Loss of Neprilysin Function Promotes Amyloid Plaque Formation and Causes Cerebral Amyloid Angiopathy (original) (raw)
Abstract
Cerebral deposition of the amyloid β protein (Aβ), an invariant feature of Alzheimer’s disease, reflects an imbalance between the rates of Aβ production and clearance. The causes of Aβ elevation in the common late-onset form of Alzheimer’s disease (LOAD) are largely unknown. There is evidence that the Aβ-degrading protease neprilysin (NEP) is down-regulated in normal aging and LOAD. We asked whether a decrease in endogenous NEP levels can prolong the half-life of Aβ in vivo and promote development of the classic amyloid neuropathology of Alzheimer’s disease. We examined the brains and plasma of young and old mice expressing relatively low levels of human amyloid precursor protein and having one or both NEP genes silenced. NEP loss of function 1) elevated whole-brain and plasma levels of human Aβ40 and Aβ42, 2) prolonged the half-life of soluble Aβ in brain interstitial fluid of awake animals, 3) raised the concentration of Aβ dimers, 4) markedly increased hippocampal amyloid plaque burden, and 5) led to the development of amyloid angiopathy. A ∼50% reduction in NEP levels, similar to that reported in some LOAD brains, was sufficient to increase amyloid neuropathology. These findings demonstrate an important role for proteolysis in determining the levels of Aβ and Aβ-associated neuropathology in vivo and support the hypothesis that primary defects in Aβ clearance can cause or contribute to LOAD pathogenesis.
Cerebral accumulation of extracellular deposits of the amyloid β protein (Aβ) is an early and necessary feature of Alzheimer’s disease (AD). Aβ is derived by sequential proteolytic cleavages of β-amyloid precursor protein (APP) by the β- and γ-secretases. Aβ deposition can occur in the brain parenchyma, forming amyloid plaques, and in the walls of brain blood vessels, referred to as cerebral amyloid angiopathy (CAA). There is emerging evidence that small, soluble oligomers of secreted Aβ may cause electrophysiological and behavioral abnormalities.1 The marked elevation of Aβ levels in AD brain compared with healthy controls, ranging from ∼50 to ∼1500-fold, indicates an imbalance between the rates of Aβ production and clearance. Overproduction of Aβ has been implicated in less than 1% of the entire AD population, namely those who have early-onset, autosomal dominant AD caused by mutations in the genes encoding APP or the γ-secretase catalytic components presenilin-1 and presenilin-2. In contrast, patients with the more common late-onset form of AD (LOAD) are not known to overproduce Aβ, suggesting that deficits in clearance of the peptide could be responsible for the cerebral Aβ accumulation that precipitates LOAD.
Newly generated Aβ is rapidly cleared from the brains of APP transgenic mice (half-life, ∼2 hours)2,3 and from the central nervous system in humans.4 Genetic knockout of each of four different zinc metallopeptidases that degrade Aβ in vitro has been shown to elevate endogenous murine brain Aβ in vivo: neprilysin (NEP),5,6 insulin-degrading enzyme (IDE),7,8 and endothelin-converting enzymes 1 and 2.9 Rat brains infused with thiorphan, which inhibits several metallopeptidases, were also found to have elevated levels of endogenous Aβ.10 Mouse and rat Aβ differ from the human peptide at three amino acids and do not readily aggregate into the oligomers and extracellular amyloid fibrils that seem to be neurotoxic in AD. Because only rodent Aβ was examined in these gene deletion and protease inhibitor studies, the effect of the proteases on human Aβ monomers and oligomers, AD-type amyloid plaques, and CAA could not be determined. Of note, a recent study11 reported that brains from 2-year-old NEP knockout mice contained small electron-dense deposits within enlarged axons associated with myelin degeneration. Although this histological finding is interesting and deserves further investigation, these deposits, possibly composed of murine Aβ, do not have the morphology, extracellular location, or associated neuropathology of the amyloid plaques of AD or APP transgenic mice, making it difficult to interpret the relevance of these granular lesions to AD. Another recent study demonstrated that genetic inactivation of the cysteine protease cathepsin B elevates plaque load in human APP transgenic mice.12
Overexpression of human NEP or IDE in the brains of APP transgenic mice has been shown to lower Aβ levels, decrease amyloid plaque load, and prevent premature lethality.13,14,15 This body of work suggests that increased Aβ proteolysis could be therapeutic in AD, but artificial overexpression does not follow normal anatomical and cell-type distributions or temporal expression patterns, and it does not involve the expression of different natural isoforms of the proteins. For example, in the overexpression studies, high levels of a single mRNA splice form of either NEP or IDE was expressed predominantly or exclusively in neurons in certain brain regions, whereas natural IDE and NEP are each expressed as several mRNA splice forms in the central nervous system,16,17 occur in other cell types besides neurons,16,18 and are higher in some brain regions than others.17,19 As a result, it is difficult to draw conclusions about a protease’s natural role from such overexpression studies. Furthermore, overexpression clearly does not model the potential hypofunction of Aβ proteases that could occur in LOAD.
A role for decreased Aβ proteolysis in LOAD is suggested by the findings that NEP and IDE protein levels are lower in AD brains than controls19,20 and that endogenous NEP levels have been found to be decreased in the cerebrospinal fluid of patients with LOAD or mild cognitive impairment (which often progresses to AD).21 Human genetic studies have also suggested a role for faulty Aβ proteolysis in LOAD.22
In this study, we examined whether isolated hypofunction of an endogenous Aβ-degrading protease can increase the steady-state levels and half-life of human Aβ in vivo and thus promote the development of AD-type amyloid pathology. We generated mice expressing relatively low levels of human APP and having one or both copies of the NEP gene disrupted. In these animals, we systematically assessed human Aβ40 and Aβ42 levels in brain and plasma at different ages, cerebral amyloid plaque burden, the presence of CAA, the relative levels and species of Aβ oligomers, and the basal levels and half-life of interstitial fluid (ISF) Aβ in the hippocampus in vivo.
Materials and Methods
Animals and Collection of Plasma and Tissue
The transgenic mouse line J9 (gift of L. Mucke, University of California, San Francisco, CA) expresses a human APP minigene with the KM670/671NL and V717F AD-causing mutations driven by a platelet-derived growth factor promoter.23 J9 mice in a C57BL/6 background were bred to mice with targeted deletion of the NEP gene that were also in this background (gift of B. Lu, Children’s Hospital, Boston, MA)24 to generate mice expressing human APP that were either null (−/−) or heterozygous (+/−) for NEP. Genotyping was performed by polymerase chain reaction, and the genotypes of all animals used in experiments were confirmed by APP and NEP immunoblotting. Mice were sacrificed by CO2 inhalation followed by decapitation, and then blood was collected and brains removed. Blood was immediately mixed with a protease inhibitor cocktail including ethylenediamine tetraacetic acid and centrifuged, and the supernate (plasma) was saved. One cerebral hemisphere was flash-frozen in liquid nitrogen for biochemical analysis, and the other was fixed in 10% formalin, dehydrated, and embedded in paraffin for sectioning.
