Golgi Localization of Syne-1 (original) (raw)
Abstract
We have previously identified a Golgi-localized spectrin isoform by using an antibody to the β-subunit of erythrocyte spectrin. In this study, we show that a screen of a λgt11 expression library resulted in the isolation of an ∼5-kb partial cDNA from a Madin-Darby bovine kidney (MDBK) cell line, which encoded a polypeptide of 1697 amino acids with low, but detectable, sequence homology to spectrin (37%). A blast search revealed that this clone overlaps with the 5′ end of a recently identified spectrin family member Syne-1B/Nesprin-1β, an alternately transcribed gene with muscle-specific forms that bind acetylcholine receptor and associate with the nuclear envelope. By comparing the sequence of the MDBK clone with sequence data from the human genome database, we have determined that this cDNA represents a central portion of a very large gene (∼500 kb), encoding an ∼25-kb transcript that we refer to as Syne-1. Syne-1 encodes a large polypeptide (8406 amino acids) with multiple spectrin repeats and a region at its amino terminus with high homology to the actin binding domains of conventional spectrins. Golgi localization for this spectrin-like protein was demonstrated by expression of epitope-tagged fragments in MDBK and COS cells, identifying two distinct Golgi binding sites, and by immunofluorescence microscopy by using several different antibody preparations. One of the Golgi binding domains on Syne-1 acts as a dominant negative inhibitor that alters the structure of the Golgi complex, which collapses into a condensed structure near the centrosome in transfected epithelial cells. We conclude that the Syne-1 gene is expressed in a variety of forms that are multifunctional and are capable of functioning at both the Golgi and the nuclear envelope, perhaps linking the two organelles during muscle differentiation.
INTRODUCTION
The spectrin superfamily is comprised of a variety of functionally and structurally similar cytoskeletal proteins that include several of isoforms of spectrin (Bennett, 1990), dystrophin (Koenig et al., 1988), utrophin (Blake et al., 1996), and α-actinin (Davison et al., 1989). All of these proteins are functionally similar in that they are involved in the linkage of membranes with cytoplasmic structures, thereby providing structural support for the cell surface and, in some cases, the formation of distinct membrane domains. The structural similarity of these proteins lies in the conservation of two distinct structural motifs: 1) a highly conserved amino terminal actin binding site (Byers et al., 1989), which facilitates cytoskeletal assembly by linking spectrin family members to actin filaments; and 2) an ∼106 amino acid spectrin repeat domain, present in multiple copies (Davison et al., 1989; Koenig and Kunkel, 1990; Pascual et al., 1997), which dictates the elongated shape of these proteins. Aside from these two conserved motifs, however, spectrin superfamily members are otherwise structurally diverse, and this diversity likely forms the basis for differences in function.
Functional diversity of spectrin superfamily members is exemplified by differential tissue and subcellular distributions of these proteins. Tissue-specific isoforms of spectrin and dystrophin are found in variety of tissue types, with major isoforms in muscle (dystrophin/spectrin;Bonilla et al., 1988;Bloch and Morrow, 1989), brain (dystrophin/spectrin; Lazarides and Nelson, 1983; Lidov et al., 1990), blood cells (spectrin;Bennett, 1990), and epithelial tissues (spectrin; Nelson and Veshnock, 1987; Nelson and Hammerton, 1989). Although differential tissue distribution of superfamily members implies distinct tissue-specific functions for these proteins, the fact that individual cells may possess multiple isoforms, each with their own distinct plasma membrane localizations, indicates an additional level of functional diversity (Lazarides and Nelson, 1983). Moreover, additional studies have revealed that spectrin isoforms localize to intracellular sites other than the plasma membrane, such as the Golgi complex (Beck et al.,1994,1997; Devarajan et al., 1996) and the nuclear envelope (Apel et al., 2000;Zang et al., 2001), an observation that implies an even greater potential spectrum of functions for the spectrin superfamily.
Evidence for the existence of a Golgi-localized membrane cytoskeleton has come from the identification of Golgi-specific isoforms of the membrane skeleton proteins spectrin and ankyrin (for review, seeBeck and Nelson, 1998). Initial studies involved the use of antibodies raised against the erythrocyte isoform of spectrin that were found to react with a Golgi-specific antigen in nonerythroid cells (Beck et al., 1994). In addition, a high molecular mass (271-kDa) variant of erythrocyte spectrin has been reported to localize to the Golgi complex (Stankewich et al., 1998). Two distinct isoforms of Golgi-specific ankyrins have also been identified. One of these, Ank195, is a 195-kDa Golgi-specific protein that cross-reacts with erythroid ankyrin-specific antibodies (Beck et al., 1997). The other, AnkG119, is a truncated form of the major brain ankyrin AnkG (Devarajan et al., 1996).
The existence of two distinct isoforms of Golgi ankyrin suggests the possibility of multiple forms of the Golgi membrane cytoskeleton, each perhaps facilitating a unique function. Therefore, to gain a complete understanding of Golgi membrane cytoskeletal functions, Golgi-specific isoforms of membrane skeleton proteins must first be definitively identified and characterized at the molecular level. To achieve this aim we have set out to clone a Golgi-specific isoform of spectrin by screening a λgt11 expression library with an erythrocyte spectrin-specific antibody (βSpec-1) that was previously shown to cross-react with a Golgi-specific antigen in a variety of nonerythroid cells. We chose this approach over the use of polymerase chain reaction (PCR) amplification because we recognized that the great sequence diversity of spectrin superfamily members would likely render the latter approach ineffective. This screen has resulted in the isolation of a partial 5-kb cDNA clone that expresses a polypeptide that interacts with the Golgi with the same characteristics as the Golgi-specific spectrin previously identified with the βSpec-1 antiserum. A blast search revealed that the MDBK clone is a bovine homolog of spectrin-related protein Syne-1B/Nesprin-1β (Apel et al., 2000; Zang et al., 2001), which has previously been reported to bind to the acetylcholine receptor and localize to the nuclear envelope. We further show that Syne-1B/Nesprin-1β represents an alternative transcript of an ∼25-kb parent transcript. Expression of dominant negative inhibitory fragments of Syne-1 results in altered Golgi morphology, suggesting that one of the functions of this novel spectrin family member is to maintain the structural organization and cytoplasmic distribution of the Golgi complex.
MATERIALS AND METHODS
Cell Culture, Cell Extracts, and Immunofluorescence Microscopy
Madin-Darby canine kidney (MDCK strain J;Mays et al., 1995), human embryonic kidney (HEK) cells (293), Madin-Darby bovine kidney (MDBK), COS-1, bovine kidney epithelial cells and LB10 cells were grown in high-glucose Dulbecco's modified Eagle's (DME) medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA). Cultures were maintained at 37°C with 5% CO2 in air. For aluminum fluoride pretreatment experiments, washed cells were pretreated with 30 mM sodium fluoride and either 50 or 250 μM aluminum chloride for 20 min at 37°C and then incubated with 5 μM brefeldin A (BFA) for an additional 10 min. 293 cell extracts were prepared from confluent cultures grown on 10-cm2 dishes. Immunofluorescence staining and detergent extractions were performed as described previously (Beck et al., 1994,1997).
Library Screening
Library screening was performed using the 5′-Stretch cDNA expression system from BD Biosciences Clontech (Palo Alto, CA). This library was constructed from cDNAs, expressed as β-galactosidase fusion proteins in λgt11, derived from cultured MDBK cells. The screening procedure was performed according to the manufacturer's instructions (BD Biosciences Clontech). Positive plaques were excised, extracted, and the resulting phage stocks were subjected to two successive rounds of additional screening. A large-scale liquid culture of λgt11 clone 4 was prepared and DNA was isolated with Lambda kits (QIAGEN, Santa Clara, CA) as described by the manufacturer. The insert was excised by digesting with _Eco_RI (Promega, Madison, WI) and subcloned into Bluescript (Stratagene, La Jolla, CA) for sequencing.
To identify additional 5′ sequences of human Syne-1, we performed 5′-rapid amplification of cDNA ends (5′-RACE) with primers complementary to the 5′ end of the human sequence for the partial bovine clone. Human sequences corresponding to this region were derived from the human clone KIAA1262, which overlapped with the 5′ end of the bovine cDNA. 5′-RACE PCR reactions were performed using the SMART race cDNA amplification system (BD Biosciences Clonetech), according to the manufacturer's instructions. Typically, nested primers were used. The first round of PCR was performed with the 3′-most primer and the product was diluted 1:50 and used as template for a second round of amplification by using the 5′-most primer. PCR products were then subcloned using the TOPO TA cloning system (Invitrogen) and sequenced. The resulting sequences where then submitted to the Human Genome Database at the National Center for Biotechnology Information. The submitted sequences were found to be identical to predicted exons residing within contigs of genomic DNA from chromosome 11. The bovine Syne-1 cDNA was also found to be homologous with portions of these contigs. Additional 5′ sequence was then identified by predicting exons within the entire genomic contig that were upstream of the exons corresponding to the RACE products. The sequences of these predicted 5′ exons were then used to generate new PCR primers and the process was repeated.
