Regulation of PRAK Subcellular Location by p38 MAP Kinases (original) (raw)
Abstract
The p38 mitogen-activated protein kinase (MAPK) pathway plays an important role in cellular responses to inflammatory stimuli and environmental stress. p38 regulated/activated protein kinase (PRAK, also known as mitogen-activated protein kinase activated protein kinase 5 [MAPKAPK5]) functions downstream of p38α and p38β in mediating the signaling of the p38 pathway. Immunostaining revealed that endogenous PRAK was predominantly localized in the cytoplasm. Interestingly, ectopically expressed PRAK was localized in the nucleus and can be redistributed by coexpression of p38α or p38β to the locations of p38α and p38β. Mutations in the docking groove on p38α/p38β, or the p38-docking site in PRAK, disrupted the PRAK-p38 interaction and impaired the ability of p38α and p38β to redistribute ectopically expressed PRAK, indicating that the location of PRAK could be controlled by its docking interaction with p38α and p38β. Although the majority of PRAK molecules were detected in the cytoplasm, PRAK is consistently shuttling between the cytoplasm and the nucleus. A sequence analysis of PRAK shows that PRAK contains both a putative nuclear export sequence (NES) and a nuclear localization sequence (NLS). The shuttling of PRAK requires NES and NLS motifs in PRAK and can be regulated through cellular activation induced by stress stimuli. The nuclear content of PRAK was reduced after stimulation, which resulted from a decrease in the nuclear import of PRAK and an increase in the nuclear export of PRAK. The nuclear import of PRAK is independent from p38 activation, but the nuclear export requires p38-mediated phosphorylation of PRAK. Thus, the subcellular distribution of PRAK is determined by multiple factors including its own NES and NLS, docking interactions between PRAK and docking proteins, phosphorylation of PRAK, and cellular activation status. The p38 MAPKs not only regulate PRAK activity and PRAK activation-related translocation, but also dock PRAK to selected subcellular locations in resting cells.
INTRODUCTION
Mitogen-activated protein kinases (MAPKs) are activated in response to a variety of extracellular stimuli (Nishida and Gotoh, 1993; Davis, 1994; Waskiewicz and Cooper, 1995; Cohen, 1996;Kyriakis and Avruch, 1996;Su and Karin, 1996;Brunet and Pouyssegur, 1997;Robinson and Cobb, 1997;Nebreda and Porras, 2000). The p38 group of kinases belongs to the MAPK family and plays an important role in cell proliferation, cell differentiation, cell death, development, and immune responses (Ono and Han, 2000). p38 MAPKs exert their function partially through their downstream kinases (Ono and Han, 2000). There are at least six protein kinases that can be regulated by p38α and/or p38β. These downstream kinases of p38s include MAPK-activated protein kinase 2 (MAPKAPK2 or MK2; Stokoe et al., 1992), MAPKAPK3 (Ludwig et al., 1996), MAPK-interacting kinase 1 (MNK1;Fukunaga and Hunter, 1997), MNK2 (Waskiewicz et al., 1997), p38-activated/regulated protein kinase (PRAK or MAPKAPK5;New et al., 1998;Ni et al., 1998), and mitogen- and stress-activated protein kinase (MSK;Deak et al., 1998;New et al., 1999). Several downstream targets of this family of kinases have been discovered, which include small heat shock protein, cAMP response element binding protein (CREB; Iordanov et al., 1997), serum response factor (SRF;Heidenreich et al., 1999), and the basic helix-loop-helix (HLH) protein E47 (Neufeld et al., 2000). Knockout mice of MAPKAPK2 have been generated and the mice have shown an ability to survive endotoxin shock (Kotlyarov et al., 1999). The lack of MAPKAPK2 results in changes in translation efficiency and the mRNA stability of different cytokines (Winzen et al., 1999).
To precisely transduce signaling, the proteins in the signaling pathway need to recognize and interact with specific upstream and downstream partners. A number of scaffold or anchor proteins were found to facilitate the efficiency and secure the fidelity of signaling transductions (Le Cabec et al., 1997; Schaeffer et al., 1998; Whitmarsh and Davis, 1998; McDonald et al., 2000). At the submolecular level, great advances have been made in recent years enabling the identification of domain structures for protein-protein interactions (Hunter, 2000). The structure of a given domain determines what the other domain(s) can interact with. A common docking (CD) domain required for the interaction with upstream and downstream kinases has been identified in MAP kinases including p38α (Tanoue_et al.,_ 2000). Mutagenesis studies reveal that this domain does not determine the specificity of the interaction, and a sequence further upstream serves as a determinant of specificity (Hotchkiss et al., 1999). Recently, crystal structures of p38α bound to docking sites on its substrate or activator have been determined. Structural analysis reveals that the CD domain does not directly interact with the substrate or the activator, and two new residues on the docking groove have been identified to be critical for binding (Chang et al., 2002). The domain structures of MAPKAPK2 were recently studied. Both the nucleus location sequence (NLS) and the nucleus export sequence (NES) were identified in MAPKAPK2 (Engel et al., 1998). MAPKAPK2 was primarily localized in the nucleus and transported into the cytoplasm upon extracellular stimulation with a number of different stress stimuli (Ben-Levy et al., 1998b; Engel et al., 1998). Translocation of MAPKAPK2 into the cytoplasm may allow the kinase to reach its cytosolic substrates. Interestingly, the nuclear export of MAPKAPK2 also serves as a mechanism to bring the p38α that docked to MAPKAPK2 into cytoplasm (Ben-Levy et al., 1998b). The export of MAPKAPK2 into the cytoplasm requires the phosphorylation of T317 by p38α, but not of T205 within the activation loop. The nuclear export of MAPKAPK2 is sensitive to leptomycin B, an inhibitor of nucleus export that binds to chromosomal region maintenance 1 (CRM1) protein, a nuclear export receptor for proteins carrying the leucine-rich NES (Ossareh-Nazari et al., 1997; Engel et al., 1998; Kudo et al., 1998).
PRAK can be activated in response to cellular stress and proinflammatory cytokines. PRAK activity was regulated by p38α and p38β in vitro and in vivo through phosphorylation. T182 within the activation loop of PRAK has been determined to be the regulatory phosphorylation site (New et al., 1998). Small heat shock protein 27 (Hsp27) and the regulatory light chain of myosin II have been shown to be the potential substrates of PRAK (New et al., 1998). PRAK may play a role in balancing other MAPK pathways because overactivation of PRAK can inhibit Ras mediated cell proliferation and gene activation (Tanoue et al., 2001). Current available data of PRAK show almost no difference between PRAK and MAPKAPK2 in their activation profile and substrate specificity. However, PRAK should have different functions in comparison with MAPAK2 because it cannot compensate for MAPKAPK2 deficiency in cells.
Because of the importance of subcellular location in protein function, we studied the regulation of PRAK subcellular localization. We found that PRAK is localized predominantly in the cytoplasm and is constantly shuttled between the cytoplasm and the nucleus. We have mapped domain sequences that are required for PRAK localization and translocation and have shown that the location of PRAK in resting and activated cells is determined by multiple factors.
MATERIALS AND METHODS
Reagents
SB203580 was purchased from Calbiochem (San Diego, CA). Anisomycin, sodium arsenate, M2 anti-Flag antibody and immunoprecipitation beads, myelin basic protein (MBP), antifade mounting solution, and leptomycin B were purchased from Sigma-Aldrich (St. Louis, MO). TNF-α was purchased from R&D systems (Minneapolis, MN). Polyclonal antibodies against green fluorescent protein (GFP) were purchased from ClonTech (Palo Alto, CA). FITC-labeled secondary antibodies were purchased from BD PharMingen (La-Jolla, CA), and the polyclonal antibodies against p38α, p38β, PRAK, and MAPKAPK-2 were generated from New Zealand white rabbits by stepwise subcutaneous and muscle injections of each of the affinity-purified recombinant proteins described previously (New et al., 1998).
Construction of Protein Expression Vectors
All of the his-tagged recombinant proteins were cloned downstream of a T7 RNA polymerase promoter in the pETm1vector through direct insertion of PCR fragments containing full-length coding regions of different genes as describe before (Malinin et al., 1997). The GFP-C1 vector from ClonTech is used for all of the GFP-fusion constructs, and the target genes are constructed downstream and in-frame to the GFP. HA-tagged PRAK is in the pcDNA3 vector as described elsewhere (New et al., 1998), and Flag-tagged p38α and p38β are also cloned in the pcDNA3 vector, but with a Flag-tag sequence 5′ to the target genes (Ge et al., 2002). The cDNA of a p38β splicing variant (also called p38β2 or p38–2; Stein et al., 1997) encoding 364 amino acids was used. Various mutations in PRAK and p38α or p38β in different vectors are created by using the Quick Change kit (Stratagene, La Jolla, CA) with designed corresponding pairs of mutagenic oligonucleotides. Each mutation was confirmed by DNA sequencing of the whole target gene.
