Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial (original) (raw)
. Author manuscript; available in PMC: 2007 Aug 8.
Published in final edited form as: Am J Clin Nutr. 2006 Nov;84(5):1033–1042. doi: 10.1093/ajcn/84.5.1033
Abstract
Background
Weight loss, through caloric restriction (CR) or increases in exercise energy expenditure (EX), improves glucose tolerance and insulin action. However, EX may further improve glucoregulation through weight-loss independent mechanisms.
Objective
To assess the hypothesis that weight loss through EX improves glucoregulation and circulating factors involved in insulin action, to a greater extent than does similar weight loss through CR.
Design
Sedentary 50- to 60-year-old men and women (body mass index=23.5–29.9 kg/m2) were randomized to 12-month EX (n=18) or CR (n=18) weight loss interventions or to a healthy lifestyle (HL) control group (n=10). Insulin sensitivity index (ISI) and the glucose and insulin areas under the curve (AUCs) were assessed by oral glucose tolerance test (OGTT). Adiponectin and tumor necrosis factor-α (TNFα) were assessed in fasting serum. Fat mass was determined by DXA.
Results
Yearlong energy deficits were not different between EX and CR as evidenced by body weight and fat mass changes. ISI increased, and the glucose and insulin AUCs decreased in the EX and CR groups and remained unchanged in the HL group but did not differ between EX and CR. Marginally significant increases in adiponectin, and decreases in the TNFα-to-adiponectin ratio, occurred in the EX and CR groups but not in the HL group.
Conclusions
EX- and CR-induced weight losses are both effective for improving glucose tolerance and insulin action in non-obese, healthy, middle-aged men and women; however, it does not appear that exercise training-induced weight loss results in greater improvements than those that result from CR.
Keywords: aging, caloric restriction, exercise training, glucose tolerance, weight loss, overweight humans
INTRODUCTION
Reductions in body weight and abdominal fat, induced by restricting caloric intake (CR) or by increasing exercise energy expenditure (EX), improve insulin action and glucose tolerance (1), which are often impaired in overweight and obese individuals (2;3). In addition to weight loss induced by an energy deficit, exercise induces increases in muscle insulin sensitivity and responsiveness that are independent of weight loss (4). Previous studies have compared the effects exercise training to those of CR-induced weight loss, however, exercise training, in these studies was accompanied by an increase in energy intake such that little or no weight loss occurred (5–7). While these studies provide information about the weight-loss independent benefits of exercise training, they may understate the beneficial effects of exercise training since exercise training in the absence of changes in energy intake results in weight loss (8;9). The purpose of the present study was to test the hypothesis that exercise training-induced weight loss results in greater improvements in glucose tolerance and insulin action than those that result from similar weight loss through CR. We also assessed changes in circulating glucoregulatory factors [i.e. adiponectin, tumor necrosis factor-α (TNFα), cortisol, and free fatty acids (FFA)] that might contribute to changes in insulin action to gain preliminary insights regarding the mechanisms for improvements in glucoregulation induced by EX or CR. The data reported in this paper were obtained as part of an investigation (CALERIE - Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) of the feasibility of CR in healthy volunteers.
SUBJECTS AND METHODS
Participants
Fifty- to sixty-year-old men and women, with body mass index (BMI) values of 23.5 – 29.9 kg/m2 were recruited from the Saint Louis metropolitan area. Although the selection criterion included the high end of the range for normal BMI, only nine enrolled subjects (five in the EX group and four in the CR group) were below 25.0 kg/m2. Candidates for the study were excluded if they had: 1.) a history of diabetes or a fasting blood glucose value ≥ 126 mg/dL, 2.) a history or clinical evidence of coronary artery disease, stroke, or lung disease, 3.) a resting blood pressure ≥ 170 mmHg systolic and/or 100 mmHg diastolic, or 4.) a recent history or evidence of malignancy.
Furthermore, all candidates had to be non-smokers and sedentary (defined as exercising less than 20 minutes per day, twice per week during the 6 months before baseline testing) and not taking medications that could affect study outcomes. Women had to be postmenopausal. After screening and before baseline testing, participants were randomly assigned (stratified for sex) to weight loss by CR, weight loss by EX, or healthy lifestyle control (HL) groups in a 2:2:1 ratio. All participants gave their informed written consent to participate in the study, which was approved by the Human Studies Committee and the General Clinical Research Center Advisory Committee at Washington University School of Medicine.
Calorie Restriction Intervention
The objective of the CR intervention was to decrease calorie intake by 16% during the first 3 months and by 20% during the remaining 9 months. Initial calorie intake was assumed to be equal to total daily energy expenditure (TEE) as determined by the doubly labeled water (DLW) method over two consecutive 2-week assessment periods. Calorie intake prescriptions were calculated as baseline TEE minus the desired magnitude of CR (i.e. 16 or 20% of TEE). The participants met with the study dietitians on a weekly basis during which body weight was measured and consultation was provided to maximize compliance with the CR prescription. The participants frequently recorded their food consumption. The dietitians used these records, qualitatively, as a basis for personalized dietary changes that would help the participants achieve the prescribed caloric restriction. The general strategy was to encourage reductions in portion size and to substitute foods with low calorie density for those with high calorie density.
Exercise Training Intervention
The goal of the exercise training intervention was to induce the same calorie deficit as was induced by the CR intervention by holding energy intake constant at baseline levels and increasing exercise energy expenditure by 16% of baseline TEE for the first 3 months and by 20% for the subsequent 9 months. Exercise energy expenditure goals were given to the participants during weekly meetings with exercise trainers. The participants exercised, either in our facility or on their own, while using wrist watch-type heart rate (HR) monitors (S610, Polar Electro Oy, Kempele, Finland) which stored exercise-specific data for gross energy expenditure, HR, exercise duration, and the number of exercise sessions performed. Maximal oxygen uptake (V̇O2max ), maximal HR, and body weight, which the monitors use to estimate gross energy expenditure, were measured and updated every three months. Because the study goals were based on net exercise energy expenditure, while the Polar HR monitors quantified gross energy expenditure, the number of calories that would have been expended during the exercise time, if the participant did not exercise, was added to the net exercise energy expenditure goal, according to the following formula, to give the prescribed gross exercise energy expenditure goal:
Prescribed gross exercise energy expenditure (kcal/wk)=(TEEweek×G)+(T×TEEmin)
Where TEEweek and TEEmin are baseline DLW-based estimates of TEE in kcal per week and kcal per minute, respectively. G is the goal for the increase in energy expenditure expressed as a decimal (i.e. 0.16 or 0.20), T is the estimated time, in minutes per week, required for the participant to achieve the weekly gross exercise energy expenditure goal. For the first week of the intervention, T was fixed at 630 minutes (equivalent to 90 minutes daily). Thereafter, T was estimated as the amount of time that would have been required during the previous week to exactly meet the exercise energy expenditure prescription if all of the exercise was performed at the measured average rate of gross exercise energy expenditure [where average rate of gross exercise energy expenditure = gross exercise calorie expenditure (kcal/wk) ÷ exercise duration (min/wk)]. For post hoc reporting, average net exercise energy expenditure (kcal/day) was calculated as average gross exercise energy expenditure minus the product of the baseline TEE (kcal/min) and the average exercise duration (min/day).
