Staufen- and FMRP-Containing Neuronal RNPs Are Structurally and Functionally Related to Somatic P Bodies (original) (raw)
. Author manuscript; available in PMC: 2007 Aug 29.
Summary
Local control of mRNA translation modulates neuronal development, synaptic plasticity, and memory formation. A poorly understood aspect of this control is the role and composition of ribonucleoprotein (RNP) particles that mediate transport and translation of neuronal RNAs. Here, we show that staufen- and FMRPcontaining RNPs in Drosophila neurons contain proteins also present in somatic “P bodies,” including the RNA-degradative enzymes Dcp1p and Xrn1p/Pacman and crucial components of miRNA (argonaute), NMD (Upf1p), and general translational repression (Dhh1p/Me31B) pathways. Drosophila Me31B is shown to participate (1) with an FMRP-associated, P body protein (Scd6p/trailer hitch) in FMRP-driven, argonautedependent translational repression in developing eye imaginal discs; (2) in dendritic elaboration of larval sensory neurons; and (3) in bantam miRNA-mediated translational repression in wing imaginal discs. These results argue for a conserved mechanism of translational control critical to neuronal function and open up new experimental avenues for understanding the regulation of mRNA function within neurons.
Introduction
Localized translation of mRNAs has emerged as a major mechanism for regulating dynamic intracellular processes such as those involved in early embryonic development and synapse plasticity (Johnstone and Lasko, 2001; Martin, 2004). In the specific cases of growthcone guidance and synapse plasticity, temporally and spatially restricted repression of mRNA translation allows subcellular locations within a single neuron to transiently achieve different molecular and functional properties. This allows growth-cone turning in specific directions or, potentially, synapse-specific alterations required during learning and memory (Martin, 2004; Richter and Lorenz, 2002). Similarly, in dendrites, translationally repressed RNAs mobilized by synaptic stimulation are translated through control mechanisms that may include polyadenylation of mRNAs at stimulated synapses (Richter and Lorenz, 2002). It is likely that such locally translated mRNAs influence dendritic growth as well as maintain protein synthesis-dependent forms of synaptic plasticity (Ye et al., 2004; Martin, 2004).
Translational repression often occurs in cytoplasmic, ribonucleoprotein (RNP) particles. In the mammalian nervous system, staufen-containingRNPsare thought to mediate translational repression and/or mRNA transport of dendritically localized mRNAs (Kiebler and Bassell, 2006). These granules often contain the fragile X mental retardation protein (FMRP), a translational repressor that negatively regulates dendritic growth (Nimchinsky et al., 2001), as well as mRNAs translationally regulated at synapses (Knowles et al., 1996; Kohrmann et al., 1999; Krichevsky and Kosik, 2001; Mallardo et al., 2003; Kanai et al., 2004). However, the compositional diversity, cellular functions, and underlying mechanisms of staufen-containing RNPs remain largely unknown.
The shared presence of staufen (Stau) and an associated protein, barentsz (Btz), on maternal and neuronal RNPs suggests a compositional similarity between at least two classes of RNA storage/transport granules (Kiebler et al., 1999; Macchi et al., 2003; Mallardo et al., 2003). This hypothesis is further supported by roles for Stau in both maternal and neuronal mRNA transport (St Johnston et al., 1991; Tang et al., 2001) and for FMRP (dFMR1 in Drosophila) in translational repression during Drosophila oocyte development (Costa et al., 2005). While additional shared components may soon be identified using biochemistry combined with proteomics (Elvira et al., 2006; Kanai et al., 2004), there is currently limited information on how far biochemical and functional similarities between neuronal and maternal RNPs extend.
Recently, a third class of conserved somatic cytoplasmic RNPs, termed cytoplasmic RNA processing bodies (“P bodies”; also termed GW182 or DCP bodies), have been described in yeast, C. elegans, and mammalian cells. P bodies contain nontranslating mRNAs and multiple proteins involved in mRNA degradation and translational control (Kiebler and Bassell, 2006). While first described as sites of mRNA decapping and 5′to 3′exonucleolytic degradation (Cougot et al., 2004; Sheth and Parker, 2003), P bodies have recently been shown to function in conventional and miRNA-mediated translational control as well as mRNA storage (Brengues et al., 2005; Coller and Parker, 2005; Liu et al., 2005a; Pillai et al., 2005). Indeed, shared features of yeast mRNA turnover and translational pathways are indicated by the observation that two proteins that accumulate with mRNA in P bodies, Dhh1p and Pat1p, promote both mRNA decapping and translational repression (Coller and Parker, 2005). Similarities between P bodies and maternal RNPs are further suggested by the known functions of Dhh1p-orthologous, DEAD-box RNA helicases (Me31B, CGH-1, and Xp54) in maternal RNA granules of Drosophila, C. elegans, and Xenopus oocytes, respectively (Coller et al., 2001; Ladomery et al., 1997; Nakamura et al., 2001; Navarro et al., 2001). Together, these observations led us to hypothesize that many RNA granules will share a core composition and function.