Sequencing and Sequence Analysis
Automated DNA sequencing was performed using an ABI Prism 377 DNA sequencer (Applied Biosystems, Foster City, CA). Multiple sequence alignments were made using the CLUSTAL W program. Spectrin repeats were originally identified with the ProfileScan Server of the Swiss Institute for Bioinformatics (http://www.isrec.isb-sib.ch/software/PFSCAN_form.html). This server uses “pfscan” program to search protein sequences against database of profiles.
Antibodies
The βspec-1 and Eank-2 antisera, which react with Golgi antigens in MDCK and MDBK cells (Beck et al.,1994,1997), were raised against canine erythrocyte spectrin and ankyrin as described previously (Beck et al., 1994). The following antibodies were purchased: mouse monoclonal anti-Golgi p58 (Sigma-Aldrich, St. Louis, MO), mouse monoclonal anti-βCOP (Sigma-Aldrich), mouse monoclonal anti-HA epitope tag (Babco, Richmond, CA), and mouse monoclonal anti-clathrin adapter γAP-1 (Sigma-Aldrich). Antibodies were raised against a bacterially expressed glutathione_S_-transferase (GST) fusion protein composed of amino acids 1–469 of the bovine clone. To construct this fusion protein, nucleotides 368–839 of MDBK clone 4 were amplified by PCR by using forward and reverse primers complementary to the appropriate flanking sequences. The forward primer was modified by adding a _Xho_I site to the 5′ end. To the reverse primer we added a stop codon followed by a _Not_I site. The amplified PCR product was purified, digested with _Xho_I and_Not_I, and ligated into a modified pGEX-3 ×-M1 (kindly provided by Dr. Yih-Tai Chen, Stanford University, Stanford, CA) previously digested with _Xho_I and _Not_I (Promega). The resulting construct encoded a fusion protein containing an amino-terminal GST in frame with the GS1.5 region of MDBK clone 4. The protein was expressed in Escherichia coli strain XL1-blue. Peptide antibodies were prepared against the following peptides: EAKASSPEMDISADC (SN357-1 and SN357-2) and EESGEEGTNSEISSC (SN119 and SN120). Peptide synthesis, conjugation to KLH, and antibody production was performed at SynPep (Dublin, CA).
Ectopic Expression of Syne-1 Fragments
For ectopic expression of various regions of Syne-1, epitope-tagged constructs were prepared using the identical strategy described above for construction of GST fusion proteins. PCR products digested with _Xho_I and _Not_I where ligated with a modified pCDM8 (Invitrogen) previously digested with _Not_I and _Xho_I. The modified pCDM8 (kindly provided by Dr. Yih-Tai Chen, Stanford University) encodes a 5′ epitope tag derived from the influenza hemagglutinin protein (Chen et al., 1993). The resulting constructs encoded fusion proteins containing an amino-terminal HA epitope tag in frame with various bSyne-1 segments. For transient transfections with constructs subcloned into pCDM8, 0.5 μg of DNA was mixed with 100 μl of serum-free DME (Invitrogen) containing 5 μl of LipofectAMINE reagent (Roche Diagnostics, Indianapolis, IN). After incubation for 45 min at 22°C, 800 μl of serum-free DME was added to bring the total volume to 1 ml. This mixture was added to MDBK cells plated on collagen-coated cover slips or 293 cells grown on plastic culture dishes. After a 6-h incubation at 37°C, the cells were washed in serum-free DME and incubated at 37°C for 36–48 h in DME containing 5% fetal bovine serum.
RESULTS
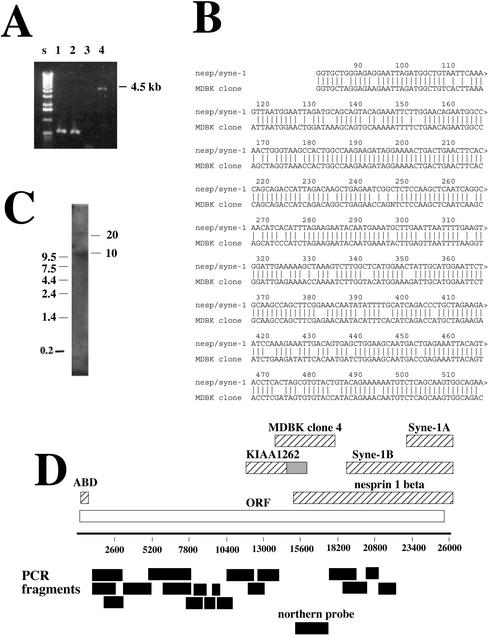
A screen of 1.2 × 106 possible clones from the MDBK library by using the βspec-1 antiserum resulted in the isolation of four positive clones. Their sizes were determined by PCR amplification of lambda DNA isolates (Figure 1A). Only one of these clones, clone 4 (Figure 1A), was large enough (5 kb) to be considered a candidate spectrin homolog. Sequencing of clone 4 identified a single uninterrupted open reading frame of 1594 amino acids. Because the open reading frame started immediately at the 5′ end of the cDNA and extended throughout the length of the clone we concluded that the 4.5-kb lambda clone 4 represented a partial cDNA missing additional 5′ and 3′ sequences.
Figure 1.
Cloning of a Golgi-specific spectrin family member. A λgt11 expression library constructed from MDBK cDNA was screened with the antiserum βSpec-1. (A) λ DNA was isolated from four positive clones and PCR amplification of the insert (MDBK cDNA) was performed with primers complementary to λ genome sequences flanking the insert site. Only one clone was large enough to be considered a spectrin (clone 4, 4.5 kb, lane 4). (B) Sequence alignment of a portion of MDBK clone 4 with the 5′ end of Syne-1B/Nesprin-1β. The homology begins at nucleotide 81 of Syne-1B/Nesprin-1β. (C) A Northern blot of poly-A+ MDBK RNA with a probe derived from clone 4 (see D for the region used to construct the probe) revealed two transcripts of ∼10 and ∼25 kb. (D) A schematic of the predicted full-length cDNA for Syne-1, including regions homologous to the MDBK clone isolated by antibody screening. KIAA1262, including its untranslated region (gray bar), is shown. Also shown are the full-length Nesprin-1β, Syne-1A, the N-terminal actin binding domain (ABD), and the partial sequence initially reported for Syne-1B. To determine whether MDBK cells do indeed express this full-length sequence, RT-PCR was performed on HEK-293 cell extracts by using primer pairs that defined several overlapping segments of the clone (black bars). All RT-PCR reactions generated single reaction products of correct sizes (our unpublished data).
A blast search of the sequence database revealed significant homology of the MDBK cDNA with two known cDNAs: KIAA1262, a partial cDNA of a human gene with unknown function that overlapped with the 5′ end of MDBK clone 4; and Syne-1B/Nesprin-1β, a novel spectrin family member previously shown to associate with the nuclear envelope (Apel et al., 2000;Zang et al., 2001). Sequence comparison of the bovine clone with both of these cDNAs revealed a high level of homology at both the nucleotide (85%,Figure 1B) and amino acid level (Table 1), indicating that the MDBK clone represents the bovine homolog of KIAA1262 and Syne-1/Nesprin-1β. As was reported for Syne-1/Nesprin-1β (Apel et al., 2000;Zang et al., 2001), the MDBK cDNA (referred to herein as bSyne-1, GenBank accession numberAF525163) showed a low but detectable homology with conventional spectrin as well as other spectrin family members (Table 1). Sequence comparisons revealed that bSyne-1 is as distantly related to erythroid α-spectrin as it is to dystrophin, the spectrin family member having the lowest overall homology to α-spectrin (17% identity and 39% homology). However, bSyne-1 is no more closely related to dystrophin than it is to any other spectrin family member (17% identity and 38% homology;Table 1), suggesting that it is not a dystrophin isoform. It also possesses very low overall sequence homology with a previously identified Golgi-specific β-spectrin (Stankewich et al., 1998). In contrast, the Golgi β-spectrin is highly homologous to erythroid β-spectrin (62% identity and 77% homology), whereas bSyne-1 shows the same low level of homology with erythroid β-spectrin as is does with α-spectrin (Table 1). Analysis of the primary amino acid sequence of bSyne-1 therefore indicates that although the clone was isolated by cross-reactivity with a spectrin-specific antibody, it is only distantly related to known spectrin family members and likely represents a novel spectrin superfamily member.
Table 1.
