Transcriptional Analysis of the Genetic Element pSSVx: Differential and Temporal Regulation of Gene Expression Reveals Correlation between Transcription and Replication (original) (raw)
Abstract
pSSVx from Sulfolobus islandicus strain REY15/4 is a hybrid between a plasmid and a fusellovirus. A systematic study performed by a combination of Northern blot analysis, primer extension, and reverse transcriptase PCR revealed the presence of nine major transcripts whose expression was differentially and temporally regulated over the growth cycle of S. islandicus. The map positions of the RNAs as well as the clockwise and the anticlockwise directions of their transcription were determined. Some genes were clustered and appeared to be transcribed as polycistronic messengers, among which one long transcriptional unit comprised the genes for the plasmid copy number control protein ORF60 (CopG), ORF91, and the replication protein ORF892 (RepA). We propose that a termination readthrough mechanism might be responsible for the formation of more than one RNA species from a single 5′ end and therefore that the nine different RNAs corresponded to only seven different transcriptional starts. Three transcripts, ORF76 and two antisense RNAs, countertranscribed RNA1 (ctRNA1) and ctRNA2, were found to be specifically expressed during (and hence correlated to) the phase in which the pSSVx copy number is kept under stringent control, as they were completely switched off upon the onset of the induction of replication.
Whereas major studies on viral systems in Bacteria and Eukarya have shed sufficient light on basic and regulated gene expression in these organisms, very little regarding the elucidation of the mechanisms of viral gene expression and regulation in hyperthermophilic Archaea has been addressed. A few archaeal viruses have been investigated in depth at molecular and physiological levels (23, 25, 48, 53). These viruses are known to be highly unusual in terms of morphology and genome structure/sequence (38, 39, 40, 41, 45). A survey of the extrachromosomal elements in the hyperthermophilic crenarchaeon Sulfolobus (64, 65) has revealed the existence of many new viruses, with the Fuselloviridae being the most common family among the Sulfolobales to date (37). Sulfolobus spindle-shaped virus 1 (SSV1) is the best-studied member of this family, and it behaves as a temperate virus both in Sulfolobus shibatae and in the nonnatural but related Sulfolobus solfataricus host (53); infection, integration of DNA into the host chromosome, and the production of virions cause apparently no phenotype change but do cause a significant growth retardation of the host cells, which can be visualized as turbid plaques on plated lawns of indicator host cells around propagation foci (53, 65).
Transcription studies conducted on SSV1 have pointed out that the copy number of the episomal DNA as well as the virus titer remain essentially constant in the unirradiated host (48). DNA replication increases after induction by UV or other DNA-damaging agents and seems to be mediated by transcription at the promoter Tind (48, 53). Furthermore, the structural genes of SSV1 are constitutively and coordinately transcribed in nonirradiated cells, and the amount of these transcripts increased in an essentially identical fashion upon UV irradiation (48). This transcriptional analysis has provided the basics for an early definition of the consensus sequences of archaeal promoters (49) and terminators (50) in both constitutive and UV-inducible transcripts. Nevertheless, the molecular mechanisms responsible for regulation of transcription and DNA replication remain undefined.
A comprehensive analysis of gene expression has been reported for the Sulfolobus islandicus rod-shaped viruses SIRV1 and SIRV2 and carried out during the infection of nonnatural Sulfolobus host cells (23). Transcription begins simultaneously at multiple start sites promptly after infection for both viruses, suggesting that the expression of these genes is not regulated temporally. This simple pattern of transcription is consistent with the stable carrier state of these rudiviruses in host cells. Interestingly, a Sulfolobus transcription activator factor, Sta1, has been shown to be a host protein and to trigger transcription initiation from SIRV1 promoters in vitro (24).
The two distinct genetic elements SSV2 and pSSVx coexist in the same Sulfolobus islandicus strain REY 15/4 host (65) and represent the only known two-virus system in the Archaea (2). These viruses belong to the family Fuselloviridae, and they are also able to infect Sulfolobus solfataricus and spread into infected cultures (3, 11). SSV2 and pSSVx do not induce cell lysis of their hosts in the entire life cycle, but they impose strong inhibition of the growth of their host upon infection (11, 64). Whereas SSV2, like SSV1, is an autonomous virus, pSSVx needs SSV2 as a helper for virus particle generation (2). At the sequence level, the pSSVx genome contains two open reading frames (ORFs), which are conserved in the Fuselloviridae family (2, 57); the remaining genome sequence is typically plasmidic, with the putative minimal replicon shared with members of the pRN plasmid family, such as pRN1 (21), pRN2 (22), pHEN7 from different S. islandicus species (36), pDL10 from Acidianus ambivalens (26), and several defective integrated plasmids occurring in Sulfolobus genomes (54). This conserved region includes ORFs encoding proteins named CopG (a copy number control protein), RepA (a replication initiator protein), and PlrA (a putative plasmid regulatory protein) (31). More recently, another SSV-type virus satellite, pSSVi, was also found to interact with its helper SSV1 and SSV2 viruses and to inhibit host growth (61). pSSVx and pSSVi share a common genome architecture since they have comparable numbers of ORFs that are similar in length and relative position. However, most of the ORFs of pSSVx and pSSVi are different: pSSVx encodes all the three highly conserved ORFs of the pRN family, whereas the pSSVi genome contains only one homologous ORF, CopG. Furthermore, the putative Rep protein of pSSVi is unrelated to the ORFs encoded by all the known genetic elements since it contains no polymerase/primase domain, but it shows low similarity to an ORF of the integrated element pSA2 identified in the Sulfolobus acidocaldarius genome (9, 55). The only ORF that is conserved between these two viruses is c68 in pSSVx and c56 in pSSVi (61). This indicates that the two systems are distantly related.
In a previous study, it was demonstrated that the DNA replication of SSV2 and pSSVx is actively induced during growth in the natural host REY15/4 (11). This kind of physiological induction represents a unique feature among the crenarchaeal viruses and therefore proposes the pSSVx/SSV2-host system as a novel and intriguing model for study. In fact, the closely related SSV1 is inducible by UV irradiation or mitomycin C treatment at the level of both the replication and transcription of its genes (8, 53).
Herein, we studied the relationship between viral gene expression and replication induction during the growth of S. islandicus by using pSSVx as a model system. The results of this investigation revealed that the expression of pSSVx genes is differentially and temporally regulated in the transition from the state in which a tight copy number control is exerted to the phase where the induction of replication occurs. The complexity and variability of the transcription map must have resulted from multiple transcriptional and posttranscriptional control mechanisms.
MATERIALS AND METHODS
Enzymes and chemicals.
Restriction and modification enzymes were purchased from Roche. Synthetic oligonucleotides were supplied by MWG Biotech. Radioactive materials were obtained from Perkin-Elmer.
Growth conditions and extraction of nucleic acids.
S. islandicus REY 15/4 (65) was grown at 80°C and pH 3.2 in TYS, a glycine-buffered Brock's basal mineral medium supplemented with 0.1% yeast extract (Difco)-0.2% sucrose and with 0.1% tryptone (Oxoid). The optical density at 600 nm (OD600) of the liquid cultures was monitored, and samples were collected at different growth stages (time ranging from 6 to 42 h) for extrachromosomal DNA (12) and total RNA preparations. All the experiments were repeated for three independent cultures grown under identical conditions. Total RNA was extracted by the guanidine thiocyanate method (52). DNA contamination was removed by DNase I (AMbion) according to the manufacturer's instructions, but the procedure was repeated three times. Concentration of the RNA was determined by the absorbance at 260 nm, and purity was estimated by the ratio of the absorbance values at 260 and 280 nm.
Northern blot analysis.
For Northern blot analysis, total RNA (15 μg) was run on denaturing, formaldehyde-containing 1.0 to 1.6% (wt/vol) agarose gels and transferred onto a nylon membrane (Hybond-XL; Amersham-Pharmacia). Hybridizations with double-stranded probes (obtained by PCR amplification with the oligonucleotides listed in Table S1 in the supplemental material) were carried out at 65°C for 16 h in a solution containing 7% sodium dodecyl sulfate (SDS), 1 mM EDTA, 1% albumin serin bovin, and 0.5 M phosphate buffer (pH 7.2) (10). The filters were washed twice in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.5% SDS for 20 min at 65°C, once in 1× SSC-0.5% SDS for 20 min at 65°C, and once in 0.1× SSC-0.5% SDS for 15 min at 65°C. In order to reuse the membrane for subsequent hybridization experiments, the probe was removed by 10 min of boiling in 0.1% SDS. Hybridizations with single-stranded oligonucleotides (listed in Table S2 in the supplemental material) were performed using buffer containing 5× SSC, 5× Denhardt's reagent, 0.05 M sodium phosphate (pH 6.5), 0.1% SDS, and denatured, fragmented salmon sperm DNA (100 μg/ml) at the appropriate temperature overnight. Filters were washed once for 15 min in washing buffer (1× SSC, 0.1% SDS) at room temperature and twice for 20 min in washing buffer at 55°C and were finally rinsed in 2× SSC.