Our exercise technician-trainers stayed in close contact with the participants, providing advice, encouragement, and weekly exercise prescription updates. The participants were weighed, and data from their HR monitors was downloaded on a weekly basis. They were also questioned about any exercise sessions that were performed but not recorded on the HR monitors and were asked to rank order the times spent performing various exercise modes (i.e. walking, cycling, etc.) during the preceding week.
Healthy Lifestyle Intervention
Participants in the healthy lifestyle control (HL) group did not receive instructions to change either exercise or diet behaviors. These participants were offered advice for eating a healthy diet, but only received it if they requested it. Furthermore, all HL group participants were provided with passes to off-site yoga classes to use as they desired. Although the frequencies of dietary consultations and yoga class attendance were not documented, both were minimal.
OGTT and Fasting Blood Draw
Two-hour, 75-gram OGTTs were performed at baseline and at the end of the intervention as described previously (10). All OGTTs were started between 7am and 9am. The participants in the exercise group, as well as those in the CR and HL groups, were instructed to refrain from exercise for at least 48 hours prior to the baseline and final OGTTs. Plasma glucose was measured by the glucose oxidase method (YSI Stat Plus, Yellow Springs, OH) and insulin by double antibody radioimmunoassay (11) Total areas under the curve (AUCs) were calculated for the OGTT plasma glucose and insulin responses using the trapezoidal rule (12). An index of insulin sensitivity (ISI) was calculated according to the method of Matsuda and Defronzo (13). Serum from fasting blood samples was assessed for concentrations of FFA (NEFA C, Wako Chemicals USA, Richmond, VA), adiponectin (B-Bridge International, Sunnyvale, CA), TNFα, (Quantakine High Sensitive, R&D Systems, Minneapolis, MN), and cortisol (Cortisol RIA DSL-2100, Diagnostic Systems Laboratories Inc., Webster, TX).
Body weight and composition
Body weight and composition were measured at baseline, 1, 3, 6, 9, and 12 months. Body weight was measured in duplicate in the morning, after an overnight fast, while the participant was wearing only underwear and a hospital gown. Body weight for each time point was calculated as the mean of multiple (up to 5 for baseline and up to 3 for each follow-up) weekly weights. Fat mass, fat-free mass, and percent body fat were measured by dual-energy X-ray absorptiometry (DXA, Delphi W, Hologic Corporation, Waltham MA, software version 11.2). DXA was also used to quantify truncal and abdominal fat mass. The trunk was defined as the region above the iliac crests and below the inferior aspect of the mandible after exclusion of the upper extremities. The abdomen was defined as the region between the 12th thoracic vertebra and the inferior end of the sacroiliac joint. Body composition data for each subject at each time point were calculated as the mean of up to 3 assessments at baseline and 1 to 2 assessments at follow-up time points except for abdominal fat mass, which were based on single assessments at each time point and were only available for baseline and 12-months.
Energy intake
Energy intake was quantified at baseline, 1, 3, 6, 9, and 12 months using a DLW/DXA approach and by seven-day food records. Each DLW-based assessment of TEE was two weeks in duration and was performed by the Schoeller method (14;15). Duplicate TEE assessments were made at baseline and single assessments were made at all time points thereafter. Baseline energy intake was assumed to equal TEE since body weight was stable. For follow-up assessments, average energy intake for each 3-month segment of the intervention was calculated as the average of the TEE measurements made during the 3-month segment with adjustments for change in total body energy stores as determined using DXA-based measures of body composition during the same 3-month interval. For the estimation of changes in total body energy stores, fat and fat free mass were assumed to contain 9.3 and 1.1 kcal/gram, respectively. Energy intake was also determined using food diaries, which were analyzed for energy intake using Nutrition Data System for Research nutrition analysis software (versions 4.05, 4.06, and 5.0, Nutrition Coordination Center, University of Minnesota, Minneapolis, MN).
Aerobic capacity
V̇O2max was determined at baseline and 12 months by indirect calorimetry during an incremental treadmill exercise test to exhaustion as described elsewhere (16).
Physical activity levels
A modified version of the Stanford 7-day Physical Activity Recall Questionnaire (PAR) (17;18) was administered at baseline, 1, 3, 6, 9, and 12 months. Physical activities were classified as light, moderate, hard, or very hard based on provided examples of activities and these categories were assumed to require 1.5, 4.0, 6.0, and 10.0 resting metabolic rate equivalents (METs), respectively. Since PAR data are provided in the present report as an index of absolute physical activity levels (i.e. time weighted averages of light, moderate, hard, and very hard activities) the data are presented as MET-hr/day. We chose to not present the data in terms of calorie cost of activity since the calorie cost of physical activity decreases with weight loss, even if habitual activities remain constant.
Statistical Analyses
All analyses were performed with the inclusion of all subjects who provided follow-up OGTT data. Baseline characteristics were compared between groups using chi-square tests for sex, Fisher’s exact test for race, and analysis of variance for all quantitative variables with subsequent Tukey tests for post-hoc comparisons. For outcomes where only baseline and final data were available, paired t-tests or Wilcoxon’s signed rank tests were used to assess within group changes and analysis of covariance (ANCOVA) was used for the comparison of final values between groups after adjustment for the initial values. Post-hoc comparisons among groups were performed using Tukey tests. For outcomes where data were available from five or more time points, analyses were performed with mixed model repeated measures analyses of variance. When interactions between group and time point were significant, contrasts assessing the equality of changes from baseline to 1 yr were examined using Tukey tests. Associations between changes in selected variables were assessed using Pearson correlations from which the effects of initial ISI values were parsed out. The Fisher’s z transformation was used to compare partial correlation coefficients. Analyses were performed using SAS software, version 9.1.3 of the SAS System for Linux (SAS Institute Inc., Cary, NC, USA). All statistical tests were two-tailed, and significance was accepted at P ≤ 0.05. Data are presented as arithmetic mean ± SD unless noted otherwise.