Immunohistochemistry and Thioflavin-S Staining
Immunohistochemistry and counterstaining with Harris hematoxylin was performed as previously described14 on 10-μm sagittal brain sections using the sensitive anti-Aβ polyclonal antibody R1282 (1:1000).25 Additional sections were deparaffinized, hydrated, incubated with 1% thioflavin-S for 8 minutes, and washed in 80% and 95% ethanol and water. Quantification of Aβ and thioflavin-S staining by computer-assisted image analysis were each performed on the hippocampi from two sections (∼300 μm apart) from each mouse in the 13-month cohort, as described.26
Preparation of Brain Extracts
Three biochemical pools of Aβ were sequentially extracted from the fresh frozen cerebral hemispheres: aqueous or Tris-buffered saline (TBS)-soluble pool, detergent Nonidet P-40 (NP-40)-soluble pool, and the guanidine hydrochloride-soluble (GuHCl) pool. Each hemisphere was first homogenized in 5 volumes (w/v) (“initial hemisphere-volumes”) of ice-cold TBS extraction buffer [25 mmol/L Tris-HCl, pH 7.4, 140 mmol/L NaCl, 3 mmol/L KCl, 5 mmol/L ethylenediamine tetraacetic acid, 2 mmol/L 1,10-phenanthroline, and complete ethylenediamine tetraacetic acid-free protease inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany)] in a Potter-Elvehjem tissue grinder (Wheaton Science Products, Millvale, NJ) using a motorized rotor for the pestle (20 strokes at ∼3500 rpm). After centrifugation at 100,000 × g for 1 hour, the resulting supernate was saved as the TBS extract, and the pellet was resuspended in 5 initial hemisphere-volumes of NP-40 extraction buffer (TBS extraction buffer with 1% NP-40). The pellet was sonicated, then homogenized as above, and spun at 100,000 × g for 1 hour, and the clear supernate saved as the NP-40 extract. Of note, there was a small, separate, viscous cloudy layer between the supernate and pellet. This cloudy layer and the remaining pellet were then homogenized together in 6.25 mol/L GuHCl (in 50 mmol/L Tris-HCl, pH 8.0) in 5 initial hemisphere-volumes and placed on a nutator for 2 hours at room temperature. The samples were spun at 20,800 × g for 20 minutes, the small GuHCl-insoluble pellet was discarded, and the supernate was kept as the GuHCl extract.
Enzyme-Linked Immunosorbent Assay (ELISA) and Immunoblotting on Brain Homogenates
Human Aβ40 and Aβ42 levels in the brain extracts and plasma were analyzed by sandwich ELISAs, as previously described,27 using monoclonal C-terminal-specific antibodies 2G3 and 21F12 for capture of Aβ40 and Aβ42, respectively, and the human-specific biotinylated monoclonal antibody 3D6 (against Aβ1–5) for detection. ELISA antibodies were gifts from Elan Pharmaceuticals, South San Francisco, CA. All samples were assayed in duplicate and averaged to give final values. Outlying values were detected by Grubb’s test (extreme studentized deviate) and not included in the final analyses. For routine immunoblotting, membranes were probed with the following antibodies: affinity-purified C9 (1:2000)28 for APP and its proteolytic derivatives C83 and C99; 8E5 (1:2000) (Elan Pharmaceuticals), which is specific for human APP; 56C6 (1:100) (Novocastra Laboratories, Newcastle on Tyne, UK) for NEP; and IDE-1 (1:3000)29 for IDE. Densitometry for C9, 8E5, and 56C6 Western blots was performed using the NIH public software ImageJ (http://rsb.info.nih.gov/ij/). To detect Aβ oligomers in the brain homogenates, an immunoprecipitation (IP)-Western protocol was used as previously described.30 For IP, 100 μl of GuHCl brain extract diluted in 4.9 ml of Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) or 350 μl of undiluted NP-40 brain extract was incubated with the anti-Aβ polyclonal antibody R1282 (1:50). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed on the immunoprecipitate using NuPAGE Novex 4 to 12% Bis-Tris minigels (Invitrogen). Immunoblotting for Aβ was performed with monoclonal antibodies 2G3, 21F12, or 4G8 (against Aβ17–24) (Signet Laboratories, Dedham, MA), all at 1:1000. Detection was performed with the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE). Immunoblots were stripped with 25 mmol/L glycine, 1% sodium dodecyl sulfate, pH 2, and complete removal of antibody was confirmed by re-imaging before probing the membrane with a new primary antibody.
In Vivo Microdialysis
The basal concentration and half-life of brain ISF Aβ1-x in the hippocampus of awake, freely moving mice were measured as previously described.3,31 This technique samples soluble molecules within the extracellular fluid that are smaller than 38 kd, the molecular mass cutoff of the microdialysis probe membrane. Under isoflurane volatile anesthetic, a guide cannula (BR-style; Bioanalytical Systems, Indianapolis, IN) was cemented into the left hippocampus (bregma, 3.1 mm, 2.5 mm lateral to midline, and 1.2 mm below dura at a 12° angle). Two-millimeter microdialysis probes were inserted through the guides so the membrane was contained entirely within the hippocampus (BR-2, 38-kd molecular weight cutoff membrane; Bioanalytical Systems). The microdialysis perfusion buffer was artificial cerebrospinal fluid containing 0.15% bovine serum albumin that was filtered through a 0.1-μm membrane. Flow rate was a constant 1.5 μl/minute. Samples were collected every 60 minutes with a refrigerated fraction collector into polypropylene tubes and assessed for Aβ1-x by sandwich ELISA at the completion of each experiment as previously described.3 The basal concentration of ISF Aβ was defined as the mean concentration of Aβ from hours 8 to 12 after probe insertion. The actual in vivo concentration of measurable ISF Aβ was calculated based on the concentration of Aβ1-x collected at 1.5 μl/min, assuming an 11.5% recovery of Aβ from the ISF.3 After basal ISF Aβ levels were established, mice were treated by subcutaneous injection with 3 mg/kg body weight of LY411575, a potent, blood-brain barrier-permeable γ-secretase inhibitor (Eli Lilly and Co., Indianapolis, IN). Microdialysis samples were collected for an additional 8 hours following treatment. To determine Aβ half-life, time points between drug delivery and when Aβ concentrations plateaued were analyzed (typically 6 hours post-treatment). For first-order processes, the elimination rate of a molecule from the body is directly related to the slope of the semilog plot of the percent of the basal concentration versus time: a = −_K_e/2.3, where a represents the slope and _K_e represents the elimination rate constant. The half-life (_t_1/2) can be expressed in terms of the elimination rate constant: _t_1/2 = 0.693/_K_e.
Following each experiment, animals were sacrificed, and probe placement was verified histologically with Cresyl violet staining. Brain sections from each mouse were also stained with antibody 3D6 to confirm the lack of Aβ deposition within the hippocampus.