MDBK clone 4 homology with spectrin superfamily members
| α-Spectrin (α1Σ1) homology % identity (% similarity) | MDBK clone 4 homology % identity (% similarity) | |
|---|---|---|
| α-Spectrin (α1Σ1)a | 100 (100) | 17 (37) |
| α-Spectrin (α2Σ1)b | 55 (72) | 17 (37) |
| α-Actinin | 31 (53) | 20 (39) |
| β-Spectrin | 26 (47) | 17 (38) |
| Golgi β-spectrin | 25 (44) | 17 (37) |
| Dystrophin | 17 (39) | 17 (39) |
| MDBK clone 4 | 17 (37) | 100 (100) |
| Syne-1 | 17 (37) | 85 (90) |
Syne-1/Nesprin-1β has been show to be expressed in multiple forms (Apel et al., 2000;Zang et al., 2001). Syne-1B/Nesprin-1β is expressed as an ∼10-kb transcript with broad tissue distribution (Apel et al., 2000; Zang et al., 2001), whereas Syne-1A/Nesprin-1β is a muscle specific 4.5-kb transcript that corresponds to a C-terminal portion of Syne-1B (Apel et al., 2000;Figure 1D). As mentioned above, the MDBK clone 4 cDNA encodes a single open reading frame that overlaps with the 5′ end of Nesprin-1β, implying that either the proposed transcription start site for Nesprin-1β is inaccurate or that Nesprin-1β actually represents a truncated alternate transcript of a larger parent molecule. To examine the expression of bSyne-1 in kidney epithelial cells, we performed a Northern blot of MDBK cDNA with a probe derived from the MDBK clone 4 sequence (Figure 1D). As was previously reported for Syne-1B/Nesprin-1β (Apel_et al._, 2000; Zang_et al._, 2001), a prominent ∼10-kb transcript was detected (Figure 1C). We also detected a less abundant ∼25-kb species, indicating the existence of an additional, previously unidentified transcript of the Syne-1 gene.
The identification of a 25-kb Syne-1 transcript, together with the fact that the original bovine cDNA was a continuous open reading frame lacking a 5′ start site, necessitated a search for upstream sequences. To accomplish this, we used an approach that used a combination of 5′-RACE and comparison of the resulting new 5′ sequences to the human genome database (see MATERIALS AND METHODS). This allowed us to “walk” along the sequence in the 5′ direction. Using this approach we detected an additional 15 kb of potential mRNA sequence upstream of the original MDBK clone (Figure 1D). The final 5′-RACE experiment gave rise to a sequence encoding a putative 5′ untranslated region with no significant open reading frame. The coding region initiates with a Kozak consensus site. Importantly, just downstream from this putative start site we identified a region with high homology to the actin binding domain found in many spectrin-like proteins (Figure 2). Because actin binding domains are located at the extreme amino-termini of all known spectrin family members, this result confirmed that we had identified the 5′ end of Syne-1. Using human genome sequence data, we constructed a hypothetical cDNA that was 26,089 nucleotides in length and encoded a polypeptide of 8406 amino acids (Figure 1D). To verify that the predicted transcript for human Syne-1 (hSyne-1, GenBank accession number BK000543) is expressed in intact cells, we performed reverse transcription-polymerase chain reaction (RT-PCR) with HEK-293 cell cDNA by using primers designed to detect overlapping fragments of hSyne-1. Additionally, each of these PCR products was subjected to one round of DNA sequencing to demonstrate that the appropriate sequence was amplified (data not shown). In all cases, the primer pairs generated fragments of the appropriate size (Figure 1D).
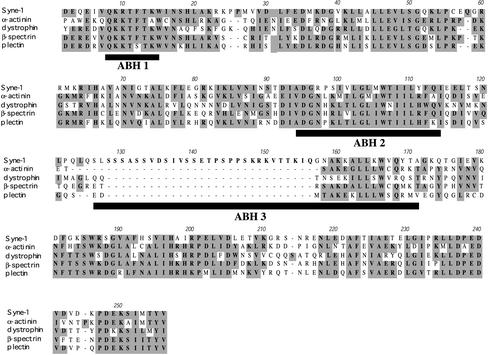
Figure 2.
The Syne-1 actin binding domain. A 257 amino acid region of the sequence of hSyne-1 near its amino terminus is aligned with the actin binding domains of human erythroid β-spectrin and three other spectrin-related proteins: α-actinin, dystrophin, and plectrin. Conserved amino acids are highlighted. Note how this region of hSyne-1 possesses high homology with the actin binding domains of the other spectrin family members. Also shown are the positions of the three α-helical segments of the utrophin actin-binding domain, which have been shown to interact directly with actin (black bars, ABH 1, ABH 2, and ABH 3). The putative Syne-1 actin binding domain differs from other actin binding domains in that it contains a unique 28 amino acid proline and serine-rich insert at the start of the third actin binding helix (ABH 3)
All spectrin family members are related structurally by the presence of a conserved amino-terminal actin binding domain (Byers et al., 1989), as well as multiple, sequential copies of a 106 amino acid spectrin repeat domain (Koenig et al., 1988; Pascual et al., 1997). Therefore, to find additional evidence that the sequences predicted from human genome data were contiguous with our MDBK clone, we examined the amino acid sequence of these upstream segments to identify spectrin-like domains. As mentioned above, at the extreme amino terminus of hSyne-1 we detected an ∼250 amino acid region with extensive homology to a spectrin-actin binding domain (Figure 2). Throughout this region we found good correspondence between the amino acid sequence of hSyne-1 with that of other spectrins. If fact, the homology within this region was much greater than the overall homology between Syne-1 and other family members. However, the putative actin binding domain of hSyne-1 differed from that of the other spectrins in that it contained a serine- and proline-rich insert (Figure 2). Importantly, this insert lies within one of the three helical segments of the utrophin actin binding domain that have been shown to associate directly with actin. This suggests that Syne-1 may have altered actin binding activity. When the hSyne-1 amino acid sequence was submitted as a query to the ProfileScan Server of the Swiss Institute for Experimental Cancer Research (see MATERIALS AND METHODS), numerous spectrin repeat domains were detected along the entire length of the molecule (our unpublished data). Importantly, spectrin repeats were found not only in the regions corresponding to Syne-1A and B, as has been reported previously (Apel et al., 2000;Zang et al., 2001), but they were also present within the MDBK clone 4 sequence as well as the 5′ upstream sequences predicted from human genome data (our unpublished data). This confirms that all of these sequences are derived from the same gene. Because all of these features of the hSyne-1 sequence upstream of our original bovine clone are consistent with a spectrin-like protein, and because RT-PCR results show overlapping identity with the original sequence, we conclude that the upstream sequence identified by our 5′-RACE approach is indeed contiguous with MDBK clone 4.
Functional Analysis of Syne-1
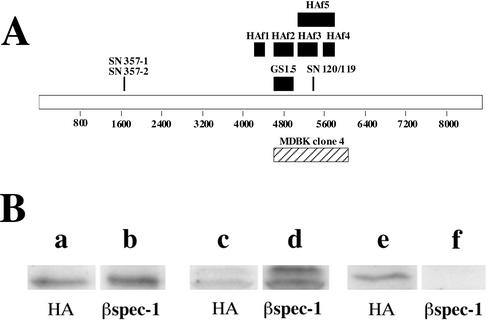
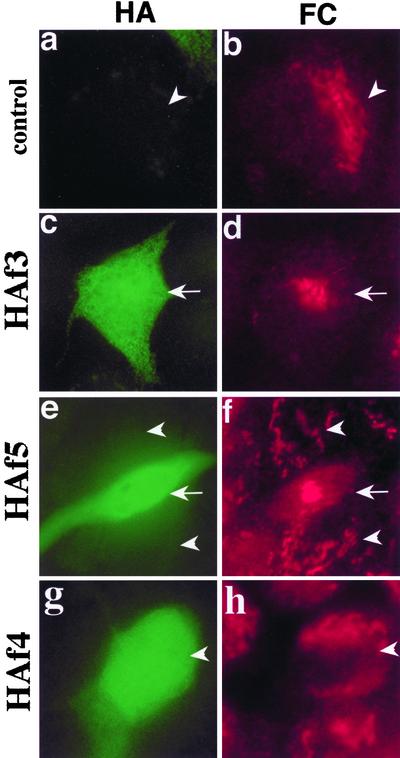
Because the initial screen of the λgt11 library was performed with an antibody raised against erythrocyte β-spectrin, we were surprised by the low level of sequence homology of the isolated cDNA with spectrin. To verify that the antibody used in the initial screening does indeed react with the bSyne-1 protein, we expressed three epitope-tagged fragments of bSyne-1 (HAf3, HAf4, and HAf5; Figure 3A) in 293 cells and analyzed extracts of these cells by PAGE and immunoblotting (Figure 3B). The electrophoretic mobilities of these three fragments were determined by immunoblotting with an epitope-tag specific antibody (Figure 3B, a, c, and e). On immunoblotting with the βspec-1 antibody, we found that two of these fragments, HAf5 and the N-terminal portion of HAf5, HAf3 (Figure 3B, d and e), cross-reacted with the spectrin-specific antiserum. In contrast, HAf4, the c-terminal half of HAf5, did not react with βspec-1 (Figure 3B, f). It should be noted that because of relatively low transformation frequency, neither of the ectopically expressed fragments could be detected in these extracts by general protein stain (Coomassie Blue and Ponceau S; our unpublished data), indicating that the reactivity with the βspec-1 antibody was not due to nonspecific binding to an exceptionally abundant protein. Furthermore, the specificity of the βspec-1 antibody is also demonstrated by the absence of reactivity with HAf4, which was expressed at levels roughly equivalent to the other two fragments. These results indicate that despite the low-level homology with spectrin family members the MDBK protein does indeed react with the spectrin-specific antibody.
