primary cilia are deflected by fluid flow. Xiao et al. (17) found cilium-like structures in MC3T3-E1 osteoblasts and MLO-Y4 osteocytes. To establish that these structures extended from the centrosome and were in fact primary cilia, these cells were stained for acetylated α-tubulin, which is enriched in ciliary microtubules (33), and the centrosomal component CEP135 (34). Both MC3T3-E1 and MLO-Y4 cells possess acetylated α-tubulin-positive primary cilia extending from the centrosome (Fig. 1 A and B and Table 1). To determine whether primary cilia protrude from the surface of bone cells (35), MC3T3-E1 cells were stained for acetylated α-tubulin and 5-chloromethylfluorescein diacetate (CMFDA) to label the cell surface, and confocal image stacks were collected. Three-dimensional projections of these stacks demonstrated primary cilia extending beyond the cell surface (Fig. 1C). When imaged in side view with Hoffman modulation (36), primary cilia were seen as 4- to 9-μm-long rods projecting from the apical surface of MC3T3-E1 cells (Fig. 1D). These cilia deflected upon application of ≈0.03 Pa steady flow and recoiled after cessation of flow [see supporting information (SI) Movie 1]. That primary cilia project from the surface of bone cells and deflect during flow indicates that they have the potential to sense fluid flow.
Fig. 1.
Primary cilia project from the apical surface of bone cells and bend during fluid flow. (A and B) Primary cilia stained with anti-acetylated α-tubulin (red) extend from centrosomes stained with anti-CEP135 (green) in MC3T3-E1 osteoblasts (A) and MLO-Y4 osteocytes (B). DNA is stained with DAPI (blue). (C) Maximum projection of a confocal z series shows a primary cilium marked by acetylated α-tubulin (red) extending from the apical surface marked by CMFDA (green) of an MC3T3-E1 osteoblast. DNA is stained with DAPI (blue). (D) Side view of a primary cilium (arrowheads) extending from the apical surface of an MC3T3-E1 osteoblast before (Upper) and during (Lower) application fluid flow from left to right. Images are inverted frames from SI Movie 1. (Scale bars: 5 μm.)
Table 1.
Percentage of cells with primary cilia under various conditions
| Condition | Cells with primary cilia, % | |
|---|---|---|
| MC3T3-E1 | MLO-Y4 | |
| No treatment | 60.8 ± 8.4 | 62.0 ± 15.2 |
| Chloral hydrate | 10.2 ± 6.1 | 6.8 ± 2.2 |
| Control siRNA | 58.3 ± 7.9 | 47.8 ± 2.1 |
| Polaris siRNA | 35.6 ± 13.9 | 25.5 ± 8.0 |
Abrogation of Primary Cilium Formation and Function.
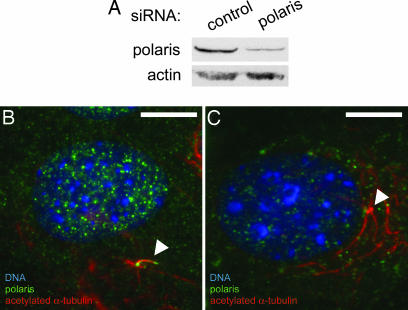
Primary cilia were abrogated by using two independent approaches. Bone cells were treated with chloral hydrate (see Methods), which removes primary cilia in kidney cells (26). Chloral hydrate treatment caused a 90% reduction in the fraction of cells with primary cilia as scored by acetylated α-tubulin staining (Table 1). We also prevented primary cilium formation by siRNA-mediated depletion of the IFT component polaris, which is required for primary cilium biogenesis and function (37). Depletion of polaris was confirmed by Western blotting and immunofluorescence (Fig. 2) and caused ≈50% reduction in the fraction of cells with primary cilia (Table 1). siRNA transfection efficiencies of 82.7 ± 4.5% of cells were obtained, as assayed by Cy3-labeled siRNA, indicating that some transfected cells still possessed levels of polaris sufficient to grow ciliary axonemes.
Fig. 2.
RNAi of polaris prevents primary cilium formation in cultured bone cells. (A) Western blot showing reduced levels of protein in MC3T3-E1 osteoblasts transfected with polaris siRNA (right lane) relative to cells transfected with control siRNA (left lane). Western blot for actin is a loading control. (B) Control MC3T3-E1 osteoblast showing polaris (green) at the primary cilium (arrowhead) marked by acetylated α-tubulin (red). DNA is stained with DAPI (blue). (C) Polaris siRNA-treated MC3T3-E1 osteoblast showing reduced polaris staining (green) and the absence of a primary cilium extending from the centrioles (arrowhead). Stable cytoplasmic microtubules are marked by acetylated α-tubulin (red) and DNA is stained with DAPI (blue). (Scale bars: 10 μm.)
Primary Cilia Are Required for Osteogenic and Bone Resorptive Responses to Dynamic Fluid Flow.