In this work, we provide experimental support for a model in which neuronal staufen-containing RNPs (also referred to here as “staufen RNPs” or “staufen granules”) share fundamental organization with maternal RNA granules and somatic P bodies. Staufen RNPs visualized in Drosophila are shown to contain not only maternal translational control and RNA-transport molecules but also components of miRNA, nonsense-mediated decay (NMD), and RNA-turnover pathways present on somatic P bodies. Additionally, we present functional data showing that Me31B/Dhh1p, a protein present in neuronal staufen granules, P bodies, and maternal RNA granules, functions (1) together with another dFMR1- associated,Pbodyprotein(trailerhitch/Scd6p) indFMR1- driven, argonaute-dependent translational repression in the developing eye disc; (2) dendritic elaboration in larval sensory neurons, a process previously shown to be regulated by translational repressor proteins pumilio (Pum), nanos (Nos), and dFMR1; and (3) in bantam miRNA-mediated translational repression in the developing wing imaginal disc. Thus, in addition to documenting broadly conserved composition and function of RNA granules in neuronal, germline, and somatic cells, we identify Me31B as novel component (to our knowledge) of thedFMR1 pathway, which acts as a critical regulator of dendritic morphogenesis and microRNA function in vivo.
Results
Neuronal Staufen Granules in Drosophila
To identify and characterize Drosophila RNPs involved in neuronal translation control, we combined a primary cell-culture system (Kraft et al., 1998) with microscopic localization of transgenically expressed Stau, a highly conserved protein of maternal RNPs and mammalian neuronal granules (Ferrandon et al., 1994; Kiebler et al., 1999). A Stau:GFP fusion protein expressed in Drosophila ventral ganglion neurons is concentrated in puncta within neurites of 3- to 4-day-old primary cultures of dissociated larval ventral ganglia, with large puncta observed in the cell body (Figure 1A; see Figure S1 in the Supplemental Data available online). Of 292 granules analyzed in nine Stau:GFP-expressing cells, 56.5% of granules were within 1 μm of branch points and 33.9% were away from branch points (Figure 1A and inset). This observed localization of staufen granules is consistent with the previously proposed role for translational regulation in controlling dendritic branching in Drosophila (Ye et al., 2004). In vivo, panneuronally expressed Stau:GFP revealed similar particles within peripheral nerves exiting the larval central nervous system as well as in cell bodies within the ventral ganglion (Figure 1B).
Figure 1. Drosophila Neurons Have Ribonucleoprotein Particles Containing Stau, dFMR1, Btz, and a Dendritically Targeted mRNA.
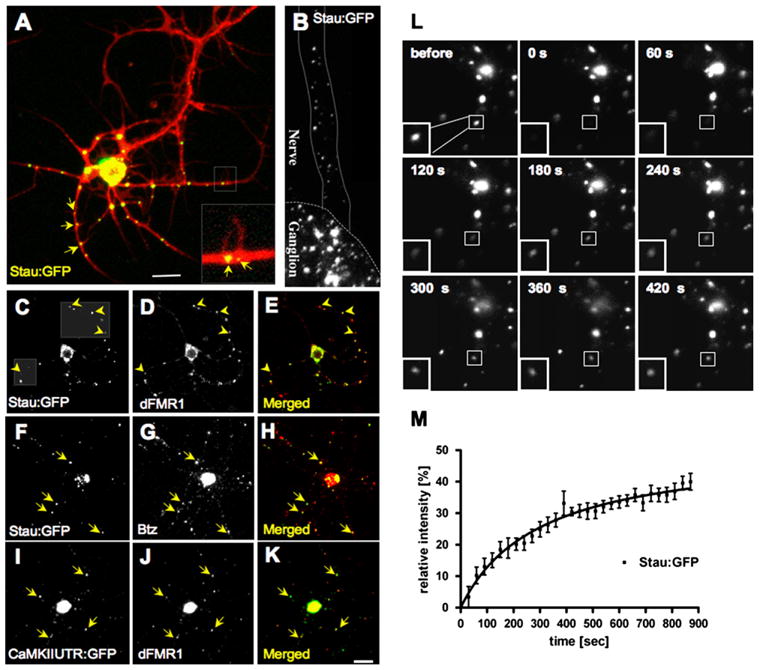
(A) Stau:GFP (green) in cultured Drosophila motor neurons counterstained with a anti-HRP antibody (red). The inset shows Stau:GFP puncta at the base of small neurite branches (arrows). These puncta show occasional bidirectional movement within neurites (Movies S1 and S2).