Figure 3.
Erythroid spectrin-specific antibodies react with ectopically expressed Syne-1. (A) A schematic map of the amino acid sequence of the predicted Syne-1 gene illustrating the location of MDBK clone 4 (hatched bar). Also shown are the positions of the various epitope-tagged fragments of Syne-1 (HAf 1–5, black bars) used in these studies, as well as the positions of the peptides (SN357-1, SN357-2, SN-120, and SN119) and fusion protein (GS1.5) used to raise antibodies to Syne-1. (B) Epitope tagged (influenza HA tags) constructs encoding various regions of Syne-1 were expressed in HEK-293 cells. Cell extracts were prepared and analyzed by SDS-PAGE and immunoblotting with either the epitope tag specific antibody (HA, lanes a, c, and e) or the erythroid β-spectrin antibody (βspec-1, lanes b, d, and f) originally used to clone MDBK clone 4: lanes a and b, cells expressing HAf3; lanes c and d, cells expressing HAf5; lanes e and f, cells expressing HAf4.
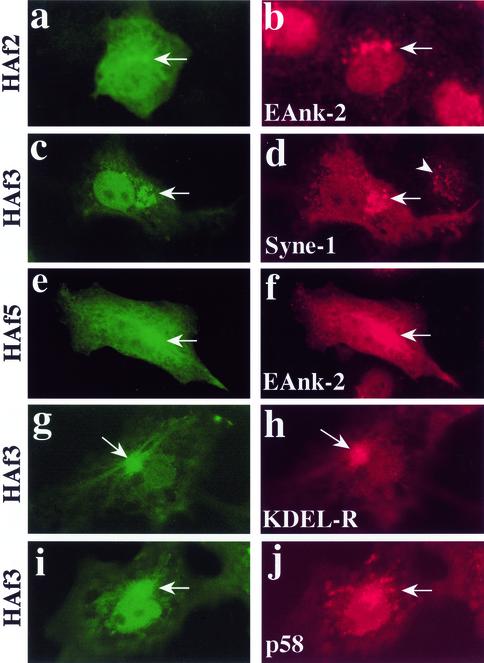
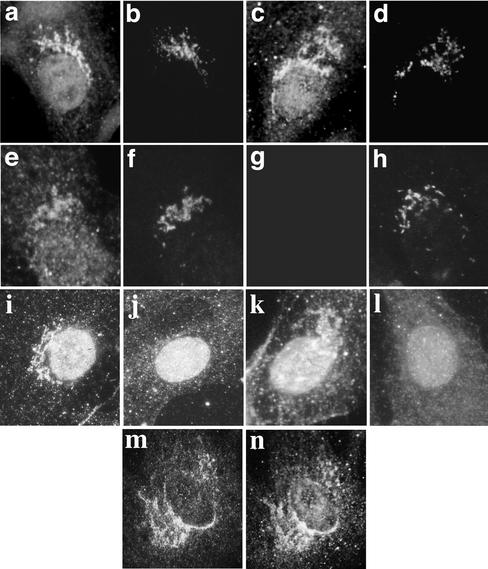
To establish whether bSyne-1 is a Golgi resident protein, we first expressed recombinant fragments of bSyne-1 in intact MDCK cells and human LB10 epithelial cells and determined their subcellular distributions by indirect immunofluorescence microscopy (Figure 4). Recombinant fragments (HAf1-5,Figure 3A) were epitope tagged with eight amino acids derived from the influenza HA protein. Transient transfections of MDCK cells were performed and the localization of recombinant proteins was determined by staining with HA-specific antibodies (Figure 4). Golgi localization was observed with HAf2 (Figure 4a), HAf3 (Figure 4, c, g, and i), and HAf5 (Figure 4e). Note that HAf5 contains the sequence of HAf3 within it (Figure 3). With all of these fragments, we observed distinct localization to perinuclear Golgi structures (Figure 4, arrows) that costained with a variety of Golgi markers, including a Golgi-specific ankyrin antibody EAnk-2 (Figure 4, b and f), KDEL receptor (Figure 4h), and the cis Golgi/intermediate compartment marker p58 (Figure 4j). In addition to the Golgi localized signal, we also observed diffuse cytoplasmic localization with these fragments, consistent with a cytoplasmic pool of protein in equilibrium with a Golgi bound fraction. Somewhat less obvious Golgi localization was observed with the HAf2 fragment (Figure 4a) than with HAf3. Typically, Golgi-specific staining was more difficult to detect with HAf2 fragment due to higher cytoplasmic staining compared with that observed with HAf3, suggesting that the HAf2 fragment binds Golgi with a lower affinity. Golgi localization was not observed with HAf4, which gave a diffuse cytoplasmic signal, or HAf1, which localized to discrete cytoplasmic puncta that did not correspond to any known organelle (our unpublished data). These results demonstrate a capacity for Syne-1 to bind and localize to the Golgi complex. Furthermore, they also identify at least two distinct Golgi binding sites on Syne-1, a low-affinity site in the vicinity of amino acids 4606–4945, and a high-affinity site within amino acids 5015–5410. It should be noted that we did not observe nuclear envelope localization with any of the fragments tested.
Figure 4.
Ectopically expressed fragments of Syne-1 identify Golgi binding determinants. To demonstrate Golgi localization for Syne-1, we expressed epitope-tagged fragments HAf2 (a and b), HAf3 (c, d, g, h, i, and j) and HAf5 (e–f) in MDCK (a–f) or COS-1 (g–j) cells and localized them by indirect immunofluorescence microscopy. Twenty-four to 48 h after transfection, cells where double stained with the HA epitope tag antibody (a, c, e, g, and i) and with antibodies to a Golgi-specific ankyrin (Eank-2, b and f), Syne-1 (d), the _cis_-Golgi marker KDEL receptor (KDEL-R, h) or the Golgi intermediate compartment marker p58 (j). HAf2, HAf3, and HAf5 were prominently localized to perinuclear Golgi structures (arrows) which were stained with Golgi marker antibodies. When the anti-Syne-1 antibody SN120 was used to stain cells transfected with HAf3, transfected cells (d, arrow) showed a higher reactivity to the antibody than untransfected cells (d, arrowhead), confirming that the epitope for this antibody is present in HAf3.
As an alternative approach to demonstrate Golgi localization for Syne-1, we prepared a polyclonal antiserum, GS1.5, to a recombinant bSyne-1 fragment that corresponds to the HAf2 region (Figure 3A). We also prepared antibodies to two different peptides derived from the Syne-1 sequence (Figure 3A, SN 357, SN119, and SN120). When these antibodies were used to stain MDBK and LB10 cells by indirect immunofluorescence (Figure 5), we found that the predominant feature of the resulting staining patterns were reticular, perinuclear Golgi structures. These immunoreactive structures costained with antibodies to two markers of the Golgi complex, p58 (Figure 5, b and n) and the Golgi-specific coat protein β-COP (Figure 5d). To test the specificity of the GS1.5 antiserum, we affinity purified the antibody by using two different affinity columns. The first was composed of recombinant HAf2, the antigen used to prepare the antiserum. Material bound to this column was eluted and tested for Golgi staining activity (Figure 5e). As expected, Golgi staining was observed with antibody affinity purified against the original antigen. In contrast, Golgi-specific immunoreactivity was not observed with the eluate from an affinity column composed of purified erythrocyte spectrin (Figure 5g), indicating that the GS1.5 antiserum is specific for bSyne-1 and does not cross-react with erythroid spectrin. Thus, the ability of the GS1.5 antiserum to stain the Golgi complex correlates only with its ability to bind bSyne-1. Golgi staining observed with peptide antibodies SN120 and SN119 was also specific because it could be blocked by preabsorption with peptide (Figure 5, j and l). Furthermore, it is worth noting that the SN120 antibody was raised against a peptide that resides within the HAf3 fragment (Figure 3A) and that when we transfected MDBK cells with HAf3 and stained them with SN120 we observed increased intensity of both cytoplasmic and Golgi-specific staining in transfected cells (Figure 4d, arrow) verses nontransfected cells (Figure 4d, arrowhead). This indicates that the SN120 does indeed react with the antigen to which it was raised, even when that antigen was presented in fixed cells prepared for immunofluorescence microscopy. Finally, specific Golgi staining was not only observed with antibodies raised against epitopes that resided within the original MDBK clone 4. We also observed specific Golgi staining with two separate antibodies (SN357-1, our unpublished data; and SN357-2, Figure 5m) raised against a peptide derived from a site far upstream from the MDBK clone 4 homology region (Figure 3A).