Cell Culture and Transfection
Hela cells and HEK293 cells were maintained in high-glucose Dulbecco's modified Eagle's medium plus 10% FBS at 37°C in a humidified 5% CO2 atmosphere. In the case of timed microscopic photography of living cells, room temperature and a normal laboratory atmosphere was applied. The transfection of protein expression vectors was done by using Lipofectamine 2000 (Invitrogen, San Diego, CA) following the vender's protocol. Briefly, Hela or HEK293 cells were freshly seeded to 70% confluence 1 d before transfection. The total DNA, 1–2 μg, was mixed with 3 μl of Lipofectamine 2000 in 200 μl of nonserum medium for 30 min before dripping onto the cells. Analysis of transfected gene expression took place within 24 h.
Immunostaining and Fluorescent Microscopy
Antisera containing polyclonal IgG to p38α, p38β, PRAK, and MAPKAPK-2 were subjected to antigen-specific affinity purification as follows: 0.45-μm nitrocellulose membrane saturated with purified recombinant protein (600 μg protein in 0.8 ml) was air-dried completely. Then the membrane was incubated in 1 ml of buffer A (5% BSA, 10 mM Tris, 0.15 M sodium chloride, and 0.2% NP-40, pH 7.4) for 30 min, and placed in fresh buffer A for another 5 min. The membrane was incubated with the corresponding antiserum (1 ml) for 2 h with gentle agitation and then washed three times with 1× PBS. The membrane bound antibodies were eluted in 200 μl of elution buffer (100 mM glycine, pH 2.5, adjusted with hydrogen chloride) and immediately neutralized by adding 0.1 ml of 1 M Tris (pH 8.0). The concentration of purified antibodies was ∼0.1 mg/ml.
For immunostaining, differently treated cells growing on glass chamber slides were fixed with 4% of 1× PBS-buffered formaldehyde for 10 min, followed by incubation in methanol/acetone (1:l) at -20°C for 5 min. After washing with PBS, the slides were incubated in blocking buffer (10% goat serum, 0.1% Tween 20 in PBS) for 1 h followed by incubation with the corresponding purified first antibody (1:200) in fresh blocking buffer for 1 h. The slides were washed with wash buffer (1× PBS plus 0.1% Tween 20) twice and one more wash in wash buffer containing 10% BSA. The slides were incubated in blocking buffer containing the FITC-labeled secondary antibody for 50 min followed by washing three times in wash buffer and three times in water. Slides were then mounted with antifade solution, and the cover slips were sealed with nail polish.
GFP-fusion fluorescent-emitting cells or FITC-labeled cells were viewed on an inverted Axiovet 200M microscope, and images were captured through a 100× objective lens by a Carl Zeiss Vision digital camera (Carl Zeiss, Oberkochen, Germany).
For quantitative fluorescence analysis, photos were captured in the z-Plane at a 0.3-μm intervals and then the volume was deconvoluted by using a regulated-inversion filter program. A quantitative program measuring intensities of fluorescent particles was used. Individual cells were selected, and the cytoplasmic and nuclear fluorescent intensities were measured separately. After normalizing the background in the same field, the value of nuclear intensity of one cell is then divided by the cytoplasmic intensity of the same cell to obtain LI (localization index;Chan and Chan, 1999). Forty individual cells from four different views of microphotographs were subjected to this measurement. The mean of LI and the SD of LI from two differently treated groups of cells were calculated and compared.
Coimmunoprecipitation and Western Analysis
Mixture of two different plasmid DNAs (1:1) totaling 3 μg was cotransfected in HEK293 cells with Lipofectamine 2000. Twenty-four hours after transfection, the cells were lysed by chilled 1× lysis buffer (New England Bio-Labs, Beverly, MA) and then collected in a microfuge tube. Cell lysate was incubated with 20 μl of M2 beads (anti-Flag) for 4 h at 4°C followed by stringent washing several times. Samples were boiled to fully denature the proteins for 5 min followed by SDS-PAGE gel analysis. To detect coimmunoprecipitated proteins, Western blots were performed as described (New et al., 1998). Anti-GFP antibodies were used to probe the coimmunoprecipitated proteins, and the antiflag antibodies were then used to probe the Flag-tagged proteins.
Immune Complex Kinase Assay
Cells, after 24 h of transfection or cotransfection of the kinase expression vectors, were treated with or without TNF-α for a period of time as indicated and then washed in cold PBS buffer and lysed in 0.8 ml of chilled 1× lysis buffer. Cell lysates were collected in microfuge tubes and centrifuged for 15 min at 4°C. The supernatants were transferred to new tubes and incubated with 20 μl of anti-GFP beads (Santa Cruz) or anti-Flag M2 beads at 4°C for 4 h. The beads bound with kinase samples were centrifuged and washed in 1× PBS buffer twice and in 1 × kinase buffer (New England Bio-Labs) once. At this point, total amount of immune complex of anti-GFP or M2 beads, from each sample, was split into two equal volume parts. One part was used for kinase assay and the other part saved for Western analysis to justify the amount of each immune complex obtained through the process. The kinase reaction for PRAK were carried out in a total volume of 60 μl in 1× kinase buffer containing the immune complex, 10 μCi of [γ-32P]ATP, and 10 μg of Hsp 27 prepared as before (New et al., 1998) at 37°C for 40 min with shacking. The reaction was terminated by adding 30 μl of SDS loading buffer. The activities of immunoprecipitated p38α and p38β with their corresponding mutants were assayed as that of PRAK described above except that 10 μg of MBP was used as kinase substrate. The kinase reaction samples were boiled for 5 min before SDS-PAGE, and the extent of protein phosphorylation was analyzed by phosphoimaging. The control Western analysis with anti-GFP antibody or with anti-Flag antibody was performed as described above.
RESULTS
Subcellular Location of PRAK
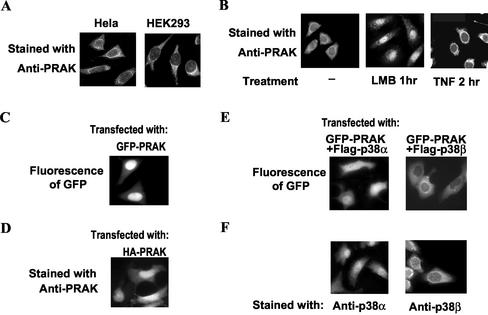
To better understand the regulation and function of PRAK, we used immunostaining to determine the subcellular location of PRAK. Anti-PRAK polyclonal antibodies, raised with recombinant PRAK, were affinity-purified with immobilized-PRAK protein and used in the immunostaining. As shown inFigure 1A, a predominantly cytoplasmic stain of PRAK was exhibited in HEK293 (293) and Hela cells, with the brightest staining shown peripherally around the nucleus and extending to the distal cytoplasm.
Figure 1.
The subcellular locations and cytoplasm-nucleus shuttling of endogenous PRAK. (A) HEK293 and Hela cells were fixed and immunostained with anti-PRAK primary antibodies and FITC-labeled secondary antibody. A representative microimage for each stain was shown. PRAK is predominantly located in cytoplasm of resting cells. (B) HEK293 cells were treated with nothing (-), LMB (3 ng/ml) for 1 h, or TNF-α (100 nM) for 2 h and then fixed and stained with anti-PRAK antibody. An apparent accumulation of PRAK in the nucleus was observed when nuclear export was blocked by LMB (middle panel). TNF stimulation reduced nuclear PRAK (right panel, see text for the statistic analysis). (C) HEK293 cells were transfected with expression vector of GFP-PRAK. GFP-PRAK was visualized under a fluorescent microscope 24 h after transfection. Ectopically expressed GFP-PRAK was localized in nucleus. (D) HEK293 cells were transfected with expression vector of HA-PRAK. The cells were fixed 24 h after transfection and immunostained with anti-PRAK primary antibodies and FITC-labeled secondary antibody. Ectopically expressed HA-PRAK was localized in nucleus. (E) HEK293 cells were transfected with expression vector of GFP-PRAK together with flag-p38α or flag-p38β. GFP-PRAK was visualized under a fluorescent microscope 24 h after transfection. Coexpression of p38α and p38β relocalized GFP-PRAK. (F) HEK293 cells were fixed and immunostained with anti-p38α or anti-p38β antibodies and FITC-labeled secondary antibody.