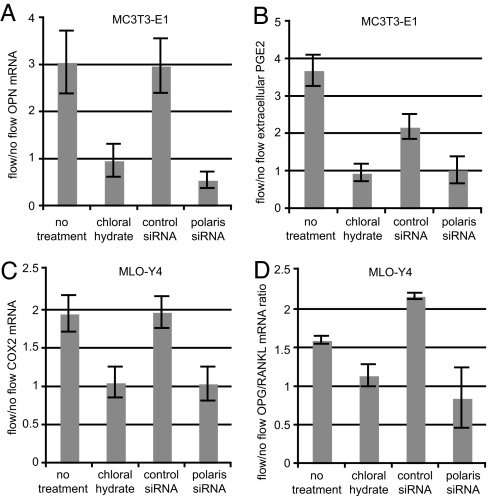
Osteoblasts and osteocytes respond to dynamic fluid flow with characteristic increases in gene expression and cytokine release. For example, bone-forming osteoblasts up-regulate the expression of osteopontin (OPN), a bone matrix protein, in response to flow (14). We measured the levels of OPN mRNA in MC3T3-E1 osteoblasts after exposure to oscillatory fluid flow. In untreated cells, exposure to flow resulted in a 3-fold increase in OPN mRNA levels (P < 0.01), whereas in cells treated with chloral hydrate, exposure to flow did not result in increased levels of OPN mRNA (Fig. 3A). OPN mRNA levels increased 4.4-fold in chloral hydrate-treated cells exposed to 10 nM vitamin D (Sigma, St. Louis, MO) (38), demonstrating that chloral hydrate does not inhibit OPN gene expression nonspecifically (data not shown). In cells treated with control siRNA, exposure to flow resulted in a 3-fold increase in OPN mRNA (P < 0.005), whereas in cells treated with siRNA targeting polaris, exposure to flow did not result in increased OPN mRNA levels and in fact caused a 50% reduction in OPN mRNA (P < 0.05) (Fig. 3A).
Fig. 3.
Primary cilia are required for cellular responses to dynamic fluid flow in osteoblasts and osteocytes. (A) Flow-induced OPN mRNA levels in MC3T3-E1 osteoblasts with and without primary cilia. Cells were exposed to 1-Pa 1-Hz oscillatory fluid flow for 1 h, and OPN mRNA levels were quantified by real-time RT-PCR and normalized to 18S rRNA. (B) PGE2 release for MC3T3-E1 osteoblasts with and without primary cilia. Extracellular PGE2 levels were quantified by ELISA and normalized to total protein. (C) COX2 mRNA levels for MLO-Y4 osteocytes as quantified by real-time RT-PCR and normalized to18S rRNA. (D) OPG/RANKL mRNA ratios for MLO-Y4 osteocytes. [Error bars: SEM (n ≥ 6).]
The cytokine prostaglandin E2 (PGE2) is produced by osteoblasts and osteocytes after mechanical loading in vivo and regulates bone metabolism (39). Because PGE2 is released by cultured osteoblasts and osteocytes in response to dynamic fluid flow (40, 41), we asked whether flow-induced PGE2 release requires primary cilia. In untreated MC3T3-E1 cells, exposure to flow resulted in a 3.7-fold increase in PGE2 release (P < 0.001), whereas in cells treated with chloral hydrate, exposure to flow resulted in no change in PGE2 release (Fig. 3B). As a control, MC3T3-E1 and MLO-Y4 cells were treated with chloral hydrate, and they displayed 13-fold and 3.3-fold increases in PGE2 release, respectively, after 40-min exposure to 200 μM A23187 calcimycin (Sigma), a calcium ionophore known to induce PGE2 release (42) (data not shown). In cells treated with control siRNA, exposure to flow resulted in a 2.2-fold increase in PGE2 release (P < 0.01), whereas in cells treated with siRNA targeting polaris, exposure to flow did not result in increased PGE2 release (Fig. 3B). In MLO-Y4 cells, increases in PGE2 release with flow were also abrogated by treatment with chloral hydrate or polaris siRNA (data not shown).
We next examined whether primary cilia mediate flow-induced changes in cyclooxygenase 2 (COX2) gene expression. COX2 converts arachidonic acid to prostaglandin H2, which is subsequently converted into PGE2 in bone cells (43). In untreated cells, exposure to flow resulted in a 2-fold increase in COX2 mRNA levels (P < 0.005), whereas in cells treated with chloral hydrate, exposure to flow did not result in increased levels of COX2 mRNA (Fig. 3C). In cells treated with control siRNA, exposure to flow resulted in a 1.9-fold increase in COX2 mRNA (P < 0.005), but this increase was eliminated in cells treated with siRNA targeting polaris (Fig. 3C). This increase was also observed in MC3T3-E1 cells and was lost with chloral hydrate or polaris siRNA treatment (data not shown).