Figure 5.
Antibodies specific for Syne-1 stain the Golgi complex. To confirm that Syne-1 is a Golgi localized protein, peptide antibodies raised against two peptides derived from Syne-1 (EESGEEGTNSEIS for antibodies SN120 and SN119; i and k respectively; and EAKASSPEMDISAD for antibody SN357-2, m), as well as an antibody to a bacterially expressed fragment of Syne-1 (GS1.5; a, c, e, and g) were prepared and used to localize Syne-1 by indirect immunofluorescence microscopy. MDBK (a–l) or LB10 (m and n) cells were fixed and double stained with antibodies specific for Syne-1 (a, c, e, i, k, and m) and the Golgi markers p58 (b, f, h, and n) or β-COP (d). The Syne-1 antibodies prominently stained reticular, perinuclear structures that costained with Golgi markers. The specificity of the GS1.5 antiserum was evaluated by affinity purification on either immobilized erythrocyte spectrin (spectrin-Sepharose; g) or immobilized Syne-1 fragment (GS1.5-Sepharose; e). Antibodies eluted from these affinity resins were tested for reactivity with a Golgi antigen by indirect immunofluorescence (e and g). Double staining with the Golgi marker p58 (f and h) is also shown. Although a Golgi-reactive antibody was eluted from the GS1.5 resin (e), specific Golgi staining was not detected when the GS1.5 antibody was affinity purified on spectrin-Sepharose (g). To test the specificity of the peptide antibodies SN120 and 119, these antibodies were preadsorbed with peptide before staining (j and l). Preadsorbtion specifically blocked the ability of these antibodies to stain perinuclear Golgi structures. Note that nuclear staining persists after preadsorbtion (j and l). Nuclear staining was not observed with peptide antibody SN357-2, which reacted predominantly with perinuclear Golgi structures (m) that costained with p58 (n)
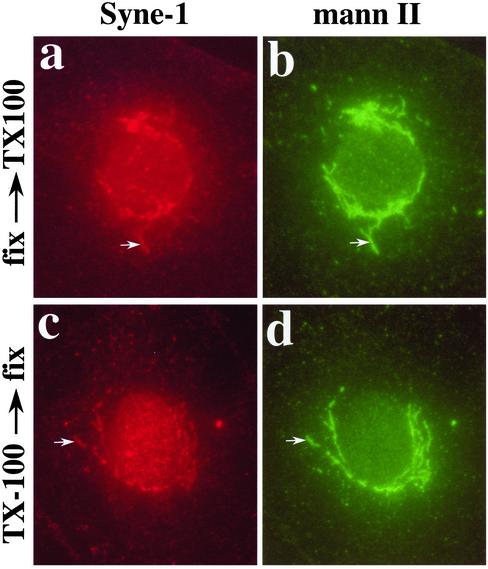
The antibody used to identify bSyne-1, βspec-1, had been previously shown to react with a Golgi-specific spectrin isoform (Beck et al., 1994). Having established that Syne-1 is a Golgi-localized protein structurally similar to spectrin at the level of primary sequence data, we next set out to determine whether Syne-1 shares functional properties with the Golgi-specific spectrin previously identified with the βspec-1 antiserum. First, it had previously been shown that Golgi spectrin, identified with βspec-1, associates with the Golgi complex in the form of a detergent insoluble structure that was referred to as a Golgi ghost (Beck et al., 1997). To localize Syne-1 to a detergent-insoluble Golgi ghost, we extracted cells in Triton X-100 before fixation and examined the distribution of Syne-1 by immunofluorescence microscopy (Figure 6). We have previously shown that although the extraction conditions used in these experiments are sufficient to fully extract proteins and lipids from the Golgi, both Golgi spectrin and ankyrin remain associated with a cytoplasmic structure that is morphologically identical to the Golgi complex (Beck et al., 1997). When we examined the distribution of Syne-1 in cells extracted under identical conditions, we found that the protein remained localized to a cytoplasmic structure (Figure 6c) that costained with the Golgi marker mannosidase II (Figure 6d) and was morphologically equivalent to the Golgi of nonextracted cells (Figure 6, a and b).
Figure 6.
Localization of Syne-1 to a detergent-insoluble Golgi ghost. We have shown previously that Golgi spectrin and Golgi ankyrin localize to a detergent-insoluble structure that maintains the basic morphology of the Golgi complex. We refer to these structures as detergent-insoluble Golgi ghosts. To determine whether Syne-1 also localizes to Golgi ghosts, normal rat kidney (NRK) cells were extracted with Triton X-100 (0.5%, 10 min, 20°C) either before (c and d) or after fixation (a and b). Cells were then double stained with GS1.5 antibody (a and c) and antimannosidase II, which we have previously shown to act as a detergent-resistant Golgi marker (Beck et al., 1997). The extraction conditions used have been previously shown to be sufficient to extract ceramide-labeled lipid in the _trans_-Golgi, unassembled cytoplasmic tubulin, as well as the integral membrane protein TGN 38 (Beck et al., 1997). The GS1.5 antiserum was able to stain Golgi ghosts in extracted cells (c) that colocalized with mannosidase II and was virtually identical in morphology to the Golgi of control cells (a and b) extracted after fixation.
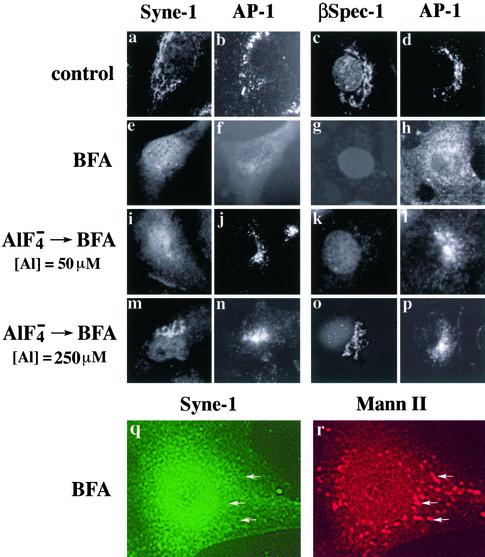
Previous studies have also shown that the Golgi-specific spectrin isoform identified with βspec-1 (which we refer to here as Golgi spectrin) associates with Golgi membranes in a BFA-sensitive manner (Beck et al., 1994,1997). Like Golgi spectrin, bSyne-1 rapidly dissociates from Golgi membranes after 10-min treatment with BFA (Figure 7, e and q), as revealed by a diffuse cytoplasmic staining (Figure 7e) and a lack of colocalization with the Golgi marker mannosidase II (Figure 7, q and r). BFA-induced dissociation of both Golgi spectrin and bSyne-1 is fully reversible upon washout of BFA (data not shown). Furthermore, the effects of BFA on the distributions of Golgi spectrin and the bSyne-1 are blocked by aluminum fluoride (Figure 7, o and m). This effect, which was also observed with other BFA-sensitive coat proteins such as AP-1 (Figure 7j; Wong and Brodsky, 1992), suggests a role for GTPases in the regulation of Golgi membrane skeleton assembly. Interestingly, the concentrations of aluminum fluoride required to affect the assembly of both bSyne-1 and Golgi spectrin (Figure 7, m and o) differed from those affecting the activity of AP-1 (Figure 7, j and l). A fivefold higher concentration of aluminum fluoride was required to protect Golgi spectrin and bSyne-1, suggesting that although Golgi spectrin and other coat proteins (i.e., clathrin and coatamer coats) may be regulated in a similar manner, the actual agents that facilitate this regulation are different. Importantly, in all cases the bSyne-1 behaved identically to the Golgi-localized spectrin originally identified with the βspec-1 antiserum, indicating that these two molecules are functionally equivalent.
Figure 7.
Syne-1 associates with the Golgi in a BFA and aluminum fluoride sensitive manner. MDBK cells were untreated (control, a–d) or treated with 5 μM BFA for 10 min (e–h, q, and r); aluminum fluoride (50 μM aluminum) for 20 min before treatment with BFA (i–l); or aluminum fluoride (250 μM aluminum) for 20 min before treatment with BFA (m–p). Fixed cells were double stained with antibodies to either Syne-1 (GS1.5; a, e, i, and m) and AP-1 (b, f, j, and n); Golgi spectrin (βspec-1; c, g, k, and o) and AP-1 (d, h, l, and p); or Syne-1 (SN120; q) and mannosidase II (r). When MDBK cells are treated with BFA for 10 min, a uniform cytoplasmic distribution of Syne-1, distinct from the Golgi pattern seen in control cells (a) was observed. Identical results were observed by staining with antibodies to erythroid β-spectrin and the Golgi-associated clathrin coat protein AP-1 (f and h). Under these conditions, cells double stained with antibodies to syne-1 and the Golgi marker mannosidase II revealed that Syne-1 no longer localized to Golgi membranes (q and r, arrows), as we have shown previously for the antigen recognized by the βspec-1 antiserun (Beck et al., 1997). Aluminum fluoride pretreatment blocked the effect of BFA on the distribution of Syne-1 and AP-1, but the effects of aluminum fluoride were concentration dependent. At 50 μM aluminum, BFA insensitivity was observed for the clathrin coat protein AP-1 (j and l) but not for Syne-1 (i) or the βspec-1–reactive epitope (k). However, at 250 μM aluminum fluoride both Syne-1 (m) and spectrin (o) were rendered insensitive to BFA.