Results
Quantification of Cerebral APP and NEP in the Mouse Models
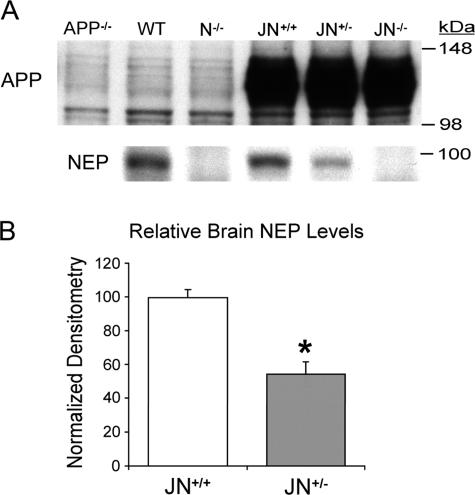
NEP is a type II transmembrane glycoprotein located predominantly on the cell surface that has been found to degrade several peptides beside Aβ in vitro, including enkephalins, substance P, and atrial natriuretic peptide. NEP, which is expressed at lower levels in brain than kidney and lung, is found in the central nervous system on pre- and postsynaptic neuronal membranes and smooth muscle cells of the cerebral vasculature.32,33 To create an amyloid-forming mouse model with an endogenous deficit in Aβ clearance, we crossed mice with disruption of the NEP gene24 with the J9 mouse line that expresses comparatively low levels of human APP with both the KM670/671NL (“Swedish”) and V717F (“Indiana”) AD-causing mutations.23 We examined three age-matched cohorts (3, 6, and 13 months). Each cohort contained the J9 controls with both copies of the NEP gene (referred to as JN+/+ mice) and mice expressing the J9 APP transgene but with either one or both NEP genes silenced (JN+/− and JN−/−, respectively). The newly generated JN+/− and JN−/− lines are viable, fertile, and grossly normal. Routine necropsy and histological examination on male and female JN−/− mice revealed no abnormalities in the heart, lungs, trachea, salivary glands, intestines, pancreas, liver, kidney, skeletal muscle, uterus, prostate gland, or lymph nodes. Protein levels of human APP (Figure 1A), total APP, and the APP C-terminal fragments (CTFs) C83 and C99 were indistinguishable in age-matched JN+/+, JN+/−, and JN−/− brains by immunoblotting and densitometry (data not shown), suggesting that APP processing to Aβ does not differ among the three mouse lines. JN+/+ brains had NEP levels indistinguishable from those of wild-type mice, whereas NEP was absent in the JN−/− brains (Figure 1A). Disruption of one copy of the NEP gene resulted in approximately half (54%) the level of NEP protein in the JN+/− brains compared with JN+/+ at 6 months of age (P < 0.00005) (Figure 1B). There were no differences in cerebral IDE levels in the JN+/+ and JN−/− brains by immunoblotting (data not shown).
Figure 1.
Cerebral APP and NEP levels in NP-40 brain fractions. A: A representative Western blot comparing levels of human APP (antibody 8E5) and endogenous NEP (antibody 56C6) in equal amounts of brain protein from the three JN lines at age 6 months. Control mice are APP knockout (APP−/−), wild-type (WT), and NEP knockout (N−/−). Densitometric comparison of the APP and CTF bands (not shown) among the three JN lines on three separate Western blots (n = 16 samples) revealed no significant difference. B: Comparison of relative NEP protein levels by densitometric analysis of quantitative Western blots (n = 13 JN+/+ and 14 JN+/− brains on three separate Western blots. For each blot, densitometry scores were normalized to the mean JN+/+ score, allowing combination of data sets). Bars represent the mean normalized densitometry score ± SEM. *P < 0.00005 by Student’s two-tailed _t_-test.
NEP Hypofunction Markedly Increases Hippocampal Amyloid Plaque Burden
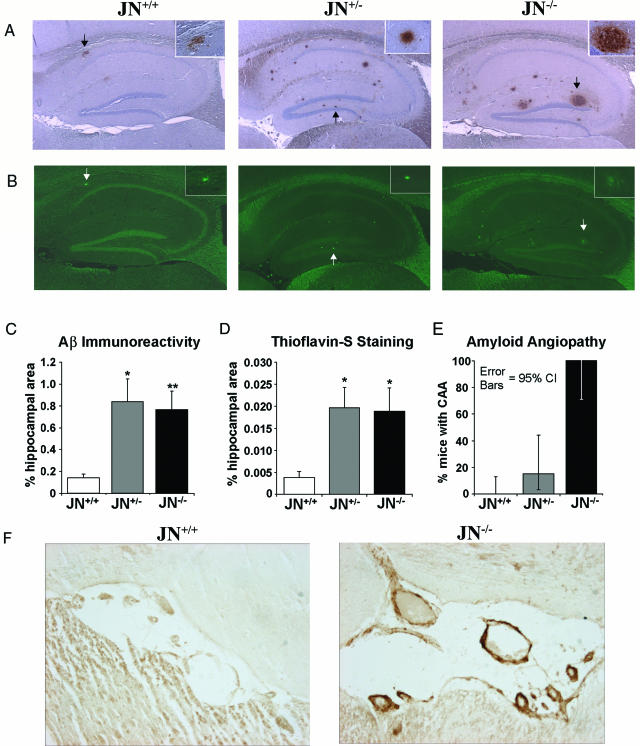
We first investigated whether decreased neprilysin activity, as we hypothesized may occur in LOAD, could affect the levels of a key defining pathological feature of AD, the amyloid plaque. JN+/+ control mice predominantly develop plaques in the hippocampal region, and, in our colony, ∼85% of the JN+/+ controls developed at least one hippocampal plaque by age 13 months. We performed Aβ immunohistochemistry on four sagittal sections (two pairs of adjacent sections, with each pair ∼300 μm apart) from one cerebral hemisphere of each mouse in our 13-month-old cohort of JN+/+, JN+/−, and JN−/− mice. There was a striking increase in Aβ plaque burden in the animals partially or completely deficient in NEP expression (Figure 2A). Although the vast majority of plaques were located in the hippocampal region, 35% of JN+/+, 44% of JN+/−, and 60% of JN−/− mice had at least one (but never more than a few) extrahippocampal plaques, usually in the frontoparietal cortex or corpus callosum. To compare objectively the plaque burdens among our three mouse lines, we used image analysis software to measure the percentage of the hippocampal area that stained positively for Aβ. The JN+/− and JN−/− hippocampi had ∼490% (P < 0.0005) and ∼440% (P ≤ 0.00001) more area with plaques than the JN+/+ controls, respectively (Figure 2C). Interestingly, there was no significant difference in plaque burden between the JN+/− and JN−/− brains. By visual inspection, it appeared that the increased plaque burden in the NEP-deficient animals was due to an increase in both the number and size of the plaques. Blinded plaque counts and estimation of plaque size on the JN+/+ and JN−/− sections confirmed that the actual number of plaques was indeed greater in the JN−/− brains (∼210%, P < 0.0005) and that a larger percentage of these plaques was classified as large as compared with the JN+/+ plaques. Seventy percent of the 13-month-old JN+/+ mice had five or fewer plaques, whereas none of the JN−/− animals had fewer than six plaques. No plaques were detected in any of the mouse brains from the 3- and 6-month-old cohorts.
Figure 2.
Disruption of NEP increases cerebral Aβ pathology at 13 months. A: Aβ immunoreactivity to antibody R1282 in sagittal sections of hippocampi (magnification, ×2) from mice representative of each line. Each section is from a mouse with the median degree of pathology for its genotype. The insets (magnification, ×20) show plaque morphology. B: Thioflavin-S staining of sections adjacent to those in A demonstrates deposition of fibrillar Aβ. Arrows in A and B mark a representative plaque in each brain positively stained by both the anti-Aβ antibody and thioflavin-S. Mean percentage of the hippocampal area with positive Aβ (C) or thioflavin-S (D) staining as determined by computer-assisted image analysis for each experimental group. Bars represent the group mean ± SEM (n = 20 JN+/+, 11 JN+/−, and 8 JN−/−; *P < 0.0005; **P = 0.00001 compared with JN+/+). F: Blood vessels in the hippocampal fissure on sagittal brain sections stained by Aβ immunohistochemistry reveals prominent CAA in the JN−/− mouse. E: Graph represents the percentage of mice with CAA by genotype and the 95% confidence intervals (CI) on these proportions as determined by the modified Wald method. The JN−/− CI does not overlap with those of the JN+/+or JN+/− mice. n = 22 JN+/+, 13 JN+/−, and 8 JN−/−.