The signal intensity of the hybridization with the labeled ribosomal 16S sequence (amplified by PCR from the total DNA of REY 15/4) was used as a reference for normalization on replica filters with 1.5 μg of total RNAs blotted after electrophoresis. The relative abundance of each transcript was evaluated by quantifying the radioactive signals using a Molecular Dynamics Bio-Rad PhosphorImager (Quantity One software).
The Random Prime DNA Labeling kit (Roche Applied Science) was used to label double-stranded DNA probes with radioactive [α-32P]dATP (Perkin-Elmer), and unincorporated [α-32P]dATP was removed from the labeled probe by gel filtration using Nick columns (Amersham Biosciences). T4 polynucleotide kinase (Roche Applied Science) was employed to label the 5′ ends of the oligonucleotides according to the manufacturer's instructions. The purification of the radiolabeled oligonucleotides was performed by chromatography on a Sep-pack C18 column according to a method described previously by Sambrook and Russell (52). The sizes of the autoradiographic signals were determined using RNA molecular weight standards (Promega and Roche).
Primer extension and reverse transcriptase PCR (RT-PCR) analyses.
The oligonucleotides used in the primer extension analysis are listed in Table S2 in the supplemental material. Total RNA (30 to 40 μg) and 5′-labeled primer (1 × 105 cpm pmol−1) were coprecipitated with 2.5 volumes of ice-cold ethanol for 30 min at −80°C. After centrifugation, the pellets were resuspended in the reverse transcription buffer purchased from Roche and denatured for 3 min at 65°C, frozen in dry ice, and thawed on ice. The reaction mixtures were incubated for 30 min at 37°C for annealing. Deoxynucleoside triphosphates (final concentration, 2 mM each) and 15 units of RNase inhibitor (Promega) were added. The primer was extended with 8 units of avian myeloblastosis virus reverse transcriptase (Roche) at 48°C for 1 h. The resulting products were separated on denaturing 6% polyacrylamide gels, and DNA sequencing reaction products, obtained using the same primers, were used as reference ladders.
RT-PCR was performed on 1 to 2 μg of total RNA by using avian myeloblastosis virus reverse transcriptase; after synthesis, the enzyme was removed from cDNAs by phenol extraction and ethanol precipitation. The relative abundance of the mRNAs was evaluated by withdrawing aliquots of the amplicons after 20, 25, 30, 35, and 40 reaction cycles with Taq DNA polymerase (Roche Molecular Biochemicals, Germany). As a test for DNA contamination, PCR amplifications were carried out on the RNA preparations without the reverse transcriptase step. The 16S gene was used to normalize the mRNA fraction used in the RT-PCR experiments. The limit of sensitivity for each set of template and primers was established by performing the PCR on diluted plasmid solutions down to one or two copies in each sample.
Bioinformatics analysis.
The prediction of the secondary structures of RNAs was performed using the program available at http://www.bioinfo.rpi.edu/applications/mfold/cgi-bin/rna-form1.cgi.
Data banks (nonredundant) were screened for homologs to ORFs of pSSVx with the programs BLAST, PSI-BLAST, and CDD. Sequence alignments were performed with ClustalW, and predictions of the secondary structures of proteins were carried out with different algorithms and programs [GOR(IV) (16), AGADIR, JPred, PredictProtein, and NNPredict] that are provided at the Expasy website.
RESULTS
pSSVx replication and gene transcription.
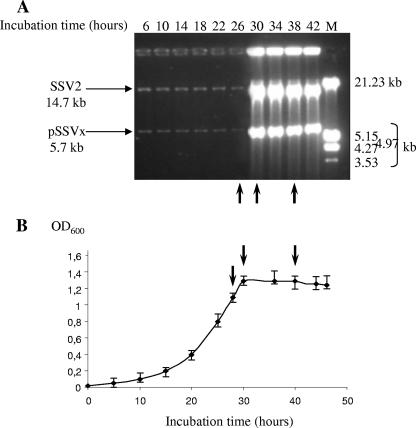
As previously reported, the replication induction of the SSV2 and pSSVx viruses sets in at the late exponential growth phase of both the natural host S. islandicus and stabilized SSV2 lysogens cured from pSSVx, namely, in the presence or the absence of pSSVx (11). As shown in Fig. 1, pSSVx parallels SSV2 in the accumulation of the episomal DNA, with both elements rapidly reaching a maximum within 4 h, around the 30th incubation hour of the cell culture. To address whether the nine ORFs of pSSVx that were previously predicted were transcribed and whether their expression was differentially regulated, transcriptional analysis was initially conducted on all the predicted ORFs throughout the growth phases. However, results are reported only for the growth stages (lanes 1, 2, and 3 of all the Northern blot and primer extension analyses presented) that more significantly highlighted the variation of gene expression profiles before (0.95 OD600 units, 26th hour) (lane 1), during (1.3 OD600 units, 30th hour) (lane 2), and 8 h after (1.3 OD600 units, 38th hour) (lane 3) the pSSVx copy number had reached the highest number of copies/cell. The results relative to earlier growth phases are not shown because no appreciable changes were observed in transcription patterns up to 0.95 OD600 units. In fact, phase 1 is representative of the entire exponential growth/plasmid replication/gene transcription condition up to the 26th incubation hour. On the other hand, 8 h after the cultures had reached 1.3 OD600 units, S. islandicus cells were in the mid-stationary phase of growth and had not yet entered the stationary/death phase (11).
FIG. 1.
Time-dependent replication of the episomal SSV2 and pSSVx genomes in S. islandicus cells and relative growth curve. (A) Episomal DNAs were prepared from aliquots of a single culture of S. islandicus, withdrawn at the time indicated, digested with ClaI (one cut for both SSV2 and pSSVx genomes), and analyzed by agarose gel electrophoresis. The size of the linearized fragments is indicated. M indicates molecular weight markers (kb). (B) OD600 values of the culture were measured and plotted versus the incubation time. The values are the averages of independent experiments performed on three different cultures, and standard deviations are indicated for optical densities. Total RNAs used in the transcriptional analysis (cf. lanes 1 to 3 of Northern blot and primer extension experiments) were isolated from cultures harvested at 26th, 30th, and 38th time points, as indicated by the arrows.
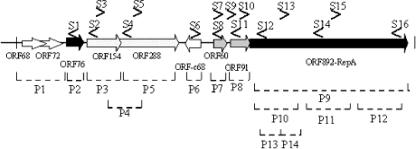
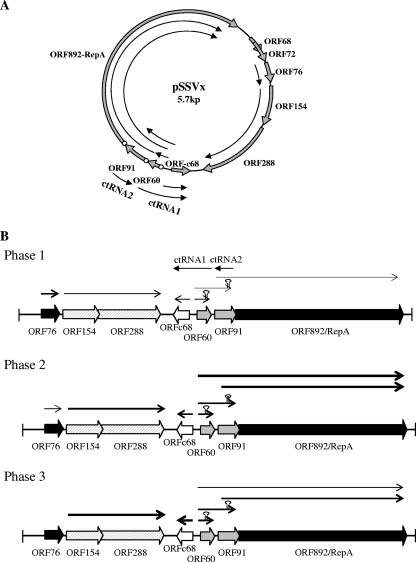
In the Northern blot experiments, double-stranded PCR products (P1 to P14) (primers are listed in Table S1 in the supplemental material) as well as single-stranded oligonucleotides (S1 to S16) (see Table S2 in the supplemental material) were used to cover the whole pSSVx genome as graphed in Fig. 2. In some cases, the interpretation of the data obtained from hybridization with double-stranded probes required further investigation using single-stranded ORF-specific probes. Furthermore, RT-PCR was performed to confirm Northern blot analysis or resolve specific ambiguities as well as to identify low-abundance mRNAs. Primer extension analyses were conducted to define the 5′ ends of the individual transcripts identified in the Northern blot analysis and, consequently, to determine whether multiple and overlapping transcription units initiate from a single site. The antisense direction of transcription was also tested by primer extension and RT-PCR experiments with oligonucleotides mapping at various positions on the noncoding sequence of the pSSVx ORFs. Three different sets of genes on the basis of the transcriptional patterns and their interconnection were identified.
FIG. 2.