RESULTS
Participants
Sample sizes for recruitment and for each stage and arm of the study are diagramed in Figure 1. Of the 48 participants who started the study, two dropped out before the follow-up oral glucose tolerance test and were therefore not included in the analyses for the present report. Sex and racial group representations were not different in the EX, CR, and HL groups (67, 61, and 60% female, respectively, p=0.92; 89, 94, and 70% white, respectively, p=0.32). Participants in the exercise group were slightly older than those in the CR (p=0.0006) and HL (p=0.02) groups (59 ± 3, 55 ± 3, and 56 ± 3 years, respectively). Inclusion of age as a covariate in the outcome analyses did not affect the study findings (data not shown), therefore, age was not included as a covariate in the reported data.
Figure 1.
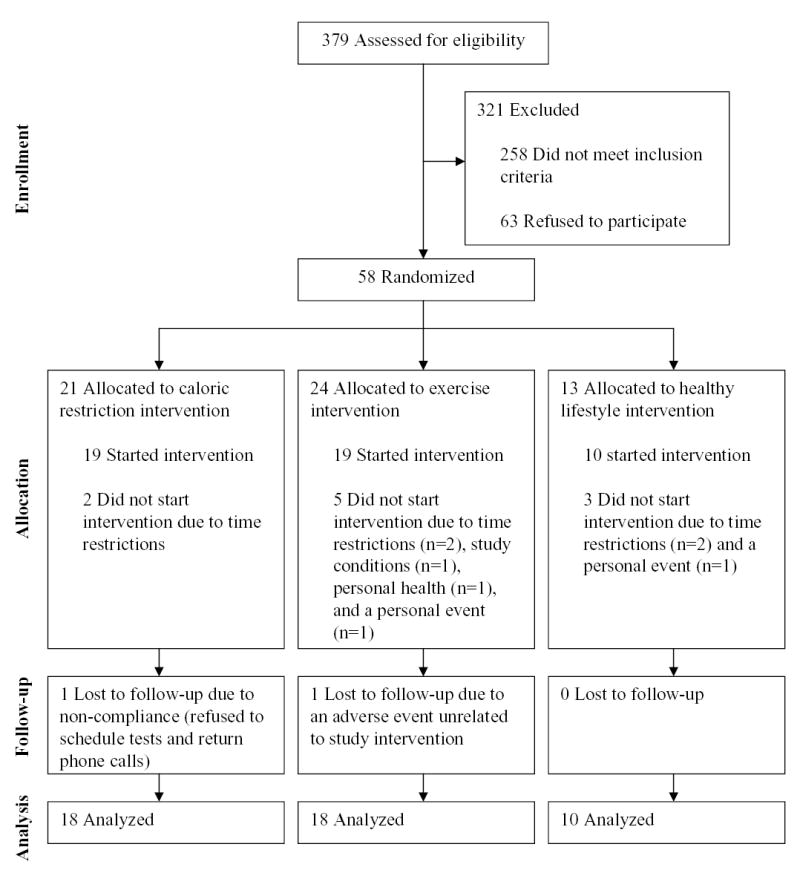
Consort diagram indicating sample sizes at each stage and in each arm of the study.
Physical Activity Levels
Physical activity, as assessed with the 7-day PAR, increased from baseline during the intervention in the exercise group and remained unchanged in the CR and HL groups (Figure 2). The interaction between group and time for physical activity levels was significant (p=0.0497) after accounting for baseline physical activity levels.
Figure 2.
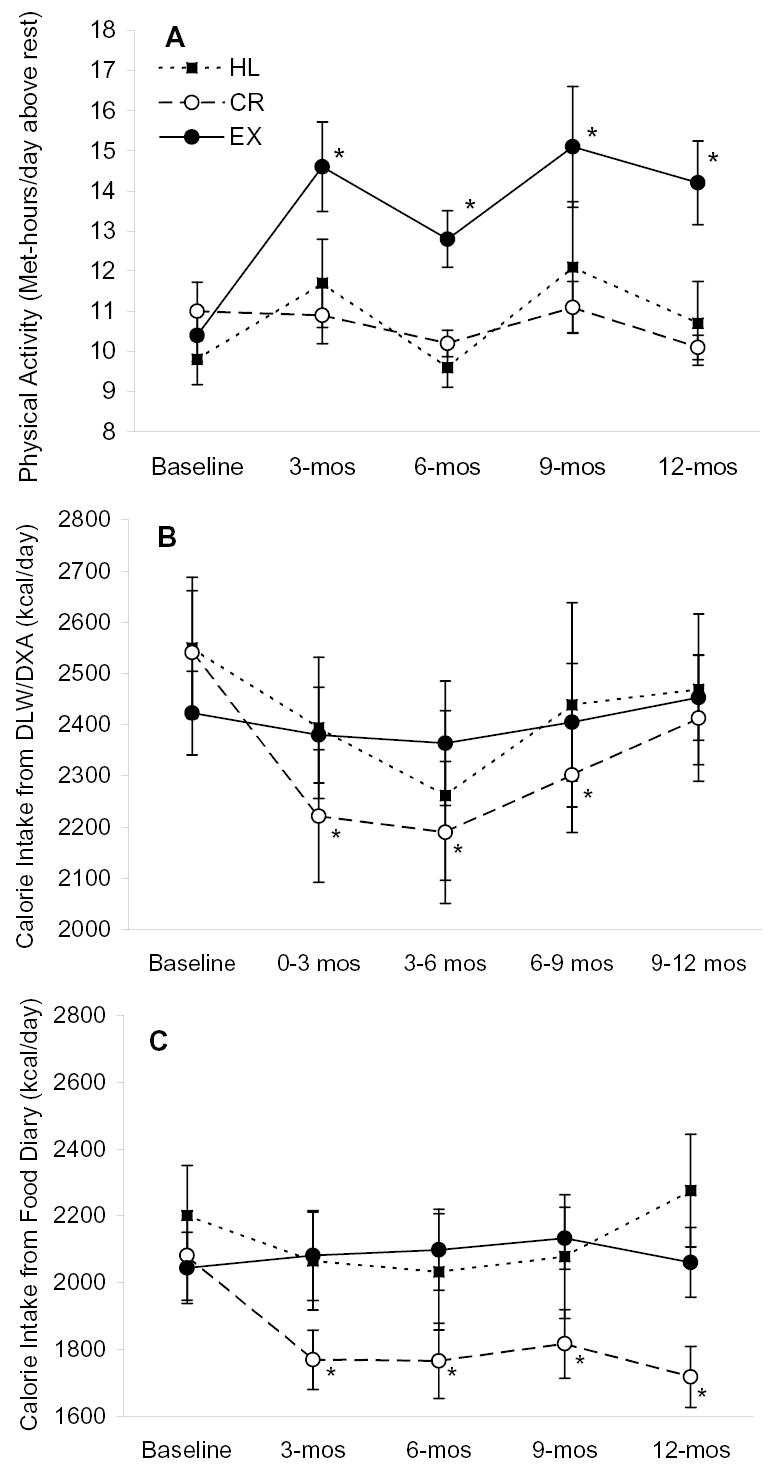
Physical activity and energy intake (mean ± SE) before and during the intervention. Panel A is physical activity level determined by physical activity recall questionnaire, Panel B is energy intake determined by DLW/DXA, and panel C is energy intake determined by food diary. Baseline values were not different among groups for any of the outcomes according to ANOVA. Interactions between study group and time were significant for data in panel A (p=0.0497), panel B (p = 0.01) and panel C (p=0.003) by repeated measures mixed-model ANOVAs that included baseline values as a covariate. * P ≤ 0.05 versus baseline within group by repeated measures, mixed-model ANOVA and Tukey tests. Sample sizes are 18, 18, and 10 for the EX, CR, and HL groups, respectively.