To characterize better the Aβ-immunoreactive plaques, we stained with thioflavin-S, which detects fibrillar Aβ in a β-pleated sheet conformation. As expected, only a subgroup of the Aβ-immunoreactive plaques in all three mouse lines was stained by thioflavin-S (Figure 2B). Partial and total NEP deficiencies both resulted in ∼400% (P < 0.001) increases in the fraction of hippocampal area with fibrillar Aβ compared with animals with wild-type NEP levels (Figure 2D). Again, there was no significant difference between JN+/− and JN−/− staining (P = 0.43). We conclude that even a ∼50% decrease in NEP levels causes a dramatic increase in plaque deposition.
CAA Develops Only in NEP-Deficient Mice
We next examined whether decreased clearance of Aβ can modulate CAA, another pathological feature of AD. Remarkably, Aβ immunohistochemistry revealed that none of the 22 JN+/+ control animals had CAA at 13 months, whereas all (eight of eight) of the JN−/− mice had vascular Aβ deposition (Figures 2, E and F). In the JN+/− animals, 15% (2 of 13) had CAA, suggesting a possible gene-dose effect. The CAA was located almost exclusively in the hippocampal fissure. No CAA was observed in the 3- or 6-month-old cohorts.
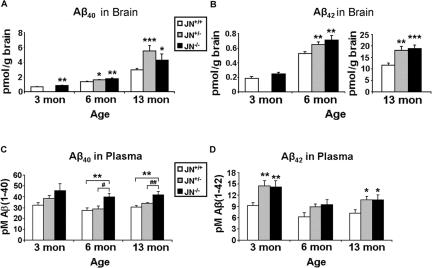
NEP Hypofunction Elevates Aβ40 and Aβ42 in Brain and Plasma
After β-secretase cleavage of APP, the γ-secretase complex cleaves the membrane-retained C-terminal fragment (C99) in a manner that generates Aβ peptides of slightly varying lengths. The two most abundant and best-studied forms contain 40 (Aβ40) or 42 (Aβ42) residues, the latter being the far more amyloidogenic peptide. To determine the impact of NEP deficiency on the levels of human Aβ40 and Aβ42 in vivo at different ages, we measured these peptides in brain and plasma from the 3-, 6-, and 13-month-old animal cohorts using human Aβ40- and Aβ42-specific ELISAs. Aβ40 levels in the JN−/− GuHCl-extracted brain fractions were 28% (P < 0.01), 31% (P < 0.005), and 45% (P < 0.05) higher than those of the JN+/+ controls at ages 3, 6, and 13 months, respectively (Figure 3A). Brain Aβ levels were only measured in JN+/− brains at ages 6 and 13 months, and at these ages they had 19% (P < 0.05) and 87% (P < 0.0005) more Aβ40 than the JN+/+ brains, respectively. Complete absence of NEP resulted in elevations of GuHCl-extractable Aβ42 of 36% at 6 months (P < 0.01) and 63% at 13 months (P < 0.0005), whereas disruption of only one NEP gene elevated the peptide by 24% at 6 months (P < 0.01) and 56% at 13 months (P < 0.005) (Figure 3B). There were no significant differences between the JN−/− and JN+/− brains in either Aβ40 or Aβ42 levels. The Aβ42/Aβ40 ratio increased dramatically with age from ∼0.3 at 3 months to ∼5 at 13 months, and this relative increase in Aβ42 accumulation was similar among the three genotypes. We also measured Aβ40 and Aβ42 levels in TBS and NP-40 JN+/+ and JN−/− brain extracts (prepared as described in Materials and Methods) from our three age cohorts. Aβ levels were higher in most of the JN−/− extracts, but only some of the Aβ elevations reached statistical significance. In the absence of NEP, Aβ40 levels were ∼25% elevated in the NP-40 brain extracts at 3 and 6 months and ∼50% elevated in the TBS extracts at 6 months (P < 0.05). JN−/− Aβ42 levels in the TBS extracts were ∼150% greater than the JN+/+ levels at 13 months (P < 0.05).
Figure 3.
Quantification of Aβ40 and Aβ42 in brain and plasma at 3, 6, and 13 months in NEP-deficient mice. ELISAs specific for human Aβ40 (A and C) and Aβ42 (B and D) were performed on the GuHCl brain extracts (A and B) and plasma (C and D). Bars represent the mean values ± SEM (n = 8 to 18 mice per group). No JN+/− brains were examined at 3 months. Because of the tremendous increase in cerebral Aβ42 with age, the 13-month Aβ42 brain data are shown with a differently scaled y axis than the 3- and 6-month data (compared with JN+/+, *P < 0.05; **P ≤ 0.01; ***P < 0.0005. JN+/− compared with JN−/−, #P < 0.05; ##P < 0.01).
Plasma Aβ42 is elevated in patients with early-onset familial AD34 and also in some cohorts of patients with LOAD or those destined to develop LOAD several years later.35,36 Plasma Aβ40 and Aβ42 levels have also been shown to be heritable traits in LOAD.37 To determine whether an elevation in plasma Aβ as occurs in LOAD can be caused by faulty proteolytic clearance of the peptide in vivo, we measured plasma Aβ40 and Aβ42 by ELISA in mice of our three age-matched cohorts. Compared with the JN+/+ controls, plasma Aβ40 was elevated in the JN−/− animals by 42% (P < 0.05), 46% (P < 0.01), and 37% (P < 0.001) at 3, 6, and 13 months, respectively (Figure 3C). In contrast to what was observed with cerebral Aβ, disruption of only one NEP gene did not significantly elevate plasma Aβ40. As a result, at 6 and 13 months, JN+/− plasma Aβ levels were significantly lower than those of the JN−/− animals (P < 0.05). In contrast, ∼50% higher levels of Aβ42 occurred in both the JN−/− and JN+/− plasma, compared with the JN+/+ plasma at all three ages, with the 3- and 13-month-old cohorts reaching statistical significance (Figure 3D).
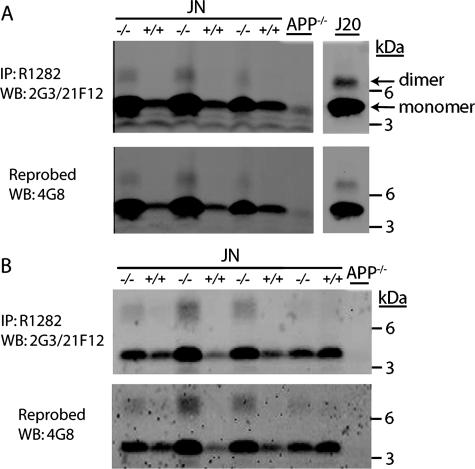
Aβ Oligomers Are Elevated in the Brains of Mice Lacking NEP
Standard Aβ ELISAs detect monomeric Aβ, whereas the potentially pathogenic Aβ oligomers are not clearly measured by this method.38,39 To investigate the effect of NEP deficiency on the levels and species of Aβ oligomers in the brain, we performed a sensitive IP-Western blot analysis for Aβ in GuHCl and NP-40 fractions from 13-month-old JN+/+ and JN−/− brains. After IP with a polyclonal antibody (R1282) to Aβ, we immunoblotted with monoclonal antibodies specific for the C terminus of Aβ40 (2G3) and Aβ42 (21F12) (Figure 4, A and B, top). We confirmed that the observed bands were species of Aβ by reprobing the blots with the monoclonal antibody 4G8 that recognizes the midregion of Aβ (Figure 4, A and B, bottom). In the GuHCl and NP-40 brain fractions, we observed Aβ monomer as the predominant species, but low levels of dimer were detected in the JN−/− brains. Both Aβ monomer and dimer levels were elevated in the GuHCl and NP-40 extracts of the JN−/− brains relative to the JN+/+ controls. This increase in JN−/− Aβ dimer was confirmed by densitometry (P < 0.05, not shown). Thus, NEP loss of function led to the cerebral accumulation of both Aβ monomers and dimers.
