DEFECTIVE PRESENTATION OF ENDOGENOUS ANTIGENS BY A MURINE SARCOMA: Implications for the Failure of an Anti-Tumor Immune Response (original) (raw)
. Author manuscript; available in PMC: 2007 Aug 21.
Published in final edited form as: J Immunol. 1991 Aug 15;147(4):1453–1459.
Abstract
MHC class I-restricted CTL play a central role in the immune response against methylcholanthrene (MCA)-induced sarcomas in mice. We, therefore, hypothesized that MCA-induced tumors may evade immune recognition by failing to present Ag to CD8+ CTL. Of a number of previously described MCA-induced sarcomas, one, MCA 101, fails to induce CTL, is nonimmunogenic, and grows rapidly and lethally in nonimmunosuppressed recipients. To better understand the nonimmunogenicity of MCA 101 we examined its ability to present foreign Ag to CTL. Unlike immunogenic sarcomas, MCA 101 failed to present endogenously synthesized influenza virus Ag to influenza virus-specific CTL. The deficiency in presentation of endogenous Ag by MCA 101 was attributed to a markedly reduced rate of synthesis of class I molecules because up-regulation of class I synthesis by IFN-γ greatly increased the presentation of influenza A virus Ag. Despite low levels of cell surface class I expression, MCA 101 presented exogenous peptide Ag to anti-influenza CTL with efficiency similar to immunogenic MCA sarcoma cell lines. These findings could not be attributed to deficiencies in class I assembly or transport, as has been suggested by others who have studied mutant cells with defective Ag presentation. Furthermore, our studies suggest that some tumor cells can escape recognition by CTL and subsequent immune eradication by suppressing presentation of endogenous Ag.
Murine MCA2 sarcomas differ widely in their ability to elicit a specific immune response (1, 2). MHC class I-restricted CD8+ T cells are an essential element of this immune response (3-9). MCA sarcomas function as specific immunogens in vivo, protecting an animal against subsequent tumor challenge by the immunizing sarcoma but not by other independently derived syngeneic sarcomas (2, 6, 8, 9). The immune response against murine MCA-induced sarcomas is abrogated by the systemic administration of anti-CD8+ antibody (8).Class I-restricted CD8+ CTL can be generated that specifically lyse human and murine tumor cells in vitro (6, 10, 11). In vivo CD8+ CTL can eliminate established tumor in both mouse and man (3, 4, 6, 8, 9, 12-14). Thus, CTL play a critical role in host rejection of tumor. Some murine MCA-induced tumors are classified as nonimmunogenic because they fail to induce CTL and rapidly kill their hosts (8).
Knowledge of the nature of Ag recognized by tumorspecific CTL has increased over the past several years. It is now well established that CTL specific for SV40-transformed cell lines recognize antigenic fragments of the T Ag (15). Boon and Van Pel (16) have characterized stable mutants of P815 cells that, unlike the parental cells, fail to form progressive tumors due to their enhanced ability to elicit CTL. These CTL recognize antigenic fragments of altered proteins expressed by the mutants(16).Thus, anti-tumor CTL can recognize Ag that are processed in the same manner as foreign Ag for presentation in association with class I molecules.
There has been considerable progress in understanding how Ag are processed by cells and presented for CTL recognition. Class I molecules are composed of a membrane glycoprotein of 45 kDa non-covalently associated with _β_2-microglobulin. Proteins located in the cytosol appear to be cleaved into fragments of approximately 9 residues(17). These peptides are then transported into the exocytic compartment, possibly by specialized proteins encoded within the MHC that are members of the P-glycoprotein family (18-21). Binding to class I molecules occurs in the exocytic pathway. either in or between the endoplasmic reticulum and the early Golgi complex (22, 23). Solution of the 3-dimensional structure of class I molecules revealed a groove in the molecule thought to bind Ag (24). Addition of synthetic peptides that correspond to the natural antigenic determinants bypasses the normal route of Ag processing, by binding to class I molecules at the cell surface (25). Mutant cell lines have been identified, such as RMA-S, which fail to present endogenously synthesized proteins to CTL (26, 27). Such mutants are believed to be deficient in their ability to generate Ag or transport Ag into the exocytic compartment (28). In these cell lines, class I H chains fail to properly associate with _β_2-microglobulin and are transported at a slower rate to the plasma membrane. These cells, however, present exogenously provided peptide Ag at normal or supernormal levels despite the relatively low levels of steady state expression of cell surface class I molecules (29). It has been proposed that the ability of mutants to present exogenous peptides is due to their expression of equal or greater amounts of class I molecules with nonoccupied binding grooves (30).
In the present study, we have correlated the immunogenicity of seven syngeneic, murine MCA-induced tumor cell lines and a colon adenocarcinoma with their ability to present endogenously synthesized foreign Ag to specific CTL. We found that the only cell line of the eight that consistently failed to induce CTL responses demonstrated a near absolute deficit in presenting endogenously synthesized influenza Ag to CTL. We then investigated the nature of the deficiency in this cell line. These findings have potential implications in explaining the escape of tumors from immune recognition.
MATERIALS AND METHODS
Tumors
MCA-induced sarcomas (101, 102, 105, 106, 203, 205, and 207) were generated in our laboratory in 8-wk-old female C57BL/6n mice (Animal Production Colonies, Frederick Cancer Research Facility, National Institutes of Health, Frederick, MD) by intramuscular injection of 0.1 ml of 0.1% MCA in sesame seed oil. MC 38 is a colon adenocarcinoma of C57BL/6n origin (12). RMA is a lymphoma and RMA-S is a mutant derived from RMA by Kärre and colleagues (31). Tumor lines were passaged in C57BL/6n mice and all tumors were of early passage (passage 3 to 7) except for MC 38 (passage number unknown). Tumors were harvested from mice, triple-enzyme digested with 0.1% collagenase, 0.002% DNase, and 0.01% hyaluronidase (all from Sigma, St. Louis, MO) and maintained in monolayer culture in CM containing RPMI 1640, 10% heat-inactivated FCS, 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate (all from Biofluids, Rockville. MD), 5 × 10-5 M2-ME (Aldrich Chemical Co., Milwaukee, WI). and 0.03% (100 mM) glutamine (NIH media unit, Bethesda. MD). For some experiments, 200 U/ml of recombinant murine IFN-γ (Genentech, South San Francisco, CA) were added.