Strategy for transcriptional mapping of pSSVx. The location and the orientation of the oligonucleotides used in this analysis are given above the linearized map and are represented by arrows (S1 to S16). The bottom part of the figure shows the DNA double-stranded probes (P1 to P14) depicted as dotted lines. The sequences of all the oligonucleotides used in this study are listed in Tables S1 and S2 in the supplemental material.
Gene set 1.
The first set of genes includes genes that were considered not to be transcribed under the growth conditions and the detection procedures used, as in the case of the two short, partially overlapping ORFs named ORF68 and ORF72. These results reinforce the previous prediction based on sequence analysis that the region in which these putative ORFs reside is indeed involved in DNA replication initiation since it contains a long inverted repeat sequence with a tendency to form a stable stem-and-loop structure (2).
Gene set 2.
The second set of genes includes genes transcribed in single species, namely, as mono- or bicistronic units; this is the case for ORF76 and ORF154/ORF288, respectively.
(i) ORF76.
The most conserved gene in crenarchaeal plasmids is plrA (ORF76 on the pSSVx genome), whose structural homologs are also found in all conjugative and cryptic plasmids except pOAR1, pIT3 (43), and pSSVi (61). The homolog in plasmid pRN1, ORF80, encodes a leucine zipper DNA-binding protein (29). Structural homology was also confirmed at the functional level: the recombinant protein encoded by ORF76 was shown to bind its own promoter in an identical fashion (our unpublished results). However, the precise function of this protein in vivo remains obscure.
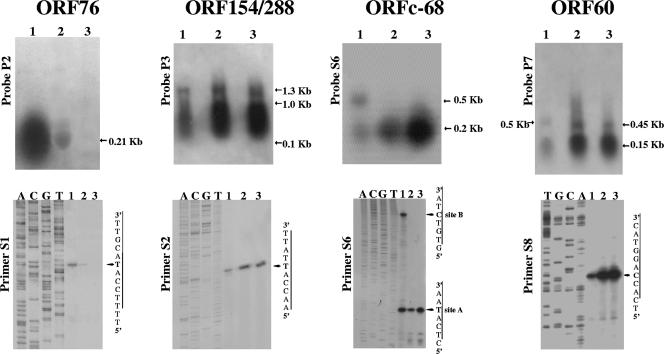
A DNA probe containing the ORF76 sequence hybridized to a single and monocistronic transcript of the expected size of 200 nucleotides (nt) (Fig. 3). The initiation site of ORF76 transcription was determined using primer extension analysis. A single start site was revealed, and it corresponded to the first nucleotide, A, in the ATG translation start codon of the gene (Fig. 3), confirming the result of the Northern analysis. However, ORF76 expression was subjected to down-regulation during host growth: the strong signal detected in RNAs prepared from the cells harvested in phase 1 and containing ca. 1 of copy pSSVx per chromosome turned out to be 50-fold weaker in phase 2, i.e., after 4 h of incubation, following a reversed trend with respect to the copy number of pSSVx, which increased ca. 50-fold (compare Fig. 3 with Fig. 1A). Moreover, this signal disappeared completely after incubation for eight more hours (phase 3). It is worth noting that the complete inhibition of ORF76 transcription occurs during the process of pSSVx replication induction, namely, when the control mechanisms more typically viral and imposed by SSV2 induction become predominant.
FIG. 3.
Transcriptional analysis of ORFs 76, 154-288, c68, and 60. Total RNAs isolated from phases 1, 2, and 3, which highlight the variation of gene expression before (0.95 OD600 units, 26th incubation hour) (lane 1), during (1.3 OD600 units, 30th hour) (lane 2), and after (1.3 OD600 units, 38th hour) (lane 3) pSSVx has reached the highest number of copies/cell, respectively, were analyzed by Northern blot and primer extension experiments. The DNA probes and the oligonucleotides used for Northern hybridizations and primer extension experiments, respectively, are indicated (see Fig. 2 for the location of the probes and the primers). The cDNA products were electrophoresed with the sequence ladder generated by the same primer on the noncoding strand of the relative genes. The mapped transcriptional start sites are indicated by the arrow.
(ii) ORF154 and ORF288.
The partially overlapping ORF154 and ORF288 exhibited high sequence similarity to tandem ORFs present in the genomes of SSV viruses with identity scores of 63% to 75% for the homologs of ORF154 and 31% to 49% for those of ORF288. They have been previously indicated as being proteins necessary for specific pSSVx DNA recognition and prepackaging processes (2, 57). In Northern hybridization analysis, a signal of ca. 1.3 kb was identified (Fig. 3) when a 462-bp sequence corresponding to ORF154 was used as the probe (probe P3) (Fig. 2); the size of the transcript matched the length of a bicistronic mRNA coding for both ORF154 and ORF288. The same results were obtained when the filter was reprobed with probe P5 (Fig. 2), a radiolabeled ORF288 sequence (data not shown). The transcription start site was mapped by primer extension, and a single initiation nucleotide was identified, which corresponded to A in the ATG translation start codon of ORF154 (Fig. 3). The same results were obtained also with a primer designed against ORF288 (not shown). Therefore, ORF154 and ORF288 were transcribed only as a bicistron as predicted from sequence analyses. Furthermore, the abundance of this bicistronic mRNA increased in proportion to the pSSVx DNA copies (about 40-fold) and was mostly degraded as indicated by the broad lower-molecular-weight signal (ranging from 100 to 1,000 nt) in the Northern experiments (Fig. 3).
Gene set 3.
Gene set 3 is characterized by a complex transcriptional network involving four genes, ORF892 (RepA) (encoding a putative replication protein) and the adjacent ORFc68, ORF60, and ORF91, which are likely to be involved in regulating rep gene expression.
(i) ORFc68.
ORF c68 is the only reverse-oriented putative pSSVx gene and has no homologs in the crenarchaeal family of pRN plasmids (2). It is highly similar (42% identity and 53% similarity) to ORFc56 of pSSVi (61) and also finds its homologs in integrated elements of the Sulfolobus tokodaii (20) and Sulfolobus acidocaldarius (9) genomes, although the biological function is still unknown. However, an in-depth inspection performed with the PSI-BLAST and CDD searches and secondary structure predictions (16) highlighted the presence of a SpoVT/AbrB-like domain in the translated protein. This motif is present in a family of prokaryotic factors demonstrated to be repressors, activators, or ambiactive, depending on the specific case (18, 62).
Two differently sized transcripts of about 200 and 500 nt (Fig. 3) were detected in RNA prepared from cultures harvested in phase 1 by a 32P-radiolabeled ORFc68 single-strand sequence (primer S6) (Fig. 2). These two transcripts must have originated from the same strand since they were detected by means of hybridization with a specific single-stranded DNA. The 200-nt transcript, which corresponded in size to the ORFc68 gene, became the predominant transcription unit in the transition from phase 1 to phase 2. More precisely, the content of this transcript increased gradually by 10-fold in passage from phase 1 to phase 2 and 20-fold from phase 2 to phase 3, namely, even after the pSSVx copy number had reached its maximum (Fig. 3, lanes 1 to 3). This expression profile suggests an up-regulation for ORF c68 specifically in the latest growth phase analyzed and the complete transcription repression of the 500-nt species as soon as pSSVx has reached the maximum copy number. The differential expression of the 200- and 500-nt transcripts was confirmed by the mapping of their 5′ termini. In fact, the primer extension performed with primer S6 revealed two major transcriptional start sites only from the RNA prepared in phase 1: the first 5′ end corresponded to nucleotide A in the ATG translation start codon, and the second one (which was more precisely mapped with primer S7) (not shown) localized the start at the position complementary to nucleotide +5 of the ORF91 coding sequence (Fig. 3). Therefore, the longer transcript comprised the complete antisense ORF60 in addition to ORFc68, and hence, it was named countertranscribed RNA1 (ctRNA1). ctRNA1 shows the propensity to form a stable stem-and-loop structure at its 5′ terminus (Fig. 4C), as predicted for the complementary region near the 3′ end of ORF60. Similar structures have previously been described as mediating the formation of sense-antisense complexes between RNA molecules that are transcribed from regions upstream of the rep gene in bacterial plasmids (6, 7, 12).