Figure 4.
NEP-deficient mice have elevated levels of Aβ dimers at 13 months. After Aβ IP with antibody R1282, Western blotting (WB) was performed with a mix of the C-terminal-specific antibodies 2G3 (Aβx-40) and 21F12 (Aβx-42), then stripped and reprobed with antibody 4G8 (Aβ 17–24) to confirm specificity. A: IP-Western on GuHCl brain extracts from JN+/+, JN−/−, APP−/−, and a transgenic mouse line (J20)53 expressing the same APP minigene that is in our experimental animals but at much higher levels. B: IP-Western on NP-40 brain extracts. The lower, dominant band is Aβ monomer, and the higher band seen only in JN−/− lanes is Aβ dimer.
NEP Deficiency Prolongs the Half-Life of Human Aβ and Increases Its Basal Levels in Brain ISF
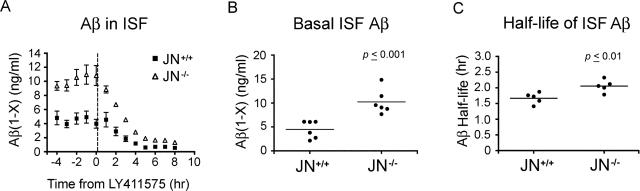
We demonstrate above that whole-brain Aβ levels are elevated in mice lacking NEP in all three age groups. Presumably, in the 13-month-old animals, much of the measured total cerebral Aβ is associated with the extracellular plaques and CAA, but no plaques or CAA were found in the 3- and 6-month-old groups. It is not known if NEP degrades Aβ intracellularly (eg, in the secretory pathway or after endocytosis) and/or extracellularly in vivo, and it is unclear in which of these compartments soluble, non-plaque-associated Aβ is elevated when NEP is disrupted. Furthermore, to date, no potential proteolytic Aβ clearance mechanism has been explicitly shown to affect the half-life of Aβ in vivo. It is important to confirm that any proposed clearance mechanism actually influences Aβ levels via modulating the peptide’s half-life and not by affecting its production, particularly given the numerous actions and ligands of the proposed Aβ transport receptors and the multiple substrates of the proteases. To examine the effect of NEP deficiency on the basal levels and half-life of extracellular soluble Aβ in the brains of living animals, we used an in vivo microdialysis technique3 to assess hippocampal ISF Aβ levels. For these experiments, we studied JN+/+ and JN−/− mice at age 4.5 months, before the onset of plaque deposition or CAA. ISF was collected from awake, freely moving mice every 60 minutes for 12 hours, and human Aβ1-x levels were quantified by ELISA. We measured the basal concentrations of ISF Aβ in our mice for 4 hours; then, after the subcutaneous injection of a potent γ-secretase inhibitor (LY411575) to eliminate new Aβ production, we assessed clearance of the ISF Aβ (Figure 5A). We found that mean basal Aβ levels were elevated 128% in the JN−/− hippocampal ISF compared with JN+/+ (P < 0.001) (Figure 5B) and that all of the JN−/− values were higher than those of the JN+/+, indicating that NEP naturally regulates the levels of extracellular Aβ. Using a semilog plot of the percent basal Aβ concentration versus time, the half-life of the cerebral ISF Aβ was calculated (see Materials and Methods). Animals normally expressing NEP had an ISF Aβ half-life of 1.7 hours, whereas those lacking the protease showed a significant 23% prolongation of the Aβ half-life to 2.1 hours (P < 0.01) (Figure 5C), demonstrating that NEP does, in fact, regulate the levels of Aβ in vivo through clearance of the peptide.
Figure 5.
Basal levels and half-life of hippocampal ISF Aβ are increased in NEP-deficient mice. Hippocampal ISF was assessed in awake, unrestrained 4.5-month-old JN+/+ and JN−/− mice via a microdialysis probe every hour for 12 hours and Aβ1-x measured by ELISA. A: Data points represent the mean ISF Aβ concentrations ± SEM at the specified times of five or six animals. After the first 4 hours, mice were injected subcutaneously with LY411575, a γ-secretase inhibitor, to prevent generation of new Aβ (denoted by dashed vertical line just after time point 0). B: Each data point denotes the mean Aβ concentration of the five ISF samples removed from a single mouse between time points −4 and 0 hours before administration of LY411575. The horizontal lines denote the mean basal ISF Aβ concentrations for each group (4.48 and 10.22 ng/ml for JN+/+and JN−/−, respectively; P = 0.001). C: ISF Aβ concentrations for samples collected from time points 0 to 5 hours were used to calculate the ISF Aβ half-life for each animal (closed circles) as described in Materials and Methods. The mean ISF Aβ half-life for each genotype group is designated by the horizontal line (1.7 and 2.1 hours for JN+/+and JN−/−, respectively; P = 0.01).
Discussion
Here, we show that hypofunction of endogenous NEP 1) dramatically increases cerebral amyloid plaque burden, 2) allows the development of amyloid angiopathy, 3) elevates brain and plasma levels of human Aβ40 and Aβ42 in both young and old animals, 4) raises the concentration of Aβ dimers, 5) increases the levels of extracellular soluble Aβ measured in awake living animals, and 6) prolongs the half-life of brain ISF Aβ. Importantly, we find that even a 50% reduction in the levels of an Aβ-degrading protease has major pathophysiological consequences. The main significance of these findings is twofold. First, they demonstrate the important natural role of Aβ proteolysis in determining monomeric and oligomeric Aβ levels and Aβ-associated neuropathology in vivo. Second, they lend strong biological support to the emerging human genetic and biochemical evidence that defects in Aβ clearance may underlie at least some cases of LOAD.
Natural Role of NEP in Aβ Clearance
Isolated disruption of a single protease, NEP, caused a 400 to 500% elevation in cerebral Aβ plaques, one of the essential diagnostic lesions of AD. Both diffuse (Aβ-immunopositive, thioflavin-S-negative) and fibrillar (Aβ-immunopositive, thioflavin-S-positive) plaque burdens were increased, and this was attributable to increases in both the number and size of the plaques. It seems that none of the other Aβ clearance machinery, including central nervous system Aβ-export proteins and other Aβ-degrading proteases, could effectively compensate for the NEP deficiency. The lack of Aβ deposition in the 3- and 6-month-old JN−/− brains suggests that additional, non-NEP clearance mechanisms may have some role. Although there was no change in IDE levels in JN−/− brains, there could be increases in other routes of Aβ catabolism in response to the NEP deficiency, which would lead the present study to underestimate the extent of NEP’s role in Aβ clearance. The brain’s inability to compensate effectively for even a partial deficit of NEP-mediated Aβ proteolysis demonstrates the important role this zinc metalloprotease has in modulating Aβ levels and associated pathology in vivo.