Pulsing of tumor cells with exogenous Ag
Amino acids 365-380 of the nucleoprotein gene product of the influenza A/PR/8/34 virus have the sequence IASNENMETHESTLE-amide (single letter amino acid symbols) (32). This peptide was synthesized and purified to >98% purity by HPLC by Multiple Peptide Systems (San Diego, CA). Tumor cells (3 × 106) in a volume of 1 ml of CM were “pulsed” with concentrations of peptide ranging from 0.01 _μ_M to100 _μ_M. This peptide pulsing was done during 51Cr labeling for 90 min at 37°C. Cells were then washed three times with HBSS (Biofluids).
Viruses and infection of tumor lines
Tumor cells (3 × 106) were washed three times in RPMI 1640 then placed in 1 ml of RPMI 1640 with 0.1% BSA (Sigma) and 30 mM HEPES (Biofluids) at pH 6.8. Cells were then infected with one of the following viruses for 90 at 37°C: 5 HA units per cell of PR8, 20 plaque-forming units per cell of wild-type Vac, or 20 plaque-forming units per cell of various Vac recombinant constructs containing one of the following influenza genes: NP, NS1, HA, and PA (33). The nucleocapsid gene from the vesicular stomatitis virus inserted into a vaccinia recombinant was used as a control (34). After infection, cells were incubated in CM for 3 h at 37°C, then labeled with 51Cr, as described below. Viral infection by PR8 of target cells was verified by their ability to each rosette >5 human RBC. The production of the viral HA Ag was quantified by FACS with anti-HA mAb and goat anti-mouse FITC-labeled antibody (Becton Dickinson. Mountain View, CA). Production of viral proteins not expressed on the cell surface was verified by cytoplasmic staining: the cell lines were allowed to adhere to the bottom of a flat-bottomed 96-well plate (Costar, Cambridge, MA), then washed three times with PBS (Biofluids). Cell membranes were rendered permeable by fixation with 50% ethanol/50% acetone(v/v). Cells were then washed four times with PBS. One of three murine mAb (HB-65 anti-NP, NS1-1A7 anti-NS1 protein, or H28-E23 anti-HA Ag) (35) was then added with overnight incubation. Cells were then washed two times with PBS. Horseradish peroxidase-linked rat anti-mouse antibody (Jackson Immuno Research Laboratories, West Grove, PA) was added with incubation for 1 h. Plates were then developed using _o_-phenylenediamine dihydrochloride (Sigma).
Effector cells
Polyclonal responder populations were generated by in vivo priming of female 6-wk-old C57BL/6n mice with 200 _μ_l of PR8 viral allantoic fluid (diluted 1:10) which represented 20 HA units. After at least 2 wk, spleens were removed, dispersed to single cell suspensions with a Dounce homogenizer. and cultured in Iscove’s modified medium with 7.5% heat-inactivated FCS (Biofluids). Splenocytes were then cultured for 7 days in the absence of IL-2 either with synthetic peptide at 1 _μ_g/ml (~0.6 _μ_M) or with PR8-infected spleen cells. Allogeneic effector cells were generated by a MLC with DBA/2 splenocytes cultured with irradiated C57BL/6n splenocytes in the presence of 20 Cetus units IL-2/ml (Cetus Corp., Emeryville. CA) in CM and cultured for at least 5 days. LAK cells were generated as described previously (5). Briefly, fresh splenocytes from C57BL/6n mice were cultured in 1000 Cetus units of recombinant human IL-2/ml of CM for 3 to 5 days.
FACS analysis
Cell surface class I expression was assayed by FACS analysis by using a FACScan 440 (Becton Dickinson). Cultured tumor cell lines were harvested with 0.02% EDTA, washed, then stained for 30 min with culture supernatant from one of the following mAb: 28.14.8s (anti-Db), 28.8.6s (anti-Kb and -Db) (36), obtained from D. Sachs (Immunology Branch, National Cancer Institute, Bethesda, MD), H28-E23 (anti-HA Ag) or with the appropriate isotype matched control antibody (Becton Dickinson) followed by goat anti-mouse FITC-conjugated antibody (Boehringer Mannheim Biochemicals, Indianapolis, IN). FACS analyses were standardized with Calibrite flow cytometer beads (Becton Dickinson).
51Cr-release assay
Four-hour 51Cr-release assays were per-formed as previously described (6). Briefly, 2 × 106 tumor targets in 0.5 ml of CM were labeled with 200 _μ_Cl of 51Cr (New England Nuclear, Boston, MA) for 90 min. In experiments where peptide was exogenously provided, tumor lines were “pulsed” with synthetic peptide during 51Cr labeling as described by Townsend et al. (25). When we studied endogenous presentation, cells were infected with virus before 51Cr labeling, as detailed above. Labeled tumor cells were coincubated with anti-viral effector CTL lines for 4 h. Supernatants were harvested and counted with a gamma counter (LKB Instruments, Gaithersburg. MD). Percent lysis was calculated as follows: (experimental cpm – spontaneous cpm) / (maximal cpm – spontaneous cpm] × 100.