Exercise Training Volume and Mode
According to HR monitor data, net exercise energy expenditure for participants in the exercise group averaged 228 ± 131 kcal per day over the 12-month intervention and this was accomplished in 5.8 ± 2.5 exercise sessions per week at an average exercise duration of 62 ± 18 minutes per session. These estimates of exercise quantity are conservative since the participants did not record 13.5 ± 16.8% of the exercise sessions on the HR monitors according to the weekly questionnaires. Exercise intensity was 71 ± 9% of maximal HR measured during the most recent V̇O2max test. Walking, elliptical machine exercise, cycling, and running were the most commonly used exercise modes.
Aerobic Capacity
Baseline V̇O2max did not differ among the EX, CR, and HL groups whether expressed in absolute terms or relative to body weight (Table 1). V̇O2max increased from baseline to 12 months in the exercise group whether expressed in absolute or relative terms (Table 1). V̇O2max decreased from baseline to 12 months in the CR group, however, this reduction was more than fully offset by the reduction in body weight such that relative V̇O2max increased by the end of the intervention (Table 1). Neither absolute nor relative V̇O2max changed from baseline to 12 months in the HL group (Table 1).
Table 1.
Aerobic capacity before and after intervention.
| EX (n = 16 a) | CR (n = 17 a) | HL (n = 9 a) | Among group P | |
|---|---|---|---|---|
| V̇O2max, mL·min−1 | ||||
| Baseline | 1969 ± 591 | 2111 ± 527 | 2183 ± 685 | |
| Final | 2265± 726† | 1995 ± 569 | 2062 ± 641 | <0.0001 |
| Change | 296 ± 272* | −116 ± 121* | −122 ± 237 | |
| V̇O2max, mL·kg−1·min−1 | ||||
| Baseline | 25 ± 6 | 27± 4 | 26 ± 5 | |
| Final | 32 ± 9† | 28 ± 5 | 25 ± 5 | <0.0001 |
| Change | 7 ± 5* | 2 ± 2* | −1 ± 3 |
Energy Intake
According to DLW-based estimates, energy intake during the intervention in the exercise and HL groups was not different from baseline (Figure 2). Energy intake in the CR group was significantly lower than baseline during months 0–9. During the last three months of the study, energy intake in the CR group was not statistically different from baseline (p = 0.10). The interaction between group and time for the DLW-based estimates of energy intake was significant (p=0.01) after accounting for baseline energy intake.
According to the 7-day food records, calorie intake in the exercise and HL groups was not statistically different from baseline during the intervention. Energy intake in the CR group was lower than baseline at all time points during the intervention (Figure 2). The interaction between group and time for the food record-based estimates of energy intake was significant (p=0.003) after accounting for baseline energy intake.
Body Weight and Composition
Total body weight, BMI, total fat mass, truncal fat mass, and abdominal fat mass all decreased in the EX and CR groups but remained unchanged in the HL group (Table 2). As a result, the final values for weight and all of the body composition measures were lower in the EX and CR groups than in the HL group after adjusting for baseline values. Body weight did not change during the 3 weeks prior to the final OGTT in both the EX group (3 weeks prior to OGTT = 70.4 ± 9.6 kg, day of OGTT = 69.8 ± 9.0 kg) and the CR group (3 weeks prior to OGTT = 70.7 ± 11.0 kg, day of OGTT = 70.8 ± 11.0 kg).
Table 2.
Body weight and body composition before and after intervention.
| EX (n = 18) | CR (n = 18) | HL (n = 10) | Among group P | |
|---|---|---|---|---|
| Weight, kg | ||||
| Baseline | 76.5 ± 10.5 | 78.9 ± 9.4 | 81.9 ± 11.3 | |
| Final | 69.9 ± 8.9† | 70.6 ± 11.1† | 80.7 ± 12.3 | 0.0003 |
| Change | −6.6 ± 5.5* | −8.2 ± 4.8* | −1.2 ± 2.1 | |
| BMI, kg/m2 | ||||
| Baseline | 27.1 ± 1.9 | 27.1 ± 2.5 | 27.9 ± 1.3 | |
| Final | 24.8 ± 2.6† | 24.2 ± 2.8† | 27.4 ± 1.8 | 0.0002 |
| Change | −2.3 ± 1.7* | −2.9 ± 1.7* | −0.5 ± 0.8 | |
| Total fat mass, kg | ||||
| Baseline | 25.7 ± 5.7 | 26.4 ± 5.4 | 26.5 ± 3.3 | |
| Final | 20.1 ± 7.6† | 20.1 ± 6.7† | 26.2 ± 3.3 | 0.0009 |
| Change | −5.6 ± 4.9* | −6.3 ± 3.8* | −0.4 ± 1.7 | |
| Trunk fat mass, kg | ||||
| Baseline | 13.0 ± 3.0 | 13.4 ± 3.2 | 14.0 ± 2.2 | |
| Final | 9.7 ± 4.0† | 9.9 ± 3.8† | 13.8 ± 2.7 | 0.004 |
| Change | −3.3 ± 3.1* | −3.5 ± 2.2* | −0.2 ± 0.9 | |
| Abdominal fat mass, kg | ||||
| Baseline | 7.9 ± 1.9 | 8.5 ± 2.0 | 8.2 ± 1.3 | |
| Final | 6.0 ± 2.6† | 6.4 ± 2.6† | 8.2 ± 1.5 | 0.009 |
| Change | − 1.9 ± 2.1* | − 2.1 ± 1.6* | − 0.02 ± 0.7 |
Body weight, total fat mass, energy intake, aerobic capacity, and physical activity data are included in the present paper as indicators of compliance and to help in the interpretation of results for the primary outcomes. These outcomes have also been published elsewhere (19).