PRAK Is Constantly Shuttling between Nucleus and Cytoplasm
To determine if PRAK traffics between the cytoplasm and the nucleus, we treated 293 (Figure 1B) and Hela (our unpublished results) cells with leptomycin B (LMB), a specific inhibitor of nuclear export that interferes with the binding of the leucine-rich NES to the export receptor exportin 1 (Ossareh-Nazari et al., 1997). PRAK was found to rapidly accumulate in the nucleus of the LMB-treated cells (Figure 1B, middle panel), implying constant trafficking of PRAK between the cytoplasm and the nucleus under normal conditions. However, when the cells were stimulated with tumor necrosis factor-α (TNF) for 2 h, the nuclear stain of PRAK appeared to be reduced (Figure 1B). To statistically assess the effect of TNF on translocation of PRAK, we measured the intensity of FITC stain in the nucleus and cytoplasm of each individual cell from a total of 40 cells treated with or without TNF-α, respectively, using an interactive particle-volume measuring software program (Carl Zeiss). The ratio of nuclear over cytoplasmic florescence intensity was operationally defined as the LI (Chan et al., 1996;Chan and Chan, 1999) and calculated for each cell measured. The LImean ± LIsd for TNF-treated cells is 0.15 ± 0.03, whereas for non–TNF-treated cells is 0.31 ± 0.04. The value of LIsd from the two groups is close, but the value of LImean from TNF-treated cells is substantially smaller than that of nontreated cells. TNF stimulation increases PRAK nucleus export and/or decreases PRAK transport into the nucleus.
Docking of PRAK to Proteins such as p38α and p38β Plays a Key Role in the Subcellular Localization of PRAK
Fusion of GFP with a protein of interest has proven to be very useful in studying the subcellular location of the protein. To investigate PRAK translocation, we made use of the GFP-PRAK fusion system. As shown inFigure 1C, in contrast to endogenous PRAK, GFP-PARK is mainly located in the nucleus of 293 cells. The same result was obtained when using Hela cells (our unpublished results). To exclude the possibility that the nuclear localization of GFP-PRAK is caused by GFP, we transfected GFP expression vectors into the cells and observed the diffused distribution of GFP in both the nucleus and the cytoplasm (our unpublished results). We also expressed HA-tagged PRAK in 293 (Figure 1D) and Hela (our unpublished results) cells and stained these cells with the anti-PRAK antibody. The HA-tagged PRAK in the transfected cells was detected in the nucleus, which is consistent with the GFP-PRAK subcellular localization and suggests that ectopically expressed PRAK is in different subcellular locations than the endogenous protein.
It is well accepted that cellular transport systems play a key role in sorting the different proteins into their distinct subcellular compartments. However, the subcellular location of a given protein may also be influenced by its interacting proteins. The nuclear location of ectopically overexpressed PRAK may be caused by the fact that there is insufficient amount of corresponding PRAK-interacting proteins to dock PRAK, thus causing PRAK molecules to be sorted into the nucleus. To test this hypothesis, we examined whether the upstream kinases of PRAK, p38α, and p38β that interact with PRAK have any effect on the location of GFP-PRAK. This was done by coexpressing GFP-PRAK with p38α or p38β in 293 (Figure 1E) and Hela cells (our unpublished results). Coexpression of p38α with GFP-PRAK caused GFP-PRAK to redistribute to both the nuclear and cytoplasmic locations (Figure 1E, left panel), whereas coexpression of p38β with GFP-PRAK resulted in GFP-PRAK to predominate in the cytoplasm(Figure 1E, right panel). Therefore, the location of GFP-PRAK appears to be controlled by coexpression of both p38α and p38β, because p38α is located in the nucleus and the cytoplasm (Figure 1F, left panel), and p38β is located predominantly in the cytoplasm (Figure 1E, right panel). Collectively, the results shown in Figure 1, C–F, demonstrate that the docking of PRAK to other proteins could significantly affect the location of PRAK and that p38α and p38β are among the molecules that can dock to PRAK.
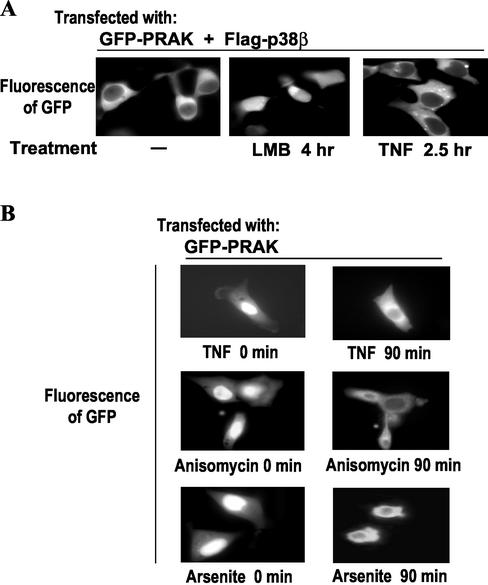
Shuttling of Endogenous PRAK Can Be Mimicked by GFP-PRAK
Because docking of GFP-PRAK to p38β closely mimics endogenous PRAK in subcellular locations, we examined whether the trafficking of GFP-PRAK in this system is similar to that of endogenous PRAK. We coexpressed GFP-PRAK and p38β in 293 cells (Figure 2A) and treated these cells with LMB (Figure 2A, middle panel) or TNF (Figure 2A, right panel). Similar to what we observed in Figure 1B with endogenous PRAK, an increase in the nuclear GFP-PRAK concentration became apparent when nuclear export was inhibited, though some of the GFP-PRAK still remained in the cytoplasm (Figure 2A, middle panel). As in the case of the endogenous PRAK (Figure 1B, right panel), TNF treatment reduced the nuclear GFP-PRAK concentration even when p38β was coexpressed (Figure 2A, right panel). These results indicate that GFP-PRAK, coexpressed with p38β, behaves similarly to endogenous PRAK in terms of constant shuttling within resting cells and enhanced exporting of GFP-PRAK from the nucleus to the cytoplasm in TNF-treated cells. Taking advantage of the nuclear location of GFP-PRAK (Figure 1C), we compared the effects of other stimuli that are known to cause cellular stress similar to that of TNF on the nuclear exportation of PRAK. As shown inFigure 2B, TNF, anisomycin, and arsenite all induced export of GFP-PRAK from the nucleus to the cytoplasm. These data are consistent with the results obtained when using endogenous PRAK (Figure 1B, right panel and our unpublished results).
Figure 2.
Translocation of GFP-PRAK. (A) Expression vectors of GFP-PRAK and flag-p38β were cotransfected into HEK293 cells. Twenty-four hours after the transfection, the cells were treated with or without LMB or TNF-α for various times as indicated. GFP-PRAK in the same individual cells was monitored by fluorescent microscopy. (B) HEK293 cells transfected with GFP-PRAK expression vector were subjected to a treatments of TNF-α (top panel), ainsomycin (15 μg/ml; middle panel), and arsenite (250μM; bottom panel). The redistribution of GFP-PRAK in the same individual cells was monitored by fluorescent microscopy.
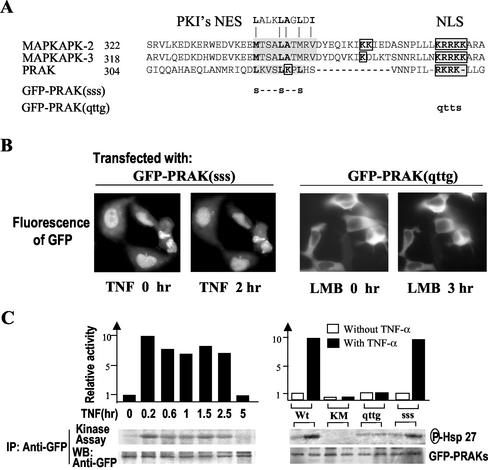
PRAK Contains a Typical Leucine-rich NES and a Bipartite NLS
Aligning the primary protein sequences of PRAK, MAPKAPK2, and MAPKAPK3 reveals that PRAK contains similar NES and NLS (Figure 3A). The major difference between PRAK and MAPKAPKs in these sequences is that one part of NLS in PRAK is located inside the NES part. The putative NES in PRAK is highly conserved to the prototypic NES sequence from the inhibitor of protein kinase A, PKI (Wen et al., 1995; Figure 3A). To determine whether the sequences found in PRAK truly function as signals for nuclear export and import, we assessed the requirement of the NES and NLS in nuclear import and export of PRAK by the creation of site-specific mutations in the two motifs (Figure 3A). The NES mutant was made by changing the three leucines (L) in the putative NES of PRAK to serines (S) and termed PRAK(sss) (Figure 3A). The NLS mutant was made by converting the stretch of four basic amino acids arginine-lysine-arginine-lysine (RKRK) to glutamine-threonine-threonine-glycine (QTTG;Figure 3A) and named PRAK(qttg). When GFP-PRAK(sss) was expressed in 293 cells, it located in the nucleus. TNF treatment could not drive this protein out of the nucleus (Figure 3B, right panel), indicating that the putative NES was indeed required for PRAK export from the nucleus. However, GFP-PRAK(qttg) was found to be exclusively localized in the cytoplasm, and LMB failed to accumulate GFP-PRAK(qttg) in the nucleus (Figure 3B, right panel), confirming that the predicted nuclear location sequence is functional in PRAK.
