Fibrin Fibers Have Extraordinary Extensibility and Elasticity (original) (raw)
. Author manuscript; available in PMC: 2007 Aug 20.
Published in final edited form as: Science. 2006 Aug 4;313(5787):634. doi: 10.1126/science.1127317
Blood clots stem the flow of blood, which is essentially a mechanical task. Hence, there has been a longstanding interest, initiated by Ferry et al. (1), in elucidating the mechanical properties of clots. The macroscopic properties are well known (2), and these can be related to clotting disorders. Nevertheless, the underlying microscopic mechanisms that give rise to these macroscopic properties are not understood (3). Clots form when soluble fibrinogen is converted to fibrin monomers that polymerize to form a branched network of fibrin fibers. The mechanical properties of any branched network depend on the network architecture and the mechanical properties of the individual fibers. The fibrin network architecture has been well-characterized in microscopy images (2). In contrast, aside from the bending modulus for small strains (4) and the radius dependence of the rupture force in air (5), the mechanical behavior of single fibrin fibers is unknown. Yet, exactly this knowledge is needed to construct and test mechanical models of blood clots.
We have developed an atomic force–fluorescence microscopy technique to study the mechanical properties of single fibrin fibers (6) (fig. S1). The tip of the atomic force microscope (AFM) was used to stretch fibers that were suspended across 12 μm-wide channels; the fluorescence microscope was used to image this stretching process. We selected and analyzed fibers that bridged the grooves at an approximate right angle and that were well anchored on the ridges (i.e., strain was not due to fiber slipping). Thus, our experimental design yielded a well-defined geometry. We determined the extensibility and elastic limit of fibers formed in the presence and absence of factor XIIIa (FXIIIa) (6). FXIIIa induces covalent cross-links between the γ chains [along the fiber axis (7)] and between the α chains. In this study, γ-γ cross-linking was 50 to 75% complete, and α-α cross-linking was 25 to 35% complete. Samples without FXIIIa showed no cross-linking.
All fibers showed extremely large extensibilities and recovered elastically from very large strains [definitions in (6)]. Figure 1, A to D (movie S1), depicts the rupture of a fiber. The fiber did not break at the AFM tip, indicating that the fiber ruptured due to stretching between the tip and the anchoring point. Uncross-linked fibers extended 226 ± 52% (36 fibers), and cross-linked fibers extended 332 ± 71%, or 4.32 times their original length (29 fibers). The most extreme fibers could be extended over six times their length [over 500% strain (movie S3)]. These extensibilities are the largest of any protein fiber (table S1). After rupture, the fiber arms contract to nearly their original length, indicating mainly elastic deformations.
Fig. 1.
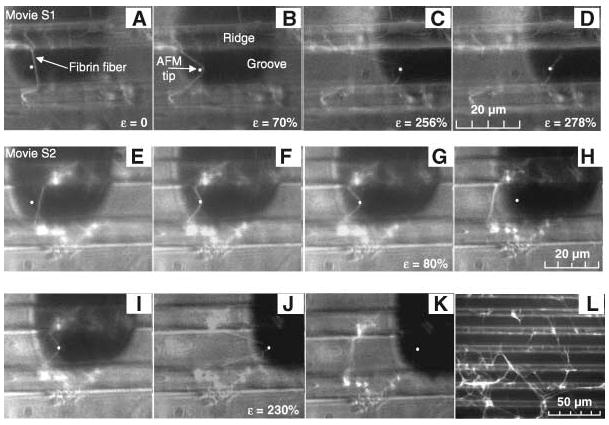
(A to D) Extensibility. A fiber suspended between two ridges (brighter horizontal bands) is stretched with the AFM tip (white dot) until rupture. The AFM cantilever appears as a dark, 35-μm-wide rectangle. The lower and upper segments of the fiber break at 183% strain and 278% strain, respectively. (E to H) Elastic deformation. A fiber was strained 80%, from which it snapped back to its original length. (I to K) Permanent deformation. The same fiber was strained to 230% (J), suffering some permanent lengthening (K). (L) Representative clot used for fiber manipulations.
We tested the elastic limit by stretching fibers to a certain strain and releasing the applied force (Fig. 1, E to K, and movie S2). Uncross-linked fibers could be stretched 2.2 times their length (120%) and recover elastically. Remarkably, cross-linked fibers could be stretched over 2.8 times their length (180% strain) and still recover without permanent damage (movies S4 and S5).
Fibrin fibers displayed extreme and different mechanical properties relative to other protein polymers (table S1). Highly regular, nearly crystalline fibers, such as actin filaments and microtubules, show small extensibilities. Despite the nearly crystalline arrangement of fibrin monomers along the fiber axis, fibrin fibers show extraordinarily large extensibilities. This apparent conflict suggests the fibrin monomers can extend substantially and reversibly while maintaining the most important fibrin-fibrin interactions within a fiber.
Furthermore, the effect of cross-linking is unusual in fibrin. In collagen, spider silk, and keratin fibers, cross-linking makes fibers stiffer and less extensible. The increased extensibility and elasticity we observed for cross-linked fibrin indicates the cross-links are directional, along the fiber axis. Thus, in physiological conditions, the fast-forming γ-γ cross-links along the axis may enhance elasticity and prevent rupture of the nascent fibers.
Whereas fibers have over 330% extensibility, whole fibrin film networks have extensibilities of “only” 100 to 200% (1). Because a network has two mechanisms to extend, first aligning then stretching fibers, it is extraordinary that individual fibrin fibers have the larger extensibility. These data suggest clot rupture does not arise from the rupture of individual fibers, as previously assumed. Rather, the branch points yield first. Further analysis may nevertheless show that the remarkable extensibility and elasticity of individual fibers influence the mechanical behavior of clots formed in vivo.
Supplementary Material
Supplemental Methods, Figures, Tables, and References
Supplemental Movie 1
Supplemental Movie 2
References and Notes
- 1.Ferry JD, Morrison PR. J Am Chem Soc. 1947;69:388. doi: 10.1021/ja01194a066. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Mockros LF, Weisel JW, Lorand L. Biophys J. 1999;77:2813. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisel JW. Biophys Chem. 2004;112:267. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Collet JP, Shuman H, Ledger RE, Lee ST, Weisel JW. Proc Natl Acad Sci USA. 2005;102:9133. doi: 10.1073/pnas.0504120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guthold M, et al. Biophys J. 2004;87:4226. doi: 10.1529/biophysj.104.042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Materials and Methods. Science. available on Online. [Google Scholar]
- 7.McKee PA, Mattock P, Hill RL. Proc Natl Acad Sci USA. 1970;66:738. doi: 10.1073/pnas.66.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.We thank M. C. Stahle, J. L. Moen, O. V. Gorkun, and L. Carroll for advice and technical assistance. We acknowledge support from NIH grant nos. P41 EB002025 (R.S.), R01 HL31048 (S.T.L.), R41 CA10312 (M.G.); Research Corporation grant no. RI0826 (M.G.); and American Cancer Society grant no. IRG-93-035-6 (M.G.).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods, Figures, Tables, and References
Supplemental Movie 1
Supplemental Movie 2