One of the striking findings of this study was that by 13 months all of the JN−/− mice developed CAA, whereas none of the JN+/+ animals did. Thus, in our animal model, NEP hypofunction was necessary for the development of CAA, and the absence of NEP activity was sufficient. This finding cannot be attributed simply to higher whole-brain Aβ concentrations, because the 13-month JN+/− brains had elevated levels of Aβ40 and Aβ42 that were statistically indistinguishable from those in the JN−/− brains, but the prevalence of CAA was much higher in the JN−/− than JN+/− animals. In addition, some of the lower individual JN−/− whole-brain Aβ values actually overlapped with the higher JN+/+ measurements, and yet the CAA phenotype sorted by genotype without exception. It has been suggested that Aβ in the ISF that drains along arterioles may be the proximal source of the peptide that deposits as CAA.40 Supporting this hypothesis is the finding that the magnitude of the JN−/− elevation in ISF Aβ levels (128%) was significantly greater than the JN−/− increase in whole-brain Aβ (∼60%), and all of the JN−/− individual ISF Aβ values were higher than the JN+/+ values. Unlike the whole-brain Aβ levels, the ISF Aβ values were predictive of which animals would develop CAA. Only the mice with the highest ISF Aβ concentrations, which were those lacking NEP expression, had Aβ deposition in the vasculature.
Notably, whereas the JN+/− brains had a rise in amyloid plaque burden equivalent to that of the JN−/− brains, their prevalence of CAA was significantly lower than that of the JN−/−, showing that partial hypofunction of NEP does not affect all amyloid pathology equally. This could potentially be due to the subcellular or anatomical distribution of NEP relative to the distribution of other Aβ clearance machinery. Perhaps compensation for a ∼50% reduction in NEP levels occurs in the vasculature (where the Aβ export receptors are located), but not in the brain parenchyma. Another possibility is that even though the JN+/− whole-brain NEP protein levels were decreased by ∼50%, they may have been disproportionately reduced in the brain parenchyma and relatively preserved in the cerebral vasculature, possibly because of distinct cell type-specific capabilities for compensatory up-regulation of expression of the remaining NEP gene. The only other situation in which the JN+/− mice were able to compensate for the silencing of one NEP gene was plasma Aβ40 levels. CAA is composed predominantly of Aβ40,41 so perhaps the clearance mechanisms that allowed the JN+/− mice to maintain plasma Aβ40 levels equal to those of the controls also allowed them to avoid the onset of CAA. The finding that a partial loss of NEP activity affects plasma Aβ40 differently than brain Aβ40 and brain and plasma Aβ42 suggests that mechanisms of Aβ clearance may be specific to the anatomical location and isoform of the peptide.
Further investigation is required to understand why the increases in plaque pathology and cerebral Aβ levels were indistinguishable between the JN+/− and JN−/− brains. One potential explanation is that some compensatory Aβ clearance mechanisms may come into play only at higher Aβ concentrations. If cerebral Aβ in the JN−/− or both the JN−/− and JN+/− animals reached a sufficiently high level that these other clearance routes became active, then this could diminish the differences in plaque load and Aβ levels between the two genotypes. It could also be the case that the degradation of Aβ by NEP in the JN+/− brain parenchyma is actually reduced by more than the ∼50% reduction in protein levels would suggest, perhaps because of the presence of an endogenous inhibitor or competition by elevated levels of other NEP substrates. A potential technical reason for the similar JN+/− and JN−/− cerebral Aβ levels is that the ELISA preferentially, if not exclusively, detects Aβ monomer.38,39 Because the Aβ that remains aggregated in the presence of 6.25 mol/L GuHCl, such as the oligomers examined in this study (Figure 4A), is not measured by the ELISA, this causes an underestimation of the amount of total cerebral Aβ. The JN−/− brains may contain more total Aβ than the JN+/− brains, but a larger portion of the Aβ is above the peptide’s critical concentration of aggregation and thus exists in forms not reflected by the ELISA results.
We found that cerebral Aβ was elevated in NEP-deficient mice in all three age groups. NEP can degrade soluble Aβ but not aggregated fibrils of the peptide.42 Most of the GuHCl-extracted brain Aβ measured in the 13-month-old animals probably existed as protease-resistant extracellular deposits (ie, plaques and CAA) in vivo. Therefore, the elevated Aβ deposition in the 13-month-old brains lacking NEP was presumably secondary to decreased clearance of soluble Aβ, resulting in higher concentrations of the peptide and increased aggregation. In contrast, cerebral Aβ in the younger animals, which lacked any immunohistochemically detectable amyloid deposits, exists mainly in a nonparticulate form, at least some of which is apparently susceptible to proteolysis by NEP, because its level rose in the absence of NEP. The 128% elevation in Aβ levels in JN−/− ISF supports the notion that NEP degrades soluble Aβ in vivo, because the microdialysis technique we used only measures soluble Aβ species.
There is growing evidence that soluble oligomers of Aβ can cause neurophysiological and behavioral abnormalities in the absence of plaques,1 recommending a pathogenic role for these small Aβ assemblies in AD. Our work suggests that NEP may naturally regulate the levels of cerebral Aβ oligomers, specifically dimers, in vivo. This is in agreement with recently published work that demonstrated an increase in Aβ dimer levels in brain homogenates from young, pre-plaque APP transgenic mice lacking NEP.43
From our immunoblotting (Figure 1) and microdialysis (Figure 5) experiments, we can conclude that NEP deficiency slows the clearance of extracellular Aβ without inducing an increase in Aβ generation. To date, this is the only Aβ protease that has been explicitly shown to affect the half-life of Aβ in vivo. In a simple one-compartment system, a 23% prolongation of the Aβ half-life would be expected to increase the basal Aβ levels by the same amount. In our model, we found that the rise in basal ISF Aβ levels was approximately fivefold greater than the prolongation in the peptide’s half-life. One potential explanation for this discrepancy, which assumes that the half-life of ISF Aβ is shorter than that of intracellular Aβ, is that the absence of intracellular NEP contributes to the elevation of basal ISF Aβ levels by increasing the amount of the peptide secreted into the ISF, but during the relatively short time frame of the half-life experiments, clearance of already secreted Aβ was predominantly measured. This explanation is intriguing, because it raises the possibility of a major intracellular role for NEP in Aβ catabolism in vivo. This concept is supported by previous in vitro work in which NEP overexpressed in Chinese hamster ovary cells stably transfected with human APP was shown capable of degrading intracellular Aβ.44 The increase in Aβ oligomer levels in the JN−/− brain homogenates also supports an intracellular role for NEP. Aβ monomers seem to assemble first into oligomers intracellularly,30 so the increase in oligomer levels is presumably secondary to an increase in intracellular Aβ monomer concentration resulting from the absence of NEP.