Figure 3.
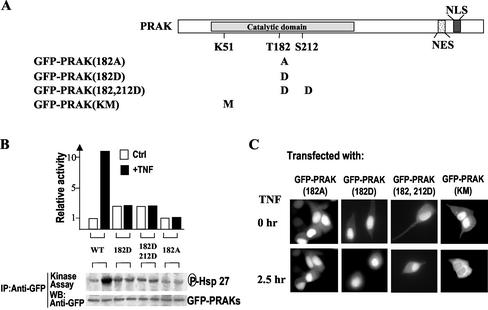
Requirement of NES and NLS for nuclear export and import of PRAK. (A) Sequence alignment NES and NLS found in PRAK, MAPKAPK2, and MAPKAPK3. The nuclear export sequence (NES) is shown shaded and the nuclear localization sequence (NLS) is both boxed and shaded. A prototypic NES sequence from PKI is shown on the top of the sequence alignment. The mutation sites of GFP-PRAK(sss) and GFP-PRAK(qttg) are indicated. (B) HEK293 cells were transfected with expression vector of GFP-PRAK(sss) or GFP-PRAK(qttg). Twenty-four hours after transfection, the cells were treated with or without TNF or LMB for time periods as indicated. The distributions of GFP were analyzed by fluorescent microscopy. (C) HEK293 cells were transfected with expression vector of GFP-PRAK, GFP-PRAK(KM), GFP-PRAK(qttg), or GFP-PRAK(sss). Twenty-four hours after transfection, the cells were treated with or without TNF for different time periods (left panel) or 2 h. GFP-PRAK and its mutants were immunoprecipitated with anti-GFP antibodies. The kinase activity in the immunoprecipitates was determined by in vitro kinase assay using Hsp27 as substrate. Equal amounts of GFP-PRAK in the immunoprecipitates were determined by Western blotting with anti-GFP antibodies.
We next determined kinase activities of GFP-PRAK(sss) and GFP-PRAK(qttg) in comparison with that of GFP-PRAK and GFP-PRAK(KM), a kinase dead mutant, by using immune complex kinase assay. Small heat shock protein (Hsp27) was used as substrate in the kinase assays. The kinase activity of GFP-PRAK was enhanced 8–10-fold in response to TNF stimulation, lasting for at least 2.5 h (Figure 3C, right panel). Wild-type GFP-PRAK had some basal kinase activity, whereas a mutation in its ATP pocket [K51 to M mutant, GFP-PRAK(KM)] eliminated kinase activity in both resting and stimulation conditions (Figure 3C, right panel). The NES mutant [GFP-PRAK(sss)] had similar basal activity as GFP-PRAK and can be activated by TNF stimulation (Figure 3C, right panel). The NLS mutant [GFP-PRAK(qttg)] retained basal activity but cannot be regulated by TNF stimulation. Because NLS in PRAK overlaps with the p38 docking site (Engel et al., 1998;Tanoue et al., 2001) and see Figure 6A), the unresponsiveness of NLS mutant to TNF stimulation could be caused by a defect in translocation to the nucleus or a defect in interacting with p38.
Figure 6.
The basic cluster of amino acids in the docking site on PRAK but not the regulatory phosphorylation site on PRAK or activity of PRAK, determines the docking interaction with p38. (A) A schematic representation of PRAK shows the relative position of the catalytic domain, NES, NLS, and the docking site. The docking site overlaps with NLS. (B) Coimmunoprecipitation assays of various GFP-PRAK mutants with flag-p38α or flag-p38β were performed as described in Figure 5B. (C) The effects of different mutations in PRAK on the redistribution of GFP-PRAK by the coexpression of flag-p38α or flag-p38β was evaluated using the same method as in Figure 5C.
The Relation among T182 Phosphorylation, Kinase Activity, and Location of PRAK
It has been demonstrated that the nuclear export of MAPKAPK2 is regulated by the phosphorylation on T317 located in the C-terminal of the kinase domain. Sequence alignment of PRAK and MAPKAPK2 (Engel et al., 1998;New et al., 1998) revealed that PRAK does not have that corresponding phosphorylation site. This indicates that a different regulation mechanism may govern the nuclear exports of PRAK and MAPKAPK2. It is known that T182 and S212 of PRAK can be phosphorylated by p38, and the phosphorylation of T182 upregulated PRAK kinase activity (New et al., 1998). To address whether the phosphorylation and kinase activity of PRAK affect the location and translocation of PRAK, we used a series of PRAK mutants. The mutants are illustrated inFigure 4A. The kinase activities of PRAK(182D), PRAK(182, 212D), and PRAK(182A) were studied previously (New et al., 1998). Here we measured activities of GFP fusion proteins of these PRAK mutants. T182 mutations (either D or A mutant) abolished the responsiveness of PRAK to TNF stimulation (Figure 4B), because these mutants can no longer be phosphorylated by p38 at T182 (New et al., 1998). GFP-PRAK(182D) and GFP-PRAK(182, 212D) had a slightly higher basal activity than GFP-PRAK and GFP-PRAK(182A), suggesting that the D replacement somewhat mimic the negative charge of phosphorylation (New et al., 1998). GFP-PRAK(KM) is kinase-dead but it still can be phosphorylated by p38 (Figure 3C and our unpublished results). All of these mutants were localized in the nucleus when expressed in 293 cells (Figure 4C, top panels), indicating that in resting cells these mutations do not influence the transport of ectopically expressed PRAK into nucleus. However, TNF stimulation cannot lead to nucleus export of the T182 mutants (Figure 4C, bottom panel), indicating a requirement of T on the 182 site in the stimulation-mediated nuclear export of PRAK. Because T182 was phosphorylated after stimulation with TNF (New et al., 1998), it is most likely that phosphorylation on T182 is required for the nuclear export of PRAK. Being retained in the nucleus of the 182D mutant after stimulation, suggests that the D mutant cannot fully mimic the function of the phosphorylation on T182. In contrast to the T182 mutants, GFP-PRAK(KM) is capable of translocation into the cytoplasm after TNF treatment (Figure 4C, bottom panels), suggesting that PRAK kinase activity is not required for nuclear export of PRAK.
Figure 4.
Nuclear export of PRAK requires the regulatory phosphorylation site T182 but not kinase activity of PRAK. (A) A schematic primary structure of PRAK indicates the position of the catalytic domain and the motifs of NES and NLS. Mutations that altered the phosphorylation sites (T182, S212) or ATP pocket (K51) were shown under the structure. (B) HEK293 cells were transfected with the expression vector of different GFP-PRAK mutants as indicated. Twenty-four hours after transfection, the cells were treated with or without TNF for 2 h. The kinase activity of GFP-PRAK mutants was analyzed by immunokinase assay as in Figure 3C. (C) HEK293 cells were transfected with the expression vector of different GFP-PRAK mutants as indicated. Twenty-four hours after transfection, the cells were treated with or without TNF for 2 h. The locations of GFP-PRAK were analyzed by fluorescent microscopy. TNF-induced nuclear export of GFP-PRAK was impaired by mutations on T182 site but not by a mutation in ATP pocket.