We hypothesized that primary cilia might also play a role in bone resorption. The osteoprotegerin (OPG)/receptor activator of NF-κB ligand (RANKL) signaling system regulates the formation rate of bone-resorbing osteoclasts (12, 44, 45). RANKL binds to receptor activator of NF-κB (RANK) on preosteoclasts and promotes osteoclast differentiation, which is inhibited by OPG, a soluble decoy receptor of RANKL. Both osteocytes and osteoblasts produce OPG and RANKL, but MLO-Y4 cells can support osteoclast formation, whereas MC3T3-E1 cells cannot (46). Thus, we examined OPG/RANKL ratios in MLO-Y4 cells. In untreated cells, exposure to flow resulted in a 1.6-fold increase in the ratio of OPG/RANKL mRNA (P < 0.005), whereas in cells treated with chloral hydrate, exposure to flow did not result in significant changes in the ratio of OPG/RANKL mRNA (Fig. 3D). In cells treated with control siRNA, exposure to flow resulted in a 2.2-fold increase in the ratio of OPG/RANKL mRNA levels (P < 0.02), and this increase was eliminated in cells treated with siRNA targeting polaris (Fig. 3D).
Ca2+ Flux in Bone Cells During Flow Is Independent of Primary Cilia and Polycystin 2.
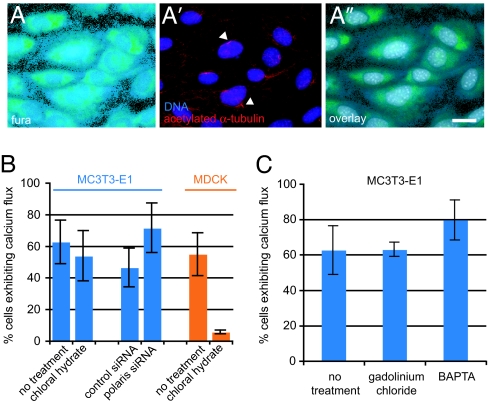
Deflecting primary cilia in kidney cells causes Ca2+ influx through the stretch-sensitive Ca2+ channel polycystin 2, triggering intracellular Ca2+ release (24, 27, 47). We and others (14, 48) have shown that bone cells respond to fluid flow with increases in intracellular Ca2+. Thus, we sought to determine whether flow-induced Ca2+ flux in bone cells was mediated by primary cilia. On the basis of the observation that ≈50% of MC3T3-E1 cells exhibit Ca2+ flux during flow (14) and that ≈60% of these cells possess primary cilia (Table 1), we hypothesized that only cells possessing primary cilia display Ca2+ flux during flow. To test this hypothesis, Ca2+ flux was measured during flow in individual MC3T3-E1 osteoblasts (Fig. 4A). The same cells were then stained for acetylated α-tubulin and scored for primary cilia (Fig. 4A′). Unexpectedly, 64 of 172 cells that lacked primary cilia displayed Ca2+ flux during flow, and there was not a significant correlation (P = 0.11, χ2 test) between possession of a primary cilium and Ca2+ flux. Also, many cells (114/207) that possessed primary cilia did not exhibit Ca2+ flux during flow. Similarly, no correlation between primary cilia and Ca2+ flux was found in MLO-Y4 osteocytes, with 73/150 cells without cilia exhibiting Ca2+ flux (P = 0.44, χ2 test) and 99/178 cells with cilia not displaying Ca2+ flux during flow.
Fig. 4.
Ca+2 flux during flow in bone cells is independent of primary cilia. (A) Fura 2-AM fluorescence in MC3T3-E1 osteoblasts during in vivo Ca+2 imaging. (A′) Staining of primary cilia (arrowheads) marked by acetylated α-tubulin (red) in the same field of cells after fixation; DNA is stained with DAPI (blue). (A″) Overlay of Fura 2-AM and DAPI fluorescence shows that the fields of cells are identical. (Scale bar: 10 μm.) (B) Percentage of MC3T3-E1 osteoblasts (blue bars) and MDCK cells (orange bars) exhibiting Ca+2 flux during flow (see Methods). (C) Percentage of MC3T3-E1 osteoblasts exhibiting Ca+2 flux during flow after various treatments. [Error bars: SEM (n ≥ 4).]
In kidney cells, primary cilium-mediated Ca2+ flux propagates between cells via gap junctions (24). Thus, cells without primary cilia might exhibit Ca2+ flux during flow because of propagation of Ca2+ from neighboring cells, rather than in direct response to flow. To eliminate this confounding effect, cells were scored for Ca2+ flux and the presence of primary cilia while gap junctions were blocked using 30 μM 18α-glycyrrhetinic acid (Sigma) (49). Cells that lacked cilia still responded to flow (102/159 cells) after gap junction inhibition, and there was no significant correlation between the presence of primary cilia and Ca2+ flux (P = 0.11, χ2 test), indicating that cell–cell communication could not account for the observed lack of correlation.