Insulin Action and OGTT insulin concentrations
ISI increased in the exercise and CR groups but not in the HL group. As a result and after adjusting for baseline values, ISI was greater in the exercise and CR groups than in the HL group at the end of the intervention (Table 3). The final adjusted ISI means were not different between the exercise and CR groups. Likewise, fasting insulin and insulin AUC decreased in the exercise and CR groups but not in the HL group, such that the final adjusted values were significantly lower in the exercise and CR groups than in the HL group. Because of baseline differences in ISI, we evaluated the OGTT insulin data after adjusting for baseline ISI, however, this did not change the statistical significance of the OGTT insulin findings.
Table 3.
Indices of glucose tolerance and insulin action before and after intervention.
| EX (n = 18b) | CR (n = 18) | HL (n = 10) | Among group P | |
|---|---|---|---|---|
| ISI a | ||||
| Baseline | 4.5 ± 2.3 | 7.7 ± 4.8 | 4.2 ± 2.8 | |
| Final | 7.4 ± 2.9† | 9.7 ± 3.7† | 4.5 ± 2.9 | 0.001 |
| Change | 3.0 ± 2.7* | 2.0 ± 3.9* | 0.3 ± 1.4 | |
| Fasting insulin, μU/mL a | ||||
| Baseline | 8.2 ± 4.7 | 6.8 ± 6.1 | 9.0 ± 3.4 | |
| Final | 5.6 ± 3.7† | 4.4 ± 2.8† | 10.3 ± 5.3 | 0.01 |
| Change | −2.7 ± 5.0* | −2.5 ± 3.9* | 1.3 ± 3.2 | |
| Insulin AUC, ×103 min·μU/mL | ||||
| Baseline | 9.1 ± 4.5 | 5.5 ± 2.9 | 10.2 ± 4.7 | |
| Final | 5.7 ± 2.9† | 4.2 ± 1.5† | 8.8 ± 3.6 | 0.001 |
| Change | −3.4 ± 3.0* | −1.3 ± 2.1* | −1.4 ± 2.1 | |
| Fasting glucose, mg/dL | ||||
| Baseline | 96.1 ± 5.8 | 94.7 ± 8.7 | 94.0 ± 9.2 | |
| Final | 94.7 ± 6.4 | 89.4 ± 7.3 | 92.1 ± 10.4 | 0.06 |
| Change | −1.4 ± 4.6 | −5.3 ± 6.3 | −1.9 ± 6.5 | |
| Glucose AUC, ×103 min·mg/dL | ||||
| Baseline | 18.4 ± 2.5 | 18.4 ± 3.4 | 17.6 ± 3.4 | |
| Final | 16.0 ± 1.9 | 15.9 ± 2.2 | 16.9 ± 3.0 | 0.11c |
| Change | −2.4 ± 2.5 | −2.5 ± 2.5 | −0.7 ± 1.5 |
Oral Glucose Tolerance
Although fasting glucose decreased in the CR group but not the exercise or HL groups, the final fasting glucose values were not statistically different among the groups after adjusting for baseline values (Table 3) and these results were unaffected after including baseline ISI as a covariate. Final glucose AUC values were not statistically different among study groups after adjusting for baseline values. However, after adding baseline ISI as a covariate, the p-value of 0.11 for glucose AUC (Table 3) became significant (p=0.03) and there were significant differences between both intervention groups and the control group.
Adiponectin, TNFα, cortisol, and FFA
Serum adiponectin concentration tended to increase in the exercise (p=0.06) and the CR (p=0.07) groups and decreased significantly in the HL group (p=0.05) (Table 4). At the end of the intervention, adiponectin levels were higher in the exercise and CR groups than in the HL group but were not different between EX and CR. Final adjusted serum TNFα concentrations were not different among study groups. The TNFα to adiponectin ratio tended to decrease in the exercise group (p=0.06) and decreased significantly in the CR group but remained unchanged in the HL group. As a result, the final adjusted TNFα to adiponectin ratio values were significantly lower in the exercise and CR groups than in the HL group. Final values for the TNFα to adiponectin ratio were not different between the exercise and CR groups. Final serum cortisol concentrations, after adjusting for baseline values, did not differ among the three study groups. Serum FFA did not change in any of the study groups and the final adjusted values were not different among groups. None of the statistical results for outcomes in reported in Table 4 were affected by the inclusion of baseline ISI as a covariate.
Table 4.
Circulating adiponectin, TNFα, cortisol, and FFA concentrations before and after the intervention.
| EX (n = 18) | CR (n = 18) | HL(n = 10) | Among group P | |
|---|---|---|---|---|
| Adiponectin, μg/mL | ||||
| Baseline | 11.0 ± 3.8 | 13.3 ± 6.8 | 8.6 ± 3.0 | |
| Final | 12.9 ± 4.7† | 15.5 ± 6.8† | 6.7 ± 1.6 | 0.005 |
| Change | 1.9 ± 4.0 | 2.2 ± 4.7 | −1.9 ± 2.6* | |
| TNFα, pg/mL | ||||
| Baseline | 1.34 ± 0.70 | 1.13 ± 0.52 | 1.17 ± 0.89 | |
| Final | 1.09 ± 0.56 | 0.93 ± 0.46 | 1.11 ± 0.70 | 0.53 |
| Change | −0.25 ± 0.56 | −0.20 ± 0.26 | −0.06 ± 0.41 | |
| TNFα: adiponectin ratio, ×10−8 | ||||
| Baseline | 13.2 ± 7.7 | 11.0 ± 6.7 | 13.8 ± 7.9 | |
| Final | 10.1 ± 7.3† | 7.2 ± 4.0† | 15.7 ± 6.5 | 0.0009 |
| Change | −3.0 ± 6.4 | −3.9 ± 4.1* | 1.9 ± 5.2 | |
| Cortisol, μg/mL | ||||
| Baseline | 11.9 ± 4.1 | 15.4 ± 4.7 | 12.8 ± 2.7 | |
| Final | 11.8 ± 4.9 | 11.4 ± 3.2 | 10.6 ± 3.1 | 0.30 |
| Change | −0.1 ± 4.4 | −4.0 ± 4.1 | −2.2 ± 4.2 | |
| FFA, μmol/L | ||||
| Baseline | 646 ± 210 | 569 ± 231 | 603 ± 207 | |
| Final | 586 ± 254 | 501 ± 203 | 616 ± 175 | 0.39 |
| Change | −60 ± 336 | −68 ± 306 | 13 ± 152 |
To gain insight into the mechanism of improvements in ISI that resulted from exercise and CR, the correlations between changes in ISI, after parsing out the effects of baseline ISI, and the changes in adiponectin, TNFα, and the TNFα to adiponectin ratio were assessed. No significant difference was evident between the EX and CR groups with respect to the correlations between change in adiponectin and the change in ISI (EX: r = +0.47; CR: r = +0.17; p=0.37 for EX vs. CR). Likewise, the correlations for change in TNFα and change in ISI were not different between groups (EX: r = −0.34; CR: r = +0.33; p=0.06 for EX vs. CR). In contrast, the correlation between the change in the TNFα to adiponectin ratio and the change in ISI was stronger in the EX group than in the CR group (EX: r = −0.67, p=0.005; CR: r = 0.005, p=0.98; p = 0.03 for EX vs. CR; Figure 3).
