ADP-Ribosylation Factor/COPI-dependent Events at the Endoplasmic Reticulum-Golgi Interface Are Regulated by the Guanine Nucleotide Exchange Factor GBF1 (original) (raw)
Abstract
ADP-ribosylation factor (ARF) mediated recruitment of COPI to membranes plays a central role in transport between the endoplasmic reticulum (ER) and the Golgi. The activation of ARFs is mediated by guanine nucleotide exchange factors (GEFs). Although several ARF-GEFs have been identified, the transport steps in which they function are still poorly understood. Here we report that GBF1, a member of the Sec7-domain family of GEFs, is responsible for the regulation of COPI-mediated events at the ER-Golgi interface. We show that GBF1 is essential for the formation, differentiation, and translocation of pre-Golgi intermediates and for the maintenance of Golgi integrity. We also show that the formation of transport-competent ER-to-Golgi intermediates proceeds in two stages: first, a COPI-independent event leads to the formation of an unstable compartment, which is rapidly reabsorbed in the absence of GBF1 activity. Second, the association of GBF1 with this compartment allows COPI recruitment and leads to its maturation into transport intermediates. The recruitment of GBF1 to this compartment is specifically inhibited by brefeldin A. Our findings imply that the continuous recruitment of GBF1 to spatially differentiated membrane domains is required for sustained membrane remodeling that underlies membrane traffic and Golgi biogenesis.
INTRODUCTION
Transport between the endoplasmic reticulum (ER) and the Golgi is mediated by transport intermediates generated by three recognizable traffic stages: the formation of transport intermediates at the ER, maturation and movement of such intermediates toward the Golgi, and coalescence at the incoming_cis_-face of the Golgi. The differentiation of ER-Golgi transport intermediates requires the sequential action of two cytosolic coat complexes, COPII and COPI (Aridor et al., 1995; Rowe et al., 1996; Scales et al., 1997). COPII recruitment is coupled to the differentiation of specialized ER subdomains called endoplasmic reticulum exit sites (ERESs) (Bannykh and Balch, 1997). Protein export from the ER seems to occur exclusively from ERESs. Morphologically, ERESs are characterized by COPII-coated tubular ER elements adjacent to a collection of vesicular tubular clusters (VTCs) coated with COPI (Bannykh et al., 1996). VTCs represent a subsequent stage of transport, and one ERES generates multiple VTCs during its existence (Lippincott-Schwartz et al., 1998). Association of COPI with ERESs occurs after these membranes have been first differentiated by the activity of COPII (Rowe et al., 1996).
COPI binding seems to be required at multiple stages of ER-Golgi transport. Experimental evidence indicates that the initial requirement for COPI association is to differentiate VTCs adjacent to ERES. Specifically, when COPI binding to membranes is inhibited, cargo proteins and resident Golgi proteins fail to exit the ER and be incorporated into ERESs (Dascher and Balch, 1994;Lippincott-Schwartz et al., 1989; Peters et al., 1995; Rowe et al., 1996). Additional COPI function is required after VTCs leave the ERES region and initiate their microtubule-dependent movement toward the Golgi (Lippincott-Schwartz et al., 1998).
Recent findings indicate that the association of COPI with membranes is likely to result from rapid cycles of COPI binding and dissociation (Presley et al., 2002). The transient nature of COPI association implies that COPI must be continuously recruited from the cytosol and suggests that the machinery that recruits COPI must be present on all COPI-coated membranes. COPI is recruited to membranes by a small GTPase of the ARF family (Rothman and Wieland, 1996). ARFs cycle between a GDP-bound inactive state and a GTP-bound active state. Inactive ARF is largely cytosolic, and ARF activation promotes its recruitment to a membrane and allows it to interact with membrane associated downstream effectors (Chavrier and Goud, 1999). ARF binds GDP with high affinity and to become active must interact with a guanine nucleotide exchange factor that stimulates the exchange of GDP for GTP (Chavrier and Goud, 1999; Jackson and Casanova, 2000).
ARF-GEFs share a highly conserved 200 amino acid region, termed the Sec7 domain, which is sufficient to catalyze the exchange of GDP for GTP on ARFs in vitro (Jackson and Casanova, 2000). ARF-GEFs are subdivided into two major classes based on size and sequence similarities (Jackson and Casanova, 2000). The small GEFs (<100 kDa) have no orthologs in _Saccharomyces cerevisiae_, suggesting a function specific for higher eukaryotes. The large (>100-kDa) ARF-GEFs are conserved from yeast to humans, suggesting an evolutionary conserved role. Large GEFs include the yeast Gea1p, Gea2p, and Sec7p and their mammalian orthologs GBF1 (Gea1/2), BIG-1, and BIG-2 (Sec7). All large yeast GEFs seem to function at the ER-Golgi system, because various temperature-sensitive alleles of GEA1, GEA2, and SEC7 have defects in ER-Golgi and intra-Golgi transport (Franzusoff et al., 1992; Peyroche et al.,1996,2001;Spang et al., 2001). In mammals, BIG1 and 2 have been associated with post-Golgi transport (Shinotsuka et al.,2002a,b;Zhao et al., 2002b). In contrast, the function of GBF1 is still poorly understood. The finding that GBF1 overexpression prevents brefeldin A (BFA)-induced Golgi disassembly and that it cycles between the Golgi and the ER is consistent with its possible function in ER-Golgi traffic (Claude et al., 1999; Kawamoto_et al_., 2002; Zhao_et al._, 2002b).
Herein, we report that GBF1 regulates ARF/COPI dynamics at the ER-Golgi interface and acts by promoting the recruitment of COPI to an unstable post-ERES compartment. GBF1 is essential for transport between the ER and the Golgi and for the maintenance of Golgi integrity.
MATERIALS AND METHODS
Antibodies and Cell Reagents
BFA was from Sigma-Aldrich (St. Louis, MO). Polyclonal antibodies against p115 and GM130 were described previously (Nelson et al., 1998). Rabbit polyclonal anti-GBF1 antibodies were generated by immunization with purified his-tagged construct encoding the last 107 amino acids of mouse GBF1 and affinity purified as described previously (Nelson et al., 1998). Anti-giantin and anti–ERGIC-53 monoclonal antibodies were provided by Dr. Hans-Peter Hauri (University of Basel, Basel, Switzerland). Polyclonal anti-Mann II antibodies were provided by Dr. Marilyn Farquhar (University of California, San Diego, La Jolla, CA). Polyclonal anti-Sec31 antibodies were a kind gift from Dr. Wanjin Hong (Institute of Molecular Biology, Singapore). The following commercially available antibodies were used: monoclonal anti-myc (Invitrogen, Carlsbad, CA), polyclonal anti-his and monoclonal anti-HA (Santa Cruz Biotechnology, Santa Cruz, CA), monoclonal anti-p115 and anti-GM130 (Transduction Laboratories, Lexington, KY), polyclonal anti-β-COP (Oncogene Science, Cambridge, MA); polyclonal anti-HA (Zymed Laboratories, South San Francisco, CA). Secondary antibodies conjugated with Texas Red-X, Oregon Green, or Alexa385 were from Molecular Probes (Eugene, OR).
DNA Constructs
A partial human GBF1 cDNA (KIAA0248) was obtained from The Kazusa DNA Research Institute (Chiba, Japan). KIAA0248 is 5634 base pairs and lacks 0.5 kb of open reading frame. The missing fragment was amplified from a Human lung cDNA library (kindly provided by Dr. Cary Wu, University of Alabama at Birmingham, Birmingham, AL). The polymerase chain reaction product was then subcloned into the KIAA0248 clone by using the internal _Eco_RI site at base 1124 and an engineered external _Xho_I site.