To test whether Ca2+ flux during flow can occur independently of primary cilia in bone cells, MC3T3-E1 osteoblasts were treated with either chloral hydrate to remove primary cilia or siRNA targeting polaris to prevent cilium formation and then assayed for Ca2+ flux during flow. Neither treatment significantly altered the fraction of cells exhibiting Ca2+ flux relative to control cells (Fig. 4B). Previous work (24, 26) establishing the role of the primary cilia in flow-induced Ca2+ flux used kidney cells exposed to constant flow. Our finding that Ca2+ flux in response to dynamic flow is independent of primary cilia in bone cells suggests either that kidney and bone cells differ in their primary cilium-mediated responses to fluid flow or that steady and dynamic flow cause different cellular responses. To distinguish between these possibilities, constant flow was applied to MC3T3-E1 cells after chloral hydrate treatment. As was the case for untreated cells, a high percentage of these cells exhibited Ca2+ flux (data not shown). Also, a greatly reduced percentage of MDCK kidney cells treated with chloral hydrate displayed Ca2+ flux during dynamic flow (Fig. 4B), similar to experiments using steady flow (26). These data suggest that the mechanisms responsible for cilium-mediated mechanosensation in MC3T3-E1 bone cells and MDCK kidney cells are indeed distinct.
We next tested whether polycystin 2 is involved in Ca2+ response in bone cells. In kidney cells, removal of extracellular Ca2+ or treatment with the stretch-activated ion channel inhibitor gadolinium chloride (25, 50) eliminates flow-induced Ca2+ flux. In contrast, we found that treating MC3T3-E1 osteoblasts with 10 μM gadolinium chloride (Sigma) for 30 min or removing extracellular Ca2+ by pretreating flow medium with 90 μM BAPTA (Sigma) did not eliminate flow-induced Ca2+ flux (Fig. 4C). Thus, extracellular Ca2+ entry and polycystin 2 apparently do not play a role in flow-induced Ca2+ response in bone cells. These data, in combination with our findings that bone cells lacking primary cilia exhibit Ca2+ flux during flow, indicate that primary cilia act as flow sensors in bone cells by a mechanism that is different from that of kidney cells.
Discussion
Many tissues are sensitive to mechanical stimuli, and this sensitivity is essential for their physiological functioning. However, the mechanisms by which cells sense and respond to mechanical stimuli are understood in remarkably few cases. In bone, this question is compelling because the deformations due to loading are small, yet loading is required in order to maintain the structure and function of the skeleton. In this study, we present evidence that primary cilia act as mechanosensors in bone cells, providing a potential mechanism by which mechanical stimuli are translated into osteogenic and bone resorptive responses. We demonstrate that cilium-mediated mechanotransduction in bone differs from that described in other tissues, particularly kidney epithelium, with respect to the molecular events that initiate intracellular signaling. We also show that that primary cilia sense dynamic flow, in addition to the constant flow that has been examined in the context of the kidney. Our evidence that the primary cilium is a mechanosensor in bone highlights it as a potential therapeutic target for efforts to prevent bone loss during disease and disuse.
We first show that primary cilia protrude from the apical surface of bone cells and are deflected during fluid flow. These results, in combination with scanning electron microscopy data showing that primary cilium-like structures are external in MLO-Y4 osteocytes (17), indicate that bone cell primary cilia possess physical properties consistent with a flow-sensing function. However, it is important to note that these in vitro experiments do not reproduce the complex 3D environment of osteocytes and osteoblasts. It is possible that bone cell primary cilia interact with extracellular matrix proteins, as has been shown in cartilage (51), and if this were the case, integrins on the primary cilium could translate deformations of the extracellular matrix into intracellular signals, amplifying mechanical stimuli. Further experiments will be required in order to characterize the structural and molecular environment of the primary cilium in bone tissue.
It should be noted that the methods we used to abrogate primary cilia have important limitations. Chloral hydrate removes primary cilia, possibly by disrupting the junction of the cilium and the basal body (52), but is also known to disrupt mitosis (53). Although chloral hydrate could thus have nonspecific effects on cell physiology, it is currently one of the only chemical methods for removing primary cilia from vertebrate cells. We used a protocol (26) in which chloral hydrate was removed for 24 h before experiments were performed and found that 24 h after drug removal, cells possessed normal morphology and cytoplasmic microtubules but low numbers of primary cilia (Table 1). Primary cilia did eventually recover, returning to pretreatment numbers 72 h after drug removal (data not shown). After polaris siRNA treatment, only 50% fewer cells had primary cilia than control cells (Table 1), but a complete loss of flow response was observed in these cells (Fig. 3). A possible explanation for this discrepancy is that the efficiency of IFT might decrease upon reduction of polaris protein levels, preventing delivery of functional ciliary components to some fraction of the remaining cilia. Indeed, ciliary localization of polycystin 2 is lost after moderate RNAi of another IFT component, IFT20, even though the ciliary axonemes appear normal (54), suggesting that axoneme growth is not sufficient for ciliary function. However, the consistency of the results we obtained by using these two independent methods to abrogate primary cilia, combined with control experiments (see Results), allowed us to confidently assess the role of primary cilia in sensing flow in bone cells.