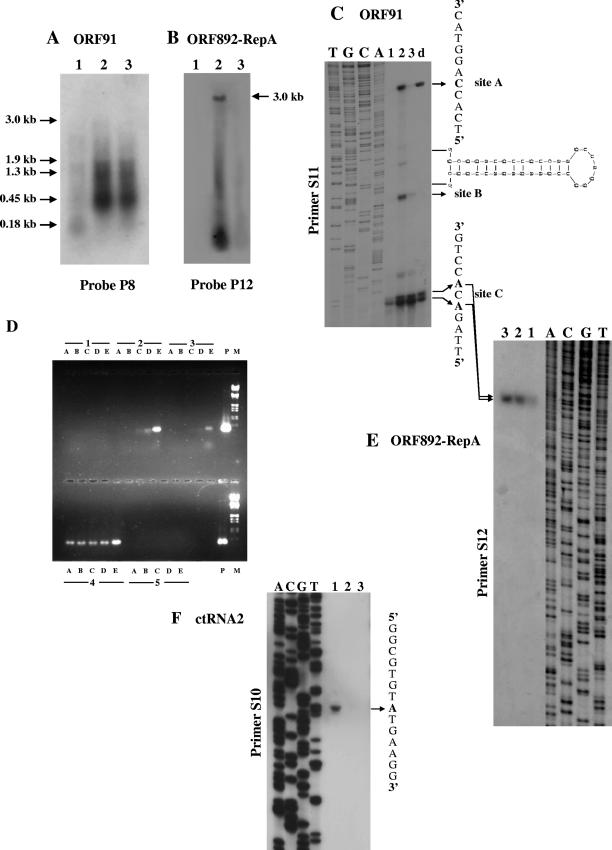
FIG. 4.
Transcriptional analysis of the region comprising ORF91 and ORF892 (RepA). (A and B) Northern blots performed with P8 and P12 probes. (C and E) Primer extension analysis using S11 and S12 as primers for detection of the 5′ ends of ORF91 and ORF892 (RepA), respectively. Site A signal, produced by primer S11 designed on the ORF91 noncoding sequence, coincides with the 5′ end of ORF60 transcript. Site B resulted in an artifact caused by the stalling of reverse transcriptase. Lanes 1, 2, and 3 represent the primer extension products obtained with RNA prepared at phases 1, 2, and 3, respectively. Lane d, cDNA products obtained under more stringent RNA denaturation conditions. The potential stem-and-loop structure at the 5′ of ctRNA1 was determined by computer-assisted RNA secondary structure analysis, and its location within the ORF60 sequence is indicated. (D) RT-PCR amplification products of the RNA prepared from growth phases 1, 2, and 3, as indicated in the Northern blot analysis, for the detection of the ORF60/ORF91/ORF892 (RepA) transcript. 4 and 5 represent the positive (PCR) and the negative (RT-minus PCR) controls, respectively. Lanes A, B, C, D, and E are the amplification products that were obtained after 20, 25, 30, 35, and 40 cycles, respectively, using primers S7 and S12. Lanes P and M were loaded with the PCR amplification products as positive controls and molecular weight markers, respectively. (F) Primer extension analysis using primer S10 designed on the ORF91 coding sequence detected the transcription start site of a countertranscript (named ctRNA2) at position +220 in the ORF91 sequence. For the location of the primers, see the scheme depicted in Fig. 2.
(ii) ORF60.
ORF60 encodes a protein exhibiting low (20 to 25%) but significant sequence identity to CopG proteins that play a role as transcriptional regulators in the copy number control of several broad-host-range bacterial plasmids (2, 13). The secondary structure predictions performed with the different algorithms available at the Expasy proteomic server predict that, like CopG proteins, the deduced product of ORF60 is small, compact, and homodimeric protein with each subunit exhibiting a ribbon-helix-helix arrangement.
Sequence analysis suggested that the transcription of ORF60 should yield a transcript of about 180 nt as a monocistronic mRNA. This transcript was indeed detected during all the growth phases (Fig. 3) and increased by about 30-fold during pSSVx replication induction (phase 2). Thereafter, it remained approximately constant up to the following 8 h of incubation (phase 3), indicating a direct dependence of ORF60 transcription on pSSVx replication due merely to the increased gene dose. Furthermore, the ORF60 double-stranded probe detected two additional transcripts that were larger than the monocistronic mRNA. The 460-nt mRNA was assignable to the transcription of a bicistron also including the ORF91 sequence (Fig. 3, lanes 2 and 3). The 500-nt transcript was the same ctRNA1 revealed by Northern analysis of ORFc68 (lane 1). The detection of only the monocistronic ORF60 and the bicistronic ORF60/ORF91 transcripts with a single-strand oligonucleotide (primer S8) designed on the coding strand of ORF60 (data not shown) provided evidence that ctRNA1 originated from a different strand and was divergently regulated. Primer extension analysis (Fig. 3) indicated that the initiation transcription site of ORF60 is located 18 nt downstream of the putative translation start nucleotide indicated previously by Arnold et al. (2). Despite the resulting downstream shift (+27 nt) of the translation start codon ATG, the name ORF60 was not changed to ORF51 for the sake of clarity and uniformity.
ORF91 and ORF892 (RepA).
ORF91 is situated in a partially overlapping tandem array with ORF892 (RepA), encoding a putative polymerase/primase, which is shared by all members of the pRN1 family (30). This genetic arrangement recalls the cop/rep organization present in most bacterial plasmids (12). Nevertheless, any search for structural motifs typical of copy number control proteins, such as those found in the CopG protein encoded by ORF60, did not yield any match in data banks with the ORF91 protein. However, the putative ORF91 protein contains a putative Zn motif similar to that found in nucleic acid (single- and double-stranded RNA and/or DNA) binding proteins from plant RNA viruses (carlaviruses) and transcriptional activators of late genes in both coliphages and relative satellites (19). Hybridization with a probe containing the ORF91 sequence (probe P8) (Fig. 2) did not reveal any defined signal representing the monocistronic transcript (about 270 nt), indicating that the ORF91 monotranscript would be relatively scarce if it was ever transcribed autonomously (Fig. 4A). Rather, the major transcript (about 450 nt) detected as a strong signal in phases 2 and 3 was coincident with the signal produced by the ORF60 probe and hence was confirmed to be bicistronic. Although this transcript was barely detectable by Northern blotting in phase 1, its presence was confirmed by RT-PCR experiments (data not shown). The content of the bicistronic mRNA increased about 50-fold in the passage from phase 1 to phase 2 and remained constant thereafter. A smaller messenger (about 180 nt) was evident (Fig. 4A, lane 1) in phase 1 and undetectable thereafter. This transcript originated from the noncoding strand of ORF91 and hence represents the second countertranscript tRNA (ctRNA2) mapped on pSSVx. In fact, primer extension (Fig. 4F) and RT-PCR amplification (data not shown) allowed the fine mapping of the transcription start site (at the position complementary to nt +220 of ORF91) and of the 3′ end (located between positions +29 and +52). In addition, the presence of discrete multiple mRNAs up to 3,000 nt (Fig. 4A, lanes 2 and 3) also suggested a complex transcription association of ORF91 with ORF892 (RepA). A defined and distinguishable transcript comprising the entire ORF892 (RepA) message (approximately 3,000 nt) could be detectable only in phase 2 by probe P12, containing the 3′ regions of the ORF892 (RepA) sequence (Fig. 4B). The continuous smear ending with an even more intense signal at low molecular weight was revealed even if cells were harvested at shorter time ranges (30 min) around the pSSVx induction peak (not shown). In fact, all signals, including this continuous smear, dramatically dropped after 1 to 2 h (and almost completely disappeared in phase 3, i.e., after 8 h) (Fig. 4B, lane 3). The transcript accounting for the entire coding sequence was also detected (1 to 2 copies/cell) in the preceding growth phases but only by RT-PCR, thus confirming that the ORF892 (RepA) gene was always transcribed and sensibly overexpressed only during the brief period of plasmid replication induction. Moreover, the positive amplification of the cDNA of a larger transcript comprising ORF91 mRNA confirmed the hypothesis that the two overlapping ORFs can be cotranscribed in a large bicistronic mRNA (data not shown). Mapping of the transcription start site(s) for ORF91 and ORF892 (RepA) clarified these complex transcription patterns conclusively. Primer extension experiments for ORF91 performed with primer S11 produced three major cDNAs (sites A, B, and C) (Fig. 4C). The band indicated as “site A” is coincident with transcription initiation of ORF60 transcript. “Site C” consists of two major bands corresponding to positions +2 and +4 of ORF91, thus giving rise to two ORF91 transcripts (indicated with the same *ORF91 name) that are untranslatable at their 5′ ends. The intermediate signal (site B) coincident with nt −52 is located in the ORF60 sequence in the proximity of no significant sequence for transcriptional regulation. Therefore, it was assumed to be an artifact caused by the stalling of reverse transcriptase. This site is indeed the beginning of the region with a high propensity to form stem-and-loop structures complementary to that identified in ctRNA1, as shown in Fig. 4C. In fact, it disappeared when more stringent RNA denaturation was performed before cDNA synthesis (Fig. 4C, lane d). Primer extension experiments performed with a primer for ORF892 (RepA) (primer S12) revealed a single transcription start site coinciding with the major “site C” signal detected for the ORF91 transcript (Fig. 4E). Taken together, these results indicate that the ORF91 sequence can reside in at least two different transcriptional units, one containing the complete ORF91 translationally coupled to ORF60 and the other beginning with an ORF91 pseudomessage (*ORF91), which does not allow translation at its 5′ terminus. By analogy with the gene organization of the rep locus in some bacterial plasmids (12), a more complex tricistronic mRNA containing all three ORFs was searched. RT-PCR experiments produced a specific amplicon when performed with primers designed to detect this hypothetical ORF60/ORF91/ORF892 (RepA) transcriptional unit and revealed its narrow time range induction during the active replication of pSSVx (Fig. 4D). This result demonstrated that the transcripts *ORF91/ORF892 (RepA) (about 2,950 nt) and ORF60/ORF91/ORF892 (RepA) (about 3,100 nt) both contributed to all the signals, including the one centered at 3,000 nt detected in the Northern blot analysis (Fig. 4B). By comparing the relative intensities of the Northern blot and primer extension experiments, we concluded that an overall 100-fold increase in the ORF892 (RepA) messages (in all the transcript species) was reached in the passage from phase 1 to phase 2.