To generate GBFmyc, wtGBF1 was amplified by polymerase chain reaction and subcloned into pcDNA4.0/TO/myc-his (Invitrogen). E794K was generated by a single nucleotide mutation with the QuickChangeXL mutagenesis kit according to manufacturer's instructions (Stratagene, La Jolla, CA). E794K-green fluorescent protein (GFP) was generated by subcloning E794K into pEGFP-C2 (BD Biosciences Clontech, Palo Alto, CA). All constructs were verified by sequencing at the University of Alabama at Birmingham Sequencing Facility.
VSVG ts045-GFP and p58-GFP were kind gifts from Dr. Jennifer Lippincott-Schwartz (National Institutes of Health, Bethesda, MD). ARF1-T31N was a gift from Dr. Julie Donaldson (National Institutes of Health). GFP-GalTase was kindly provided by Dr. Brian Storrie (Virginia Tech, Blacksburg, VA).
Analysis of tsO45 VSV-G Transport
HeLa cells grown on coverslips and transfected with VSV-G-GFP (ts045). Cells were then incubated at 40°C for 16 h to accumulate the misfolded G protein in the ER. Transport of G protein was initiated by incubating the cells at 32°C. Cells were fixed at different times and processed for immunofluorescence.
Immunofluorescence Microscopy
Cells grown on coverslips were washed in phosphate-buffered saline (PBS), fixed in 3% paraformaldehyde for 10 min, and quenched with 10 mM ammonium chloride. Cells were permeabilized with 0.1% Triton X-100 in PBS. The coverslips were then washed with PBS and blocked in PBS, 2.5% goat serum, 0.2% Tween 20 for 5 min followed by blocking in PBS, 0.4% fish skin gelatin, and 0.2% Tween 20. Cells were incubated with primary antibody 1 h at room temperature. Coverslips were washed with PBS, 0.2% Tween 20 and incubated with secondary antibodies for 45 min. Coverslips were washed as described above and mounted on slides in 9:1 glycerol/PBS with 0.1%_p_-phenylenediamine.
Quantification of Colocalization
Double-labeled images were acquired and analyzed for signal overlap. Using Adobe Photoshop 6.0, structures were individualized by drawing a rectangle around it and the percentage of colocalization was calculated. A structure is defined by a signal greater than 50 (0–255 range) and an area ≥4 pixels2 (0.162 μm in the 50× objective).
Time-Lapse Imaging
HeLa cells grown on glass coverslips were sealed into a silicon rubber chamber placed on a glass slide and containing buffered medium with 25 mM HEPES, pH 7.5. Images were acquired in an Olympus IX70 inverted epifluorescence microscope, equipped with a 40×, 1.35 numerical aperture objective and a cooled charge-coupled device (Photometrics, Tucson, AZ) for 12-bit detection. IpLab Spectrum software (Signal Analytics, Vienna, VA) was used to control image acquisition and manipulation.
RESULTS
GBF1 Localizes to ERES and post-ERES Transport Intermediates
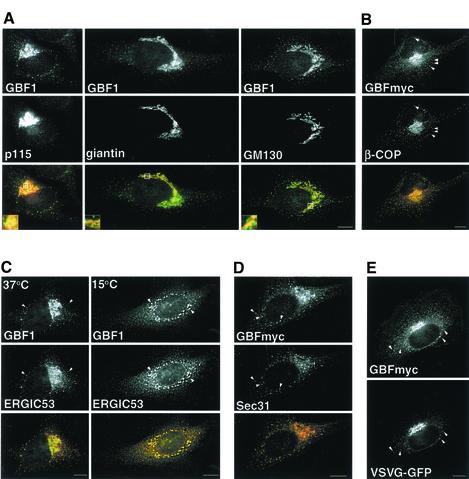
It has been previously shown that GBF1 localizes to the _cis_-side of the Golgi and that it cycles between the ER and the Golgi (Zhao et al., 2002b). In agreement, we found that GBF1 overlaps extensively with the_cis_-Golgi protein p115, but only partially with giantin and GM130, two proteins concentrated in the more medial regions of the Golgi (Figure 1A) (Nakamura et al., 1995; Shima et al., 1997). In addition to the Golgi complex, GBF1 also localizes to peripheral punctate structures throughout the cells (Figure 1A, arrowheads). As expected for a GEF that regulates ARF and COPI recruitment, the majority (80 ± 6.8%) of GBF1-positive peripheral structures are also positive for β-COP (Figure 1B, arrowheads), suggesting GBF1 is involved in COPI recruitment at these sites. To determine the identity of the peripheral structures containing GBF1, we compared the distribution of GBF1 to those of VTC or ERES markers. As shown inFigure 1C, GBF1 colocalizes significantly with ERGIC53 (46.9 ± 6.6%) in peripheral sites shown previously to represent VTCs (Hauri et al., 2000). Colocalization with ERGIC53 is maximal at 15°C (74.0 ± 10.0%), in agreement with previous results (Figure 1C) (Zhao et al., 2002b). Interestingly, a significant fraction of the peripheral GBF1 also localizes to ERES, as suggested by its overlap with an ERES marker, the COPII subunit Sec31 (Figure 1D). Quantitative analysis shows that 66 ± 1.5% of peripheral structures positive for GBF1 also contain Sec31, and another 13% of GBF1-containing structures are located in proximity to Sec31-positive structures.
Figure 1.
GBF1 localizes to the _cis_-Golgi, to ERES, and to COPI-coated cargo-containing pre-Golgi intermediates. (A) HeLa cells were stained with antibodies to GBF1 and p115, GM130, or giantin. Insets show higher magnification of boxed areas. GBF1 colocalizes significantly with p115 but only partially with giantin and GM130 (B) GBFmyc-transfected cells were stained with anti-COPI (β-COP) and anti-myc. Throughout this study we used either endogenous GBF1 or exogenously expressed myc-tagged GBF1, which behaves like wild type at low levels of expression; see supplemental material). (C) Cells were stained with anti-GBF1 and anti-ERGIC53 antibodies in control cells or in cells incubated at 15°C for 2 h. Arrowheads indicate peripheral structures that contain both GBF1 and ERGIC53. (D) GBFmyc-transfected cells were stained with anti-myc antibodies and antibodies to COPII (anti-Sec31). GBFmyc colocalizes significantly with Sec31 (arrowheads). (E) Cells were cotransfected with GBFmyc and VSV-G-GFP, incubated for 16 h at 42°C, and shifted to 32°C for 30 min. Cells were stained with anti-myc antibodies. GBFmyc is detected in pre-Golgi intermediates that contain VSV-G-GFP (arrowheads). The red signal in the merged panels correspond to GBF1 (in all figures). Bars, 10 μm.
Most of the peripheral GBF1-positive structures contain cargo en route from the ER to the Golgi. Cells were cotransfected with a myc-tagged GBF1 (GBFmyc) and VSV-G-GFP and incubated at the restrictive temperature (40°C) for 16 h, and then shifted to the permissive temperature for 10 min to allow transport of VSV-G protein out of the ER. As shown inFigure 1E, VSV-G can be detected in peripheral punctate sites containing GBF1, confirming that, like β-COP, GBF1 is present in peripheral VTCs en route to the Golgi.
It has been shown recently that ARF and COPI are continuously binding and dissociating from membranes, suggesting that an ARF-GEF must be present in all the compartments in which COPI is present (Presley et al., 2002). Our data demonstrate that at steady state, GBF1 localizes to the Golgi, to ERES, and to COPI-coated pre-Golgi intermediates containing cargo, suggesting a role for GBF1 in the regulation of ARF/COPI recruitment at these compartments.