(B) View of a Drosophila larval ventral ganglion and emerging nerve from an animal expressing Stau:GFP in the nervous system.
(C–E) Confocal image pair and merged image of a cultured motor neuron labeled for Stau:GFP (C) and endogenous dFMR1 (D). Dashed boxes show regions optimized for displaying faint spots: the yellow arrowheads show that particles appearing red on the merged image (E) in fact contain Stau:GFP (green).
(F–H) Cell double labeled with Stau:GFP (F) and endogenous Btz (G).
(I–K) Drosophila CaMKII mRNA (I) visualized by ms2-tagged CamKII mRNA combined with MCP:GFP detection (Ashraf et al., 2006) is present on dFMR1-positive particles (J).
(L) In FRAP experiments in live cultured motor neurons expressing Stau:GFP, images of a staufen granule were recorded “before,” immediately after bleaching (“0 sec”), and once every 30 s during the course of recovery.
(M) For each time point, fluorescence intensity within a small region of interest (ROI) was measured and plotted on the graph after normalization to a paired “unbleached” spot. From the data set (n = 6 cells; 11 spots), a fluorescence recovery curve was calculated using nonlinear regression. Rectangles frame the bleached particle; ROIs, not shown, were smaller and closer to spot dimensions. Scale Bar, 10 μm.
To determine whether these Stau:GFP particles were similar to mammalian RNPs involved in neuronal mRNA regulation, we asked if they contained other established components of mammalian neuronal RNPs. As shown in Figure 1, Stau:GFP-containing granules were strongly labeled by antibodies against dFMR1 (Figures 1C–1E) or Btz (Figures 1F–1H). Stau:GFP and dFMR1 colocalized extensively but not completely in wild-type and Stau:GFP- or dFMR1-overexpressing neurons (Table 1; Figure 1; Figure S2). These results indicate that dFMR1 and Stau exist substantially in the same granules but can also be observed in separate yet related particles (see Discussion).
Table 1. Percent Colocalization of P Body Components with Stau- and dFMR1-Containing Granulesa.
| Genotype | Stau:GFP Expressing | dFMR1 Overexpressing | Wild-Type (_w_1118) | Wild-Type (_w_1118) |
|---|---|---|---|---|
| Antibody used as reference | v. Stau | v. dFMR1 | v. dFMR1 | v. Stau |
| Stau | — | 80.5 (11; 462) | 77.2 (8; 408) | — |
| dFMR1 | 80.3 (19; 524) | — | — | 45.1 (17; 1223) |
| Me31B | 71.0 (12; 526) | 85.0 (4; 207) | 60.0 (14; 432) | 50.2 (8; 440) |
| Tral | 100.0 (4; 52) | 90.6 (7; 339) | 56.8 (6; 491) | NDb |
| Pcm | 88.0 (7; 117) | 72.3 (7; 343) | 60.3 (8; 401) | NDb |
| DCP1 | 92.6 (5; 81) | 85.8 (11; 318) | 75.3 (3; 97) | NDb |
| UPF1 | 90.0 (6; 130) | 80.5 (10; 394) | 58.3 (3; 96) | NDb |
| Ago-2 | 74.3 (4; 109) | 73.0 (8; 293) | NDc | NDb |
For additional evidence that staufen granules could be involved in translational repression, we also examined whether a known dendritically transported mRNA was present in these staufen/dFMR1-positive granules. Recent work has shown that Drosophila CaMKII mRNA is transported along dendrites through a process stimulated by neuronal activity (Ashraf et al., 2006). This phenomenon is analogous to activity-stimulated movement of mammalian CaMKII mRNAs in staufen-positive neuronal RNPs (Krichevsky and Kosik, 2001). To visualize CaMKII mRNA, we cultured neurons coexpressing a GFP-tagged, nuclearly targeted RNA virus capsid protein (GFP:MCP) and CaMKII mRNA, multiply tagged with binding sites for MCP (Ashraf et al., 2006). Figures 1I–1K show that CaMKII mRNA-containing puncta observed in neurites overlap with protein markers of staufen granules.