Defects in Aβ Clearance Could Be Causative of Some Cases of LOAD
NEP levels and activity have been reported to decrease with age in mouse and human brain.45,46,47,48 Indeed, numerous genes show reduced expression in the human brain after age 40, possibly because of oxidative damage of DNA in their promoter regions.49 Although it is unknown whether the observed age-associated reduction in NEP brain levels occurs by this mechanism, it is known that NEP mRNA and protein levels are, in fact, decreased in the brains, cerebral vasculature, and cerebrospinal fluid of LOAD patients.19,21,33,50,51 Importantly, it has also been shown that the levels of cortical NEP and vascular NEP in AD brain are inversely related to the levels of insoluble Aβ48 and the severity of CAA,33,52 respectively. Because brains with severe CAA had similar reductions in NEP in Aβ-free and Aβ-laden vessels, it seemed that the decrease in NEP was not simply secondary to CAA, suggesting that low NEP levels may have led to the vascular amyloid deposition. Human genetic data also suggest that primary defects in Aβ degradation could actually cause or be risk factors for LOAD.22 The present study directly demonstrates that a decrease in NEP levels can recapitulate some of the classic features of AD, including elevations in brain and plasma Aβ, thioflavin-positive amyloid plaques, and CAA. A limitation of our JN mouse model is that at the ages examined, the amyloid neuropathology occurred mainly in the hippocampal region, whereas in Alzheimer’s disease, CAA and amyloid plaques are also widely distributed throughout the neocortex. Interestingly, the patterns of Aβ elevation in the brains and plasma of our NEP-disrupted mice parallel those observed in LOAD. As in LOAD, the cerebral GuHCl-soluble Aβ40 and Aβ42 levels rose with age, with particularly dramatic increases in Aβ42 once parenchymal deposition of Aβ began, resulting in a marked rise in the Aβ42/Aβ40 ratio. In addition, the plasma Aβ42 levels of our NEP-deficient mice were higher in younger animals but declined in the older age groups with more disease, a pattern that has been reported in at least some LOAD patients.36
The JN+/− animals may be a better model than the JN−/− mice for an Aβ clearance defect that may occur in LOAD, since human brain and cerebrospinal fluid data suggest only partial (∼20 to 70%) decreases in NEP levels in LOAD subjects.19,21,50,51 Remarkably, even a ∼50% reduction in NEP expression in our animal model led to an approximately fivefold increase in plaque deposition and significant increases in cerebral Aβ40 and Aβ42. We speculate that in LOAD, a primary defect in Aβ proteolysis could raise the concentration of Aβ monomers intra- and extracellularly, leading to the generation of oligomers, and ultimately amyloid plaques and CAA. The present study, combined with the aforementioned human brain and genetic data, strongly support the emerging hypothesis that decreased Aβ clearance may cause or contribute to the development of late-onset Alzheimer’s disease.
Acknowledgments
We thank Timothy Seabrook, Marcel Meier, Matthew L. Hemming, Allen Chen, and Alice Y. Chang for technical advice; Thomas Ward, Michael Judge, and Liying Jiang for technical assistance; Lennart Mucke (Gladstone Institute, University of California, San Francisco, CA) and Bao Lu (Children’s Hospital, Boston, MA) for the APP transgenic and NEP knockout mice, respectively; Patrick May (Eli Lilly and Company, Indianapolis, IN) for LY411575; and Peter Seubert (Elan, plc, South San Francisco, CA) for antibodies 2G3, 21F12, 3D6, and 8E5.
Footnotes
Address reprint requests to Wesley Farris, M.D., Department of Neurology, Pittsburgh Institute for Neurodegenerative Diseases, 3501 Fifth Avenue, BST3-7019, Pittsburgh, PA 15260; or Dennis J. Selkoe, M.D., Center for Neurologic Diseases, Harvard Institutes of Medicine, Room 730, 77 Avenue Louis Pasteur, Boston, MA 02115. E-mail: farrisw@upmc.edu and dselkoe@rics.bwh.harvard.edu.
Supported by National Institutes of Health grants NS046324 (to W.F., S.S., and A.G.), DA07261 (to J.R.C.), AG12749 (to G.M.S. and D.J.S.), AG022476 (to X.S. and W.Q.Q.), and AG13956 (to D.M.H.).
References
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Savage MJ, Trusko SP, Howland DS, Pinsker LR, Mistretta S, Reaume AG, Greenberg BD, Siman R, Scott RW. Turnover of amyloid β-protein in mouse brain and acute reduction of its level by phorbol ester. J Neurosci. 1998;18:1743–1752. doi: 10.1523/JNEUROSCI.18-05-01743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Aβ by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Eckman EA, Adams SK, Troendle FJ, Stodola BA, Kahn MA, Fauq AH, Xiao HD, Bernstein KE, Eckman CB. Regulation of steady-state β-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J Biol Chem. 2006;281:30471–30478. doi: 10.1074/jbc.M605827200. [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BC, Eckman EA, Sambamurti K, Dobbs N, Chow KM, Eckman CB, Hersh LB, Thiele DL. Amyloid-β peptide levels in brain are inversely correlated with insulysin activity levels in vivo. Proc Natl Acad Sci USA. 2003;100:6221–6226. doi: 10.1073/pnas.1031520100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB. Alzheimer’s disease β-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J Biol Chem. 2003;278:2081–2084. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- Mouri A, Zou LB, Iwata N, Saido TC, Wang D, Wang MW, Noda Y, Nabeshima T. Inhibition of neprilysin by thiorphan (i.c.v.) causes an accumulation of amyloid β and impairment of learning and memory. Behav Brain Res. 2006;168:83–91. doi: 10.1016/j.bbr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Madani R, Poirier R, Wolfer DP, Welzl H, Groscurth P, Lipp HP, Lu B, Mouedden ME, Mercken M, Nitsch RM, Mohajeri MH. Lack of neprilysin suffices to generate murine amyloid-like deposits in the brain and behavioral deficit in vivo. J Neurosci Res. 2006;84:1871–1878. doi: 10.1002/jnr.21074. [DOI] [PubMed] [Google Scholar]
- Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, Gan L. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer’s disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Marr RA, Rockenstein E, Mukherjee A, Kindy MS, Hersh LB, Gage FH, Verma IM, Masliah E. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci. 2003;23:1992–1996. doi: 10.1523/JNEUROSCI.23-06-01992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- Iwata N, Mizukami H, Shirotani K, Takaki Y, Muramatsu S, Lu B, Gerard NP, Gerard C, Ozawa K, Saido TC. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain. J Neurosci. 2004;24:991–998. doi: 10.1523/JNEUROSCI.4792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Booze RM, Hersh LB. Tissue-specific expression of rat neutral endopeptidase (neprilysin) mRNAs. J Biol Chem. 1995;270:5723–5728. doi: 10.1074/jbc.270.11.5723. [DOI] [PubMed] [Google Scholar]
- Farris W, Leissring MA, Hemming ML, Chang AY, Selkoe DJ. Alternative splicing of human insulin-degrading enzyme yields a novel isoform with a decreased ability to degrade insulin and amyloid β-protein. Biochemistry. 2005;44:6513–6525. doi: 10.1021/bi0476578. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J Biol Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Akiyama H, McGeer EG, McGeer PL. Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of β-amyloid peptide. Neurosci Lett. 2001;297:97–100. doi: 10.1016/s0304-3940(00)01675-x. [DOI] [PubMed] [Google Scholar]