We also searched for potential countertranscripts originating from the antisense direction of ORF892 (RepA) by performing primer extension and/or RT-PCR experiments with primers S13, S15, S14, and S16 (Fig. 2), but we found no indication of such antisense transcripts.
Together with the results described above, these findings indicate that ORF91 is transcribed either as bicistronic (ORF60/ORF91 and *ORF91/ORF892 [RepA]) or tricistronic (ORF60/ORF91/ORF892 [RepA]) mRNAs. The transcription process originating from the promoter upstream of ORF60 (Pr1) extends over the whole ORF892 (RepA) sequence. Nevertheless, it can undergo premature termination at the 3′ terminus of ORF60 and/or ORF91, suggesting a typical case of attenuation control at the transcriptional level (17). The existence of the *ORF91/ORF892 (RepA) transcripts is not explainable by initiation from promoter Pr1 and processing of the tricistronic messenger at the 5′ end. In fact, intact ORF60 and ORF60/ORF91 mRNAs as well as the absence of transcripts containing the ORF91 sequence alone would exclude nuclease sensitivity of the ORF60 portion. Therefore, an additional sequence (Pr2) located in the intergenic region of ORF60/ORF91 should promote autonomous initiation of the *ORF91/ORF892 (RepA) transcripts. The 2-nt difference at their 5′ ends is similar to that described previously for some Sulfolobus promoters analyses (44, 51). In fact, transcripts differing in size by 1 to 4 nt can be generated by the slippery form of the RNA polymerase at the initiator (INR) region. The expression driven by promoter Pr2 suggests that a further control mechanism, mediated by the leader *ORF91, might occur at the level of ORF892 (RepA) expression. Transcriptional/translational leaders coupled to replication initiation protein genes were previously described for some bacterial plasmids (6, 7, 12, 27).
vp1/vp3 of the SSV2 genome.
Since pSSVx is packaged into viral particles, we also analyzed the expression of the capsid genes (vp1 and vp3) located on the SSV2 genome (57). A schematic map of the SSV2 genome is shown in Fig. S1A in the supplemental material.
The vp1 coding sequence revealed a major transcript of about 250 nt, which represented the monocistronic vp1 mRNA, and a 500-nt message, which might correspond to the bicistronic vp1/vp3 messenger (see Fig. S1B in the supplemental material). When the filter was probed with radiolabeled vp3 sequence (see Fig. S1C in the supplemental material), only the longer transcript was highlighted, indicating that the vp1 gene was transcribed as either a monocistronic or a bicistronic messenger, while the transcription of vp3 was linked strictly to that of the vp1 gene. Interestingly, the transcription profile of vp genes was identical in the SSV2 and SSV1 viruses (47). The packaging and extrusion of SSV2 and pSSVx particles are induced at the late exponential growth phase as indicated by the sudden increase in the plaque-forming activity of the culture supernatants. The virus titer was maintained low and constant (no de novo virus production) up to the DNA replication induction; at this point, a 100-fold increase was observed, and the maximum value measured (1.0 × 105) remained invariable during the time range tested thereafter (11). Therefore, as expected, active transcription of the vp1 and vp3 genes paralleled the variation trend of the virus titer since it occurred specifically in phase 2 with a 100-fold increase, coincident with the induction of pSSVx replication, with a drastic decrease at later incubation hours, i.e., when almost no new viral particle was assembled.
Analysis of regulatory sequences.
The results of the transcriptional analyses are shown in Fig. 5, which summarizes the identified transcripts as well as their location and orientation on the pSSVx map. The temporal expression of the transcripts in the three phases is also shown.
FIG. 5.
Transcriptional map of pSSVx genes and their temporal expression. (A) The pSSVx transcripts identified by Northern blot experiments and mapped by primer extension are represented by arrows. The white circles indicate the positions of the putative SD motifs. (B) The temporal expression of the pSSVx gene in the preinduction (phase 1), induction (phase 2), and postinduction (phase 3) phases of pSSVx replication is illustrated. The transcripts detected in each phase are represented by arrows. The relative abundance of the RNA species in the three phases is highlighted by the thickness of the arrows. Differential transcription termination is indicated by the relative length of the transcripts, and putative secondary structures ( ) are shown.
) are shown.
A comparative analysis of the regions around the transcriptional initiation sites revealed the presence of sequence motifs that are generally found in the promoters of Sulfolobus genes (see Fig. S2 in the supplemental material): (i) an A/T-rich sequence (7 to 8 nt) generally centered at the position −23/−29, corresponding to the TATA box; (ii) two purines located in a consensus sequence (RNWAAW, where R is purine, W is A or T, and N is any base) immediately upstream of the TATA box and representing the transcription factor B-responsive element; and (iii) an A/T peak centered at position −10 (see Fig. S2 in the supplemental material) (35, 60). These three elements were identified in the promoter regions of ORF76, ORF154-288, ORFc68, and ORF91, whereas the remaining pSSVx promoters exhibited poorly conserved sequence motifs in one or two of these elements. In particular, T-rich sequences occupying the position of the TATA box and containing six thymidines were found upstream of the start sites of both ORF60 and ctRNA1 transcripts. These two 5′-flanking regions shared also an additional sequence (5′-CATTTT-3′) recognizable at 1 and 3 bp downstream of the six-T stretch, respectively. However, despite the nonconserved TATA consensus, the six-T boxes are preceded by canonical B-responsive element motifs, which strengthens the definition of these sequences as actual promoter elements. It is worth noting that three palindromic sequences were identified immediately upstream of and/or encompassing the TATA box of ORF60, suggesting their involvement as _cis_-acting sequences for the recruitment of further specific factors besides those required for basal transcription. A palindromic motif was also found at a distance of −5 to −14 bp from the transcriptional start site of the ORFc68 sequence, i.e., downstream of the TATA box, thus evoking the role of a negative _cis_-acting element recognizable by a transcriptional repressor(s).
In most cases, translation starts at the ATG codon, with the only exception being GTG for the ORF91. Transcripts that lack a leader sequence and a Shine-Dalgarno (SD) motif (ORF76, ORFc68, ORF154, and ORF288) as well as mRNAs endowed with typical archaeal ribosome binding sites were found. Hence, the dual nature of the archaeal translation initiation process which occurs either via a mechanism requiring ribosome binding to the SD sequence (as in Bacteria) or via a process which involves different signals downstream the translation initiation codon (4, 59, 60) is exemplified by the transcripts of the pSSVx genes. In particular, SD sequences were located in the 5′ untranslated regions of ORF60 (5′-GGTG-3′) and ORF91 (5′-GAGG-3′) as well as in the 5′ end of ORF892 (RepA) (5′-GGTG-3′). The distribution of these ribosome binding sites only partially agrees with previously reported statistical data based on archaeal genome sequence comparisons; this analysis pointed out their frequent occurrence upstream of internal genes contained in operons and only in a few cases upstream of the first genes in operons or of individually transcribed genes (4, 59). For example, transcripts starting at ORF60 that contain an SD leader, although ORF60 is the first gene of the operon and can be autonomously transcribed, and the ORF288 messenger, which does not carry any SD sequence even though it is the internal gene of the ORF154/ORF288 operon, represent the most clear-cut exceptions. Therefore, the SD motifs seem to be a specific feature of the gene cluster at the rep locus. The presence of these SD sequences might guarantee a more efficient frequency of translation initiation of the proteins that play a fundamental role in the replication, copy number balance, and/or maintenance of pSSVx.