Metabolic radiolabeling experiments
Pulse-chase experiments were performed as previously described (37). Briefly, 6 × 106 cultured tumor cells were washed three times in prewarmed PBS. They were incubated in methionine-free DMEM (Biofluids) for 30 min at 37°C, then pulsed with 100 _μ_Ci 35S-methionine (Amersham, Arlington Heights, IL) for 10 min. Ice-cold PBS was then immediately added to half of the cells. These cells represent the “pulse” portion of the experiment. Warmed CM containing 2 mg/ml of methionine (Sigma) was added to a second portion of the cells, which were then incubated at 37°C for 2 h. These cells are designated “chase.” Both groups were then lysed and precipitated with 10% TCA to equalize the number of counts in each sample (38). Class I molecules were then immunoprecipitated by using the antibody 28.14.8s. One-half of each sample was then digested overnight with 0.005 U endo H (Boehringer Mannheim Biochemicals). The other half of the sample was mockdigested. Samples were then analyzed with a 12% SDS-polyacrylamide gel, which was then treated by Amplify (Amersham), dried, and exposed to preflashed Kodak XAR-5 X-ray film (Eastman Kodak, Rochester, NY) for autoradiography.
RESULTS
MHC expression of murine MCA-induced sarcoma cell lines
Table I summarizes information about the tumor lines described in this report. MC 38, MCA 105, MCA 203, MCA 205, and MCA 207 are described as immunogenic in that they protected against subsequent tumor challenge (6, 8, 12). MCA 102 had a n intermediate phenotype: it induced a CTL response, but did not protect against subsequent challenge (8, 9). In earlier studies (7, 8) MCA 106 expressed high levels of class I and was immunogenic. More recently, MCA 106 was found to be a low class I expresser. Nevertheless, when previously performed immunization-challenge experiments were repeated, MCA 106 was found to have remained immunogenic, despite its reduced levels of class I expression. MCA 101 neither induced CTL nor protected against tumor challenge (9).
TABLE I.
Summary in vitro and in vivo characteristics of MCA-induced sarcomas
| Tumor | Anti-Tumor CTLa in Vitro | Immunogenb in Vivo | MHC Class Ic Expression | References |
|---|---|---|---|---|
| MC 38 | Yes | Yes | 423 ± 12 | 12 |
| MCA 207 | Yes | Yes | 249 ± 4 | 6 |
| MCA 205 | Yes | Yes | 110 ± 3 | 6 |
| MCA 203 | Yes | Yes | 64 ± 2 | 6 |
| MCA 105 | Yes | Yes | 32 ± 9 | 7, 8 |
| MCA 102 | Yes | No | 28 ± 3 | 7-9 |
| MCA 101 | No | No | 7 ± 1 | 7, 8 |
| MCA 106 | Yes | Yes | 4 ± 1 | 7, 8 |
As shown in Table I, the tumors differed widely in their expression of class I molecules on the cell surface. At opposite ends of the distribution are MC 38 and MCA 101 or MCA 106, which had a 100-fold difference in steady state levels of cell surface class I. Similar results were obtained by using either fresh tumor explants or cells that had been maintained in tissue culture. MHC class II molecules were not detected on the surfaces of any of these tumors by FACS analysis (data not shown) (7).
Quantitation of viral infection of tumor cell lines
As a measure of the ability of tumor cell lines to present endogenously synthesized Ag to CTL, we examined the ability of CTL to lyse cells that were infected by influenza virus. As cells vary widely in their susceptibilities to influenza virus infection (39), it was essential to establish that the cells could be infected by influenza and produce viral gene products.
Indirect immunoperoxidase assays revealed that after infection with PR8, each of the tumor cell lines abundantly expressed the NS1 protein as was evidenced by light microscopy (data not shown). This protein is synthesized in large amounts during influenza virus infection but is not a component of the viral particle. Its presence in infected cells then, indicates that viral RNA is transcribed and translated in target cells (39). To quantify the degree of infection between different cell lines, FACS analysis was performed on viable cells by using a mAb specific for the viral HA, which is normally expressed on the plasma membrane. The different demonstrated considerable variability in both the percentage of cells expressing HA, and the mean fluorescence of positive cells. It should be emphasized, however, that MCA 101 was very well infected by PR8 virus and was also highly productive of viral gene products. In fact, 97% of all MCA 101 tumor cells were found to be infected by wild-type PR8 virus as measured by their ability to rosette human RBC. FACS analysis revealed that MCA 101 was among the highest in its HA expression after infection by PR8 virus (data not shown). HA expression increased 40-fold in the 4-h incubation period after infection of cells with virus (data not shown). This indicates that most of the HA expressed by cells was synthesized by the cells, and did not simply represent free or virion-associated HA that adsorbed to the cell surface during incubation with the virus inoculum.
Presentation of endogenously synthesized influenza virus Ag
To determine the ability of MCA tumor cell lines to present endogenously synthesized proteins, we first determined the ability of polyclonal anti-PR8 CTL present in secondarily stimulated splenocyte cultures to lyse PR8-infected cells. The results of eight experiments are summarized in Figure 1. Six of the cell lines were lysed at values ranging from 20% to 60%. For these six cell lines, there was no obvious correlation between the degree of lysis and the levels of cell surface class I. For example, MCA 102 was lysed as efficiently as MC 38, which expressed far more class I at the cell surface. On the other hand, MCA 101, which expressed very low amounts of cell surface class I, was not lysed above background levels. Furthermore, MCA 106 expressed the lowest levels of class I and likewise presented Ag poorly. RMA and RMA-S were included as controls.
Figure 1.