A Role for Syne-1 in Maintaining Golgi Structure
As mentioned above, the recombinant Syne-1 fragment HAf3 localized to the Golgi (Figure 4). While performing these experiments, we noticed that the structure of the Golgi complex was often altered in cells expressing this fragment. This was evident only in MDBK cells, which possess a Golgi complex that is highly reticular in appearance and tends to form a crescent shape that follows the contour of the nucleus (Figure 5 a, c, and m). MDBK cells expressing HAf3 and HAf5 tended to have a more compacted Golgi (Figure 4, d and f). This altered Golgi morphology was more evident in cells expressing high levels of Golgi binding fragments of Syne-1 (Figure 8). Figure 8 shows several MDBK cells expressing the Syne-1 fragments HAf3, the Golgi binding fragment; HAf5, a larger fragment that contains the sequence encoding HAf3; and HAf 4, the C-terminal half of HAf4 that does not bind Golgi. We judged the expression levels of these fragments to be high in these experiments because the accumulation of cytoplasmic fragment obscured the Golgi-localized material. Significant compaction of the Golgi complex was observed in cells expressing Golgi binding Syne-1 fragments (Figure 8, d and f, arrows), whereas untransfected cells (Figure 7, b and f, arrowheads) and cells expressing HAf4 (Figure 8h), which does not bind Golgi, had Golgi complexes with normal crescent-shaped reticular morphologies. Compaction of the Golgi apparatus upon expression of HAF3 was also observed in C2C12 myoblasts but was not observed in COS-7 cells, which normally possess a more compacted Golgi complex (data not shown). We interpret these results as an indication that the Golgi binding fragments of Syme-1 act as dominant negative inhibitors of Syne-1 function at the Golgi, perhaps by competing with endogenous Syne-1 for Golgi binding sites. Furthermore, we conclude that the altered Golgi morphology observed upon expression of these fragments indicates that at least one function of Golgi-associated Syne-1 is to maintain Golgi structure and distribution. This effect is apparently cell type specific. Finally, the ability of Syne-1 fragments to alter Golgi structure also confirms our general conclusion that Syne-1 can reside and function at the Golgi.
Figure 8.
Ectopic expression of a Golgi binding fragments of Syne-1 alter the structure of the Golgi. MDBK cells were transiently transfected with epitope tagged Golgi binding fragments HAf3 (c and d), HAf5 (e and f), as well as the control fragment HAf4 (g and h). Transfected cells were stained with antibodies to HA (a, c, e, and g) and the TGN marker furin convertase (b, d, f, and h). In untransfected cells (a, b, e, and f, arrowheads) and cells transfected with the control fragment (g and h, arrowhead), the Golgi complex has a normal morphology comprised of reticular elements that surround portions of the nucleus. In cells transfected with the Golgi binding fragments HAf3 and HAf5 (c, d, e, and f, arrows) the Golgi complex collapsed into a compacted structure located near the center of the cell.
DISCUSSION
We have isolated a cDNA distantly related to spectrin by using an expression screening technique. The antibody used in our screen, βspec-1, was raised against canine erythrocyte β-spectin, and despite the low overall sequence homology of the isolated clone with other spectrin family members (Table 1), this antibody reacted with at least two distinct domains encoded by the MDBK clone (Figure 3B). Previous reports have shown that the βspec-1 antiserum reacts with a Golgi-localized protein in nonerythroid cells, including a variety of epithelial cell lines such as MDBK, the source of the cDNA used in our screen. Functionally, the MDBK clone isolated in this study behaves identically to the proposed Golgi-spectrin isoform originally identified with the βspec-1 antiserum. Like Golgi spectrin, MDBK clone 4 localizes to the Golgi complex, as indicated by two different approaches used in this study: ectopic expression of MDBK clone 4 cDNA fragments (Figure 4) and indirect immunofluorescence microscopy with four different antibody preparations (Figure 5). Syne-1 associates with Golgi membranes as a detergent-insoluble complex (Figure 6) and, like βspec-1–reactive epitope, its association with the Golgi is sensitive to BFA and aluminum fluoride treatment (Figure 7). Thus, in all of the functional tests performed herein, Syne-1 consistently behaved identically to the Golgi spectrin isoform identified with the βspec-1 antiserum, the same antiserum used to isolate the clone.
Additional evidence that Syne-1 can reside and function at the level of the Golgi complex comes from our observation that ectopic expression of Syne-1 fragments alters the morphology of the Golgi (Figure 8), suggesting that Syne-1 serves to maintain Golgi structural organization. This function is consistent with Syne-1 being a spectrin family member because it is well established that one of the functions of spectrin and spectrin-like proteins is to maintain the structural integrity of the plasma membrane. As for the mechanism whereby Golgi structure is perturbed by the Syne-1 fragments, it is interesting to note that the condensed Golgi complex that we have observed in transfected cells is remarkably similar to Golgi structure changes observed after perturbation of the actin cytoskeleton by treatment with cytochalasin D (Valderrama et al., 1998) or by expression of the transforming N-Ras mutant K61 (Babiá et al., 1999), suggesting that Syne-1 may exert its effects on Golgi structure by interacting with the actin cytoskeleton,. This is also consistent with Syne-1 being a spectrin family member with a conserved actin binding domain (Figure 2). Furthermore, it is well established that Golgi structural organization is dependent on microtubules and microtubule motor proteins such as kinesin and dynein (Thyberg and Moskalewski, 1985). We have shown previously that Golgi-specific ankyrin and spectrin are constituents of a detergent-insoluble Golgi ghost that is anchored in the cytoplasm to a drug and temperature stable population of microtubules (Beck et al., 1997). In this study, we show that Syne-1 also localizes to this structure (Figure 6), suggesting that Syne-1 could serve to affect Golgi structure by linking Golgi membranes to microtubules. In support of this, previous studies using the same β-spectrin antibody used herein to clone Syne-1, βspec-1, have shown that Golgi spectrin associates with the dynactin complex, a regulator of dynein function (Holleran et al., 1996). In addition, recent studies in our laboratory have identified a binding site on Syne-1 for the trimeric kinesin KIF3B (Fan and Beck, unpublished data), suggesting that Syne-1 could effect Golgi structure through both plus and minus end directed microtubule motors. Finally, it should be pointed out that the Syne-1–dependent Golgi structure changes that we have observed occurred in MDBK cells but not in COS cells, and that the condensed Golgi observed in transfected MDBK cells resembles the normal Golgi morphology observed in COS cells. This suggests that Syne-1 is involved in aspects of Golgi structure that may give rise to cell type-specific Golgi morphology.