The presence of Stau, dFMR1, Btz, and, in at least some cases,CaMKII mRNA in overlapping puncta indicates that these foci represent_Drosophila_ neuronal RNPs likely to function in the transport and translational regulation of neuronal mRNAs. Consistent with this hypothesis, these staufen/dFMR1-positive granules also stain with antisera against the RNA-binding protein ypsilon schachtel (Yps; Figures S3A–S3C) and the zipcode binding protein (ZBP/Imp; Figures S3D–S3F), both of which function in transport or regulation of localized mRNAs (Mansfield et al., 2002; Munro et al., 2006). Moreover, these granules also stain positive for (1) the RNAbinding proteins and translational repressors Pum and Nos, recently implicated in neuronal translational control and dendrite morphogenesis (Ye et al., 2004; Figures S3G–S3L); (2) the cap-binding translational-initiation factor, eIF4E (Sonenberg and Gingras, 1998; Figures S3M–S3O); together with (3) the eIF4E-inhibitory protein Cup, which represses translation by binding to and blocking eIF4E function (Figures S3P–S3R). The presence of Cup is consistent with translational repression of particle-associated mRNAs (Lasko et al., 2005). Control experiments (see Supplemental Data) established that the colocalization of various granule proteins described is observed in neurons of multiple genotypes: (1) wild-type control; (2) UAS-dFMR1; or (3) UAS-Stau: GFP, although images are typically shown from the bright, easily imaged neuronal granules observed in cells expressing transgenically encoded Stau:GFP or dFMR1.
The above results reveal two general properties of these granules in_Drosophila_ neurons. First, in all cases, a major class of granule exists wherein various proteins colocalize. For example, in wild-type cells, 77.2% of dFMR1-containing particles are positive for staufen and 75.3% for DCP1 (Table 1). Second, the observation that there are clearly granules that stain strongly for some but not all markers suggests there are subclasses of particles. Two additional observations suggest that these subclasses of particles are related to the major class of granules. First, they share components such as Stau or dFMR1. Second, the increased brightness of staufen granules in Stau:GFP or dFMR1-overexpressing cells (compared to wild-type) and the increase in colocalization is presumably due to growth, or fusion, of related and dynamic endogenous granules when assembly components are present in abundance. Such a model is supported by fluorescence recovery after photobleaching (FRAP) analyses (Figures 1L and 1M; see Supplementary Experimental Procedures). These experiments show that staufen granules are dynamic, allowing relatively rapid exchange of at least a fraction of Stau:GFP with the cytoplasm. Taken together, these results are most consistent with Drosophila neurons containing a family of potentially interacting RNPs with related composition and function.
Neuronal Staufen Granules Are Related to Somatic P Bodies
Recent work on yeast and mammalian P bodies has suggested that they are dynamic RNPs like neuronal staufen granules (Andrei et al., 2005) and can be sites of transient translational repression (Brengues et al., 2005; Bhattacharyya et al., 2006). Thus, we asked whether Drosophila staufen granules are related to P bodies. We first tested whether neuronal staufen RNPs contained hydrolytic enzymes that mediate removal of the m7GDP (7-methyl-GDP) cap and subsequent 5′to 3′degradation. These enzymatic events are respectively mediated by a decapping enzyme that includes the Dcp1p subunit and by the 5′to 3′riboexonuclease Xrn1p (LaGrandeur and Parker, 1998; Parker and Song, 2004). Both Dcp1p and Xrn1p are integral components of yeast and mammalian P bodies (Cougot et al., 2004; Sheth and Parker, 2003).
Remarkably, the Drosophila homologs of the degradative enzymes Dcp1p and Xrn1p (termed DCP1 and Pacman/Pcm) are clearly concentrated in staufen- and dFMR1-containing RNPs (Figures 2B–2D and 2F–2H; Figures S4A–S4F). The presence of these enzymes suggests that Drosophila staufen granules may have additional roles in the control of mRNA turnover.
Figure 2. Neuronal Staufen Granules Contain P Body Components Mediating Translation Repression and RNA Decay.