DISCUSSION
The transcriptional pattern of the pSSVx genes was analyzed over the whole host growth cycle based on the evidence that pSSVx replication, similarly to the helper SSV2, occurs at the late exponential phase of the growth of the natural host, S. islandicus (11). The results of Northern blot hybridizations revealed that seven of the nine ORFs identified are actively transcribed. Strikingly, the expression of these seven ORFs displays significant variations in the temporal control as well as in the complexity of the transcriptional patterns, suggesting a close interdependence between gene expression and pSSVx replication. In fact, these variations mirrored two distinct temporal modes of controlling replication and copy number of the plasmid/virus pSSVx: (i) in actively dividing cells (over the entire exponential phase up to the 26th hour of incubation), the replication rate is constant and the copy number is tightly controlled and maintained low (1 to 2 molecules/cell)—this feature is shared by most prokaryotic plasmids (1, 34, 42); (ii) during and after pSSVx replication induction, the plasmid-like control is displaced by the overwhelming control modes more typically viral and triggered by SSV2 induction. Because of its hybrid nature, the complexity of pSSVx gene expression and replication exemplifies a coordinated choreography that uses several interwoven viral and plasmidic themes of transcriptional mechanisms in order to drive the plasmid-to-virus transition of the copy number control. ORF76 appeared to be strictly associated to the plasmid nature of pSSVx not only because structural homologs are found in most of archaeal plasmids (29) but also because of its peculiar expression pattern. Transcriptional levels of the ORF76 gene were constant and fairly high, similarly to its homolog ORF80 on plasmid pRN1; this similarity suggests a fundamental role as a transcription factor in the balance between replication and maintenance of the appropriate copy number. Nevertheless, unlike ORF80 mRNA, which is synthesized at constant levels up to the stationary/death phase of the host growth (5), ORF76 transcript rapidly declined as soon as the SSV2-dependent induction of replication was established.
The polycistronic ORF154-288 message, which encodes the counterparts of viral proteins assumed to be involved in the specific recognition and packaging of pSSVx DNA (2, 57), is transcribed with abundance directly proportional to the pSSVx copy number (i.e., copy number of the encoding genes) over the entire growth cycle. Differently, mRNA levels of the vp1 and vp3 capside genes of SSV2 are strongly induced only during DNA replication induction and dropped thereafter. Hence, it is possible to argue that the gene dosage dependence of ORF154-288 mRNA transcription guarantees constant ratios of packaging proteins to DNA molecules; differently, capsid genes should be switched on only in the narrow time range required for nucleocapsid assembly and virus extrusion. As previously described (11), when the copy number of SSV2/pSSVx remains constant inside the cells, after the steep increase in the late log phase, the virus titer does not increase as well. Hence, the absence of neoformed virions in this phase can be traced back to the abrogation of vp gene transcription in the mid-stationary phase.
The dual nature of pSSVx as a plasmid and a virus is particularly evident in the transcriptional control of the genes responsible for both replication and copy number control. The rep locus is comprised of four ORFs: ORF c68 encodes a putative transcription factor containing an SpoVT/AbrB-like domain (18, 62), typical of a bacterial family of transcription factors. Since this kind of transcription factor gene is missing on other prokaryotic plasmids and is present on the genomes of pSSVi, of STSV1 (Sulfolobus tengchongensis spindle-shaped virus), and SIFV (Sulfolobus islandicus filamentous virus) (40), it is tempting to speculate that acquisition by the pSSVx genome of ORFc68 as well as of ORF154 to ORF288 has been crucial for the development of a viral nature and for the ability to respond to diverse viral stimuli. Unlike all pSSVx mRNAs, the ORFc68 transcript accumulates even after the plasmid copy number has reached its plateau value; ORF91 has a homolog (ORF73) only on plasmid pDL10 (26) and on sequences of elements integrated into the genomes of some Sulfolobales (54); the ORF892 (RepA) (30) and ORF60 (copG) genes (28) are conserved in the crenarchaeal pRN plasmids. Like all CopGs, the relative abundance of the ORF60 transcript strictly parallels all the variations of the plasmid copy number during the cell cultures. Despite the “plasmidic” nature of most genes in the rep locus, the transition from the plasmidic to the viral phase requires the expression of these genes to also be prone to a kind of viral control. In this respect, upon the onset of the pSSVx replication induction, sequence-specific hybridization of the gene cluster at the rep locus gives rise to multiple signals due not only to degradation (like for ORF892 [RepA]) but also to a more complex transcriptional pattern. The transcripts mapped showed either common initiation sites but different termination sites (ORF60, ORF60/ORF91, and ORF60/ORF91/ORF892 [RepA]) or different initiation sites but coterminal ends (*ORF91/ORF892 [RepA]); this picture recalls the transcriptional initiation and termination/antitermination modes of some genes that are functionally associated in clusters in phages (33) as well as eukaryal (46) and archaeal (32, 48, 63) viruses. The variation in the relative abundances of the three different transcripts originating from promoter Pr1 (ORF60, ORF60/ORF91, and ORF60/91/892 [RepA]) in the passage from preinduction to induction implies the shift of equilibrium between premature termination (more properly a bacterial mode) and antitermination (more typically a viral mode) (14) towards the latter.
Interestingly, the rep locus also generates countertranscripts, whose temporal expression was similar to that described for ORF76 and hence could be correlated to the plasmidic behavior of pSSVx. In fact, two antisense mRNAs, the 0.5-kb c68 mRNA complementary to the ORF60 transcript (ctRNA1) and the ORF91 countertranscript (ctRNA2), were detected during cell growth preceding the pSSVx replication induction and abruptly disappeared when the maximum plasmid copy number inside the cells was reached. The presence of these countertranscripts as well as the complex transcriptional pattern of the differentially expressed genes of the rep locus suggest that elongation and/or translation of transcripts starting from promoter Pr1 (ORF60, ORF60/ORF91, and ORF60/91/892 [RepA]) is affected mainly by an attenuation mechanism or translation inhibition dependent on antisense mRNAs (58). It is worth mentioning that control systems that use antisense RNAs are frequently found in plasmids characterized by different replication mechanisms (6, 7, 12). Antisense-mediated attenuation acts through the formation of a duplex that enhances transcription termination at a site located downstream by stabilizing termination stem-and-loop secondary structures on nascent mRNA. This process requires the formation of a stable base-pairing interaction (“kissing complex”) between two loops or between a loop and a single-stranded RNA tail (56). The formation of the “kissing complex” is stimulated by the presence of a structural motif (U turn) containing the consensus sequence YUNR (where Y is pyrimidine and R is purine) (15). The identification of typical U-turn motifs in the loop of secondary stem-and-loop structures of both ctRNA1 and ctRNA2 reinforces the hypothesis of their putative role in the antisense-mediated mechanism of attenuation termination.
The results obtained are depicted in the scheme of Fig. 5, which resumes synthetically the transcriptional regulation of the genes crucial for replication and copy number control. The hypothesis about the behavior of pSSVx as a plasmid could be attempted by analogy to prokaryotic systems. Nevertheless, the contribution of the single pSSVx ORFs as well as the factors responsible for the shift to a viral phase remain unclear and to be defined. The recent development of a shuttle vector based on this genetic element (3) could contribute to understanding whether single ORFs and regulatory sequences are relevant for replication, segregation, and copy number control as well as for the interplay between pSSVx and SSV2.
Supplementary Material
[Supplemental material]
Acknowledgments
We are grateful to Claudio Mussolino for helpful scientific discussion and to Gabriella Fiorentino for critically reading the manuscript.
This work was grant aided by the European Union within the framework of the Screen projects (contract QLK3-CT-2000-00649), by the Ministero dell'Università e della Ricerca Scientifica (Progetti di Rilevante Interesse Nazionale 2003, U. O. Simonetta Bartolucci), by the Danish Natural Science Research Council (FNU), and by the Danish Council for Technology and Production Sciences (FTP) to the Copenhagen laboratory. Support from the Regional Center of Competence (CRdC ATIBB, Regione Campania, Naples, Italy) is also gratefully acknowledged.
Footnotes
▿
Published ahead of print on 22 June 2007.