We also show that primary cilia are required for bone-specific cellular responses to dynamic fluid flow. We found that primary cilia are required for flow-induced increases in OPN mRNA levels in MC3T3-E1 cells and for increases in PGE2 release and COX2 mRNA levels in both MLO-Y4 and MC3T3-E1 cells, indicating that primary cilia play a role in osteogenic responses to flow. Primary cilia are also required in order to increase the ratio of OPG/RANKL mRNA after fluid flow in MLO-Y4 cells, suggesting that primary cilia may play an antiresorptive role in osteocytes. Although these results do not allow us to conclude whether primary cilia mediate bone resorption in vivo, they show that flow-induced increases in the OPG/RANKL mRNA ratio are dependent on primary cilia. Our results suggest that primary cilia may contribute to the balance between bone formation and resorption that is necessary for bone homeostasis. A complication in studying the role of primary cilia in bone homeostasis is that primary cilia have been implicated in the developmental and patterning steps that precede the formation of mineralized bone (17, 32). Thus, in vivo experiments to determine whether primary cilia play a role in mechanosensation and homeostasis in bone will require the ability to manipulate the presence of primary cilia after bone has fully developed.
We have shown that in bone cells, primary cilia are responsible for changes in cellular activity after mechanical stimulation, similar to the case of kidney cells (7). However, unlike kidney cells, in which primary cilia mediate polycystin 2-dependent Ca2+ entry and intracellular Ca2+ release in response to fluid flow, bone cells that lack primary cilia exhibit Ca2+ flux in response to fluid flow. This response persists after inhibition of stretch-sensitive ion channels or chelation of extracellular Ca2+, indicating that Ca2+ entry is not required for intracellular Ca2+ flux in bone cells. Thus, our results suggest that a Ca2+ entry- and polycystin 2-independent signaling mechanism links primary cilia to downstream cellular responses in bone cells. This is consistent with previous evidence (40) that some osteogenic responses to mechanical stimulation are Ca2+-independent. In cholangiocytes, primary cilia mediate changes in cAMP levels during flow (55), and in kidney cells, proteolysis of polycystin 1 is involved in transcriptional responses to flow (7). Further experiments will be required in order to determine whether these or other signaling pathways could connect flow-induced bending of the primary cilium to the cilium-dependent cellular responses we have observed in bone cells.
It is becoming increasingly clear that primary cilia act in diverse cell and tissue types to detect a range of chemical and physical signals. One unanswered question is to what extent primary cilia act in concert with other cellular sensory systems. Our data indicate that flow-induced Ca2+ flux in bone cells is independent of primary cilia, but both Ca2+ flux and MAPK phosphorylation have been shown to be required for flow-induced increases in OPN gene expression (14), although it is not clear whether these two signals are part of the same signaling pathway. We show here that primary cilia are required for flow-induced increases in OPN mRNA levels but are not required for Ca2+ flux, which we previously demonstrated is required for the OPN response to flow. This would suggest that multiple mechanosensitive pathways act in concert to contribute to changes in OPN expression. Our results also raise the possibility that, as is the case for PDGF (20), flow might induce MAPK phosphorylation by means of the primary cilium. Furthermore, there is evidence that focal adhesions play a role in mechanosensation in bone cells (56, 57), and primary cilium-mediated signaling could be integrated with focal adhesion-mediated signaling to allow cells to incorporate multiple mechanical signals into the appropriate cellular response. In their role as mechanosensors, primary cilia could thus be part of a comprehensive array of sensory tools that allow bone cells to maintain bone homeostasis.
Methods
Cell Culture.
MC3T3-E1 and MLO-Y4 cells were cultured as described in ref. 58; MDCK kidney cells were cultured in DMEM (Invitrogen, Carlsbad, CA) with 10% FBS and 1% penicillin/streptomycin. Cells were maintained at 37°C and 5% CO2. For Ca2+ imaging experiments, cells were grown on 76 × 26 × 1.6-mm UV-transparent quartz slides (Quartz Scientific, Vancouver, WA). For PGE2 and gene expression experiments, cells were grown on 76 × 48 × 1-mm glass slides (Fisher Scientific, Waltham, MA).
Immunofluorescence.