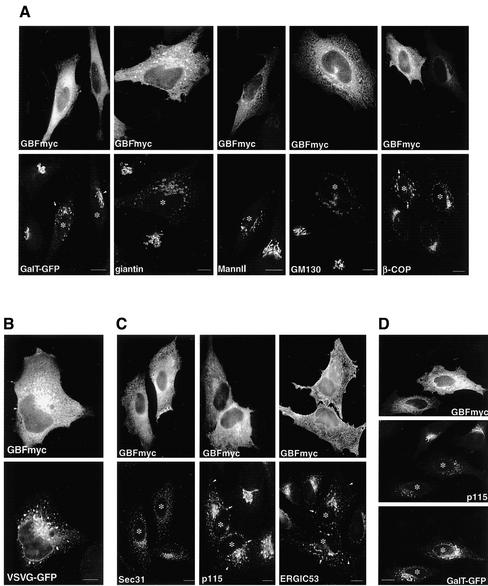
Overexpression of Wild-Type GBF1 Arrests COPI-coated Transport Intermediates at a Late pre-Golgi Step
Overexpression of a GEF is likely to increase the concentration of active GTP-ARF and to promote COPI recruitment to sites at which the GEF acts. Analysis of various Golgi markers (GalT, giantin, MannII, and GM130) in cells expressing high levels of GBFmyc shows that the Golgi redistribute from a single perinuclear Golgi complex to relatively large (1.23 ± 0.53-μm) structures found throughout the cell and concentrated around the nucleus (Figure 2A). The severity of the disrupted phenotype seems to correlate with the level of GBF1 expression. For example, the redistribution of GalT is much more severe in a cell expressing high levels of GBFmyc (Figure 2A, arrow) than in a cell expressing medium levels (Figure 2A, arrowhead). The GBFmyc-induced peripheral structures are highly enriched in β-COP, suggesting an increased COPI recruitment (Figure 2A). The distributions of all examined marker proteins, including β-COP, are analogous to that observed in cells expressing a constitutively active ARF1 mutant (ARF1-Q71L) (Dascher and Balch, 1994;Teal et al., 1994;Zhang et al., 1994;Peters et al., 1995).
Figure 2.
Overexpression of GBF1 arrests COPI-coated transport intermediates at a late pre-Golgi step. (A) HeLa cells were transfected with GBFmyc and stained with antibodies to giantin, MannII, GM130, and β-COP. In the GalT-GFP panel, cells were cotransfected with GBFmyc and GalT-GFP and stained with anti-myc antibodies. In cells expressing high levels of GBFmyc (asterisks), the Golgi redistributes to enlarged peripheral clusters. These enlarged elements are coated with COPI (β-COP panel). In the GalT-GFP panel, the arrowheads indicate a cell expressing medium levels of GBFmyc in which the Golgi is not significantly affected. The arrow shows significant Golgi disruption in a cell expressing higher levels of GBFmyc. (B) Cells were cotransfected with GBFmyc and VSV-G-GFP, incubated for 16 h at 42°C, and then shifted to 32°C for 60 min. Cells were fixed and stained with anti-myc antibodies. VSVG-GFP accumulates at enlarged peripheral and perinuclear elements. (C) GBFmyc-transfected cells were stained with antibodies to myc and antibodies to ERES (anti-Sec31) and to VTCs (anti-p115 and anti-ERGIC53). In transfetecd cells (asterisk), the distribution of ERES is not significantly affected, whereas VTC markers redistribute to peripheral enlarged elements (arrows). Arrowheads indicate the presence of small peripheral elements similar to those found in nontransfected cells. (D) Cells were cotransfected with GBFmyc and GalT-GFP and stained with anti-myc and anti-p115 antibodies. GalT-GFP localization is less affected than that of p115. Bars, 10 μm.
Because Golgi proteins undergo continuous cycling through the ER, we posited that the observed structures may represent ER-Golgi transport intermediates arrested at a late traffic stage (Lippincott-Schwartz et al., 1998). Analysis of VSV-G transport in cells expressing high levels of GBFmyc confirms this prediction (Figure 2B). VSV-G accumulates in enlarged peripheral structures analogous in morphology and distribution to those observed for Golgi markers even after long (2-h) incubations at the permissive temperature. The fact that VSV-G is able to exit the ER and accumulates at the peri-Golgi area suggests that early events in VTC formation are not inhibited by GBFmyc overexpression. In agreement, we found that the overall distribution of ERES (Sec31) is not affected by high levels of GBFmyc (Figure 2C). In contrast, dramatic changes in the distribution of peripheral VTCs (p115 and ERGIC53) are apparent in cells overexpressing GBFmyc (Figure 2C). Both p115 and ERGIC53 are present in large perinuclear structures analogous to those containing marker Golgi proteins (compareFigure 2C to A). We also detect p115 and ERGIC53 in smaller peripheral structures analogous in size to normal ERES-associated VTCs (Figure 2C, arrowheads). The data suggest that ERESs and early VTCs form normally at high levels of GBFmyc and that these early steps of ER-Golgi traffic are not significantly perturbed by GBF1-mediated increase in ARF activation.
Because overexpression of GBFmyc arrests ER-Golgi transport at a late VTC stage, redistribution of rapidly cycling proteins, like ERGIC53 and p115, should be greater than that of Golgi enzymes (Ward et al., 2001). In agreement, we found that in cells expressing high levels of GBFmyc, the redistribution of p115 is much more severe than that of GalT-GFP (Figure 2D). These results suggest that Golgi-to-ER recycling is not significantly affected by overexpression of GBFmyc.
The data suggest that an increase in GBF1 activity has limited effect on the structure and function of ERESs and early VTCs. High levels of GBFmyc do not prevent proteins from being sorted into transport intermediates that exit the ER. However, the ability of VTCs to mature and form a functional Golgi is severely compromised by GBF1-mediated increase in ARF/COPI activity.
Overexpression of Inactive GBF1 Arrests ER-Golgi Transport at an Early Step
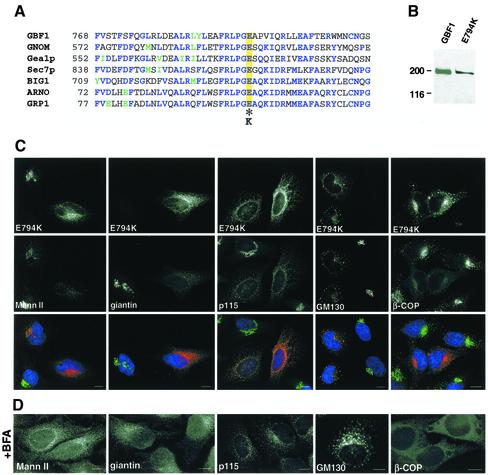
The crystal structure of the Sec7 domain has been determined and consists of 10 α-helices with a deep hydrophobic groove in the central region (Cherfils et al., 1998; Mossessova et al., 1998). A highly conserved glutamic acid residue at the edge of this groove is critical for the exchange reaction (Figure 3A) (Goldberg, 1998). A change-reversal mutation in this residue (E→K) abolishes completely the nucleotide exchange activity of all ARF-GEFs tested so far (Jackson and Casanova, 2000). It has been previously shown that the corresponding mutation in the ARF-GEF ARNO (E156K) stabilizes the interaction between the mutant GEF and ARF-GDP, suggesting that the sequestration of the cellular pool of ARF is a possible mechanism for ARF inactivation (Beraud-Dufour et al., 1998). To generate an inactive GBF1, we substituted the critical glutamic acid residue at position 794 by lysine in GBFmyc (E794K). E794K encodes a protein of the appropriate molecular mass (∼200 kDa) in transfected cells (Figure 3B).
Figure 3.