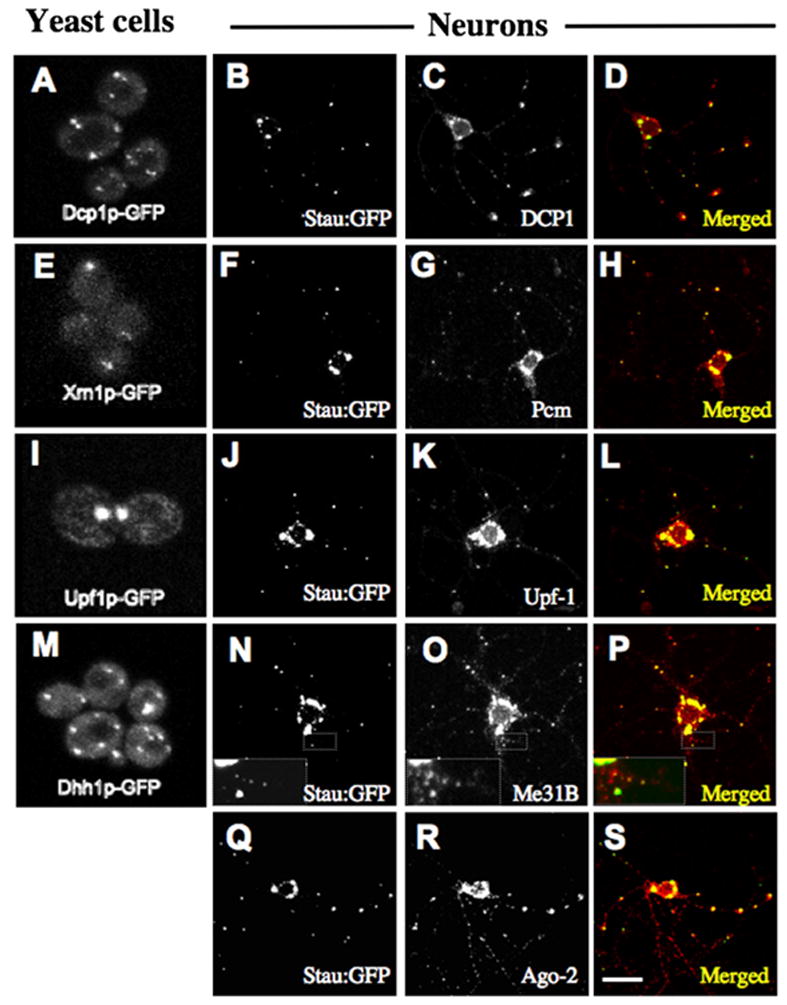
Yeast P-body proteins tagged with GFP in S. cerevisiae cells (left column) with their Drosophila orthologs localized relative to Stau:GFP in cultured Stau:GFP-expressing motor neurons. (A–D) Dcp1p/DCP1; (E–H) Xrn1p/Pcm; (I–L) Upf1p/UFP1; (M–P) Dhh1p/Me31B; (Q–S) and Ago-2 are also present on neuronal staufen granules. The inset with a magnified view of small Me31B particles in a neurite show that these also contain Stau:GFP. Scale bar, 10 μm for neurons.
We also examined the localization of other proteins known to concentrate in P bodies and promote P body formation. To date, mRNAs are known to be targeted to P bodies by three pathways: (1) the miRNA pathway by miRNAs and argonaute (Ago) proteins (Liu et al., 2005a; Pillai et al., 2005); (2) the NMD pathway, which is primarily driven by Upf1p (Sheth and Parker, 2006); and (3) a general pathway that works on bulk mRNA and is mediated by the Dhh1p and Pat1p proteins in yeast (Coller and Parker, 2005).
Association of Ago-1 and Ago-2 with dFMR1 has been argued by genetic and biochemical tests in Drosophila (Ishizuka et al., 2002; Jin et al., 2004).Wetherefore tested whether these proteins were present on staufen- and dFMR1-positive granules. While the generally poor quality of the Ago-1 antibody for immunohistochemistry (data not shown) did not allow us to easily examine its presence in Drosophila neuronal granules, Ago-2 could be visualized within these particles (Figures 2Q–2S; Figures S4J–S4L). This is consistent with recent analyses suggesting that miRNAs may function in granules such as P bodies (Pillai et al., 2005; Liu et al., 2005a, 2005b; Jakymiw et al., 2005; Chu and Rana, 2006). Thus, our observation that Ago-2 is present in dFMR1-containing staufen granules is consistent with a previously proposed role for FMRP/dFMR1 in miRNA/RNAi-mediated gene silencing (Kosik and Krichevsky, 2005).