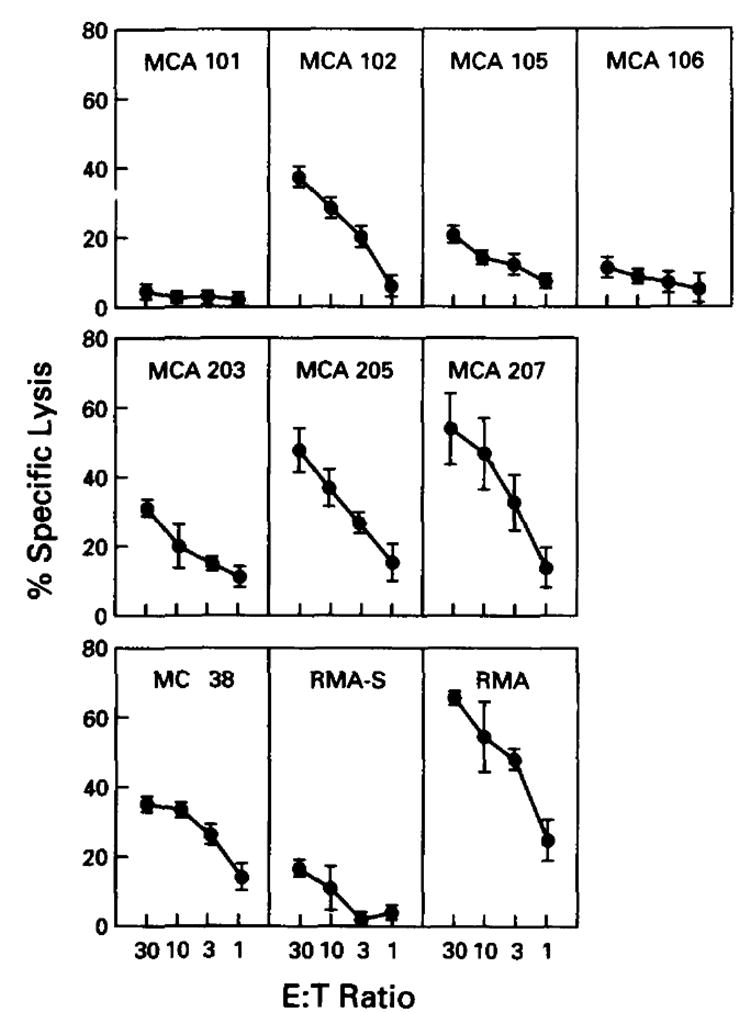
Processing and presentation of endogenously generated viral Ag by cultured tumor cell lines. Effector cells were splenocytes generated by in vivo and in vitro stimulation with PR8. Tumor cell lines were infected with wild-type influenza A virus (PR8), followed by a 4-h incubation period to allow for elaboration of viral protein. Results of eight 4-h 51Cr-release assays are depicted here. Experimental values represent the average of triplicates, with a SE of the mean of less than 4%. These experimental values are then averaged. Error bars represent SE of the mean. Each tumor was included in the following number of experiments: MCA 101 in seven, MCA 102 in four, MCA 105 in two, MCA 106 in three, MC 38 in two, MCA 203 in two, MCA 205 in five, MCA 207 In four, RMA in two, and RMA-S in two. Average lysis for controls (i.e., uninfected cells) was <5% for each tumor depicted.
We next examined the ability of MCA 101 to present individual influenza virus proteins to CTL (Fig. 2). This was accomplished by infecting cells with Vac recombinants expressing individual influenza virus proteins that are known to be recognized by H-2b-restricted CTL. This revealed that, although NP, NS1, HA, and PA were presented by MCA 207, none of the proteins were presented by MCA 101 over control levels obtained with a Vac recombinant expressing the nucleocapsid protein of vesicular stomatitis virus (Control). These findings indicated that MCA 101 was incapable of presenting a wide variety of proteins to CTL via the endogenous route.
Figure 2.
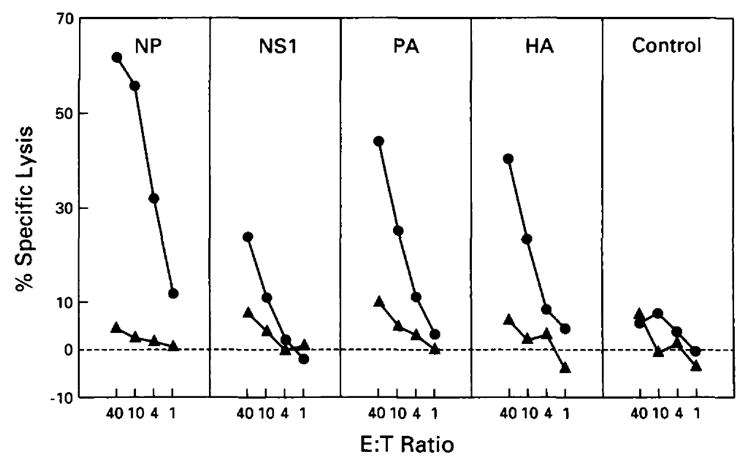
Processing and presentation of endogenous influenza virus proteins inserted into vaccinia vectors. In this experiment, MCA 101 (solid triangles) is compared with MCA 207 (solid circles). Polyclonal effectors used were splenocytes from mice immunized with PR8 virus in vivo and restimulated in vitro with wild-type PR8 influenza virus for targets infected with NS1, PA, and HA and primed with PR8 in vivo and synthetic peptide (aa 365-380) of NP in vitro for targets infected with NP and Control. Tumor lines were infected with vaccinia constructs containing the following influenza A genes: nucleoprotein (NP), nonstructural 1 (NS1), acidic polymerase (PA), and hemagglutinin (HA). The vesicular stomatitis virus gene for the Indiana nucleocapsid protein(Control) inserted into a vaccinia vector was used as a control. All experimental values represent the average of triplicates, with a SE of the mean of less than 4%. These results confirmed similar results obtained in five different experiments comparing MCA 101 and MCA 207, which used wild-type PR8 infection.
Presentation of exogenously provided influenza peptide Ag
Given the profound deficit in the ability of MCA 101 to present influenza virus proteins, it was important to determine its ability to present exogenous peptide Ag. Tumor cells were incubated with various concentrations of a synthetic peptide corresponding to amino acids 365-380 of the PR8 NP (Fig. 3). This region represents a determinant of the NP gene product binding to the Db molecule (28). Polyclonal effector cell lines directed against the peptide in the context of Db were then used to lyse pulsed tumor cell lines by polyclonal anti-PR8 CTL.
Figure 3.