Overexpression of an inactive mutant of GBF1 induces disassembly of the Golgi and arrests transport at an early step. (A) Sequence alignment of the F-G loop form seven Sec7 domain GEFs. Identical residues are indicated in blue, and homologous residues are in green. The essential glutamic acid residue is highlighted in yellow and the inactivating substitution is indicated by an asterisk. (B) HeLa lysates from control cells and from cells transfected with E794K were separated by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with anti-GBF1 (endogenous GBF1) or anti-myc antibodies (E794K). (C) E794K-transfected cells were stained with antibodies to MannII, giantin, p115, GM130, and β-COP. (D) Cells were treated with 5 μg/ml BFA for 30 min, and processed as described in C. Both in 794K-transfected cells and in BFA-treated cells, MannII and giantin redistributed to the ER, and p115 and giantin to peripheral punctuate structures, and β-COP to the cytosol. Bars, 10 μm.
E794K expression causes the complete disassembly of the Golgi, as shown by the redistribution of Golgi proteins to the ER (MannII and giantin) or to peripheral punctate structures that contain E794K (p115 and GM130) (Figure 3C). The localization of each marker protein resembles its distribution in cells treated with BFA (Figure 3D). BFA inhibits the exchange function of ARF-GEFs and induces the dissociation of COPI from membranes (Klausner et al., 1992). In E794K-transfected cells, like in BFA-treated cells, β-COP dissociates from membranes to the cytosol (Figure 3, C and D).
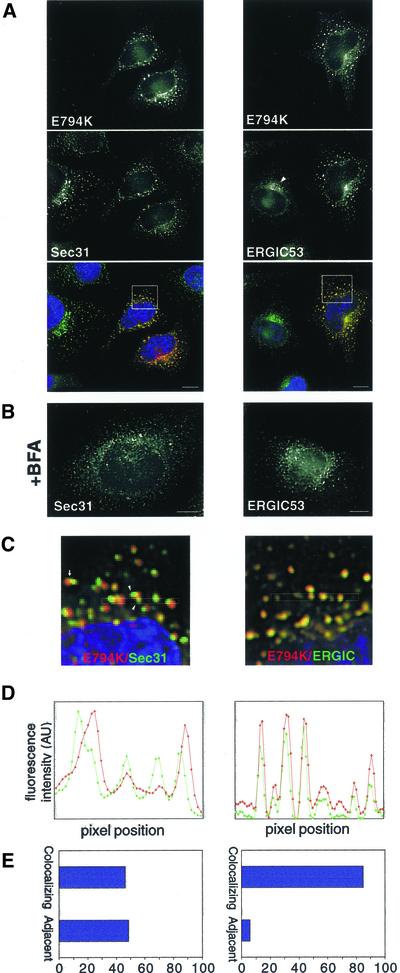
It has been shown that in contrast to its effects on COPI, BFA does not inhibit the recruitment of COPII components to ERES (Orci et al., 1993;Ward et al., 2001). Furthermore, BFA allows the formation of an adjacent tubular compartment that is devoid of COPII elements but contains ERGIC53 (and other proteins) (Lippincott-Schwartz et al., 1990; Nakamura et al., 1995; Klumperman_et al._, 1998; Nelson_et al_., 1998; Ward_et al_., 2001). In the absence of COPI, this post-ERES compartment does not differentiate into transport-competent VTCs. Expression of E794K also allows the formation of ERES and the adjacent post-ERES compartment. In E794K-expressing cells the distribution of ERES (Sec31) seems normal, and the pattern is similar to that observed in BFA-treated cells (Figure 4, A and B). Similarly, the redistribution of ERGIC53 induced by E794K is analogous to that obtained after BFA treatment (Figure 4, A and B), when ERGIC53 localizes to the tubular compartment adjacent to ERES (Lippincott-Schwartz et al., 1990; Ward et al., 2001).
Figure 4.
E794K localizes to a post-ER exit sites compartment. (A) HeLa cells were transfected with E794K and stained with anti-Sec31 or anti-ERGIC53 antibodies. (B) Cells were treated with 5 μg/ml BFA and stained as described in A. (C) Insets show a higher magnification of the boxed areas in A. The arrow marks a Sec31-positive structure adjacent to an E794K-positive structure. Arrowheads show two Sec31-positive structures associated to a single E794K positive structure. (D) Fluorescence intensity of the boxed area in C was plotted against pixel position (E) Double-labeled images were acquired, analyzed for signal overlap, and plotted. Bars, 10 μm.
E794K localizes preferentially to the post-ERES compartment. We examined the level of colocalization between E794K and Sec31, and between E794K and ERGIC53, and found that although the patterns of E794K and ERGIC53 overlap almost completely, E794K-positive structures are either completely separate or only partially overlap with Sec31-containing structures (Figures 4, C and D). Sometimes two or more Sec31 structures are associated with a single E794K structure. Quantitation analysis indicates that >85% of E794K colocalizes with ERGIC53 and <50% colocalizes with Sec31 (Figure 4E). Our results indicate that expression of E794K disassembles the Golgi and arrest transport at early VTCs that localize adjacent to ERES. It seems that further differentiation of the post-ERES compartment to VTCs is halted by E794K-induced inhibition of COPI recruitment.
Dynamics of E794K-GFP in Live Cells
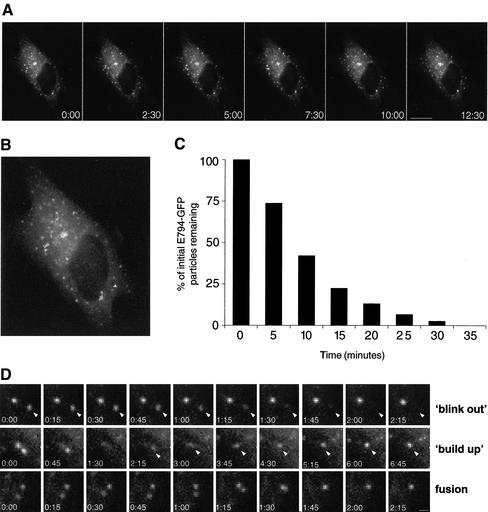
To investigate the dynamics of the post-ERES compartment in living cells, we generated a GFP-tagged construct of E794K. E794K-GFP causes Golgi disassembly and arrests transport in a manner analogous to that of E794K (our unpublished data). Time-lapse imaging shows that E794K-GFP labeled structures are relatively immobile and do not seem to change their overall distribution.Figure 5A shows a representative series of images spanning 12:30 min (see accompanying movie). Tracing of six different particles over a period of 45 min shows that most particles are practically immobile, or move over a short distance (<2.5 μm) with no particular direction (Figure 5B). Significantly, most of the structures labeled with E794K-GFP are relatively short lived and disappear with a half-life of <10 min. Quantitative analysis indicates that 86% of the structures disappear within 10 min (Figure 5C). E794K disappearance events resemble the blink-out events described after BFA treatment, suggesting E794K-GFP containing structures fuse with the ER (Sciaky et al., 1997). Alternatively, E794K-GFP may be dissociating from membranes to the cytosol. A series of images showing a blink out of a single E794K-GFP–labeled structure is shown inFigure 5D (blink-out panels). We have imaged many E794K-labeled structures in different cells and have observed blink out consistently, even when adjacent structures do not fade, suggesting that there are no major changes in the focal plane of the image (Figure 5D, blink-out panel and accompanying movie). We also show that most of the particles move very little or do not move at all, suggesting that movement out of the focal plane is a rare event. Often, we observed E794K-GFP–labeled structures forming de novo at the same place or in proximity to a blink out. As shown inFigure 5D (build-up panels), two adjacent structures undergo subsequent blink outs, and a new structure occurs in the vicinity. Fluorescence buildup of the new particle is gradual but relatively rapid, and maximal labeling of the structure is achieved within 4 min. In some cases, we observed fusion between two separate structures (Figure 5D, fusion panels). Two distinct particles ∼1.2 μm apart seem to coalesce into a single structure in ∼1.5 min and behave as a single entity until they blink out after 27 min (see accompanying movie).