A search of the protein sequence database identified a high level of homology (85%) between the MDBK cDNA and the 5′ end of a recently identified spectrin family member Syne-1B/Nesprin-1β. Syne-1B is an alternative transcript of a parent gene that was found to be expressed in at least two forms: a muscle specific form, Syne-1A, of 4.7 kb expressed in heart, skeletal muscle, and smooth muscle, and a larger ∼10-kb form, Syne-1B, that has a more general tissue distribution (Apel et al., 2000;Zang et al., 2001). We have performed multiple tissue Northern blots with probes derived from the MDBK clone 4 region of Syne-1 and observed tissue distributions identical to published distributions of the ∼10-kb Syne-1B/Nesprin-1β (our unpublished data). The observation that the MDBK clone isolated in this study encoded a continuous open reading frame that overlaps with the proposed 5′ end of Syne-1B/Nesprin-1β inferred the existence of a larger parent molecule that gives rise to both alternate transcripts. We determined the sequence of the putative precursor to Syne-1A and B by 5′-RACE PCR combined with comparison of the resulting sequences to the human genome database. This approach identified a very large (>500-kb) gene that is predicted to encode a transcript of ∼25 kb, with Syne-1B corresponding to the 3′ half of the transcript and the MDBK clone aligning roughly with the center of the molecule. We refer to this gene and its predicted product as Syne-1. Amino acid analysis identified additional spectrin repeats along the sequence extending upstream of Syne-1B, as well as a highly conserved actin binding domain at the amino terminus. While this paper was in review, two groups reported full-length sequences for Syne-1/Nesprin and related proteins in Drosophila and Caenorhabditis elegans that confirm the sequence reported here (Starr and Han, 2002 and Zhang et al., 2002)
An additional form of the Syne-1 molecule can be predicted by examining the sequence of a partial cDNA reported in the database as KIAA1262. This partial cDNA aligns with the 5′ end of MDBK clone 4 (Figure 1D). Importantly, KIAA1262 clearly represents the 3′ end of a transcript. It encodes an open reading frame that initiates at the first nucleotide but extends only to nucleotide 4089. Beyond this point, no significant open reading frame can be detected in the remaining 1500 nucleotides, indicating the presence of a 3′ untranslated region. The homology of KIAA1262 with the MDBK clone 4 begins at nucleotide 2248 and extends only to the end of the open reading frame. Examination of the predicted intron/exon structure of the Syne-1 gene reveals that the homology between KIAA1262 and the MDBK clone ends precisely at the 3′ end of a coding exon and that the putative untranslated region of KIAA1262 aligns with the adjacent downstream intron. These observations predict a fourth major form of Syne-1 that represents a premature truncation of the full-length Syne-1. We refer to this transcript as Syne-1C (Figure 9). Importantly, we have found herein that an antibody raised against a peptide derived from the putative Syne-1C region (SN357-2) also stains the Golgi (Figure 5m), suggesting that Syne-1C can reside and function at the level of the Golgi complex, although it is also possible that the Golgi staining observed with SN357-2 is due to Golgi-localized full-length Syne-1 and not Syne-1C. Thus, it seems that Syne-1 gene can be expressed in a variety of different forms, including a large full-length molecule (Syne-1), the N-terminal half alone (Syne-1C), the C-terminal half alone (Syne-1B), and a truncated muscle-specific C-terminal segment (Syne-1A). It is interesting to note that of the four forms of Syne-1, only the full-length Syne-1 and the predicted Syne-1C are expected to encode an N-terminal actin binding domain. Actin binding domains are found in all spectrin family members with the notable exception of α-spectrin, suggesting that isoforms of Syne-1 lacking actin binding sites could represent homologs of α-spectrin and serve to form heterodimers with actin binding isoforms. It is tempting to speculate that the C-terminal isoforms (Syne-1A and B) could interact with N-terminal isoforms in an anti-parallel manner, as do α- and β-spectrin. Perhaps these associations can occur as intermolecular dimmers of separate Syne-1A/B and Syne-1C chains or as an intramolecular association resulting from the folding of the full-length syne-1 in half. Such an arrangement could explain the complicated splicing of the Syne-1 gene.
Figure 9.
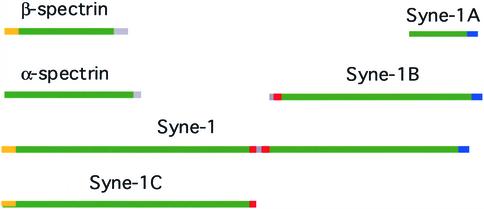
The major Syne-1 isoforms. A schematic representation of the domain structure of the major Syne-1 forms is compared with erythrocyte spectrin; spectrin repeat regions (green), actin binding domain (yellow), Golgi binding sites (red), and nuclear envelope binding (blue). Assuming that Syne-1 folds similarly to spectrin, this schematic illustrates the relative sizes of spectrin and the various Syne-1 isoforms.
The most obvious discrepancy between this study and previous reports is the subcellular localization of Syne-1. The nuclear envelope binding site for Syne-1A and Syne-1B has been localized to the extreme C termini of both molecules (Apel et al., 2000; Zang et al., 2001), whereas the two Golgi binding domains identified in this study are widely separated from nuclear envelope binding determinants (Figure 9), with both being found near the center of the full-length Syne-1 molecule, one at the N terminus of Syne-1B and the other at the C terminus of the putative Syne-1C (Figure 9). Syne-1B and the full-length Syne-1, having both Golgi and nuclear envelope binding sites, would be expected to interact with both organelles. However, because Syne-1A lacks the Golgi binding sites detected herein, we propose that it represents a nuclear envelope-specific form. One possible explanation for the discrepancy observed in this study versus other reports may lie in the specificity of the antibodies used. Previous studies showing nuclear envelope localization of Syne-1 were performed with antibodies that recognized the nuclear envelope binding Syne-1A (Apel et al., 2000; Zang et al., 2001). Because Syne-1A is the predominant isoform in muscle, and because Syne-1A contains a nuclear envelope binding site and not a Golgi binding site, it is not surprising that nuclear envelope staining predominates with this antibody. In contrast, our antibodies were raised to a region of Syne-1 that contained the Golgi binding domain. Because this domain is not present on Syne-1A, we can conclude that we are examining the distribution of other Syne-1 forms, perhaps including isoforms that do not associate with the nuclear envelope. Alternatively, as will be discussed below, the Golgi complex is known to maintain a close association with the nuclear envelope in muscle cells. In fact, at the level of the light microscope it may be difficult to distinguish Golgi and nuclear envelope staining muscle, indicating that that previous observations do not necessarily rule out Golgi localization in these cells. Other possible mechanisms that would allow Golgi localization to predominate over nuclear envelope localization in a given cell type would be the existence of isoforms that contain Golgi binding sites but no nuclear envelope binding sites, the masking of the nuclear envelope binding site by protein–protein interactions or secondary modification, or the absence of a receptor for Syne-1 on the nuclear envelope.
It is also possible that some cell types may require the activity of a molecule that can bind to both the Golgi and the nuclear envelope. For instance, there is evidence that the Golgi complex has special relationship with the nuclear envelope in muscle cells. In both cardiac and skeletal muscle, the Golgi has been found to form a uniform ring around the nucleus, a configuration that is distinct from the perinuclear, reticular Golgi found in nonmuscle cells (Tassin et al., 1985a; Kronebusch and Singer, 1987). At the ultrastructural level, the muscle cell Golgi apparatus is held at a uniform distance of ∼150–200 nm from the nuclear envelope (Tassin et al., 1985a), suggesting a specific physical connection between the two organelles. In addition, functional coupling of Golgi and nuclear envelope in muscle cells is indicated by the observation that the Golgi complex of muscle cells maintains a close association with ER exit sites located in discrete regions of the nuclear envelope (Lu et al., 2001). Finally, the observation that Golgi morphology in muscle cells is insensitive to nocodazole (Tassin et al., 1985b) suggests that mechanisms responsible for the unique cytoplasmic positioning of the muscle Golgi differ from those of other cell types. We propose that forms of Syne-1 that possess Golgi and nuclear envelope binding sites (Syne-1 and Syne-1B) would be excellent candidates for maintaining Golgi organization in muscle cells because they could serve to tether the Golgi to the nuclear envelope. Interestingly, Syne-1B is expected to be ∼1.5–2 times the size of conventional spectrin (Figure 9), provided it folds similarly. Because erythrocyte spectrin forms herterodimers of ∼100 nm, Syne-1B could form a similar rod-like structure of ∼150–200 nm, precisely the dimensions of the spacing observed between the Golgi and the nuclear envelope in muscle cells (Tassin et al., 1985). Support for such a role for Syne-1 in facilitating Golgi–nuclear envelope interactions comes from the observations that factors influencing Golgi distribution in muscle cells also effect the distribution of Syne-1. For instance, Golgi distribution in muscle is developmentally regulated. In myoblasts, the Golgi complex has a morphology and cytoplasmic distribution that is typical of fibroblasts and other epithelial cells. However, upon fusion to form myotubes, both the Golgi and centrosomes rearrange and acquire a close association with the nuclear envelope (Tassin et al., 1985a; Lu et al., 2001). Apel et al. (2000) found that Syne-1 localizes to cytoplasmic structures in undifferentiated myoblasts, whereas upon myoblast fusion, Syne-1 redistributes to the nuclear envelope. In muscle fibers, close association of the Golgi with the nuclear envelope is affected by muscle impulse activity (Ralston et al., 2001), and in some muscle fiber types the density of nuclear envelope associated Golgi elements is greater on nuclei associated with neuromuscular junctions (Ralston_et al._, 2001). Remarkably, studies examining localization of Syne-1 in intact muscle fibers have found that Syne-1 is more abundant in nuclei associated with neuromuscular synapses than in extrasynaptic nuclei (Apel et al., 2000).