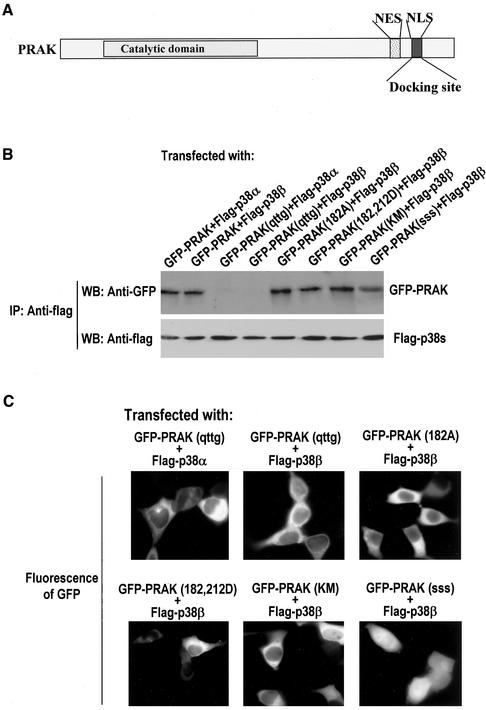
The Docking of PRAK to p38α or p38β Requires Docking Motifs on Both Proteins, But Not Kinase Activities, or Functional Phosphorylations of Either Proteins
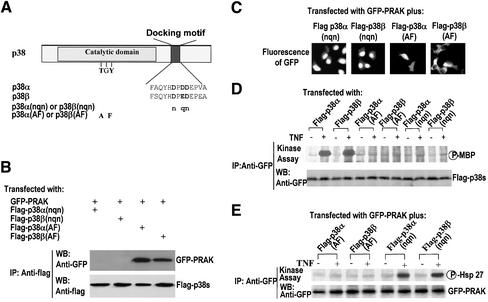
A substrate-docking motif in p38α has been shown to be required for interaction with PRAK (Tanoue et al., 2000,2001). Because the coexpression of p38α or p38β causes redistribution of GFP-PRAK and this redistribution is most likely accomplished through an interaction with PRAK, we examined whether the docking motifs in p38α or p38β and in PRAK are required for their docking interaction and the redistribution of GFP-PRAK to the cytoplasm. Mutations of the docking motif in p38α and p38β were made as shown in Figure 5A. Flag-tagged p38α(nqn), p38β(nqn), p38α(AF), or p38β(AF) were coexpressed with GFP-PRAK in 293 cells. The interaction of PRAK with the mutants of p38α or p38β was determined by coimmunoprecipitation. Apparently, mutations of the substrate docking site in p38α and p38β abolished their interaction with PRAK, respectively (Figure 5B). In contrast to the mutations in the substrate docking site, mutations on the phosphorylation sites in p38α and p38β did not affect their interaction with PRAK (Figure 5B), indicating that p38 phosphorylation and activity are not required for docking PRAK. Consistent with the interaction detected by coimmunoprecipitation, we found that p38α(nqn) and p38β(nqn) can no longer redistribute GFP-PRAK inside the cells, whereas p38α(AF) and p38β(AF) behaved similarly to wild-type proteins by docking PRAK to different subcellular locations (Figure 5C).
Figure 5.
A substrate docking motif on p38 but not the regulatory phosphorylation or activity of p38 controls the docking interaction with PRAK. (A) A schematic structure of p38α and p38β shows the positions of the catalytic domain and a substrate docking motif on p38. The key amino acid residues in these motifs were presented as bold letters. The mutation sites of p38(AF) and p38(nqn) are indicated. (B) HEK293 cells were cotransfected with various combinations of p38 and GFP-PRAK expression vectors as indicated. The cell lysates were collected 24 h after transfection and immunoprecipitated with the antiflag antibody M2. The presence of GFP-PRAK or flag-p38 in the immunoprecipitates was determined by Western blotting using antiGFP or antiflag antibodies, respectively. (C) HEK293 cells were transfected as in B and the subcellular locations of GFP-PRAK were analyzed by fluorescent microscopy. (D) The mutants of p38α or p38β were expressed in HEK293 cells as indicated. Twenty-four hours after transfection, the cells were treated with or without TNF for 2 h. The cell lysates were then collected and immunoprecipitated with the antiflag antibody M2. The kinase activity of p38 mutants was measured by immunokinase assay using MBP as substrate. The equal amount of p38α or p38β in the immunoprecipitates was determined by Western blotting. (E) HEK293 cells were cotransfected with various combinations of p38 mutants and GFP-PRAK expression vectors as indicated. Twenty-four hours after transfection, the cells were treated with or without TNF for 2 h. The cell lysates were collected and immunoprecipitated with the anti-GFP antibodies. The kinase activity of GFP-PRAK was determined by immunokinase assay as in Figure 3C.
Immune complex kinase assays were performed to measure the kinase activities of p38α, p38β, and their mutants. As expected, mutations on the phosphorylation sites or the substrate docking motif abolished kinase activities of p38α and p38β (Figure 5D). Coexpression of p38α(AF) or p38β(AF) mutant with GFP-PRAK inhibited TNF-stimulated activation of GFP-PRAK (Figure 5E), which is consistent with the dominant negative effect of these mutants (Huang et al., 1997; Wang et al., 2000; Ge et al., 2002). In contrast, the overexpression of docking motif mutants had no effect on TNF-induced PRAK activation (Figure 5E). This can be interpreted by the fact that overexpression of flag-p38α(nqn) or flag-p38β(nqn) cannot compete with endogenous p38 for substrates and upstream activators because nqn mutants of p38α and p38β cannot bind with downstream substrates (Tanoue_et al.,_ 2001 andFigure 5B) or upstream MKKs (Tanoue et al., 2000).
The docking sites in MAPKAPK2 have been mapped to the C termini of the protein and overlapped with NLS. The NLS in PRAK (Figure 6A) has also proved to be a motif essential for p38 docking in a coimmunoprecipitation experiment (Engel et al., 1998;Tanoue et al. 2001). To ascertain the function of this motif in docking, we coexpressed GFP-PRAK(qttg) with flag-p38α or flag-p38β. Coimmunoprecipitation assays show that PRAK(qttg) fails to interact with p38α or p38β (Figure 6B). In contrast, mutations of the phosphorylation sites or ATP pocket of PRAK can still be coimmunoprecipitated with p38α (our unpublished results) or p38β (Figure 6B). Mutations of NES in PRAK impaired stimulus-mediated nuclear exports of PRAK (Figure 3) but had no effect on the interaction with p38α (our unpublished results) and p38β (Figure 6B). In support of the notion that the docking interaction controls the subcellular location of PRAK, the subcellular location of GFP-PRAK(qttg) cannot be influenced by coexpressed p38α or p38β (Figure 6C). In contrast, the mutations on the phosphorylation sites or ATP pocket of PRAK did not have any effect on the relocation of PRAK by p38β (Figure 6C). Thus, the phosphorylation and activity of p38 and PRAK appear not to affect the docking interactions between the two proteins. It was observed that the cells coexpressing flag-p38β and GFP-PRAK(sss) have certain levels of GFP-PRAK(sss) in the cytoplasm (Figure 6C, bottom-right panel). This cytoplasm-retained GFP-PRAK(sss) is most likely caused by the p38β docking of this protein after its synthesis in the cytoplasm, but before its nuclear import, because nuclear GFP-PRAK(sss) cannot be exported out of the nucleus.
The results of transfection and cotransfection experiments with wild-type or mutants of PRAK and p38 are summarized in theTable 1.
Table 1.