Cells were fixed in −20°C methanol and processed for immunofluorescence as in ref. 59, using 6-11B-1 anti-acetylated α-tubulin (1:2,000, Sigma), rabbit anti-CEP135 (1:500; a gift from R. Kuriyama, University of Minnesota, Minneapolis, MN), or rabbit anti-polaris (1:500; a gift from B. Yoder, University of Alabama at Birmingham, Birmingham, AL) primary antibodies, followed by incubation with goat anti-mouse Alexa 594 and goat anti-rabbit Alexa 488 secondary antibodies (1:200; Molecular Probes, Eugene, OR). DNA was stained with DAPI (1:100,000 of a 5 mg/ml stock; Sigma).
Confocal Imaging of Primary Cilia.
Cells were stained for acetylated α-tubulin and the cell body was stained using 5 μM CMFDA (CellTracker Green; Molecular Probes). Cells were imaged on a Nikon (Florham Park, NJ) C-1 confocal microscope using a 1.4 numerical aperture ×60 violet corrected objective. Image stacks were deconvolved by using a point-spread function free adaptive algorithm (Media Cybernetics, Silver Spring, MD).
Visualization of Primary Cilia in Side View.
MC3T3-E1 cells were grown on tissue culture treated polyester membranes (Corning, Corning, NY). Membranes were excised, folded in half cell-side out, and positioned in a small flow chamber so that the folded edge was parallel to the direction of flow and aligned with the chamber edge. Cells were imaged for 20 sec (5 sec before and after flow) at 10 frames per second under ×40 magnification using Hoffman modulation contrast and exposed to a 10-sec pulse of ≈0.03 Pa steady flow. Images were contrast-enhanced using Image J software (National Institutes of Health, Bethesda, MD).
Abrogation of Primary Cilia.
To prevent cilium formation by knockdown of polaris, 20 μM siRNA targeting polaris (sequence: 5′-CCAGAAACAGATGAGGACGACCTTT-3′) or 20 μM scrambled control siRNA (Invitrogen) was transfected into cells by using XtremeGene (Roche Molecular Systems, Alameda, CA). Medium was changed 24 h after transfection, and flow experiments were performed 48 h after transfection. Neither the scrambled siRNA nor the polaris siRNA had any significant effect on overall cellular morphology. To remove primary cilia, 4 mM aqueous chloral hydrate (Sigma) was added to cells for 72 h, cells were washed three times with PBS, and fresh medium was added for 24 h before flow experiments (26).
Western Blotting.
Total cellular protein was isolated in RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA), and total protein quantity was determined by BCA assay (Pierce, Rockford, IL). Samples were separated by electrophoresis in 4–12% precast NuPAGE Bis-Tris gels (Invitrogen), transferred onto nitrocellulose membranes (Invitrogen), and probed with rabbit anti-polaris (1:5,000) and anti-actin (1:1,000; Santa Cruz Biotechnology) antibodies. Bound primary antibodies were detected by chemiluminescent detection of HRP-conjugated goat anti-rabbit antibodies.
Fluid Flow.
A previously described fluid flow device (14) was used to deliver oscillatory laminar fluid flow with a period of 1 Hz and a cell surface shear stress of 1 Pa. Shear stress was generated using a peak flow rate of 9 ml/min in the small chamber (38 × 10 × 0.28 mm) used for Ca2+ experiments and 18.8 ml/min in the large chamber (56 × 24 × 0.28 mm) used for all other experiments. For steady flow experiments, a syringe pump (KD Scientific, New Hope, PA) was used to generate flow at 9 ml/min. For calcium experiments, each group had ≥4 slides, and for gene expression and PGE2 experiments, each group had ≥6 slides. All experiments were repeated over multiple days, and for each experimental set of slides, a control set was tested in parallel to ensure that any differences detected were not due to performing flow experiments and response assays at distinct times. To compare no-flow vs. flow responses, a two-sample Student t test was used in which sample variance was not assumed to be equal. To compare observations from more than two groups, a one-way ANOVA was used followed by a Bonferroni selected-pairs multiple comparisons test.
PGE2 Assay.
After 1 h of flow, slides were removed from flow chambers, placed in dishes with 1 ml of medium, and incubated for 1 h. Medium PGE2 levels were measured by using an enzyme immunoassay kit (Amersham, Piscataway, NJ). PGE2 levels were normalized to total protein as determined by BCA assay (Pierce). All samples and standards were run in duplicate.
Real-Time RT-PCR.
Real-time quantitative RT-PCR was performed using the ABI Prism 7900 detection system. Primers and probes for 18S, OPN, COX2, OPG, RANKL, and GAPDH were obtained from Applied Biosystems (Foster City, CA). Immediately after 1-h flow, total RNA was extracted with TriReagent (Invitrogen), and cDNA was synthesized using the _Taq_Man reverse transcription kit (Applied Biosystems). cDNA samples were then amplified by real-time PCR. Amplification curves for control and experimental genes were recorded, and relative gene levels between samples were quantified by using the relative standard curve method (ABI Prism 7700 User Bulletin 2; Applied Biosystems). All samples were normalized to endogenous control 18S rRNA. All samples and standards were run in triplicate. GAPDH levels were measured as a control to verify that global gene expression levels did not increase after flow (data not shown).