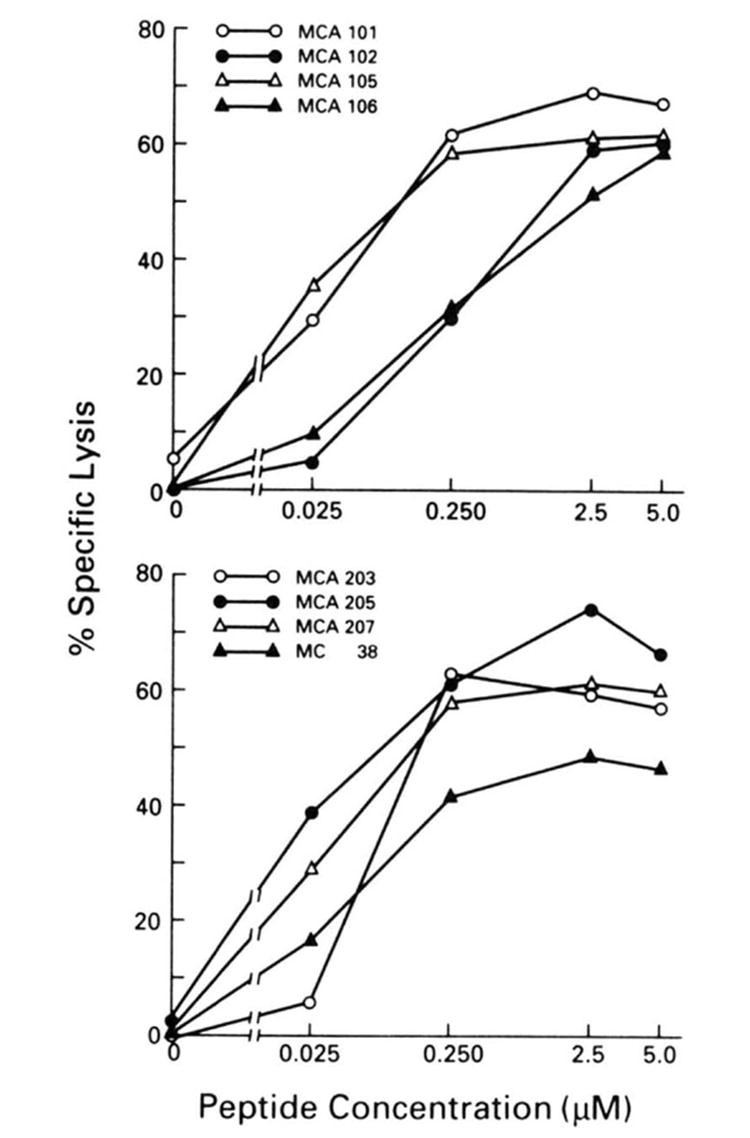
Recognition of MCA-induced tumor lines after exposure to synthetic peptide amino acids 365-380 of NP at varying concentrations. Effector cells were splenocytes from mice immunized with PR8 virus and restimulated in vitro with synthetic peptide (NP 365-380) at a concentration of 1 _μ_M. All experimental values represent the average of triplicates, with a SE of the mean of less than 4%. Each value is representative of between two and five experiments; all had similar results.
Killing of all of the cell lines reached a plateau at approximately 60% specific lysis with increasing amounts of peptide to 5 _μ_M. Increasing the peptide concentration to 100 _μ_M did not raise the killing above this level. Despite expressing very low levels of cell surface class I molecules, MCA 101 was among the most efficient of the lines at presenting exogenous peptide. This finding indicates that the failure of MCA 101 to present endogenously synthesized Ag is not due simply to its inability to be lysed by CTL, because of, for example, diminished expression of cell surface adhesion molecules that enhance the binding of CTL to target cells. Moreover, MCA 101, MCA 102, and MCA 207 differed little in their susceptibility to lysis by LAK cells (data not shown). Thus, these results could not be attributed to inherent resistance to cell mediated lysis in general.
Susceptibility to lysis by allo CTL was correlated with the level of class I expression (data not shown). The high class I-expressing MCA 207 was lysed by allo-reactive CTL, whereas MCA 101 and MCA 102 were resistant to lysis. There were no significant differences in LAK lysability. Specifically, low class I-expressing cell lines were not more LAK lysable than high class I-expressing cell lines in this series.
Defining the Ag presentation deficit in MCA 101
In expressing low levels of surface class I molecules and presenting exogenous but not endogenous Ag to CTL, MCA 101 behaved like the RMA-S and T2 mutant cell lines described by others (29). It was, therefore, important to determine whether the deficiency of class I assembly and transport exhibited by these previously described mutants also was present in MCA 101.
We first examined whether incubation of MCA 101 with the synthetic peptide enhanced the binding of a conformation-specific anti-Db mAb (B22.249) to the cell surface, as reported for RMA-S (26). Although we could reproduce enhanced binding of B22.249 to RMA-S cells, we observed no similar increase in MCA 101 (data not shown). Next, we tested whether incubation of cells at reduced temperatures (20 to 26°C) increased the binding of B22.249 to the cell surface (30). Although we could verify enhanced binding to RMA-S cells, there was no detectable effect on MCA 101 cells (data not shown).
We next biochemically characterized the biosynthesis and intracellular transport of Db molecules in MCA 101 cells. Cells were labeled for 10 min with 35S-methionine, incubated at 0°C or 37°C for 2 h, and then detergent-extracted. Db molecules were then immunoprecipitated by using the conformation-independent 28.14.8s mAb (36). To measure the intracellular transport of Db, half of the immunoprecipitates were digested with endo H, which cleaves _N_-linked oligosaccharides only when they are in the high mannose form characteristic of proteins present in the endoplasmic reticulum and cis-Golgi complex. For the sake of comparison, lysates from RMA cells treated in identical manner were also immunoprecipitated after equalizing the amount of TCA precipitable counts. This revealed (Fig. 4) that the assembly and transport of class I molecules in MCA 101 is very similar to that in RMA cells. Db H chains rapidly assemble with _β_2-microglobulin in MCA 101 cells, as in RMA cells, and a similar portion of molecules are resistant to endo H 2 h after their synthesis. It is also clear, however, that the rate of synthesis of class I is much lower in MCA 101 cells than in RMA cells. The decreased rate of Db synthesis in MCA 10 1cells is not a characteristic of MCA tumors; additional experiments (not shown) revealed that MCA 205 and MCA 207 cells expressed similar amounts of Db as RMA cells. This was consistent with previous reports that the levels of class 1 mRNA were depressed in MCA 101 cells relative to other MCA tumors (40).