Figure 5.
Dynamics of E794K-GFP in live cells. (A) Cells expressing E794K-GFP were imaged at 15-s intervals for 45 min. Images shown cover a period of 12:30 min. Bar, 10 μm. (B) Overlaid in the first frame of the accompanying movie are the tracings of six randomly chosen structures over a period of 45 min. (C) Population analysis of post-ER exit site structures' half-life. The half-life of individual particles (n = 78) was measured, grouped into 5-min intervals, and plotted. More than 50% of the initial structures disappeared during the first 10 min. (D) Analysis of post-ER exit sites' compartment behavior. (Blink out) The series show two particles, one disappearing in ∼1 min (arrowheads), whereas the other remains stable throughout. (buildup) The series shows a particle occurring in the vicinity of a blink-out event after the sequential disappearing of two particles. The series show two particles that come into proximity and fuse with each other (fusion). Bar, 1 μm.
Our results indicate that the post-ERES compartment is unstable and constantly undergoes cycles of de novo formation and disappearance. Functional GBF1 is required to stabilize this compartment and allow traffic to the Golgi.
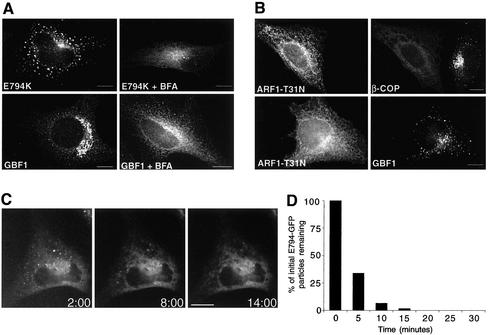
Transport between the ER and the Golgi is BFA sensitive, and it has been shown that the GEF operational at this stage is a target for BFA (Donaldson et al., 1992). In agreement, both GBF1 and E794K relocate to the ER in BFA-treated cells (Figure 6A). However, this posed a paradox. On the one hand, neither wild-type GBF1 nor E794K is sorted into the post-ERES compartment upon inhibition of COPI recruitment by BFA. On the other hand, E794K can be sorted into the post-ERES compartment even when E794K inhibits COPI recruitment (Figure 3C). These results suggest that the effects of BFA on GBF1 sorting are not due by the lack of COPI recruitment but are probably the result of BFA directly interfering with GBF1 sorting. To provide experimental evidence to support our model, we inhibited COPI recruitment without the use of BFA and analyzed the sorting of endogenous GBF1. Expression of a dominant inactive ARF1 mutant (ARF1-T31N) causes COPI release into the cytosol, and leads to Golgi disassembly, an effect similar to that of BFA (Dascher and Balch, 1994; Ward et al., 2001). We expressed ARF1-T31N in cells and analyzed its effects on β-COP and GBF1 localization. As shown inFigure 6B, β-COP is released into the cytosol in ARF-T31N–transfected cells. Significantly, GBF1 localizes to punctate structures analogous in morphology to those induced by overexpression of E794K. This finding indicates that GBF1 is sorted into the post-ERES compartment in a COPI-independent manner and that BFA directly interferes with its sorting. In agreement, analysis of the dynamics of E794K disappearance from the post-ERES compartment after BFA addition shows rapid redistribution to the ER (Figure 6C and accompanying movie). More than 90% of E794K particles disappear within the first 10 min (Figure 6D). Importantly, E794K remains in the ER and no de novo buildup of E794K is observed. It must be stressed that BFA specifically inhibits the sorting of E794K into the post-ERES compartment, but does not prevent the sorting of other proteins into this compartment. Live imaging of p58-GFP after BFA treatment confirms that a functional post-ERES compartment is maintained during BFA treatment (see supplemental movie). p58 is the rat homolog of ERGIC53 and localizes to the post-ERES compartment in BFA-treated cells (Ward et al., 2001; this study). Our results indicate that BFA allows the formation and the sorting of several proteins into a post-ERES compartment but specifically blocks the recruitment of GBF1 to this compartment.
Figure 6.
BFA directly influences E794K localization. (A) Control cells and E794K-transfected cells were incubated with 5 μg/ml BFA for 30 min and stained with anti-GBF1 and anti-myc antibodies. Sorting of E794K into the post-ERES compartment is inhibited by BFA. (B) Cells expressing a constitutively inactive mutant of ARF1 (T31N) (HA-tagged) were stained with anti-HA and anti β-COP or anti GBF1 antibodies. Although COPI recruitment to membranes is completely abolished in T31N-transfected cells, GBF1 is still recruited to post-ERES structures. (C) Dynamics of E794K-GFP in BFA-treated cells. Cells expressing E794K-GFP were treated with 5 μg/ml BFA and imaged at 20-s intervals for 45 min. Images begin 2 min after BFA addition and extent to 14 min. BFA causes redistribution of E794K to the ER and prevents reappearance. (D) Population analysis of post-ERES structures' half-life in BFA-treated cells (n = 62). The half-life of individual particles was measured, grouped into 5-min intervals, and plotted. Bar, 10 μm.
DISCUSSION
Molecular and biochemical dissection of ER-Golgi transport has revealed a crucial role for the ARF/COPI system. Assembly of a functional COPI coat is required for the formation and maturation of pre-Golgi intermediates into Golgi elements and is essential for the maintenance of a functional Golgi complex (Lippincott-Schwartz et al., 1998). COPI coats are recruited to membranes through the action of activated ARFs, which in turn are activated by GEFs. Herein, we report the identification of GBF1 as the GEF responsible for the regulation of ARF/COPI dynamics at the ER-Golgi interface. GBF1-mediated ARF activation is required for the formation of VTCs at ERESs, as well as their maturation into Golgi. GBF1 function also plays a key role in the maintenance of Golgi integrity.
Localization of GBF1
At least three GEFs have been shown to be important for transport between the ER and the Golgi in yeast: Sec7p, Gea1p, and Gea2p (Achstetter et al., 1988; Peyroche et al.,1996,1999,2001;Spang et al., 2001). The corresponding mammalian orthologs, BIG1/2 (Sec7p) and GBF1 (Gea1/2p), have been localized to distinct regions of the Golgi, and are likely to perform distinct functions (Claude et al., 1999; Mansour et al., 1999; Togawa et al., 1999; Kawamoto et al., 2002; Yamaji et al., 2000; Zhao et al., 2002b). BIG1 and BIG2 localize to the TGN and are involved in regulating the recruitment of the clathrin adaptor complexes AP-1 and GGA1 (Shinotsuka et al.,2002a,b;Zhao et al., 2002b). They do not seem to be involved in COPI events because they do not redistribute to VTCs at 15°C, and remain associated with TGN membranes in the peri-microtubule organizing center region after BFA treatment (Zhao et al., 2002b). In contrast, GBF1 is found at the _cis_-Golgi and in tubular structures likely to be VTCs, cycles between the Golgi and the ER, and redistributes to the ER in cells treated with BFA (Claude_et al_., 1999;Kawamoto et al., 2002; Zhao et al., 2002b; this study). Herein, we show that in addition to its_cis_-Golgi localization, GBF1 is present at peripheral COPI-coated structures that represent ERESs and VTCs, suggesting a role for GBF1 in COPI recruitment at several steps between the ER and the Golgi.