Subcellular location of GFP-PRAKs
| GFP-PRAK | Cotransfected with | GFP-PRAK localization |
|---|---|---|
| GFP-PRAK | — | Nucleus |
| GFP-PRAK | Flag-p38α | Nucleus and cytoplasm |
| GFP-PRAK | Flag-p38β | Cytoplasm |
| GFP-PRAK | Flag-p38α(AF) | Nucleus and cytoplasm |
| GFP-PRAK | Flag-p38β(AF) | Cytoplasm |
| GFP-PRAK | Flag-p38α(nqn) | Nucleus |
| GFP-PRAK | Flag-p38β(nqn) | Nucleus |
| GFP-PRAK(182A) | — | Nucleus |
| GFP-PRAK(182A) | Flag-p38α | Nucleus and cytoplasm |
| GFP-PRAK(182A) | Flag-p38β | Cytoplasm |
| GFP-PRAK(182D) | — | Nucleus |
| GFP-PRAK(182D) | Flag-p38α | Nucleus and cytoplasm |
| GFP-PRAK(182D) | Flag-p38β | Cytoplasm |
| GFP-PRAK(182, 212D) | — | Nucleus |
| GFP-PRAK(182, 212D) | Flag-p38α | Nucleus and cytoplasm |
| GFP-PRAK(182, 212D) | Flag-p38β | Cytoplasm |
| GFP-PRAK(KM) | — | Nucleus |
| GFP-PRAK(KM) | Flag-p38α | Nucleus and cytoplasm |
| GFP-PRAK(KM) | Flag-p38β | Cytoplasm |
| GFP-PRAK(qttg) | — | Cytoplasm |
| GFP-PRAK(qttg) | Flag-p38α | Cytoplasm |
| GFP-PRAK(qttg) | Flag-p38β | Cytoplasm |
| GFP-PRAK(sss) | — | Nucleus |
| GFP-PRAK(sss) | Flag-p38α | Nucleus |
| GFP-PRAK(sss) | Flag-p38β | Nucleus and cytoplasm |
TNF Stimulation Affects Both Nuclear Export and Import of PRAK
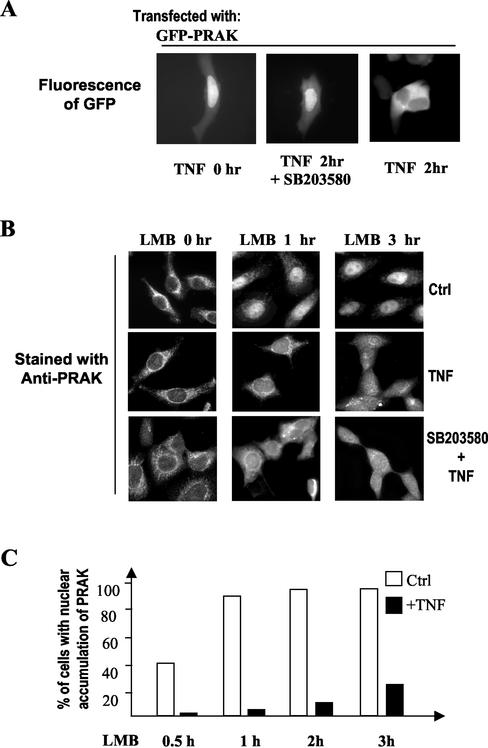
We showed earlier in Figures1B and2 that TNF stimulation led to the nuclear export of PRAK or GFP-PRAK and that the export requires T182 in PRAK (Figure 4). To determine whether p38 activity is required for the nuclear export of PRAK, we expressed GFP-PRAK in 293 cells and treated these cells with TNF in the presence or absence of SB203580. As shown in Figure 7A, SB203580 prevented the TNF-induced nuclear export of PRAK. Thus, TNF-induced nuclear export of PRAK is p38 dependent.
Figure 7.
Regulation of PRAK shuttling by TNF stimulation. (A) HEK293 cells transfected with the expression vector of GFP-PRAK were treated with or without TNF in the presence or absence of SB203580 (20 μM) for 2 h. The subcellular location of GFP-PRAK was examined by fluorescent microscopy. The p38 inhibitor SB203580 inhibited TNF-induced exportation of GFP-PRAK. (B) HEK293 cells were treated with LMB together with a vehicle (0.1% methanol and 0.1% DMSO)(Ctrl), TNF or TNF+ SB203580 for the time periods as indicated. The cells were fixed and location of PRAK was analyzed by immunostaining as inFigure 1A. (C) The relative speed of PRAK translocation from the cytoplasm into the nucleus was estimated by counting cells, whose nuclear PRAK staining was equal to, or higher than, the cytoplasmic staining (LI ≥ 1.0, see text for detail) from a population of 60 cells. The percentages of these cells in TNF-treated and untreated samples were calculated and shown.
The reduction of nuclear PRAK by extracellular stimulation can be achieved by either a decrease of nuclear import or an increase of nuclear export of PRAK. To determine whether the nuclear import of PRAK was altered when the cells were stimulated with TNF, we treated 293 cells with LMB together with or without TNF. The cells were fixed at different times after treatment and stained with the anti-PRAK antibody. The same result was obtained as was shown earlier in Figure 1. LMB treatment led to a rapid accumulation of PRAK in the nucleus (Figure 7B, top panel). TNF treatment dramatically reduced LMB-mediated nuclear localization of PRAK (Figure 7B, middle panel). We used LI ≥ 1.0, e.g., the level of nuclear PRAK was equal to or greater than the amount of cytoplasmic PRAK, as a standard for nuclear accumulation of PRAK to compare the difference in nuclear import between the cells treated with or without TNF. As shown in Figure 7C, almost all cells showed nuclear accumulation of PRAK within 1 h of LMB treatment, whereas TNF treatment reduced the cell number to <10% of the population. Longer time treatment of LMB did not appear to overcome TNF's effect because there was only 30% of the cells showing nuclear accumulation of PRAK 3 h after treatment.
To test whether TNF-induced activation of p38α and/or p38β has any role in TNF's effect on PRAK nuclear import, we treated the cells with TNF in the presence of SB203580. As shown inFigure 7B, the inhibition of p38α and p38β did not interfere with TNF-reduced nuclear import of PRAK. Thus, TNF-mediated reduction of the nuclear import of PRAK is independent of p38 activation.
DISCUSSION
We studied subcellular localization and shuttling of PRAK and concluded that 1) PRAK is predominantly localized in cytoplasm. 2) PRAK is constantly shuttled between the cytoplasm and the nucleus. The NLS and NES identified in PRAK are required for the shuttling. Because almost all PRAK proteins were accumulated in nucleus when nuclear export was inhibited, we conclude that all of the PRAK molecules are involved in the circulation between the cytoplasm and the nucleus. 3) The cytoplasmic location of endogenous PRAK is most likely caused by a docking interaction with p38β because overexpressed “free” PRAK localized in nucleus and the coexpression of p38β, a cytoplasmic PRAK interaction protein that relocalized PRAK into the cytoplasm. The docking interaction requires docking motifs identified in PRAK and p38α or p38β and is not influenced by the phosphorylation status and activity of these kinases. 4) Phosphorylation of PRAK on T182 is an event required for the nuclear export of PRAK or an event that facilitates the nuclear export of PRAK. 5) Cell activation by extracellular stimuli can alter the nuclear import and export of PRAK. Stimulation of cells with TNF reduced the nuclear import of PRAK in a p38-independent manner and increased the nuclear export of PRAK by a p38-dependent mechanism.
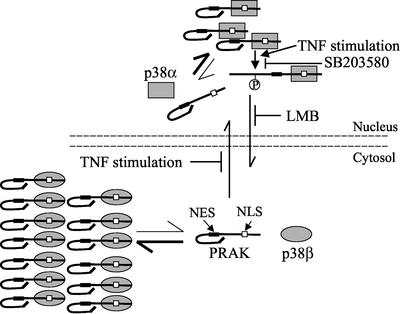
On the basis of our data, we propose a model for the regulation of PRAK localization and shuttling by p38 MAPKs (Figure 8). The majority of PRAK localized in the cytoplasm, docking by cytoplasmic p38β. The p38 docking site in PRAK overlaps with the NLS region; therefore, the interaction of PRAK with p38 likely masks this site, which may account for a mechanism in preventing the nuclear import of PRAK. It is clear that the balance is in favor of PRAK and p38β binding in the cytosol. However, there should be some remaining free PRAK. The free PRAK can be imported into the nucleus, which may account for the low level of nuclear PRAK in resting cells. Overexpressed PRAK located primarily in nucleus, suggesting that either the nuclear export signal of PRAK is not as predominant as the nuclear import signal or the NES of PRAK was masked intramolecularly or intermolecularly by other protein(s), thus preventing or reducing the speed of the nuclear exportation of PRAK. The docking interaction between PRAK and p38α should occur in the nucleus. The basal and stimulated activity of p38α should be responsible for the phosphorylation of nuclear PRAK in resting and activated cells, respectively. Nuclear exportation of PRAK may require the phosphorylation of PRAK or at least enhanced by this phosphorylation. We proposed that the phosphorylation of PRAK by p38α unmasks MES in PRAK and results in the subsequent nuclear export of PRAK. TNF stimulation reduces the nuclear import of PRAK, using an unknown mechanism, and increases the nuclear export of PRAK in a p38-dependent manner. Because most of the experiments described in this report have to use overexpression systems, the model shown in Figure 8 could be oversimplified, incomplete, and imbalanced. The docking effect of p38α and p38β on PRAK subcellular localization needs to be confirmed when p38α and p38β knockout cells are available.
Figure 8.
Regulation of subcellular location and translocation of PRAK by p38α and p38β. See text for details.
While this manuscript was in revision, Seternes et al. (2002) reported a study on PRAK (MAPKAPK5) subcellular localization. They also mapped NLS and NES motifs on PRAK and concluded that PRAK can shuttle between nucleus and cytoplasm. But different from us, they concluded that PRAK is a nuclear protein. Because the same type of cells was used by Seternes and us, the controversial results cannot be caused by cell type differences. To address this conflicting result, we have repeated our experiments with anti-PRAK antibodies from different rabbits. We have antibodies from four different rabbits that can selectively detect PRAK but not MAPKAPK2 in Western blotting analysis. We immuno-purified the different anti-PRAK antibodies and used them in immunostaining experiments. Just as in the result we obtained earlier (Figure 1A), PRAK was detected predominantly in cytoplasm by the antibodies from different rabbits (our unpublished results). The specificity of these antibodies in staining PRAK was further confirmed by their staining of ectopically expressed PRAK in nucleus (Figure 1D and our unpublished results). Thus, we have no doubt that the majority of PRAK molecules are localized in cytosol.