REFERENCES
- 1.Adamczyk, M., and G. Jagura-Burdzy. 2003. Spread and survival of promiscuous IncP-1 plasmids. Acta Biochim. Pol. 50**:**425-453. [PubMed] [Google Scholar]
- 2.Arnold, H. P., Q. She, H. Phan, K. Stedman, D. Prangishvili, I. Holz, J. K. Kristjansson, R. Garrett, and W. Zillig. 1999. The genetic element pSSVx of the extremely thermophilic crenarchaeon Sulfolobus is a hybrid between a plasmid and a virus. Mol. Microbiol. 34**:**217-226. [DOI] [PubMed] [Google Scholar]
- 3.Aucelli, T., P. Contursi, M. Girfoglio, M. Rossi, and R. Cannio. 2006. A spreadable, non-integrative and high copy number shuttle vector for Sulfolobus solfataricus based on the genetic element pSSVx from Sulfolobus islandicus. Nucleic Acids Res. 34**:**e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benelli, D., E. Maone, and P. Londei. 2003. Two different mechanisms for ribosome/mRNA interaction in archaeal translation initiation. Mol. Microbiol. 50**:**635-643. [DOI] [PubMed] [Google Scholar]
- 5.Berkner, S., and G. Lipps. 2007. Characterization of the transcriptional activity of the cryptic plasmid pRN1 from Sulfolobus islandicus REN1H1 and regulation of its replication operon. J. Bacteriol. 189**:**1711-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brantl, S. 2002. Antisense-RNA regulation and RNA interference. Biochim. Biophys. Acta 3**:**15-25. [DOI] [PubMed] [Google Scholar]
- 7.Brantl, S. 2002. Antisense RNAs in plasmids: control of replication and maintenance. Plasmid 48**:**165-173. [DOI] [PubMed] [Google Scholar]
- 8.Cannio, R., P. Contursi, M. Rossi, and S. Bartolucci. 1998. An autonomously replicating transforming vector for Sulfolobus solfataricus. J. Bacteriol. 180**:**3237-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L., K. Brugger, M. Skovgaard, P. Redder, Q. She, E. Torarinsson, B. Greve, M. Awayez, A. Zibat, H. P. Klenk, and R. A. Garrett. 2005. The genome of Sulfolobus acidocaldarius, a model organism of the Crenarchaeota. J. Bacteriol. 187**:**4992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81**:**1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contursi, P., S. Jensen, T. Aucelli, M. Rossi, S. Bartolucci, and Q. She. 2006. Characterization of the Sulfolobus host-SSV2 virus interaction. Extremophiles 10**:**615-627. [DOI] [PubMed] [Google Scholar]
- 12.del Solar, G., and M. Espinosa. 2000. Plasmid copy number control: an ever-growing story. Mol. Microbiol. 37**:**492-500. [DOI] [PubMed] [Google Scholar]
- 13.del Solar, G., A. M. Hernandez-Arriaga, F. X. Gomis-Ruth, M. Coll, and M. Espinosa. 2002. A genetically economical family of plasmid-encoded transcriptional repressors involved in control of plasmid copy number. J. Bacteriol. 184**:**4943-4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodd, I. B., K. E. Shearwin, and J. B. Egan. 2005. Revisited gene regulation in bacteriophage lambda. Curr. Opin. Genet. Dev. 15**:**145-152. [DOI] [PubMed] [Google Scholar]
- 15.Franch, T., and K. Gerdes. 2000. U-turns and regulatory RNAs. Curr. Opin. Microbiol. 3**:**159-164. [DOI] [PubMed] [Google Scholar]
- 16.Garnier, J., J. F. Gibrat, and B. Robson. 1996. GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol. 266**:**540-553. [DOI] [PubMed] [Google Scholar]
- 17.Gollnick, P., and P. Babitzke. 2002. Transcription attenuation. Biochim. Biophys. Acta 1577**:**240-250. [DOI] [PubMed] [Google Scholar]
- 18.Huffman, J. L., and R. G. Brennan. 2002. Prokaryotic transcription regulators: more than just the helix-turn-helix motif. Curr. Opin. Struct. Biol. 12**:**98-106. [DOI] [PubMed] [Google Scholar]
- 19.Julien, B., and R. Calendar. 1996. Bacteriophage PSP3 and φR73 activator proteins: analysis of promoter specificities. J. Bacteriol. 178**:**5668-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawarabayasi, Y., Y. Hino, H. Horikawa, K. Jin-no, M. Takahashi, M. Sekine, S. Baba, A. Ankai, H. Kosugi, A. Hosoyama, S. Fukui, Y. Nagai, K. Nishijima, R. Otsuka, H. Nakazawa, M. Takamiya, Y. Kato, T. Yoshizawa, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, K. Aoki, S. Masuda, M. Yanagii, M. Nishimura, A. Yamagishi, T. Oshima, and H. Kikuchi. 2001. Complete genome sequence of an aerobic thermoacidophilic crenarchaeon, Sulfolobus tokodaii strain 7. DNA Res. 8**:**123-140. [DOI] [PubMed] [Google Scholar]
- 21.Keeling, P. J., H. P. Klenk, R. K. Singh, O. Feeley, C. Schleper, W. Zillig, W. F. Doolittle, and C. W. Sensen. 1996. Complete nucleotide sequence of the Sulfolobus islandicus multicopy plasmid pRN1. Plasmid 35**:**141-144. [DOI] [PubMed] [Google Scholar]
- 22.Keeling, P. J., H. P. Klenk, R. K. Singh, M. E. Schenk, C. W. Sensen, and W. Zillig. 1998. Sulfolobus islandicus plasmids pRN1 and pRN2 share distant but common evolutionary ancestry. Extremophiles 2**:**391-393. [DOI] [PubMed] [Google Scholar]
- 23.Kessler, A., A. B. Brinkman, J. van der Oost, and D. Prangishvilli. 2004. Transcription of the rod-shaped viruses SIRV1 and SIRV2 of the hyperthermophilic archaeon Sulfolobus. J. Bacteriol. 186**:**7745-7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessler, A., G. Sezonov, J. I. Guijarro, N. Desnoues, T. Rose, M. Delepierre, S. D. Bell, and D. Prangishvili. 2006. A novel archaeal regulatory protein, Sta1, activates transcription from viral promoters. Nucleic Acids Res. 34**:**4837-4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein, R., U. Baranyi1, N. Rössler, B. Greineder, H. Scholz, and A. Witte. 2002. Natrialba magadii virus ϕCh1: first complete nucleotide sequence and functional organization of a virus infecting a haloalkaliphilic archaeon. Mol. Microbiol. 45**:**851-863. [DOI] [PubMed] [Google Scholar]
- 26.Kletzin, A., A. Lieke, T. Urich, R. L. Charlebois, and C. W. Sensen. 1999. Molecular analysis of pDL10 from Acidianus ambivalens reveals a family of related plasmids from extremely thermophilic and acidophilic Archaea. Genetics 152**:**1307-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwong, S. M., R. A. Skurray, and N. Firth. 2006. Replication control of staphylococcal multiresistance plasmid pSK41: an antisense RNA mediates dual-level regulation of Rep expression. J. Bacteriol. 188**:**4404-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipps, G., M. Stegert, and G. Krauss. 2001. Thermostable and site-specific DNA binding of the gene product ORF56 from the Sulfolobus islandicus plasmid pRN1, a putative archael plasmid copy control protein. Nucleic Acids Res. 29**:**904-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipps, G., P. Ibanez, T. Stroessenreuther, K. Hekimian, and G. Krauss. 2001. The protein ORF80 from the acidophilic and thermophilic archaeon Sulfolobus islandicus binds highly site-specifically to double-stranded DNA and represents a novel type of basic leucine zipper protein. Nucleic Acids Res. 29**:**4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipps, G., S. Rother, C. Hart, and G. Krauss. 2003. A novel type of replicative enzyme harbouring ATPase, primase and DNA polymerase activity. EMBO J. 22**:**2516-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipps, G. 2006. Plasmids and viruses of the thermoacidophilic crenarchaeote Sulfolobus. Extremophiles 10**:**17-28. [DOI] [PubMed] [Google Scholar]
- 32.Luo, Y., T. Leisinger, and A. Wasserfallen. 2001. Comparative sequence analysis of plasmids pME2001 and pME2200 of Methanothermobacter marburgensis strains Marburg and ZH3. Plasmid 45**:**18-30. [DOI] [PubMed] [Google Scholar]
- 33.Mosig, G., and D. H. Hall. 1994. Regulation of gene expression, p. 127-193. In J. D. Karam (ed.), Molecular biology of bacteriophage T4. ASM Press, Washington, DC.