Function of GBF1 in ER-Golgi Traffic
To explore the cellular function of GBF1, we used wild-type and inactive GBF1 mutant in vivo. High levels of GBF1 activity increase the rate of ARF and COPI recruitment to VTCs and arrest transport at a pre-Golgi stage. The rate-limiting step for dissociation of ARF seems to be GTP hydrolysis, and it is likely that at high levels of GBF1, the amount of active ARF exceeds the capacity of GAPs (GTPase activating protein) to inactivate it (Vasudevan et al., 1998). GBF1 overexpression does not affect function of ERES. VTCs can differentiate and relocate at least partially in the presence of high levels of GBF1, because they concentrate in the peri-microtubule organizing center region but they cannot assemble a Golgi. Our data suggest that high levels of GBF1 create a kinetic delay in the maturation of VTCs, causing a block at a late pre-Golgi stage. This finding suggests that maturation of late VTCs is probably the step most sensitive to perturbations of COPI dynamics. Our results are consistent with those obtained when COPI recruitment is stabilized by other means, such as expression of a constitutively active from of ARF1, or microinjection of anti-β-COP antibodies (Pepperkok et al., 1993; Dascher and Balch, 1994; Teal et al., 1994; Zhang et al., 1994). It is not clear what ARF isoforms GBF1 activates. In vitro observations suggest that GBF1 preferentially uses class II ARFs (ARF5) as substrate (Claude et al., 1999). However, in vivo data suggest that GBF1 can also act on ARF1 and ARF3 (Kawamoto et al., 2002). ARF1 and ARF3 belong to class I and are thought to be the main ARFs responsible for regulating ER-to-Golgi transport. It is possible that GBF1 is acting on different ARF isoforms that regulate different transport steps. Alternatively, the different ARFs might be involved in the transport of specific components or pathways. Characterization of the specificity of GBF1 will be fundamental for understanding ARF function in cells.
That GBF1 activity is essential for COPI recruitment and for the formation of VTCs is most clearly shown with the inactive E794K mutant. Expression of E794K leads to COPI dissociation from membranes and the disassembly of the Golgi. It also arrests cargo transport at the level of early VTCs that localize adjacent to ERESs. The compartment arrested by inactivating GBF1 seems analogous to that observed in cells treated with BFA. BFA binds to and stabilizes an ARF-GDP-Sec7 domain complex, trapping the exchange factor and blocking the activation of ARF molecules (Peyroche et al., 1999). In the presence of BFA, ARF activity is inhibited and COPI is not recruited to membranes. Analysis of the compartment generated by E794K (or BFA treatment) suggests that it represents the earliest defined stage in VTCs formation. We propose the term pre-COPI VTC, to define this stage in VTC maturation because it differentiates from ER exit sites in a COPI-independent manner.
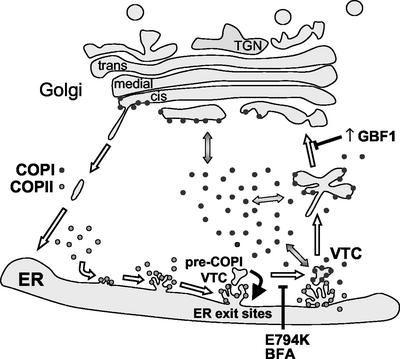
Pre-COPI VTCs differentiate by the action of the COPII coat, because they do not form when COPII recruitment is inhibited by a constitutively inactive Sar1p mutant (Ward et al., 2001). Pre-COPI VTC membranes are distinct from ERESs because they do not contain COPII markers, and contain proteins, such as ERGIC53, p115, GM130, GRASP65, syntaxin5, and membrin (Lippincott-Schwartz et al., 1990; Nakamura et al., 1995; Klumperman_et al_., 1998; Nelson_et al_., 1998; Chao_et al_., 1999; Ward_et al_., 2001). The differentiation and recruitment of specific proteins to pre-COPI VTC provides the framework for subsequent steps in VTC differentiation. Specifically, the recruitment of GBF1 to pre-COPI VTC is required to locally activate ARF and induce the spatially defined assembly of COPI coat. Association of COPI with pre-COPI VTC is required for stabilization of these structures (see below) and sorting of proteins such as MannII, Gal-T, or VSV-G into these differentiated subdomains. The COPI-mediated differentiation of pre-COPI VTC into VTCs is essential for subsequent transport to the Golgi. This model of early events in ER-Golgi traffic is diagramed in Figure 7.
Figure 7.
GBF1 regulates COPI coating at the ER-Golgi interface and defines a novel functional stage in the progressive maturation of VTCs. Expression of an inactive GBF1 mutant arrests transport, blocks COPI recruitment to membranes, and induces the collapse of the Golgi into the ER. The effect of expressing an inactive GBF1 is analogous to that of BFA. GBF1 inactivation allows the formation of an abortive post-ER exit sites compartment (pre-COPI VTCs capable of selectively sorting some transport components. In the absence of COPI recruitment, pre-COPI VTCs continuously form and disappear, presumably by fusing with the ER (red arrow). COPII-mediated differentiation of pre-COPI VTCs represents the first step in VTC formation. GBF1 regulates the subsequent association of COPI with pre-COPI VTCs, which is required for stabilization of these structures and sorting of others proteins. The COPI-mediated differentiation of pre-COPI VTCs into VTCs is essential for subsequent transport to the Golgi. Increased expression of GBF1 causes Golgi disassembly and leads to the accumulation of enlarged COPI-coated elements in a peri-Golgi region. High levels of GBF1 promote COPI recruitment and result in the stabilization of the peri-Golgi compartment, preventing its maturation into Golgi.
It is not clear whether pre-COPI VTCs are a specialized subdomain of an ER exit site or an independent compartment that forms from COPII vesicles budded from ER exit sites. Electron microscopic analysis of pre-COPI VTC structure in BFA-treated cells shows that they are morphologically similar to VTCs in nontreated cells, suggesting they may represent a separate compartment (Ward et al., 2001). However, photobleaching experiments with GFP-tagged p58 (the rat homolog of ERGIC53) show that pre-COPI VTC-localized p58-GFP exchanges rapidly with the ER pool of p58-GFP (Ward et al., 2001). Because p58 is a transmembrane protein, these data suggest that ER and pre-COPI VTC might be connected. Alternatively, it is possible that p58 rapidly moves between ER and pre-COPI VTCs via vesicles.
Initially, COPI was proposed to mediate the formation of transport vesicles carrying anterograde cargo (Lippincott-Schwartz et al., 1998; Chavrier and Goud, 1999). An alternative hypothesis proposed a role for COPI in the formation of retrograde transport vesicles that would recycle proteins from the Golgi back to the ER. However, experimental results over the last several years led to the proposal of an alternative model in which COPI plays a role in sorting proteins into membrane subdomains that subsequently bud off as anterograde or retrograde transport intermediates (Presley et al., 2002). Our data are most consistent with and extend the last model of COPI function. Our results suggest that GBF1 associates with partially differentiated membranes and through its action of recruiting COPI, further matures the membrane. The association of COPI and the continual function of GBF1 to sustain COPI association defines a spatially limited subdomain that allows the sorting and recruitment of additional proteins. Together, GBF1 and COPI facilitate the conversion of transport arrested intermediates into transport-competent intermediates. Recruitment of GBF1 to membranes represents the most proximal event in the COPI cascade. It remains to be determined whether GBF1, like ARF and COPI, is continuously cycling on and off the membrane and to define the mechanisms that target GBF1 to specific membrane sites. Time-lapse imaging analysis of wild-type GFP-GBF1 suggest that GBF1 cycles very rapidly between the cytosol and membranes, both at the Golgi region and at the ERES/VTC region (Zhao_et al_., 2002a).