Figure 3.
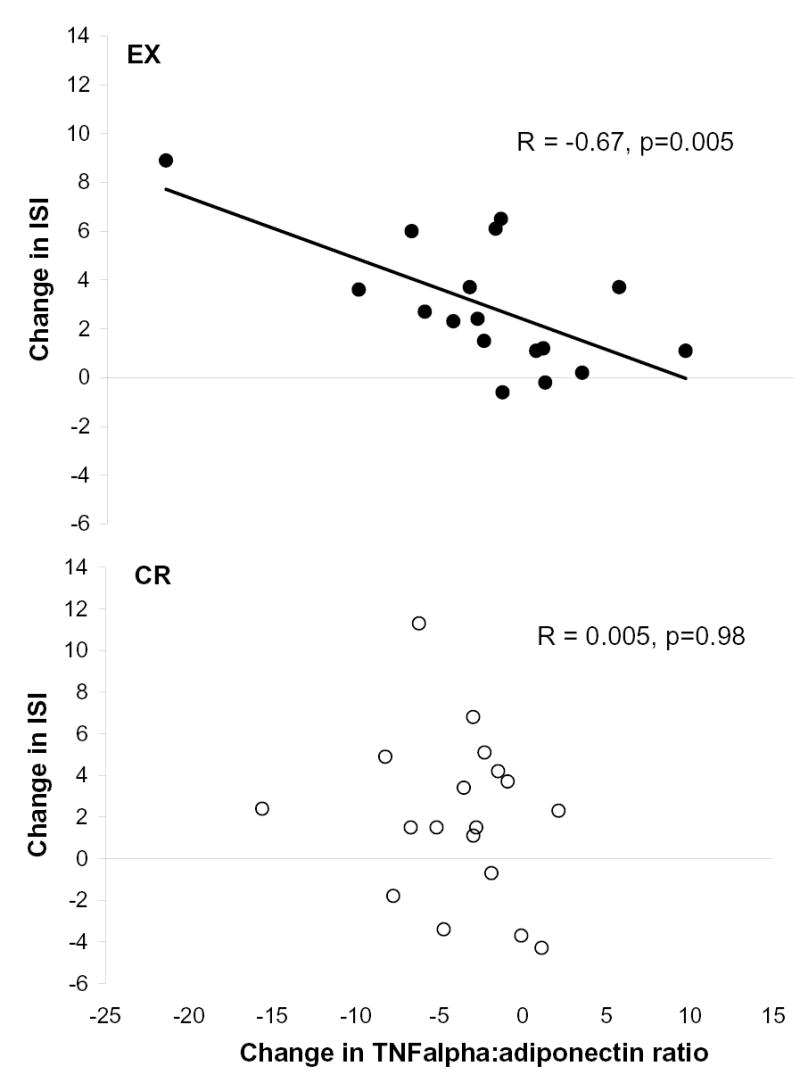
Relationships between changes in the TNFa to adiponectin ratio and the changes in insulin sensitivity index in the EX (n=17; excludes 1 subject due to technical problems during data collection) and CR (n=18) groups. Raw data were used for presentation; however, the associations were assessed using Pearson correlations after parsing out the effects of baseline ISI. The correlation in the EX group was significantly greater than that in the CR group (p=0.03) as determined using Fisher’s z transformation.
DISCUSSION
The main finding of the present study is that weight losses achieved by 12-months of exercise training with unchanged food intake, and 12-months of CR, resulted in significant improvements in glucose tolerance and insulin action that were not different between groups. Furthermore, these two interventions resulted in marginally significant improvements in circulating adiponectin and the TNFα to adiponectin ratio.
The only other direct comparisons of exercise training- and CR-induced weight loss on improvements in insulin action are those of Ross et al. (8;9) who reported enhancements in insulin action in response to exercise training-induced weight loss and CR-induced weight loss that were not different between groups. Most of the participants in the studies by Ross et al. were obese at baseline and were still overweight or obese after the intervention. In contrast, subjects in the present study were, on average, overweight (BMI 27.1±1.9 kg/m2) and none were obese and the weight losses normalized body weight (BMI < 25.0 kg/m2) in 52% of the overweight participants in the exercise and CR groups. While Ross et al. used 12- to 14-week interventions, we used a more gradual, 1-year intervention to reduce body weight. Despite the differences in baseline body weight, and the duration and intensity of the intervention, the findings of the present study are in agreement with those of Ross et al. (8;9).
There are several possible explanations for why exercise training-induced weight loss did not result in greater improvements in insulin action than those that resulted from CR, as we had hypothesized. One possibility is that the main factor responsible for the improvements is the decrease in body fat, particularly abdominal fat, which was not different between the two groups. Another possibility is that the weight-loss independent benefits of exercise training on insulin action might have worn off before the final OGTT, as these effects are lost rapidly (20;21). The exercise group participants were asked to refrain from exercise for at least 48 hours before the final OGTT and the median amount of time between the last exercise bout and the OGTT was 58 hours. In this time frame, it is unlikely that the exercise training effects would have worn off since studies on humans have shown that the myocellular adaptations that are associated with enhanced glucose uptake in trained muscle are still evident when measured 48 to 72 hours after the cessation of exercise (22–24). Furthermore, it has been reported that glucose tolerance and insulin action do not deteriorate within three days of detraining in moderately trained, middle aged men and women (20).