Figure 4.
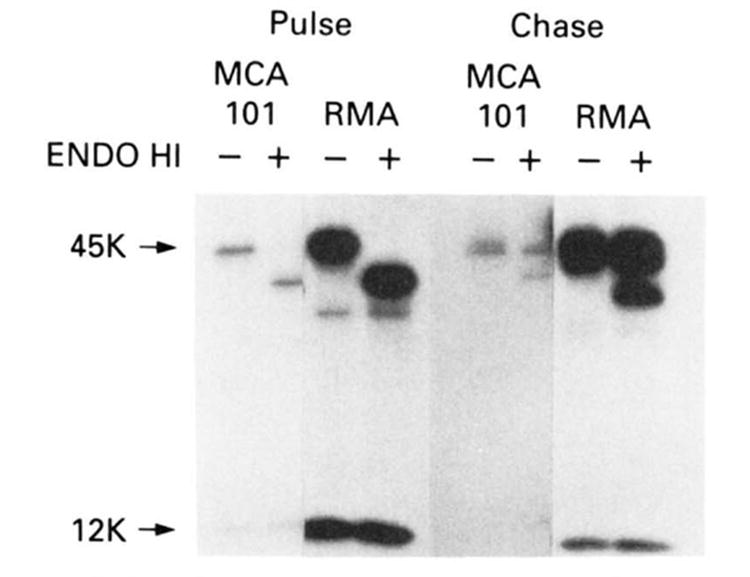
SDS-PAGE analysis of immunoprecipitated class I molecules after pulsing cells with 35S-methionine then chasing with cold methionine as described in Materials and Methods. Left lanes are with mock endo-H treatment where as right lanes show treatment with endo H. This experiment shows that class I molecules are present and transported in MCA 101. Although not readily appreciable in the present reproduction. appropriate quantities of _β_2-microglobulin were found to co-precipitate with the H chain. Control cell line is RMA. This experiment was repeated with similar results.
These findings indicate that the decreased cell surface expression of class I molecules in MCA 101 cells is due to a diminished rate of synthesis, and not to a deficiency in class I transport or assembly. Furthermore, they suggest that the deficit in presentation of endogenously synthesized Ag is due to the low levels of class I synthesis. To test this possibility, we treated cells with IFN-γ, which, by increasing the levels of class 1 mRNA in cells, increases class I biosynthesis (41).
Treatment of MCA 101 cells with IFN-γ increased the biosynthesis of Db molecules as determined by immunoprecipitation and SDS-PAGE (data not shown) and, furthermore, did not decrease the ability of influenza virus to infect these cell lines as documented by expression of HA Ag (data not shown).Pretreatment with IFN-γ restored the ability of MCA 101 to present influenza virus Ag to CTL (Fig. 5). Thus, the failure of MCA 101 to present endogenously synthesized Ag to CTL is likely due to its decreased expression of class I molecules.
Figure 5.
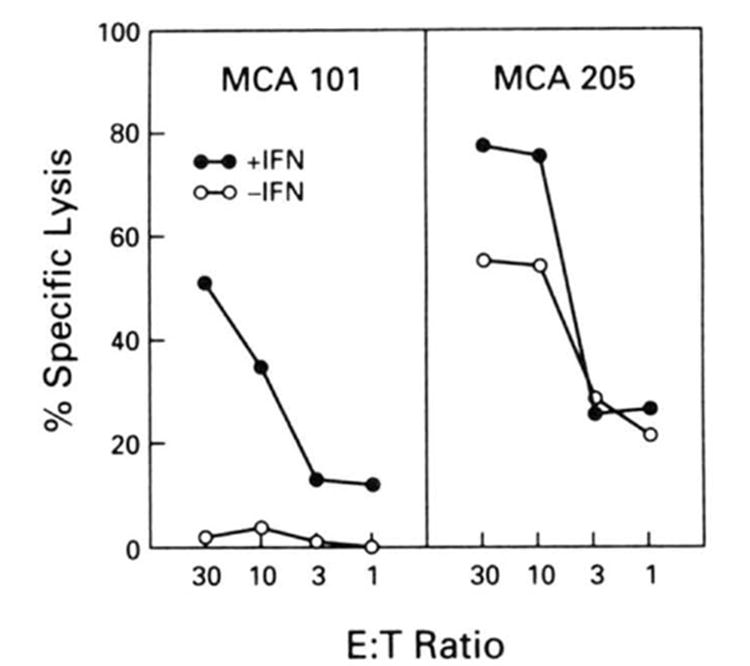
51Cr-release assay in which tumors were pretreated with 200 U/ml of IFN-γ for 48 h before the assay. Cells not exposed to IFN-γ are shown in open circles. Cells were then infected with PR8 virus. 51Cr-labeled, then subjected to killing by splenocytes from mice immunized with PR8 virus in vivo and restimulated in vitro with NP peptide (365-380). Experimental values represent the average of triplicates, with a SE of the mean of less than 4%. This experiment was repeated with similar results.
DISCUSSION
Our findings demonstrate that the nonimmunogenic murine sarcoma, MCA 101, is unable to present endogenously generated influenza virus Ag to CTL. It should be noted, however, that it remains unclear from these studies whether a complete lack of presentation occurred or whether the number of MHC-peptide epitopes was insufficient to be detected by our polyclonal responder populations. As has previously been demonstrated (40), MCA 101 down-regulates its class I expression at the level of transcription initiation. We have shown that, although class I H chain is synthesized at a reduced level, transport of this molecule from the endoplasmic reticulum to the cell surface is normal. Furthermore, presentation of endogenous Ag by MCA 101 is made possible by up-regulating class I expression with IFN-γ. These findings suggest that MCA 101 does not present endogenously generated Ag because of its low class I expression.