Dynamics of pre-COPI VTCs
In cells, pre-COPI VTCs are likely to be transient structures that rapidly acquire COPI and mature into VTCs. In E794K-transfected cells, pre-COPI VTCs are relatively short lived with half-lives that are usually <10 min. This is distinct from ERESs visualized by imaging GFP-tagged Sec13 showing that ERESs are long lived and rarely form de novo (Stephens et al., 2000). Pre-COPI VTCs are continuously disappearing, presumably by fusing with the ER. After the collapse of a pre-COPI VTCs, the adjacent ERES generally gives rise to a new pre-COPI VTC. It is likely that E794K forms an abortive pre-COPI VTC that cannot mature and is reabsorbed. It seems that GBF1 function defines the transition from pre-COPI VTC to VTC and allows the stable entry of cargo and Golgi proteins.
Is GBF1 the Target of BFA in ER-Golgi Traffic?
Because transport between the ER and the Golgi is BFA sensitive, the GEF-regulating COPI events in this pathway should be BFA sensitive. GBF1 has been originally identified as BFA-resistant GEF by its ability to confer BFA resistance to cells (Klausner et al., 1992; Claude et al., 1999; Kawamoto_et al_., 2002; Zhao_et al_., 2002b). However, it is possible that the observed resistance is a product of overexpression and not of a BFA-resistant activity. This is strongly supported by recent results showing that overexpression of BIG2, a BFA-sensitive GEF, blocks BFA-induced redistribution from membranes of ARF1 and the AP-1 complex at the TGN (Shinotsuka et al.,2002a,b). In addition, structural analyses of the residues that determine BFA resistance/sensitivity in other GEFs suggest that GBF1 could be BFA sensitive. Mutagenesis studies have shown that a critical pair of phenylalanine and alanine (FA) residues conserved in all BFA-resistant GEFs is required to confer BFA-resistant GEF activity (Peyroche et al., 1999) In contrast, BFA-sensitive GEFs contain a conserved pair of tyrosine and serine (YS) residues. Substitution of the FA pair to the YS pair converts a resistant GEF into a sensitive GEF, and vice versa (Peyroche et al., 1999). Interestingly, GBF1 contains a mixed sensitive/resistant (Y/A) sequence in that region. When the residues in the resistant FA pair were substituted individually, only the phenylalanine residue but not the alanine residue could confer BFA resistance (Peyroche et al., 1999). Because GBF1 does not contain the phenylalanine residue, it is likely a BFA-sensitive GEF. Furthermore, the yeast orthologs of GBF1 (Gea1p and Gea2p) are the major targets of BFA in the yeast secretory pathway (Peyroche et al., 1999). Our data also suggest that GBF1 might be the target for BFA at the early secretory pathway. We show that GBF1 distribution is BFA sensitive and that this is due to a direct interference of BFA in GBF1 sorting. BFA binds to a complex between a GEF and GDP-ARF, and the effect of BFA on GBF1 localization is most likely due to direct binding of BFA to the ARF-GDP-GBF1 complex. Furthermore, BFA also causes the redistribution of E794K and prevents its sorting into post-ERES compartment.
Together, our data identify GBF1 as a key molecule involved in membrane differentiation that underlies the ability of the secretory pathway to sort resident, recycling, and cargo proteins. The process involves the selective sorting of molecules into organized membrane subdomains that are defined by the recruitment of cytosolic coat proteins. The primary site of GBF1 function is at a post-ER exit site compartment that represents the first COPI assembly site. This initial ARF/COPI event defines a differentiation step required for the maturation of the unstable compartment into mobile intermediates capable of translocating to the Golgi. GBF1 activity is also required in subsequent stages of transport, indicating that GBF1 is likely to be the sole ARF-GEF regulating COPI dynamics at the ER-Golgi interface.
Supplementary Material
MBC Videos
Acknowledgments
We thank J. Lippincott-Schwartz, J. Donaldson, and B. Storrie for DNA constructs; H.P. Hauri, W. Hong, B. Glick, and M. Farquahr for antibodies; and C. Jackson and J. Lippincott-Schwartz for helpful discussion.
Abbreviations used: ARF, ADP-ribosylation factor; BFA, brefeldin A; ERES, endoplasmic reticulum exit site; GEF, guanine nucleotide exchange factor; VTC, vesicular tubular cluster.
V⃞
Online version of this article contains video material for some figures. Online version available atwww.molbiolcell.com.
References
- Achstetter, T., Franzusoff, A., Field, C., and Schekman, R. (1988). SEC7 encodes an unusual, high molecular weight protein required for membrane traffic from the yeast Golgi apparatus. J. Biol. Chem. 263, 11711-11717. [PubMed] [Google Scholar]
- Aridor, M., Bannykh, S.I., Rowe, T., and Balch, W.E. (1995). Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J. Cell Biol. 131, 875-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh, S.I., and Balch, W.E. (1997). Membrane dynamics at the endoplasmic reticulum-Golgi interface. J. Cell Biol. 138, 1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh, S.I., Rowe, T., and Balch, W.E. (1996). The organization of endoplasmic reticulum export complexes. J. Cell Biol. 135, 19-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraud-Dufour, S., Robineau, S., Chardin, P., Paris, S., Chabre, M., Cherfils, J., and Antonny, B. (1998). A glutamic finger in the guanine nucleotide exchange factor ARNO displaces Mg2+ and the β-phosphate to destabilize GDP on ARF1. EMBO J. 17, 3651-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, D.S., Hay, J.C., Winnick, S., Prekeris, R., Klumperman, J., and Scheller, R.H. (1999). SNARE membrane trafficking dynamics in vivo. J. Cell Biol. 144, 869-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier, P., and Goud, B. (1999). The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol. 11, 466-475. [DOI] [PubMed] [Google Scholar]
- Cherfils, J., Menetrey, J., Mathieu, M., Le Bras, G., Robineau, S., Beraud-Dufour, S., Antonny, B., and Chardin, P. (1998). Structure of the Sec7 domain of the Arf exchange factor ARNO. Nature 392, 101-105. [DOI] [PubMed] [Google Scholar]
- Claude, A., Zhao, B.P., Kuziemsky, C.E., Dahan, S., Berger, S.J., Yan, J.P., Armold, A.D., Sullivan, E.M., and Melancon, P. (1999). GBF1: a novel Golgi-associated BFA-resistant guanine nucleotide exchange factor that displays specificity for ADP-ribosylation factor 5. J. Cell Biol. 146, 71-84. [PMC free article] [PubMed] [Google Scholar]
- Dascher, C., and Balch, W.E. (1994). Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem. 269, 1437-1448. [PubMed] [Google Scholar]
- Donaldson, J.G., Finazzi, D., and Klausner, R.D. (1992). Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature 360, 350-352. [DOI] [PubMed] [Google Scholar]
- Franzusoff, A., Lauze, E., and Howell, K.E. (1992). Immuno-isolation of Sec7p-coated transport vesicles from the yeast secretory pathway. Nature 355, 173-175. [DOI] [PubMed] [Google Scholar]
- Goldberg, J. (1998). Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell 95, 237-248. [DOI] [PubMed] [Google Scholar]
- Hauri, H.P., Kappeler, F., Andersson, H., and Appenzeller, C. (2000). ERGIC-53 and traffic in the secretory pathway. J. Cell Sci. 113, 587-596. [DOI] [PubMed] [Google Scholar]
- Jackson, C.L., and Casanova, J.E. (2000). Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 10, 60-67. [DOI] [PubMed] [Google Scholar]
- Kawamoto, K., Yoshida, Y., Tamaki, H., Torii, S., Shinotsuka, C., Yamashina, S., and Nakayama, K. (2002). GBF1, a guanine nucleotide exchange factor for ADP-ribosylation factors, is localized to the cis-Golgi and involved in membrane association of the COPI coat. Traffic 3, 483-495. [DOI] [PubMed] [Google Scholar]