- Cook DG, Leverenz JB, McMillan PJ, Kulstad JJ, Ericksen S, Roth RA, Schellenberg GD, Jin LW, Kovacina KS, Craft S. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-ε4 allele. Am J Pathol. 2003;162:313–319. doi: 10.1016/s0002-9440(10)63822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M, Higuchi M, Takaki Y, Matsuba Y, Tanji H, Nemoto M, Tomita N, Matsui T, Iwata N, Mizukami H, Muramatsu S, Ozawa K, Saido TC, Arai H, Sasaki H. Cerebrospinal fluid neprilysin is reduced in prodromal Alzheimer’s disease. Ann Neurol. 2005;57:832–842. doi: 10.1002/ana.20494. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Gerard NP, Kolakowski LF, Jr, Bozza M, Zurakowski D, Finco O, Carroll MC, Gerard C. Neutral endopeptidase modulation of septic shock. J Exp Med. 1995;181:2271–2275. doi: 10.1084/jem.181.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkoe DJ. Aggregation of secreted amyloid β-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem. 1995;270:9564–9570. doi: 10.1074/jbc.270.16.9564. [DOI] [PubMed] [Google Scholar]
- Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, Lemere CA. Short amyloid-beta (Aβ) immunogens reduce cerebral Aβ load and learning deficits in an Alzheimer’s disease mouse model in the absence of an Aβ-specific cellular immune response. J Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Sato S, Murayama O, Murayama M, Park JM, Yamaguchi H, Takashima A. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci Lett. 2002;321:61–64. doi: 10.1016/s0304-3940(01)02583-6. [DOI] [PubMed] [Google Scholar]
- Kimberly WT, Zheng JB, Town T, Flavell RA, Selkoe DJ. Physiological regulation of the β-amyloid precursor protein signaling domain by c-Jun N-terminal kinase JNK3 during neuronal differentiation. J Neurosci. 2005;25:5533–5543. doi: 10.1523/JNEUROSCI.4883-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, Rosner MR, Selkoe DJ. Neurons regulate extracellular levels of amyloid β-protein via proteolysis by insulin-degrading enzyme. J Neurosci. 2000;20:1657–1665. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid β-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Carson JA, Turner AJ. β-Amyloid catabolism: roles for neprilysin (NEP) and other metallopeptidases? J Neurochem. 2002;81:1–8. doi: 10.1046/j.1471-4159.2002.00855.x. [DOI] [PubMed] [Google Scholar]
- Carpentier M, Robitaille Y, DesGroseillers L, Boileau G, Marcinkiewicz M. Declining expression of neprilysin in Alzheimer disease vasculature: possible involvement in cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002;61:849–856. doi: 10.1093/jnen/61.10.849. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Tang MX, Jacobs DM, Manly J, Bell K, Merchant C, Small SA, Stern Y, Wisniewski HM, Mehta PD. Plasma amyloid β-peptide 1–42 and incipient Alzheimer’s disease. Ann Neurol. 1999;46:412–416. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Honig LS, Tang MX, Manly J, Stern Y, Schupf N, Mehta PD. Plasma Aβ40 and Aβ42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61:1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- Ertekin-Taner N, Graff-Radford N, Younkin LH, Eckman C, Adamson J, Schaid DJ, Blangero J, Hutton M, Younkin SG. Heritability of plasma amyloid β in typical late-onset Alzheimer’s disease pedigrees. Genet Epidemiol. 2001;21:19–30. doi: 10.1002/gepi.1015. [DOI] [PubMed] [Google Scholar]
- Enya M, Morishima-Kawashima M, Yoshimura M, Shinkai Y, Kusui K, Khan K, Games D, Schenk D, Sugihara S, Yamaguchi H, Ihara Y. Appearance of sodium dodecyl sulfate-stable amyloid β-protein (Aβ) dimer in the cortex during aging. Am J Pathol. 1999;154:271–279. doi: 10.1016/s0002-9440(10)65273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenh C, Englund H, Lord A, Johansson AS, Almeida CG, Gellerfors P, Greengard P, Gouras GK, Lannfelt L, Nilsson LN. Amyloid-β oligomers are inefficiently measured by enzyme-linked immunosorbent assay. Ann Neurol. 2005;58:147–150. doi: 10.1002/ana.20524. [DOI] [PubMed] [Google Scholar]
- Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE. Cerebral amyloid angiopathy: amyloid β accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol. 1998;153:725–733. doi: 10.1016/s0002-9440(10)65616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravina SA, Ho L, Eckman CB, Long KE, Otvos L, Jr, Younkin LH, Suzuki N, Younkin SG. Amyloid β protein (Aβ) in Alzheimer’s disease brain: biochemical and immunocytochemical analysis with antibodies specific for forms ending at Aβ 40 or Aβ 42(43). J Biol Chem. 1995;270:7013–7016. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, Xiao Q, Hsu FF, Turk JW, Xu J, Hsu CY, Holtzman DM, Lee JM. Matrix metalloproteinase-9 degrades amyloid-β fibrils in vitro and compact plaques in situ. J Biol Chem. 2006;281:24566–24574. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]
- Huang SM, Mouri A, Kokubo H, Nakajima R, Suemoto T, Higuchi M, Staufenbiel M, Noda Y, Yamaguchi H, Nabeshima T, Saido TC, Iwata N. Neprilysin-sensitive synapse-associated amyloid-β peptide oligomers impair neuronal plasticity and cognitive function. J Biol Chem. 2006;281:17941–17951. doi: 10.1074/jbc.M601372200. [DOI] [PubMed] [Google Scholar]
- Sudoh S, Frosch MP, Wolf BA. Differential effects of proteases involved in intracellular degradation of amyloid beta-protein between detergent-soluble and -insoluble pools in CHO-695 cells. Biochemistry. 2002;41:1091–1099. doi: 10.1021/bi011193l. [DOI] [PubMed] [Google Scholar]
- Iwata N, Takaki Y, Fukami S, Tsubuki S, Saido TC. Region-specific reduction of Aβ-degrading endopeptidase, neprilysin, in mouse hippocampus upon aging. J Neurosci Res. 2002;70:493–500. doi: 10.1002/jnr.10390. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Oddo S, Sugarman MC, Akbari Y, LaFerla FM. Age- and region-dependent alterations in Aβ-degrading enzymes: implications for Aβ-induced disorders. Neurobiol Aging. 2005;26:645–654. doi: 10.1016/j.neurobiolaging.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Carter TL, Pedrini S, Ghiso J, Ehrlich ME, Gandy S. Brain neprilysin activity and susceptibility to transgene-induced Alzheimer amyloidosis. Neurosci Lett. 2006;392:235–239. doi: 10.1016/j.neulet.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Ravid R, Nordberg A: Age-dependent decline of neprilysin in Alzheimer’s disease and normal brain: inverse correlation with Aβ levels. Neurobiol Aging 2006, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the aging human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Wang DS, Lipton RB, Katz MJ, Davies P, Buschke H, Kuslansky G, Verghese J, Younkin SG, Eckman C, Dickson DW. Decreased neprilysin immunoreactivity in Alzheimer disease, but not in pathological aging. J Neuropathol Exp Neurol. 2005;64:378–385. doi: 10.1093/jnen/64.5.378. [DOI] [PubMed] [Google Scholar]
- Russo R, Borghi R, Markesbery W, Tabaton M, Piccini A. Neprylisin decreases uniformly in Alzheimer’s disease and in normal aging. FEBS Lett. 2005;579:6027–6030. doi: 10.1016/j.febslet.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Miners JS, Van Helmond Z, Chalmers K, Wilcock G, Love S, Kehoe PG. Decreased expression and activity of neprilysin in Alzheimer disease are associated with cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2006;65:1012–1021. doi: 10.1097/01.jnen.0000240463.87886.9a. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of aβ 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]