Aside from serving to maintain Golgi structural organization and distribution in muscle cells, Syne-1 could also serve additional Golgi-specific functions in muscle and nonmuscle cell types. At the plasma membrane, spectrin family members are known to function in facilitating membrane association with the cytoskeleton, the promotion of general membrane stability, and the formation of discrete membrane domains. These same functions could significantly impact a variety of Golgi-specific functions, including regulation of membrane protein compositions of discrete Golgi compartments, the sorting and segregation of newly synthesized plasma membrane proteins within the _trans_-Golgi network (Beck and Nelson, 1998), and various membrane-cytoskeleton interactions such as microtubule based vesicular transport. These latter two proposals are particularly relevant to the fact that Syne-1 was initially identified in a two-hybrid screen for acetylcholine receptor binding proteins (Apel et al., 2000). Although it is yet unclear why a nuclear envelope protein would interact with a plasma membrane receptor, an interaction with a Golgi resident protein would have more obvious implications for our understanding of acetylcholine receptor function. Finally, with the view that Syne-1 could function in muscle cells to physically couple Golgi to the nuclear envelope, it is worth reconsidering our observation that expression of Golgi binding fragments of Syne-1 in MDBK kidney epithelial cells alters Golgi structure (Figure 7). In these studies, we found that the Golgi complex, which is normally a crescent-shaped reticular structure that follows the contour of the nucleus, adopts a compacted morphology in cells expressing dominant-negative Syne-1 fragments, as if the Golgi had been pealed away from the nuclear envelope and collapsed around the centrosome. This observation suggests that even in kidney cells Syne-1 may serve to tether some Golgi elements to the surface of the nucleus and that Golgi–nuclear envelope interactions may be a widespread phenomena occurring in various cells types.
Acknowledgments
We thank Dr. Yih-Tai Chen and Karen Peterson for valuable technical assistance as well as for providing crucial reagents. We also thank Paul Primakoff, Diana Myles, and Paul Fitzgerald for critical reading of the manuscript. This research was supported by institutional funds from the University of California and by National Institutes of Health grant GM-59353-02 (to K.A.B.).
References
- Apel, E.D., Lewis, R.M., Grady, M., and Sanes, J.R. (2000). Syne-1, a dystrophin- and klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J. Biol. Chem. 275, 31986-31995. [DOI] [PubMed] [Google Scholar]
- Babiá, T., Ayala, I., Valderrama, F., Mato, E., Bosch, M., Santarén, J.F., Renau-Piqueras, J., Kok, J.W., Thomson, T.M., and Egea, G. (1999). N-Ras induces alterations in Golgi complex architecture and in constitutive protein transport. J. Cell Sci. 112, 477-489. [DOI] [PubMed] [Google Scholar]
- Beck, K., Buchanan, J., Malhotra, V., and Nelson, W.J. (1994). Golgi spectrin: identification of an erythroid beta-spectrin homolog associated with the Golgi complex. J. Cell Biol. 127, 707-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, K., Buchanan, J.A., and Nelson, W.J. (1997). Golgi membrane skeleton: identification, localization and oligomerization of a 195 kD ankyrin isoform associated with the Golgi complex. J. Cell Sci. 110, 1239-1249. [DOI] [PubMed] [Google Scholar]
- Beck, K., and Nelson, W.J. (1998). A spectrin membrane skeleton of the Golgi complex. Biochim. Biophys. Acta 1404, 153-160. [DOI] [PubMed] [Google Scholar]
- Bennett, V. (1990). Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol. Rev. 70, 1029-1065. [DOI] [PubMed] [Google Scholar]
- Blake, D., Tinsley, J., and Davies, K. (1996). Utrophin: a structural and functional comparison to dystrophin. Brain Pathol. 6, 37-47. [DOI] [PubMed] [Google Scholar]
- Bloch, R., and Morrow, J. (1989). An unusual β-spectrin associated with clustered acetylcholine receptors. J. Cell Biol. 108, 481-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla, E., Samitt, C., Miranda, A., Hays, A., Salviati, G., DiMauro, S., Kunkel, L., Hoffman, E., and Rowland, L. (1988). Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell 54, 447-452. [DOI] [PubMed] [Google Scholar]
- Byers, T., Husain-Chishti, A., Dubreuil, R., Branton, D., and Goldstein, L. (1989). Sequence similarity of the amino-terminal domain of Drosophila β spectrinto, α actinin and dystrophin. J. Cell Biol. 109, 1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.T., Holcomb, C., and Moore, H.P. (1993). Expression and localization of two low molecular weight GTP-binding proteins, Rab8 and Rab10, by epitope tag. Proc. Natl. Acad. Sci. USA 90, 6508-6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison, M., Baron, M., Critchley, D., and Wootton, J. (1989). Structural analysis of homologous repeated domains in α-actinin and spectrin. Int. J. Biol. Macromol. 11, 81-90. [DOI] [PubMed] [Google Scholar]
- Devarajan, P., Stabach, P.R., De Matteis, M.A., and Morrow, J.S. (1997). Na,K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin Darby canine kidney cells. Proc. Natl. Acad. Sci. USA 94, 10711-10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran, E.A., Tokito, M.K., Karki, S., and Holzbaur, E.L. (1996). Centractin (ARP1) associates with spectrin revealing a potential mechanism to link dynactin to intracellular organelles. J. Cell Biol. 135, 1815-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig, M., and Kunkel, L.M. (1990). Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J. Biol. Chem. 265, 4560-4566. [PubMed] [Google Scholar]
- Koenig, M., Monaco, A., and Kunkel, L. (1988). The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53, 219-226. [DOI] [PubMed] [Google Scholar]
- Kronebusch, P.J., and Singer, S.J. (1987). The microtubule organizing complex and the Golgi apparatus are co-localized around the entire nuclear envelope of interphase cardiac myocytes. J. Cell Sci. 88, 25-34. [DOI] [PubMed] [Google Scholar]
- Lazarides, E., and Nelson, W. (1983). Erythrocyte and brain forms of spectrin in cerebellum: distinct membrane-cytoskeletal domains in neurons. Science 220, 1295-1296. [DOI] [PubMed] [Google Scholar]
- Lu, Z., Joseph, D., Bugnard, E., Zaal, K.J., and Ralston, E. (2001). Golgi complex reorganization during muscle differentiation: visualization in living cells and mechanism. Mol. Biol. Cell 4, 795-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov, H., Byers, T., Watkins, S., and Kunkel, L. (1990). Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature 348, 725-728. [DOI] [PubMed] [Google Scholar]
- Mays, R., Siemers, K., Fritz, B., Lowe, A., Meer, G. V., and Nelson, W. (1995). Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK epithelial cells. J. Cell Biol. 130, 1105-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, W., and Hammerton, R. (1989). A membrane-cytoskeletal complex containing Na+, K+-ATPase, ankyrin, and fodrin in Madin-Darby canine kidney (MDCK) cells: implications for the biogenesis of epithelial cell polarity. J. Cell Biol. 108, 893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, W., and Veshnock, P. (1987). Modulation of fodrin (membrane skeleton) stability by cell-cell contact in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 104, 1527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual, J., Castresana, J., and Saraste, M. (1997). Evolution of the Spectrin Repeat. Bioessays 19, 811-817. [DOI] [PubMed] [Google Scholar]
- Ralston, E., Ploug, T., Kalhovde, J., and Lomo, T. (2001). Golgi complex, endoplasmic reticulum exit sites, and microtubules in skeletal muscle fibers are organized by patterned activity. J. Neurosci. 21, 875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewich, M., Tse, W., Peters, L., Ch'ng, Y., John, K., Stabach, P., Devarajan, P., Morrow, J., and Lux, S. (1998). A widely expressed βIII spectrin associated with Golgi and cytoplasmic vesicles. Proc. Natl. Acad. Sci. USA 95, 14158-14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr, D.A., and Han, M. (2002). Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science 298, 406-409. [DOI] [PubMed] [Google Scholar]
- Tassin, A.M., Piantrand, M., Berger, E.C., and Bornens, M. (1985a). The Golgi apparatus remains associated with microtubule organizing centers during myogenesis. J. Cell Biol. 101, 630-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin, A.M., Maro, B., and Bornens, M. (1985b). Fate of microtubule organizing centers during in vitro myogenesis. J. Cell Biol. 100, 35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyberg, J., and Moskalewski, S. (1985) Microtubules and the organization of the Golgi complex. Exp. Cell Res. 159, 1-16. [DOI] [PubMed] [Google Scholar]
- Valderrama, F., Babià, T., Ayala, I., Kok, J.W., Renau-Piqueras, J., and Egea, G. (1998). Actin microfilaments are essential for the cytological positioning and morphology of the Golgi complex. Eur. J. Cell Biol., 76, 9-17. [DOI] [PubMed] [Google Scholar]
- Wong, A.J., and Brodsky, F. (1992). 100-kD proteins of Golgi- and trans-Golgi network-associated coated vesicles have related but distinct membrane binding properties. J. Cell Biol. 117, 1171-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, Q., Skepper, J.N., Yang, F., Davies, J.D., Hegyl, L., Roberts, R.G., Weissberg, P.L., Ellis, J.A., and Shanahan, C.M. (2001). Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J. Cell Sci. 114, 4485-4498. [DOI] [PubMed] [Google Scholar]
- Zhang, Q., Ragnauth, C., Greener, M.J., Shanahan, C.M., and Roberts, R.G. (2002). The nesprins are giant actin-binding proteins, orthologous to Drosophila melanogaster muscle protein MSP-300. Genomics 80, 473-481. [PubMed] [Google Scholar]