RMA-S is a cell line that is incapable of presenting not only viral Ag, but minor histocompatibility Ag and tumor Ag as well (42). Like RMA-S, MCA 101 can present exogenously introduced peptide very efficiently, but is defective in the presentation of endogenously generated Ag. Both cell lines express relatively low steady state levels class I. The efficient presentation of exogenously provided peptide by RMA-S has been attributed to the expression of class I molecules not bound to peptide. Such “empty” molecules are thought to be more capable of binding exogenously introduced Ag (30), and are indirectly demonstrated by showing increased class I surface expression after peptide pulsing or incubation at low temperatures, two procedures thought to stabilize these molecules. In RMA-S surface expression of class I is increased either by peptide pulsing or by incubation in the cold. The above described characteristics of RMA-S clearly set it apart from MCA 101.The mechanism whereby MCA 101 presents exogenously provided peptide with such efficiency remains to be elucidated. However, the finding that the low class I-expressing MCA 101 does not express “empty” class I may provide an alternative explanation than that given for the efficiency of RMA-S in presenting exogenous peptide (30).
Our series of MCA-induced tumors vary widely in their abilities to process and present endogenously generated viral Ag. Although MCA 101 does not present endogenously generated Ag, MCA 106, MCA 105, and MCA 203 are poor presenters, whereas MCA 205 and MCA 207 are relatively more efficient. As described above, CD8+ CTL have a demonstrated role in the immune response against MCA-induced tumors (3-9). It is, thus, not surprising that many of the tumors tested have a reduced ability to present endogenously generated viral Ag. In fact, one would expect that tumor cells with Ag processing or presentation defects would enjoy a selective advantage. Decreased class I expression has been correlated with enhanced metastasis and tumor progression in some tumor types (43). Some tumors may evade a CTL response by down-regulating class I expression in a way similar to the “immunosubversive” adenovirus (38, 44). It should be noted, however, that decreased class I expression can make tumor lines more susceptible to lysis by NK cells in some cases (31, 45, 46). Thus, some of the mechanisms used by tumors to escape recognition by CTL may increase their susceptibility by other cells in the immune system.
Lack of immunogenicity as a result of defects in the presentation of endogenous Ag may have relevance to other murine as well as human tumors, especially those tumor histologies that have not been shown to elicit immune responsiveness. Ag processing and presentation assays using viral Ag may predict responsiveness or unresponsiveness to T cell-based immunotherapies. Such experiments on naturally occurring tumor cells may also be instrumental in revealing as yet undescribed Ag processing and presentation defects. Finally, such findings might be useful in the design of therapeutic strategies by the identification of deficiencies in the presentation of endogenous Ag that could be reversed.
Acknowledgments
The authors wish to thank Debbie Schulman and Ellen Bolton for assistance with FACS analysis.
Abbreviations used in this paper
HA
hemagglutinin Ag
LAK
lymphokine-activated killer cells
NP
nucleoprotein
NS1
Nonstructural 1
PA
acidic polymerase
PR8
wild-type influenza A/PR/8/34
Vac
vaccinia virus
CM
complete medium
endo H
endo-_β_-_N_-acetylglucosaminidase
References
- 1.Klein G, Sjögren HO, Klein E, Hellström KE. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960;20:1561. [PubMed] [Google Scholar]
- 2.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. JNCI. 1957;18:769. [PubMed] [Google Scholar]
- 3.Greenberg PD, Cheever MA, Fefer A. H-2 restriction of adoptive immunotherapy of advanced tumors. J Immunol. 1981;126:2100. [PubMed] [Google Scholar]
- 4.Shimizu K, Shen F-W. Role of different T cell set in the rejection of syngeneic chemically induced tumors. J Immunol. 1979;122:1162. [PubMed] [Google Scholar]
- 5.Schreiber H, Ward PL, Rowley DA, Stauss HJ. Unique tumor-specific antigens. Annu Rev Immunol. 1988;6:465. doi: 10.1146/annurev.iy.06.040188.002341. [DOI] [PubMed] [Google Scholar]
- 6.Barth RJ, Bock SN, Mulé JJ, Rosenberg SA. Unique murine tumor-associated antigens identified by tumor infiltrating lymphocytes. J Immunol. 1990;144:1531. [PubMed] [Google Scholar]
- 7.Weber JS, Jay G, Tanaka K, Rosenberg SA. Immunotherapy of a murine tumor with interleukin-2: increased sensitivity after MHC class I gene transfection. J Exp Med. 1987;166:1716. doi: 10.1084/jem.166.6.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulé JJ, Yang JC, Lafreniere R, Shu S, Rosenberg SA. Identification of cellular mechanisms operational in vivo during the regression of established pulmonary metastases by the systemic administration of high-dose recombinant interleukin 2. J Immunol. 1987;139:285. [PubMed] [Google Scholar]
- 9.Yang JC, Perry-Lalley D, Rosenberg SA. An improved method for growing murine tumor-infiltrating lymphocytes with in vivo antitumor activity. J Biol Response Modif. 1990;9:149. [PubMed] [Google Scholar]
- 10.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714. [PubMed] [Google Scholar]
- 11.Itoh K, Platsoucas CD, Balch CM. Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas: activation by interleukin 2 and autologous tumor cells, and involvement of the T cell receptor. J Exp Med. 1988;168:1419. doi: 10.1084/jem.168.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 13.Topalian SL, Solomon D, Avis FP, Chang AE, Freerksen DL, Linehan WM, Lotze MT, Robertson CN, Seipp CA, Simon P, Simpson CG, Rosenberg SA. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol. 1988;6:839. doi: 10.1200/JCO.1988.6.5.839. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. N Engl J Med. 1988;319:1676. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Tevethia MJ, Kalderon D, Smith AE, Tevethia SS. Clustering of antigenic sites recognized by cytotoxic T lymphocyte clones in the amino terminal half of SV40 T antigen. Virology. 1988;162:427. doi: 10.1016/0042-6822(88)90483-7. [DOI] [PubMed] [Google Scholar]