- Klausner, R.D., Donaldson, J.G., and Lippincott-Schwartz, J. (1992). Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116, 1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman, J., Schweizer, A., Clausen, H., Tang, B.L., Hong, W., Oorschot, V., and Hauri, H.P. (1998). The recycling pathway of protein ERGIC-53 and dynamics of the ER-Golgi intermediate compartment. J. Cell Sci. 111, 3411-3425. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Cole, N.B., and Donaldson, J.G. (1998). Building a secretory apparatus: role of ARF1/COPI in Golgi biogenesis and maintenance. Histochem. Cell Biol. 109, 449-462. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Donaldson, J.G., Schweizer, A., Berger, E.G., Hauri, H.P., Yuan, L.C., and Klausner, R.D. (1990). Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 60, 821-836. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Yuan, L.C., Bonifacino, J.S., and Klausner, R.D. (1989). Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56, 801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour, S.J., Skaug, J., Zhao, X.H., Giordano, J., Scherer, S.W., and Melancon, P. (1999). p200 ARF-GEP1: a Golgi-localized guanine nucleotide exchange protein whose Sec7 domain is targeted by the drug brefeldin A. Proc. Natl. Acad. Sci. USA 96, 7968-7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossessova, E., Gulbis, J.M., and Goldberg, J. (1998). Structure of the guanine nucleotide exchange factor Sec7 domain of human arno and analysis of the interaction with ARF GTPase. Cell 92, 415-423. [DOI] [PubMed] [Google Scholar]
- Nakamura, N., Rabouille, C., Watson, R., Nilsson, T., Hui, N., Slusarewicz, P., Kreis, T.E., and Warren, G. (1995). Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 131, 1715-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, D.S., Alvarez, C., Gao, Y.S., Garcia-Mata, R., Fialkowski, E., and Sztul, E. (1998). The membrane transport factor TAP/p115 cycles between the Golgi and earlier secretory compartments and contains distinct domains required for its localization and function. J. Cell Biol. 143, 319-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci, L., Perrelet, A., Ravazzola, M., Wieland, F.T., Schekman, R., and Rothman, J.E. (1993). “BFA bodies”: a subcompartment of the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 90, 11089-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperkok, R., Scheel, J., Horstmann, H., Hauri, H.P., Griffiths, G., and Kreis, T.E. (1993). β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell 74, 71-82. [DOI] [PubMed] [Google Scholar]
- Peters, P.J., Hsu, V.W., Ooi, C.E., Finazzi, D., Teal, S.B., Oorschot, V., Donaldson, J.G., and Klausner, R.D. (1995). Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J. Cell Biol. 128, 1003-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyroche, A., Antonny, B., Robineau, S., Acker, J., Cherfils, J., and Jackson, C.L. (1999). Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol. Cell 3, 275-285. [DOI] [PubMed] [Google Scholar]
- Peyroche, A., Courbeyrette, R., Rambourg, A., and Jackson, C.L. (2001). The ARF exchange factors Gea1p and Gea2p regulate Golgi structure and function in yeast. J. Cell Sci. 114, 2241-2253. [DOI] [PubMed] [Google Scholar]
- Peyroche, A., Paris, S., and Jackson, C.L. (1996). Nucleotide exchange on ARF mediated by yeast Gea1 protein. Nature 384, 479-481. [DOI] [PubMed] [Google Scholar]
- Presley, J.F., Ward, T.H., Pfeifer, A.C., Siggia, E.D., Phair, R.D., and Lippincott-Schwartz, J. (2002). Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature 417, 187-193. [DOI] [PubMed] [Google Scholar]
- Rothman, J.E., and Wieland, F.T. (1996). Protein sorting by transport vesicles. Science 272, 227-234. [DOI] [PubMed] [Google Scholar]
- Rowe, T., Aridor, M., McCaffery, J.M., Plutner, H., Nuoffer, C., and Balch, W.E. (1996). COPII vesicles derived from mammalian endoplasmic reticulum microsomes recruit COPI. J. Cell Biol. 135, 895-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales, S.J., Pepperkok, R., and Kreis, T.E. (1997). Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 90, 1137-1148. [DOI] [PubMed] [Google Scholar]
- Sciaky, N., Presley, J., Smith, C., Zaal, K.J., Cole, N., Moreira, J.E., Terasaki, M., Siggia, E., and Lippincott-Schwartz, J. (1997). Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J. Cell Biol. 139, 1137-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima, D.T., Haldar, K., Pepperkok, R., Watson, R., and Warren, G. (1997). Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J. Cell Biol. 137, 1211-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinotsuka, C., Waguri, S., Wakasugi, M., Uchiyama, Y., and Nakayama, K. (2002a). Dominant-negative mutant of BIG2, an ARF-guanine nucleotide exchange factor, specifically affects membrane trafficking from the trans-Golgi network through inhibiting membrane association of AP-1 and GGA coat proteins. Biochem. Biophys. Res. Commun. 294, 254-260. [DOI] [PubMed] [Google Scholar]
- Shinotsuka, C., Yoshida, Y., Kawamoto, K., Takatsu, H., and Nakayama, K. (2002b). Overexpression of an ADP-ribosylation factorguanine nucleotide exchange factor, BIG2, uncouples brefeldin A-induced adaptor protein-1 coat dissociation and membrane tubulation. J. Biol. Chem. 277, 468-9473. [DOI] [PubMed] [Google Scholar]
- Spang, A., Herrmann, J.M., Hamamoto, S., and Schekman, R. (2001). The ADP ribosylation factor-nucleotide exchange factors Gea1p and Gea2p have overlapping, but not redundant functions in retrograde transport from the Golgi to the endoplasmic reticulum. Mol. Biol. Cell. 12, 1035-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, D.J., Lin-Marq, N., Pagano, A., Pepperkok, R., and Paccaud, J.P. (2000). COPI-coated ER-to-Golgi transport complexes segregate from COPII in close proximity to ER exit sites. J. Cell Sci. 113, 2177-2185. [DOI] [PubMed] [Google Scholar]
- Teal, S.B., Hsu, V.W., Peters, P.J., Klausner, R.D., and Donaldson, J.G. (1994). An activating mutation in ARF1 stabilizes coatomer binding to Golgi membranes. J. Biol. Chem. 269, 3135-3138. [PubMed] [Google Scholar]
- Togawa, A., Morinaga, N., Ogasawara, M., Moss, J., and Vaughan, M. (1999). Purification and cloning of a brefeldin A-inhibited guanine nucleotide-exchange protein for ADP-ribosylation factors. J. Biol. Chem. 274, 12308-12315. [DOI] [PubMed] [Google Scholar]
- Vasudevan, C., Han, W., Tan, Y., Nie, Y., Li, D., Shome, K., Watkins, S.C., Levitan, E.S., and Romero, G. (1998). The distribution and translocation of the G protein ADP-ribosylation factor 1 in live cells is determined by its GTPase activity. J. Cell Sci. 111, 1277-1285. [DOI] [PubMed] [Google Scholar]
- Ward, T.H., Polishchuk, R.S., Caplan, S., Hirschberg, K., and Lippincott-Schwartz, J. (2001). Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 155, 557-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji, R., Adamik, R., Takeda, K., Togawa, A., Pacheco-Rodriguez, G., Ferrans, V.J., Moss, J., and Vaughan, M. (2000). Identification and localization of two brefeldin A-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors in a macromolecular complex. Proc. Natl. Acad. Sci. USA 97, 2567-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C.J., Rosenwald, A.G., Willingham, M.C., Skuntz, S., Clark, J., and Kahn, R.A. (1994). Expression of a dominant allele of human ARF1 inhibits membrane traffic in vivo. J. Cell Biol. 124, 289-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Claude, A., and Melancon, P. (2002a). Mol. Biol. Cell 13, 355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Lasell, T.K., and Melancon, P. (2002b). Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein Traffic Mol. Biol. Cell. 13, 119-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MBC Videos