Ca2+ Imaging.
Intracellular Ca2+ was quantified by using ratiometric imaging (14). Before exposure to flow, cells were incubated with 10 μM Fura-2-AM (Molecular Probes) for 30 min. Slides were then mounted in a small chamber and placed on a Nikon Eclipse TE-300. Flow medium consisted of MEM-α and 2% FBS. Fluorescence intensity was recorded (Metafluor; Universal Imaging, Downingtown, PA) every 2 sec for 3 min before flow and for 3 min during flow. A response was defined as a transient increase in total cellular fluorescence intensity of at least four times the maximum intensity increase recorded during the 3 min before flow.
Ca2+ Response/Cilium Correlation.
Slide backs were marked with a diamond scribe, and Ca2+ response of cells at a mark was quantified during flow as described above. After flow, slides were fixed in −20°C methanol and stained for primary cilia, again as described above. The field of cells analyzed for Ca2+ response was found and scored for the presence of primary cilia. Cells were scored blindly for Ca2+ response (Y/N) and primary cilia (Y/N), and correlation significance was tested by χ2 analysis.
Supplementary Material
Supporting Movie
Acknowledgments
We thank Linda Bonewald (University of Missouri, Kansas City, MO), Ryoko Kuriyama, and Bradley Yoder for cells and antibodies and Tim Stowe and Emily Arsndorf for help with experimental protocols. This work was supported by National Institutes of Health Grant AR45989 (to C.R.J.).
Abbreviations
COX2
cyclooxygenase 2
IFT
intraflagellar transport
OPG
osteoprotegerin
OPN
osteopontin
PGE2
prostaglandin E2
RANK
receptor activator of NF-κB
RANKL
RANK ligand.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Huang H, Kamm RD, Lee RT. Am J Physiol. 2004;287:C1–C11. doi: 10.1152/ajpcell.00559.2003. [DOI] [PubMed] [Google Scholar]
- 2.Hughes-Fulford M. Sci STKE. 2004;2004:RE12. doi: 10.1126/stke.2492004re12. [DOI] [PubMed] [Google Scholar]
- 3.Simons M, Walz G. Kidney Int. 2006;70:854–864. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto K, Takahashi T, Asahara T, Ohura N, Sokabe T, Kamiya A, Ando J. J Appl Physiol. 2003;95:2081–2088. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 5.Wong M, Siegrist M, Goodwin K. Bone. 2003;33:685–693. doi: 10.1016/s8756-3282(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 6.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 7.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Stokes IA, Aronsson DD, Dimock AN, Cortright V, Beck S. J Orthop Res. 2006;24:1327–1334. doi: 10.1002/jor.20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borer KT. Sports Med (Auckland, NZ) 2005;35:779–830. doi: 10.2165/00007256-200535090-00004. [DOI] [PubMed] [Google Scholar]
- 10.Steck R, Niederer P, Knothe Tate ML. Med Eng Phys. 2000;22:117–125. doi: 10.1016/s1350-4533(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Wang Y, Han Y, Henderson SC, Majeska RJ, Weinbaum S, Schaffler MB. Proc Natl Acad Sci USA. 2005;102:11911–11916. doi: 10.1073/pnas.0505193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CH, You L, Yellowley CE, Jacobs CR. Bone. 2006;39:1043–1047. doi: 10.1016/j.bone.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Reilly GC, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Biorheology. 2003;40:591–603. [PubMed] [Google Scholar]
- 14.You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. J Biol Chem. 2001;276:13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 15.Wheatley DN, Wang AM, Strugnell GE. Cell Biol Int. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- 16.Tonna EA, Lampen NM. J Gerontol. 1972;27:316–324. doi: 10.1093/geronj/27.3.316. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, Quarles LD. J Biol Chem. 2006;281:30884–30895. doi: 10.1074/jbc.M604772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singla V, Reiter JF. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 19.Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Hollt V. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- 20.Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Huangfu D, Anderson KV. Proc Natl Acad Sci USA. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. PLoS Biol. 2005;3:e268. doi: 10.1371/journal.pbio.0030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Am J Physiol. 2005;289:F978–F988. doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- 24.Praetorius HA, Spring KR. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 25.Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Am J Physiol. 2006;290:F1320–F1328. doi: 10.1152/ajprenal.00463.2005. [DOI] [PubMed] [Google Scholar]