Another possible explanation for why the exercise group did not demonstrate greater improvements in glucose tolerance and insulin action than the CR group is that the exercise training-induced improvement in insulin action might have been matched by a CR-specific effect that improves insulin sensitivity. One such mechanism could be a CR-specific skeletal muscle adaptation since it has been shown, in rats, that CR augments insulin stimulated glucose transport (25) through a mechanism that may be distinct from the exercise training-specific mechanism (26). If exercise training and CR increase insulin action through distinct mechanisms, the combination of CR and exercise training should result in greater improvements in insulin action than those that result from weight loss by either intervention alone. Indeed, results from most (6;7;27;28), but not all (29;30) studies that compared the effects of CR to those of CR and exercise training combined, suggest that the effects of CR and exercise training are additive.
Both exercise training- and CR-induced weight losses resulted in modestly higher serum adiponectin concentrations than those in the HL group. Although adiponectin concentration has been shown to increase after weight loss induced by medication (31) or gastrointestinal bypass surgery (32), weight loss by diet or exercise training has generally not been shown to alter adiponectin concentration (33–36). A likely explanation for this discrepancy is that others studied obese individuals while most of our participants were overweight and none were obese (BMI < 30 kg/m2). Based on cross-sectional data, adiponectin levels are associated with BMI but only when BMI is below ~29 kg/m2 (37). It seems reasonable, therefore, that changes in BMI lead to changes in adiponectin levels but only with weight losses that result in BMIs below 29 kg/m2. The inflammatory cytokine, TNFα, has been shown to decrease insulin action (38;39). In the present study, circulating TNFα did not differ in response to the interventions. However, in light of recent evidence, which suggests that TNFα and adiponectin may be reciprocally regulated (40–42), we assessed the effects of our interventions on the TNFα to adiponectin ratio. Both the exercise training and CR interventions resulted in lower values for this ratio by the end of the study.
Although changes in the TNFα to adiponectin ratio were not different between the EX and CR groups, these changes only correlated with the changes in insulin action in the EX group (Figure 3). This finding suggests that the changes in these cytokines are a more important mechanism for the improvement in insulin action in response to exercise training-induced weight loss than they are for CR. This is evidence, albeit preliminary, that some of the mechanisms are distinct for the improvements in insulin action that result from exercise training- versus CR-induced weight loss.
Two methods were used to measure energy intake in the present study. To some extent, the energy intake estimates from these methods are conflicting for the CR group (Figure 2). According to the doubly labeled water method, energy intake in the CR group was ~340 kcal/day below baseline during the first 6 months of the intervention but only ~128 kcal/day below baseline and not significantly different from baseline (p = 0.10) during the last 3 months of the intervention. In contrast, food record-based estimates of energy intake for the CR group indicate that energy intake was ~300 kcal/day below baseline throughout the intervention. In light of the difficulties of using DLW to estimate energy intake during weight loss and considering the well-known limitations of food record-based estimates of energy intake, it is difficult to know which of these estimates of change in energy intake is more accurate. It is important to note, however, that body weight and body fat mass decreased substantially in the CR group (Table 2) and remained remarkably stable during the last 3 weeks of the intervention. In the absence of increases in physical activity levels, as evidenced by the PAR data (Figure 2) and a lack of increase in absolute VO2max (Table 1), it is likely that energy intake was below baseline and stable at the end of the intervention as evidenced by the absence of weight gain.
The results of this trial have clinical ramifications, especially in light of the growing epidemic of type 2 diabetes. Although diet and exercise training can greatly reduce the incidence of type 2 diabetes (43;44), the relative contributions of exercise training and CR to these protective effects are not known. Data from the present study suggest that weight losses by exercise training and by CR are not different with respect to their abilities to improve glucose tolerance and insulin action, and presumably lower type 2 diabetes risk.
In summary, data from the present study suggest that weight loss induced by exercise training and by CR are effective means for improving glucose tolerance and insulin action in non-obese, healthy, middle-aged men and women. It does not appear that exercise training-induced weight loss provides benefits above and beyond those that can be achieved by caloric restriction alone if exercise training is discontinued for two or more days.
Acknowledgments
We are grateful to the study participants for their cooperation and to the staff of the Applied Physiology Laboratory and Nurses of the General Clinical Research Center at WUMS for their skilled assistance. The study design was developed by JOH and SK; data collection was performed and supervised by EPW, SBR, DV, LF, KSM, and JOH; data analyses and interpretation were performed by EPW, SBR, DV, KSM, KBS, and JOH; writing was performed by EPW, SBR, KSM, SK, and JOH. None of the authors had conflicts of interest.
Footnotes
This work was supported by NIH Cooperative Agreement AG20487, NIH General Clinical Research Center Grant RR00036, Diabetes Research Training Center Grant DK20579, and NIH Clinical Nutrition Research Unit Grant DK56341. EPW was supported by Institutional National Research Service Award NIH AG00078.