Previous characterization of PRAK showed its similarity to its homologue MAPKAPK2 in the activation profile and substrate specificity (New et al., 1998). Here we show that similar to MAPKAPK2, cellular stresses also stimulate nucleus export of PRAK (Figures1B and2). The major difference between these two proteins found so far is subcellular localization in resting cells (Figure 1A and our unpublished results). The difference in the subcellular localization of PRAK and MAPKAPK2 is probably caused by different docking interactions. Docking interactions may play a role in controlling the location or translocation of certain proteins. For example, the docking interaction between MEK1and ERK1 was reported to be important for the cytoplasmic location of ERK1 (Fukuda et al., 1997). The translocation of p38α from the nucleus to the cytoplasm in stressactivated cells was mediated by its binding partner, MAPKAPK2 (Ben-Levy et al., 1998b). p38α can redistribute GFP-MAPKAPK3 by interacting with it (Tanoue et al., 2001). However, the effect of docking interactions on PRAK localization shows some differences from the reported studies. MAPKAPK2 directs translocation of p38α, whereas subcellular localization of PRAK is directed by p38α or p38β (Figure 1). Also, unlike the ERK1 that anchored in the cytoplasm and was translocated to nucleus after phosphorylation, the activation of PRAK by p38β did not lead to the translocation of PRAK to the nucleus. Furthermore, the nuclear location of overexpressed ERK1 appeared to be independent of NLS-mediated translocation (Fukuda et al., 1997), whereas the accumulation of ectopically expressed PRAK is dependent on the NLS of PRAK (Figure 3). Nevertheless, our work and the works referenced above demonstrate that the docking interactions can play a very important role in determining the subcellular location of protein kinases in resting as well as in activated cells. Because docking with other proteins can play a key role in subcellular localization of a given protein, caution needs to be given when overexpression is used to study subcellular localizations of a protein.
Some proteins, such as mitochondrial proteins, never come back to the cytoplasm once their destination has been reached (Gorlich, 1998), whereas other proteins are constantly trafficking between two cellular compartments such as the nucleus and the cytoplasm (Gorlich, 1998; Murata et al., 2002). It was believed that small proteins have the potential to freely diffuse through the nuclear pore, whereas proteins >60 kDa are subjected to active and carrier mediated transport (Gorlich, 1998). PRAK is a 50-kDa protein but seems unable to freely travel between the nucleus and the cytoplasm. The nuclear import of PRAK is mediated by a process of NLS-dependent protein import, and its nuclear export requires CRM1.
PRAK functions downstream of p38 to mediated cellular responses. However, the precise role of PRAK is not clear. As with other shuttling proteins, the nucleus and cytoplasm circulation of PRAK must have a role in normal cell physiology. Alteration of shuttling in activated cells should be a part of the cellular response to extracellular stimuli, and its effects deserve further investigation. An increased export of PRAK in TNF-treated cells may permit PRAK to be activated in the nucleus, thereby phosphorylating cytoplasmic substrates. Because p38β can be activated by a similar panel of extracellular stimuli as p38α (Jiang_et al.,_ 1996; Kumar_et al.,_ 1997; Nemoto_et al.,_ 1998), we reason that cytoplasmic PRAK can be activated by cellular stress too. It is possible that the nuclear export of activated PRAK in stress-activated cells is a cellular response to compensate for a shortage of activated PRAK in the cytoplasm. But given the fact that the majority of PRAK was found in the cytoplasm, the nuclear export of a small amount PRAK may not have a significant effect if the exported PRAK executes the same function as the cytoplasmic PRAK. It is also possible that PRAK exported from the nucleus has a different function than the PRAK activated in the cytoplasm. The 182D mutant of PRAK gained certain levels of kinase activity (New et al., 1998) but could not be exported from the nucleus (Figure 4). Instead of acting as a dominant active mutant, PRAK(182D) has been reported to interfere with Ras-mediated cell proliferation (Chen_et al.,_ 2000). So the dominant negative effect of PRAK(182D) may not be caused by a defect in the kinase activity but could be caused by the defect in the nuclear export of this protein. If the nuclear export of activated PRAK is truly required for the inhibition of Ras signaling, the exported PRAK should have a different function in comparison with the cytoplasmic PRAK. The majority of MAPKAPK2 was detected in nucleus and translocated into cytosol upon cellular stress (Ben-Levy et al., 1998a; Engel et al., 1998). Because of the similarity of these two kinases in their biochemical properties, whether the MAPKAPK2 and PRAK molecules had the same function after translocation into cytosol awaits further investigation.
The family of downstream kinases of MAP kinases can be divided into several groups (Stokoe et al., 1992; Ludwig et al., 1996; Fukunaga and Hunter, 1997; Waskiewicz et al., 1997; Deak et al., 1998; New et al., 1999). It was proposed that PRAK represents a subgroup of this family because it only has 20–30% sequence identity with kinases in the family (New et al., 1998). However, comparative studies did not find a difference between PRAK and the intensively studied MAPKAPK2 (New et al., 1998;Tanoue et al., 2001). The study described here revealed that the subcellular location of PRAK is different from MAPKAPK2, thus providing a direction for studying the difference between PRAK and MAPKAPK2. Therefore, the constant shuttling between the nucleus and the cytoplasm is another important feature of PRAK and may be essential in understanding the biological function of PRAK.
Acknowledgments
We thank Tonya Thompson for excellent secretarial assistance and Dr. Sarah Stanton for critical reading of the manuscript. This work was supported by National Institutes of Health Grants AI41637 and HL07195 and Chinese National Foundation of Natural Science Grant 30128009.