- 26.Praetorius HA, Spring KR. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 27.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 28.The European Polycystic Kidney Disease Consortium. Cell. 1994;77:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 29.Pazour GJ. J Am Soc Nephrol. 2004;15:2528–2536. doi: 10.1097/01.ASN.0000141055.57643.E0. [DOI] [PubMed] [Google Scholar]
- 30.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Proc Natl Acad Sci USA. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitfield JF. J Cell Biochem. 2003;89:233–237. doi: 10.1002/jcb.10509. [DOI] [PubMed] [Google Scholar]
- 32.Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Development (Cambridge, UK) 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- 33.Piperno G, LeDizet M, Chang XJ. J Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohta T, Essner R, Ryu JH, Palazzo RE, Uetake Y, Kuriyama R. J Cell Biol. 2002;156:87–99. doi: 10.1083/jcb.200108088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alieva IB, Vorobjev IA. Cell Biol Int. 2004;28:139–150. doi: 10.1016/j.cellbi.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Roth KE, Rieder CL, Bowser SS. J Cell Sci. 1988;89:457–466. doi: 10.1242/jcs.89.4.457. [DOI] [PubMed] [Google Scholar]
- 37.Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Am J Physiol. 2002;282:F541–F552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
- 38.Shen Q, Christakos S. J Biol Chem. 2005;280:40589–40598. doi: 10.1074/jbc.M504166200. [DOI] [PubMed] [Google Scholar]
- 39.Thorsen K, Kristoffersson AO, Lerner UH, Lorentzon RP. J Clin Invest. 1996;98:2446–2449. doi: 10.1172/JCI119061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saunders MM, You J, Zhou Z, Li Z, Yellowley CE, Kunze EL, Jacobs CR, Donahue HJ. Bone. 2003;32:350–356. doi: 10.1016/s8756-3282(03)00025-5. [DOI] [PubMed] [Google Scholar]
- 41.Cheng B, Kato Y, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Endocrinology. 2001;142:3464–3473. doi: 10.1210/endo.142.8.8338. [DOI] [PubMed] [Google Scholar]
- 42.Ljunggren O, Johansson H, Ljunghall S, Lerner UH. Bone Miner. 1991;12:81–90. doi: 10.1016/0169-6009(91)90037-z. [DOI] [PubMed] [Google Scholar]
- 43.Simon AM, Manigrasso MB, O'Connor JP. J Bone Miner Res. 2002;17:963–976. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 44.Nagai M, Sato N. Biochem Biophys Res Comm. 1999;257:719–723. doi: 10.1006/bbrc.1999.0524. [DOI] [PubMed] [Google Scholar]
- 45.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Goto M, Mochizuki SI, Tsuda E, Morinaga T, Udagawa N, et al. Bone. 1999;25:109–113. doi: 10.1016/s8756-3282(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 46.Zhao S, Zhang YK, Harris S, Ahuja SS, Bonewald LF. J Bone Miner Res. 2002;17:2068–2079. doi: 10.1359/jbmr.2002.17.11.2068. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Wright JM, Qian F, Germino GG, Guggino WB. J Biol Chem. 2005;280:41298–41306. doi: 10.1074/jbc.M510082200. [DOI] [PubMed] [Google Scholar]
- 48.Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu J, Duncan RL. Am J Physiol. 2000;278:C989–C997. doi: 10.1152/ajpcell.2000.278.5.C989. [DOI] [PubMed] [Google Scholar]
- 49.Donahue HJ, Li Z, Zhou Z, Yellowley CE. Am J Physiol. 2000;278:C315–C322. doi: 10.1152/ajpcell.2000.278.2.C315. [DOI] [PubMed] [Google Scholar]
- 50.Praetorius HA, Frokiaer J, Nielsen S, Spring KR. J Membr Biol. 2003;191:193–200. doi: 10.1007/s00232-002-1055-z. [DOI] [PubMed] [Google Scholar]
- 51.McGlashan SR, Jensen CG, Poole CA. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 52.Chakrabarti A, Schatten H, Mitchell KD, Crosser M, Taylor M. Cell Tissue Res. 1998;293:453–462. doi: 10.1007/s004410051137. [DOI] [PubMed] [Google Scholar]
- 53.Lee GM, Diguiseppi J, Gawdi GM, Herman B. J Cell Sci. 1987;88:603–612. doi: 10.1242/jcs.88.5.603. [DOI] [PubMed] [Google Scholar]
- 54.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. Mol Biol Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ponik SM, Pavalko FM. J Appl Physiol. 2004;97:135–142. doi: 10.1152/japplphysiol.01260.2003. [DOI] [PubMed] [Google Scholar]
- 57.Tong L, Buchman SR, Ignelzi MA, Jr, Rhee S, Goldstein SA. Plast Reconstr Surg. 2003;111:211–222. doi: 10.1097/01.PRS.0000033180.01581.9A. and discussion 223–214. [DOI] [PubMed] [Google Scholar]
- 58.Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. J Bone Miner Res. 2000;15:209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- 59.Wong C, Stearns T. Nat Cell Biol. 2003;5:539–544. doi: 10.1038/ncb993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Movie