References
- 1.Gan SK, Kriketos AD, Ellis BA, Thompson CH, Kraegen EW, Chisholm DJ. Changes in aerobic capacity and visceral fat but not myocyte lipid levels predict increased insulin action after exercise in overweight and obese men. Diabetes Care. 2003;26:1706–13. doi: 10.2337/diacare.26.6.1706. [DOI] [PubMed] [Google Scholar]
- 2.Mykkanen L, Laakso M, Pyorala K. Association of obesity and distribution of obesity with glucose tolerance and cardiovascular risk factors in the elderly. Int.J.Obes. Relat Metab Disord. 1992;16:695–704. [PubMed] [Google Scholar]
- 3.Abbasi F, Brown BW, Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J.Am.Coll. Cardiol. 2002;40:937–43. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 4.Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J. ApplPhysiol. 2005;99:338–43. doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- 5.Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial JAMA. 1995;274:1915–21. doi: 10.1001/jama.1995.03530240025035. [DOI] [PubMed] [Google Scholar]
- 6.Dengel DR, Galecki AT, Hagberg JM, Pratley RE. The independent and combined effects of weight loss and aerobic exercise on blood pressure and oral glucose tolerance in older men. Am.J. Hypertens. 1998;11:1405–12. doi: 10.1016/s0895-7061(98)00185-x. [DOI] [PubMed] [Google Scholar]
- 7.Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J. ApplPhysiol. 1996;81:318–25. doi: 10.1152/jappl.1996.81.1.318. [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann.Intern. Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 9.Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes. Res. 2004;12:789–98. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 10.Racette SB, Weiss EP, Obert KA, Kohrt WM, Holloszy JO. Modest lifestyle intervention and glucose tolerance in obese African Americans. Obes. Res. 2001;9:348–55. doi: 10.1038/oby.2001.45. [DOI] [PubMed] [Google Scholar]
- 11.Morgan DR, Lazarow A. Immunoassay of insulin: two antibody system. Diabetes. 1963;12:115–26. [Google Scholar]
- 12.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–50. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 14.DeLany JP, Schoeller DA, Hoyt RW, Askew EW, Sharp MA. Field use of D2 18O to measure energy expenditure of soldiers at different energy intakes. J. ApplPhysiol. 1989;67:1922–9. doi: 10.1152/jappl.1989.67.5.1922. [DOI] [PubMed] [Google Scholar]
- 15.Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J. Nutr. 1988;118:1278–89. doi: 10.1093/jn/118.11.1278. [DOI] [PubMed] [Google Scholar]
- 16.Kohrt WM, Malley MT, Coggan AR, et al. Effects of gender, age, and fitness level on response of VO2max to training in 60–71 yr olds. J. ApplPhysiol. 1991;71:2004–11. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- 17.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am.J. Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 18.Sallis JF. Seven-Day Physical Activity Recall. Med.Sci. Sports Exerc. 1997;29:S89–S103. [Google Scholar]
- 19.Racette SB, Weiss EP, Villareal DT, et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006:61. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King DS, Baldus PJ, Sharp RL, Kesl LD, Feltmeyer TL, Riddle MS. Time course for exercise-induced alterations in insulin action and glucose tolerance in middle-aged people. J. ApplPhysiol. 1995;78:17–22. doi: 10.1152/jappl.1995.78.1.17. [DOI] [PubMed] [Google Scholar]
- 21.Oshida Y, Yamanouchi K, Hayamizu S, Nagasawa J, Ohsawa I, Sato Y. Effects of training and training cessation on insulin action. Int.J. Sports Med. 1991;12:484–6. doi: 10.1055/s-2007-1024718. [DOI] [PubMed] [Google Scholar]
- 22.Ebeling P, Bourey R, Koranyi L, et al. Mechanism of enhanced insulin sensitivity in athletes. Increased blood flow, muscle glucose transport protein (GLUT-4) concentration, and glycogen synthase activity. J. ClinInvest. 1993;92:1623–31. doi: 10.1172/JCI116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu M, Blomstrand E, Chibalin AV, Wallberg-Henriksson H, Zierath JR, Krook A. Exercise-associated differences in an array of proteins involved in signal transduction and glucose transport. J. ApplPhysiol. 2001;90:29–34. doi: 10.1152/jappl.2001.90.1.29. [DOI] [PubMed] [Google Scholar]
- 24.Hughes VA, Fiatarone MA, Fielding RA, et al. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol. 1993;264:E855–E862. doi: 10.1152/ajpendo.1993.264.6.E855. [DOI] [PubMed] [Google Scholar]
- 25.Cartee GD, Kietzke EW, Briggs-Tung C. Adaptation of muscle glucose transport with caloric restriction in adult, middle-aged, and old rats. Am J Physiol. 1994;266:R1443–R1447. doi: 10.1152/ajpregu.1994.266.5.R1443. [DOI] [PubMed] [Google Scholar]
- 26.Dean DJ, Brozinick JT, Jr, Cushman SW, Cartee GD. Calorie restriction increases cell surface GLUT-4 in insulin-stimulated skeletal muscle. Am J Physiol. 1998;275:E957–E964. doi: 10.1152/ajpendo.1998.275.6.E957. [DOI] [PubMed] [Google Scholar]
- 27.Rice B, Janssen I, Hudson R, Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–91. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- 28.Cox KL, Burke V, Morton AR, Beilin LJ, Puddey IB. Independent and additive effects of energy restriction and exercise on glucose and insulin concentrations in sedentary overweight men. Am J Clin Nutr. 2004;80:308–16. doi: 10.1093/ajcn/80.2.308. [DOI] [PubMed] [Google Scholar]
- 29.Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25:431–8. doi: 10.2337/diacare.25.3.431. [DOI] [PubMed] [Google Scholar]
- 30.Weinstock RS, Dai H, Wadden TA. Diet and exercise in the treatment of obesity: effects of 3 interventions on insulin resistance. Arch.Intern. Med. 1998;158:2477–83. doi: 10.1001/archinte.158.22.2477. [DOI] [PubMed] [Google Scholar]
- 31.Valsamakis G, McTernan PG, Chetty R, et al. Modest weight loss and reduction in waist circumference after medical treatment are associated with favorable changes in serum adipocytokines. Metabolism. 2004;53:430–4. doi: 10.1016/j.metabol.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Ballantyne GH, Gumbs A, Modlin IM. Changes in insulin resistance following bariatric surgery and the adipoinsular axis: role of the adipocytokines, leptin, adiponectin and resistin. Obes. Surg. 2005;15:692–9. doi: 10.1381/0960892053923789. [DOI] [PubMed] [Google Scholar]
- 33.Manigrasso MR, Ferroni P, Santilli F, et al. Association between circulating adiponectin and interleukin-10 levels in android obesity: effects of weight loss. J.Clin. EndocrinolMetab. 2005;90:5876–9. doi: 10.1210/jc.2005-0281. [DOI] [PubMed] [Google Scholar]
- 34.Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int.J.Obes. Relat Metab Disord. 2003;27:1066–71. doi: 10.1038/sj.ijo.0802387. [DOI] [PubMed] [Google Scholar]
- 35.Giannopoulou I, Fernhall B, Carhart R, et al. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–75. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Xydakis AM, Case CC, Jones PH, et al. Adiponectin, inflammation, and the expression of the metabolic syndrome in obese individuals: the impact of rapid weight loss through caloric restriction. J.Clin. EndocrinolMetab. 2004;89:2697–703. doi: 10.1210/jc.2003-031826. [DOI] [PubMed] [Google Scholar]
- 37.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem.Biophys.Res. Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 38.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor Necrosis Factor-{alpha} Induces Skeletal Muscle Insulin Resistance in Healthy Human Subjects via Inhibition of Akt Substrate 160 Phosphorylation. Diabetes. 2005;54:2939–45. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- 39.Lechleitner M, Herold M, Dzien-Bischinger C, Hoppichler F, Dzien A. Tumour necrosis factor-alpha plasma levels in elderly patients with Type 2 diabetes mellitus-observations over 2 years. Diabet. Med. 2002;19:949–53. doi: 10.1046/j.1464-5491.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- 40.Bruun JM, Lihn AS, Verdich C, et al. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285:E527–E533. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- 41.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 42.Behre CJ, Fagerberg B, Hulten LM, Hulthe J. The reciprocal association of adipocytokines with insulin resistance and C-reactive protein in clinically healthy men. Metabolism. 2005;54:439–44. doi: 10.1016/j.metabol.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N.Engl.J. Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 44.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N.Engl.J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]