- 16.Boon T, Van Pel A. T cell-recognized antigenic peptides derived from the cellular genome are not protein degradation products but can be generated directly by transcription and translation of short subgenic regions: a hypothesis. Immunogenetics. 1989;29:75. doi: 10.1007/BF00395854. [DOI] [PubMed] [Google Scholar]
- 17.Rötzschke O, Falk K, Deres K, Schild H, Norda M, Metzger J, Jung G, Rammensee H. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990;348:252. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- 18.Monaco JJ, Cho S, Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Nature. 1990;250:1723. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- 19.Deverson EV, Gow IR, Coadwell WJ, Monaco JJ, Butcher GW, Howard JC. MHC class II region encoding proteins related to the multidrug resistance family of transmembrane transporters. Nature. 1990;348:738. doi: 10.1038/348738a0. [DOI] [PubMed] [Google Scholar]
- 20.Trowsdale J, Hanson I, Mockridge I, Beck S, Townsend A, Kelly A. Sequences encoded in the class II region of the MHC related to the ‘ABC’ superfamily of transporters. Nature. 1990;348:741. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- 21.Spies T, Bresnahan M, Bahram S, Arnold D, Blanck G, Mellins E, Pious D, DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990;348:744. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- 22.Yewdell JW, Bennink JR. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989;244:1072. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- 23.Yewdell JW, Bennink JR. The binary logic of antigen processing and Presentation to T cells. Cell. 1990;62:203. doi: 10.1016/0092-8674(90)90356-j. [DOI] [PubMed] [Google Scholar]
- 24.Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987;329:512. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- 25.Townsend A, Rothbard J, Gotch FM, Bahadur G, Wraith D, McMichael AJ. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 26.Townsend A, Öhlen C, Bastin J, Ljunggren H-G, Foster L, Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989;340:443. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 27.Hosken NA, Bevan MJ. Defective presentation of endogenous antigen by a cell line expressing class 1 molecules. Science. 1990;248:367. doi: 10.1126/science.2326647. [DOI] [PubMed] [Google Scholar]
- 28.Townsend A, Elliott T, Cerundolo V, Foster L, Barber B, Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990;62:285. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- 29.Cerundolo B, Alexander J, Anderson K, Lamb C, Cresswell P, McMichael A, Gotch F, Townsend A. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature. 1990;345:449. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- 30.Ljunggren H, Stam MJ, Öhlen C, Neefjes JJ, Höglund P, Heemels M-T, Bastin J, Schumacher TNM, Townsend A, Kärre K, Ploegh HL. Empty MHC class I molecules come out in the cold. Nature. 1990;346:476. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 31.Kärre K, Ljunggren H, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 32.Gould K, Cossins J, Bastin J, Brownlee GG, Townsend A. A 15 amino acid fragment of influenza nucleoprotein synthesized in the cytoplasm is presented to class I-restricted cytotoxic T lymphocytes. J Exp Med. 1989;170:1051. doi: 10.1084/jem.170.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith GL, Levin JS, Palese P, Moss B. Synthesis and cellular location of the ten influenza polypeptides individually expressed by recombinant vaccinia viruses. Virology. 1987;160:336. doi: 10.1016/0042-6822(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 34.Yewdell JW, Bennink JR, Mackett M, Lefrancois L, Lyles DS, Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986;163:1529. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerhard W, Yewdell J, Frankel ME. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981;290:713. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- 36.Ozato K, Sachs DH. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981;126:317. [PubMed] [Google Scholar]
- 37.Yewdell JW, Yellen A, Bachi T. Monoclonal antibodies localize events in the folding, assembly, and intracellular transport of the influenza virus hemagglutinin glycoprotein. Cell. 1988;52:843. doi: 10.1016/0092-8674(88)90426-6. [DOI] [PubMed] [Google Scholar]
- 38.Cox JH, Yewdell JW, Eisenlohr LC, Johnson PR, Bennink JR. Antigen presentation requires transport from the endoplasmic reticulum. Science. 1990;247:715. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- 39.Yewdell JW, Hackett CJ. Specificity and function of T lymphocytes induced by influenza A viruses. In: Krug RM, editor. The Influenza Viruses. Plenum Publishing; New York: 1989. pp. 361–429. [Google Scholar]
- 40.Lassam N, Jay G. Suppression of MHC class I RNA in highly oncogenic cells occurs at the level of transcription initiation. J Immunol. 1989;143:3792. [PubMed] [Google Scholar]
- 41.Sugita K, Miyazaki JL, Appella E, Ozato K. Interferon increases transcription of a major histocompatibility class I gene via a 5′ interferon consensus sequence. Mol Cell Biol. 1987;7:2625. doi: 10.1128/mcb.7.7.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohlen C, Bastin J, Ljunggren HG, Foster L, Wolpert E, Klein G, Townsend ARM, Kärre K. Resistance to H-2-restricted but not to allo-H2-specific graft and cytotoxic T lymphocyte responses in lymphoma mutant. J Immunol. 1990;145:52. [PubMed] [Google Scholar]
- 43.Elliot BE, Carlow DA, Rodricks A, Wade A. Perspectives on the role of MHC antigens in normal and malignant cell development. Adv Cancer Res. 1989;53:181. doi: 10.1016/s0065-230x(08)60282-1. [DOI] [PubMed] [Google Scholar]
- 44.Jefferies WA, Burgert H. E3/19K from adenovirus 2 is an immunosubversive protein that binds to a structural motif regulating the intracellular transport of major histocompatibility complex class I proteins. J Exp Med. 1990;172:1653. doi: 10.1084/jem.172.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ljunggren H, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants: analysis of the mechanism. J Exp Med. 1985;162:1745. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ljunggren HG, Paabo S, Cochet M, Kling G, Kourilsky P, Kärre K. Molecular analysis of H-2-deficient lymphoma lines: distinct defects in biosynthesis and association of MHC class I/β-2 microglobulin observed in cells with increased sensitivity to NK cell lysis. J Immunol. 1989;142:2991. [PubMed] [Google Scholar]