- 34.Nordstrom, K. 2006. Plasmid R1 replication and its control. Plasmid 55**:**1-26. [DOI] [PubMed] [Google Scholar]
- 35.Ouhammouch, M., G. E. Langham, W. Hausner, A. J. Simpson, N. M. El-Sayed, and E. P. Geiduschek. 2005. Promoter architecture and response to a positive regulator of archaeal transcription. Mol. Microbiol. 56**:**625-637. [DOI] [PubMed] [Google Scholar]
- 36.Peng, X., I. Holz, W. Zillig, R. A. Garrett, and Q. She. 2000. Evolution of the family of pRN plasmids and their integrase-mediated insertion into the chromosome of the crenarchaeon Sulfolobus solfataricus. J. Mol. Biol. 303**:**449-454. [DOI] [PubMed] [Google Scholar]
- 37.Prangishvili, D., K. M. Stedman, and W. Zillig. 2001. Viruses of the extremely thermophilic archaeon Sulfolobus. Trends Microbiol. 9**:**39-42. [DOI] [PubMed] [Google Scholar]
- 38.Prangishvili, D., and R. A. Garrett. 2004. Exceptionally diverse morphotypes and genomes of crenarchaeal hyperthermophilic viruses. Biochem. Soc. Trans. 32**:**204-208. [DOI] [PubMed] [Google Scholar]
- 39.Prangishvili, D., and R. A. Garrett. 2005. Viruses of hyperthermophilic Crenarchaea. Trends Microbiol. 13**:**535-542. [DOI] [PubMed] [Google Scholar]
- 40.Prangishvili, D., R. A. Garrett, and E. V. Koonin. 2006. Evolutionary genomics of archaeal viruses: unique viral genomes in the third domain of life. Virus Res. 117**:**52-67. [DOI] [PubMed] [Google Scholar]
- 41.Prangishvili, D., P. Forterre, and R. A. Garrett. 2006. Viruses of the Archaea: a unifying view. Nat. Rev. Microbiol. 4**:**837-848. [DOI] [PubMed] [Google Scholar]
- 42.Praszkier, J., and A. J. Pittard. 2005. Control of replication in I-complex plasmids. Plasmid 53**:**97-112. [DOI] [PubMed] [Google Scholar]
- 43.Prato, S., R. Cannio, H. P. Klenk, P. Contursi, M. Rossi, and S. Bartolucci. 2006. pIT3, a cryptic plasmid isolated from the hyperthermophilic crenarchaeon Sulfolobus solfataricus IT3. Plasmid 56**:**35-45. [DOI] [PubMed] [Google Scholar]
- 44.Qureshi, S. A. 2006. Role of the Sulfolobus shibatae viral T6 initiator in conferring promoter strength and in influencing transcription start site selection. Can. J. Microbiol. 11**:**1136-1140. [DOI] [PubMed] [Google Scholar]
- 45.Rachel, R., M. Bettstetter, B. P. Hedlund, M. Haring, A. Kessler, K. O. Stetter, and D. Prangishvili. 2002. Remarkable morphological diversity of viruses and virus-like particles in hot terrestrial environments. Arch. Virol. 147**:**2419-2429. [DOI] [PubMed] [Google Scholar]
- 46.Rajcani J., V. Andrea, and R. Ingeborg. 2004. Peculiarities of herpes simplex virus (HSV) transcription: an overview. Virus Genes 28**:**293-310. [DOI] [PubMed] [Google Scholar]
- 47.Reiter, W. D., P. Palm, A. Henschen, F. Lottspiech, W. Zillig, and B. Grampp. 1987. Identification and characterisation of the genes encoding three structural proteins of the Sulfolobus virus-like particle SSV1. Mol. Gen. Genet. 206**:**144-153. [Google Scholar]
- 48.Reiter, W. D., P. Palm, S. Yeats, and W. Zillig. 1987. Gene expression in archaebacteria: physical mapping of constitutive and UV-inducible transcripts from the Sulfolobus virus-like particle SSV1. Mol. Gen. Genet. 209**:**270-275. [DOI] [PubMed] [Google Scholar]
- 49.Reiter, W. D., P. Palm, and W. Zillig. 1988. Analysis of transcription in the archaebacterium Sulfolobus indicates that archaebacterial promoters are homologous to eukaryotic polII promoters. Nucleic Acids Res. 16**:**1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiter, W. D., P. Palm, and W. Zillig. 1988. Transcription termination in the archaebacterium Sulfolobus: signal structures and linkage to transcription initiation. Nucleic Acids Res. 16**:**2445-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reiter, W. D., U. Hudepohl, and W. Zillig. 1990. Mutational analysis of an archaebacterial promoter: essential role of a TATA box for transcription efficiency and start-site selection in vitro. Proc. Natl. Acad. Sci. USA 24**:**9509-9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 53.Schleper, C., K. Kubo, and W. Zillig. 1992. The particle SSV1 from the extremely thermophilic archaeon Sulfolobus is a virus: demonstration of infectivity and of transfection with viral DNA. Proc. Natl. Acad. Sci. USA 89**:**7645-7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.She, Q., R. K. Singh, F. Confalonieri, Y. Zivanovic, G. Allard, M. J. Awayez, C. C. Y. Chan-Weiher, I. G. Clausen, B. A. Curtis, A. De Moors, G. Erauso, C. Fletcher, P. M. K. Gordon, I. Heikamp-de Jong, A. C. Jeffries, C. J. Kozera, N. Medina, X. Peng, H. P. Thi-Ngoc, P. Redder, M. E. Schenk, C. Theriault, N. Tolstrup, R. Charlebois, W. F. Doolittle, M. Duguet, T. Gaasterland, R. A. Garrett, M. A. Ragan, C. W. Sensen, and J. Van der Oost. 2001. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc. Natl. Acad. Sci. USA 98**:**7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.She, Q., B. Shen, and L. Chen. 2004. Archaeal integrases and mechanisms of gene capture. Biochem. Soc. Trans. 32**:**222-226. [DOI] [PubMed] [Google Scholar]
- 56.Siemering, K. R., J. Praszkier, and A. J. Pittard. 1994. Mechanism of binding of the antisense and target RNAs involved in the regulation of IncB plasmid replication. J. Bacteriol. 176**:**2677-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stedman, K. M., Q. She, H. Phan, H. P. Arnold, I. Holz, R. A. Garrett, and W. Zillig. 2003. Relationships between fuselloviruses infecting the extremely thermophilic archaeon Sulfolobus: SSV1 and SSV2. Res. Microbiol. 154**:**295-302. [DOI] [PubMed] [Google Scholar]
- 58.Tang, T. H., N. Polacek, M. Zywicki, H. Huber, K. Brugger, R. A. Garrett, J. P. Bachellerie, and A. Huttenhofer. 2005. Identification of novel non-coding RNAs as potential antisense regulators in the archaeon Sulfolobus solfataricus. Mol. Microbiol. 55**:**469-481. [DOI] [PubMed] [Google Scholar]
- 59.Tolstrup, N., C. W. Sensen, R. A. Garrett, and I. G. Clausen. 2000. Two different and highly organized mechanisms of translation initiation in the archaeon Sulfolobus solfataricus. Extremophiles 4**:**175-179. [DOI] [PubMed] [Google Scholar]
- 60.Torarinsson, E., H. P. Klenk, and R. A. Garrett. 2005. Divergent transcriptional and translational signals in Archaea. Environ. Microbiol. 7**:**47-54. [DOI] [PubMed] [Google Scholar]
- 61.Wang, Y., Z. Duan, H. Zhu, X. Guo, Z. Wang, J. Zhou, Q. She, and L. Huang. 2007. A novel Sulfolobus non-conjugative extrachromosomal genetic element capable of integration into the host genome and spreading in the presence of a fusellovirus. Virology doi: 10.1016/j.virol.2007.01.035. [DOI] [PubMed]
- 62.Yao, F., and M. A. Strauch. 2005. Independent and interchangeable multimerization domains of the AbrB, Abh, and SpoVT global regulatory proteins. J. Bacteriol. 187**:**6354-6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zillig, W., P. Palm, W. D. Reiter, F. Gropp, G. Puhler, and H. P. Klenk. 1988. Comparative evaluation of gene expression in archaebacteria. Eur. J. Biochem. 173**:**473-482. [DOI] [PubMed] [Google Scholar]
- 64.Zillig, W., D. Prangishvili, C. Schleper, M. Elferink, I. Holz, S. Albers, D. Janekovic, and D. Gotz. 1996. Viruses, plasmids and other genetic elements of thermophilic and hyperthermophilic Archaea. FEMS Microbiol. Rev. 18**:**225-236. [DOI] [PubMed] [Google Scholar]
- 65.Zillig, W., H. P. Arnold, I. Holz, D. Prangishvili, A. Schweier, K. Stedman, Q. She, H. Phan, R. A. Garrett, and J. K. Kristjansson. 1998. Genetic elements in the extremely thermophilic archaeon Sulfolobus. Extremophiles 2**:**131-140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]