References
- Ben-Levy, R., Hooper, S., Wilson, R., Paterson, H.F., and Marshall, C.J. (1998b). Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr. Biol., 8, 1049-1057. [DOI] [PubMed] [Google Scholar]
- Brunet, A., and Pouyssegur, J. (1997). Mammalian MAP kinase modules: how to transduce specific signals. Essays Biochem. 32, 1-16. [PubMed] [Google Scholar]
- Chan, P.K., Qi, Y., Amley, J., and Koller, C.A. (1996). Quantitation of the nucleophosmin/B23-translocation using imaging analysis. Cancer Lett. 100, 191-197. [DOI] [PubMed] [Google Scholar]
- Chan, P.K., and Chan, F.Y. (1999). A study of correlation between NPM-translocation and apoptosis in cells induced by daunomycin. Biochem. Pharmacol. 57, 1265-1273. [DOI] [PubMed] [Google Scholar]
- Chang, C.I., Xu, B.E., Akella, R., Cobb, M.H., and Goldsmith, E.J. (2002). Crystal structures of MAP kinase p38 complexed to the docking sites on its nuclear substrate MEF2A and activator MKK3b. Mol. Cell. Biol. 9, 1241-1249. [DOI] [PubMed] [Google Scholar]
- Chen, G., Hitomi, M., Han, J., and Stacey, D.W. (2000). The p38 pathway provides negative feedback for Ras proliferative signaling. J. Biol. Chem. 275, 38973-38980. [DOI] [PubMed] [Google Scholar]
- Cohen, P. (1996). Dissection of protein kinase cascades that mediate cellular response to cytokines and cellular stress. Adv. Pharmacol. 36, 15-27. [DOI] [PubMed] [Google Scholar]
- Davis, R.J. (1994). MAPKs: new JNK expands the group. Trends Biochem. Sci. 19, 470-473. [DOI] [PubMed] [Google Scholar]
- Deak, M., Clifton, A.D., Lucocq, L.M., and Alessi, D.R. (1998). Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17, 4426-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, K., Kotlyarov, A., and Gaestel, M. (1998). Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 17, 3363-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, M., Gotoh, Y., and Nishida, E. (1997). Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 16, 1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga, R., and Hunter, T. (1997). MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 16, 1921-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, B., Gram, H., di Padova, F., Huang, B., New, L., Ulevitch, R.J., Luo, Y., and Han, J. (2002). MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science 295, 1291-1294. [DOI] [PubMed] [Google Scholar]
- Gorlich, D. (1998). Transport into and out of the cell nucleus. EMBO J. 17, 2721-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich, O. et al. (1999). MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J. Biol. Chem. 274, 14434-14443. [DOI] [PubMed] [Google Scholar]
- Hotchkiss, R.S., Tinsley, K.W., Swanson, P.E., Chang, K.C., Cobb, J.P., Buchman, T.G., Korsmeyer, S.J., and Karl, I.E. (1999). Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc. Natl. Acad. Sci. USA 96, 14541-14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S., Jiang, Y., Li, Z., Lin, S., Ulevitch, R.J., Nemerow, G., and Han, J. (1997). Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity 6, 739-749. [DOI] [PubMed] [Google Scholar]
- Hunter, T. (2000). Signaling—2000 and beyond. Cell 100, 113-127. [DOI] [PubMed] [Google Scholar]
- Iordanov, M., Bender, K., Ade, T., Schmid, W., Sachsenmaier, C., Engel, K., Gaestel, M., Rahmsdorf, H.J., and Herrlich, P. (1997). CREB is activated by UVC through a p38/HOG-1-dependent protein kinase. EMBO J. 16, 1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., Chen, C., Li, Z., Guo, W., Gegner, J.A., Lin, S., and Han, J. (1996). Characterization of the structure and function of a new mitogen-activated protein kinase (p38β). J. Biol. Chem. 271, 17920-17926. [DOI] [PubMed] [Google Scholar]
- Kotlyarov, A., Neininger, A., Schubert, C., Eckert, R., Birchmeier, C., Volk, H.D., and Gaestel, M. (1999). MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat. Cell Biol. 1, 94-97. [DOI] [PubMed] [Google Scholar]
- Kudo, N., Wolff, B., Sekimoto, T., Schreiner, E.P., Yoneda, Y., Yanagida, M., Horinouchi, S., and Yoshida, M. (1998). Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242, 540-547. [DOI] [PubMed] [Google Scholar]
- Kumar, S., McDonnell, P.C., Gum, R.J., Hand, A.T., Lee, J.C., and Young, P.R. (1997). Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem. Biophys. Res. Commun. 235, 533-538. [DOI] [PubMed] [Google Scholar]
- Kyriakis, J.M., and Avruch, J. (1996). Protein kinase cascades activated by stress and inflammatory cytokines. BioEssays 18, 567-577. [DOI] [PubMed] [Google Scholar]
- Le Cabec, V., Calafat, J., and Borregaard, N. (1997). Sorting of the specific granule protein, NGAL, during granulocytic maturation of HL-60 cells. Blood 89, 2113-2121. [PubMed] [Google Scholar]
- Ludwig, S., Engel, K., Hoffmeyer, A., Sithanandam, G., Neufeld, B., Palm, D., Gaestel, M., and Rapp, U.R. (1996). 3pK, a novel mitogen-activated protein (MAP) kinase-activated protein kinase, is targeted by three MAP kinase pathways. Mol. Cell. Biol. 16, 6687-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinin, N.L., Boldin, M.P., Kovalenko, A.V., and Wallach, D. (1997). MAP3K-realted kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature 385, 540-544. [DOI] [PubMed] [Google Scholar]
- McDonald, P.H., Chow, C.W., Miller, W.E., Laporte, S.A., Field, M.E., Lin, F.T., Davis, R.J., and Lefkowitz, R.J. (2000). Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290, 1574-1577. [DOI] [PubMed] [Google Scholar]
- Murata, T., Yoshino, Y., Morita, N., and Kaneda, N. (2002). Identification of nuclear import and export signals within the structure of the zinc finger protein TIS11. Biochem. Biophys. Res. Commun. 293, 1242-1247. [DOI] [PubMed] [Google Scholar]
- Nebreda, A.R., and Porras, A. (2000). p38 MAP kinases: beyond the stress response. Trends. Biochem. Sci. 25, 257-260. [DOI] [PubMed] [Google Scholar]
- Nemoto, S., Xiang, J., Huang, S., and Lin, A. (1998). Induction of apoptosis by SB202190 through inhibition of p38beta mitogen-activated protein kinase. J. Biol. Chem. 273, 16415-16420. [DOI] [PubMed] [Google Scholar]
- Neufeld, B., Grosse-Wilde, A., Hoffmeyer, A., Jordan, B.W., Chen, P., Dinev, D., Ludwig, S., and Rapp, U.R. (2000). Serine/threonine kinases 3pK and MAPK-activated protein kinase 2 interact with the basic helix-loop-helix transcription factor E47 and repress its transcriptional activity. J. Biol. Chem. 275, 20239-20242. [DOI] [PubMed] [Google Scholar]
- New, L., Jiang, Y., Zhao, M., Liu, K., Zhu, W., Flood, L.J., Kato, Y., Parry, G.C., and Han, J. (1998). PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 17, 3372-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New, L., Zhao, M., Li, Y., Bassett, W.W., Feng, Y., Ludwig, S., Padova, F.D., Gram, H., and Han, J. (1999). Cloning and characterization of RLPK, a novel RSK-related protein kinase. J. Biol. Chem. 274, 1026-1032. [DOI] [PubMed] [Google Scholar]
- Ni, H., Wang, X.S., Diener, K., and Yao, Z. (1998). MAPKAPK5, a novel mitogen-activated protein kinase (MAPK)-activated protein kinase, is a substrate of the extracellular-regulated kinase (ERK) and p38 kinase. Biochem. Biophys. Res. Commun. 243, 492-426. [DOI] [PubMed] [Google Scholar]
- Nishida, E., and Gotoh, Y. (1993). The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci. 18, 128-131. [DOI] [PubMed] [Google Scholar]
- Ono, K., and Han, J. (2000). The p38 signal transduction pathway: activation and function. Cell Signal. 12, 1-13. [DOI] [PubMed] [Google Scholar]
- Ossareh-Nazari, B., Bachelerie, F., and Dargemont, C. (1997). Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278, 141-144. [DOI] [PubMed] [Google Scholar]
- Robinson, M.J., and Cobb, M.H. (1997). Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9, 180-186. [DOI] [PubMed] [Google Scholar]
- Schaeffer, H.J., Catling, A.D., Eblen, S.T., Collier, L.S., Krauss, A., and Weber, M.J. (1998). MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 281, 1668-1671. [DOI] [PubMed] [Google Scholar]
- Seternes, O.M., Johansen, B., Hegge, B., Johannessen, M., Keyse, S.M., and Moens, U. (2002). Both binding and activation of p38 mitogen-activated protein kinase (MAPK) play essential roles in regulation of the nucleocytoplasmic distribution of MAPK-activated protein kinase 5 by cellular stress. Mol. Cell. Biol. 22, 6931-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, B., Yang, M.X., Young, D.B., Janknecht, R., Hunter, T., Murray, B.W., and Barbosa, M.S. (1997). p38–2, a novel mitogen-activated protein kinase with distinct properties. J. Biol. Chem. 272, 19509-19517. [DOI] [PubMed] [Google Scholar]
- Stokoe, D., Campbell, D.G., Nakielny, S., Hidaka, H., Leevers, S.J., Marshall, C., and Cohen, P. (1992). MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 11, 3985-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, B., and Karin, M. (1996). Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. 8, 402-411. [DOI] [PubMed] [Google Scholar]
- Wang, X., McGowan, C.H., Zhao, M., He, L., Downey, J.S., Fearns, C., Wang, Y., Huang, S., and Han, J. (2000). The involvement of MKK6–p38γ cascade in γ-radiation-induced cell cycle arrest. Mol. Cell. Biol. 20, 4543-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue, T., Adachi, M., Moriguchi, T., and Nishida, E. (2000). A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol. 2, 110-116. [DOI] [PubMed] [Google Scholar]
- Tanoue, T., Maeda, R., Adachi, M., and Nishida, E. (2001). Identification of a docking groove on ERK and p38 MAP kinases that regulates the specificity of docking interactions. EMBO J. 20, 466-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz, A.J., Flynn, A., Proud, C.G., and Cooper, J.A. (1997). Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 16, 1909-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz, A.J., and Cooper, J.A. (1995). Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr. Opin. Cell Biol. 7, 798-805. [DOI] [PubMed] [Google Scholar]
- Wen, W., Meinkoth, J.L., Tsien, R.Y., and Taylor, S.S. (1995). Identification of a signal for rapid export of proteins from the nucleus. Cell 82, 463-473. [DOI] [PubMed] [Google Scholar]
- Whitmarsh, A.J., and Davis, R.J. (1998). Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem. Sci. 23, 481-485. [DOI] [PubMed] [Google Scholar]
- Winzen, R. et al. (1999). The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18, 4969-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]