The critical protein for translation repression in NMD, UPF1, is also present on staufen granules (Figures 2J–2L; Figures S4G–S4H). Finally, we observed that Me31B, a highly conserved homolog of yeast Dhh1p, is also present on these particles (Figures 2N–2P; Figures S5D–S5F). Thus, neuronal staufen- and dFMR1-positive RNPs contain critical components of three different systems of translation repression suggesting that these RNPs, like P bodies, mediate diverse RNA regulatory events.
The presence of similar proteins in staufen granules and P bodies suggests that these neuronal and somatic RNPs share a similar core biochemical composition. These data also suggest that shared proteins will be common to other types of RNA granules, including maternal RNA granules. Consistent with this view, the decapping enzymes (Dcp1 and Dcp2) have been recently reported to be present on C. elegans P granules (Lall et al., 2005). Moreover, we find both Pcm/Xrn1p and DCP1 colocalize with Me31B in maternal RNA granules in Drosophila nurse cells (Figures 3A–3F).
Figure 3. RNA Decapping and Degradative Enzymes Are Present on Maternal RNP Granules.
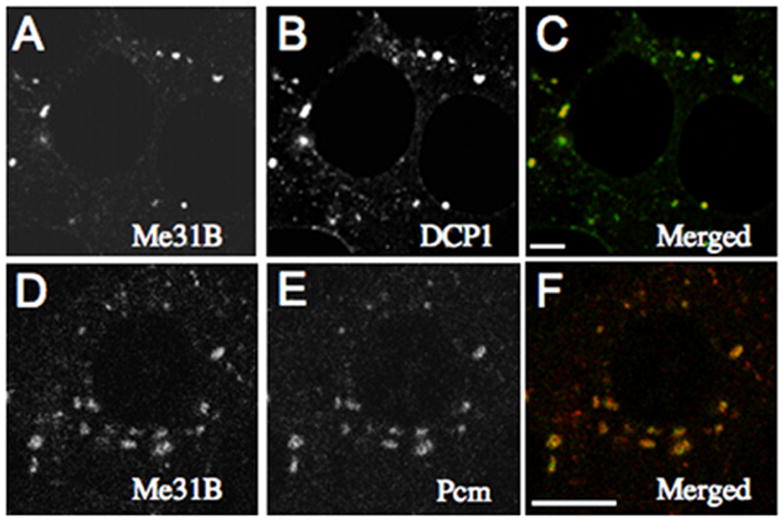
(A–C) DCP1 and (D–F) Pcm colocalize with Me31B in cytoplasmic foci in nurse cells (stage 8 is shown). (C and F) Merged images. Scale bar, 10 μm.
Trailer Hitch, a Me31B-Associated Maternal Protein, Is Present on P Bodies and Neuronal Staufen Granules
Me31B functions during oogenesis as a translational repressor of oskar mRNA in a well-studied eIF4E-Cup- Bru translational control complex (Lasko et al., 2005). This complex also contains a conserved Sm- and FDFdomain RNA-binding protein, trailer hitch (Tral). In Drosophila ovaries, Tral coimmunoprecipitates with Me31B and colocalizes with Me31B-containing maternal RNA granules (Boag et al., 2005).
Figure 4A shows that a Me31B/Tral/dFMR1 complex coimmunoprecipitates from Drosophila adult head extracts, consistent with a model in which the three proteins function together in neuronal translation control. Me31B, Tral, and dFMR1 all have a similar, ubiquitous expression pattern in the central nervous system, showing a predominantly cytoplasmic, steady-state localization (Figures S5A–S5C). In cultured Drosophila neurons, Tral also localizes to staufen- and dFMR1-containing granules (Figures 4B–4D and Figures S5G–S5I). Moreover, a GFP fusion to Scd6p, the S. cerevisiae homolog of Tral, colocalizes with Dcp2p:RFP under high cell density or nutrient starvation, conditions that enlarge yeast P bodies (Teixeira et al., 2005; Figures 4E–4G). Together, these data indicate that (1) Tral is present on Drosophila neuronal RNPs in a biochemical complex that contains Me31B and dFMR1; and (2) Scd6p, the yeast homolog of Tral, is a component of P bodies. The latter observation further extends similarities between P bodies and staufen granules.
Figure 4. Tral Is an Me31B/dFMR1-Associated Protein Present on Staufen RNPs with a Conserved Homolog, Scd6p, in Yeast P Bodies.
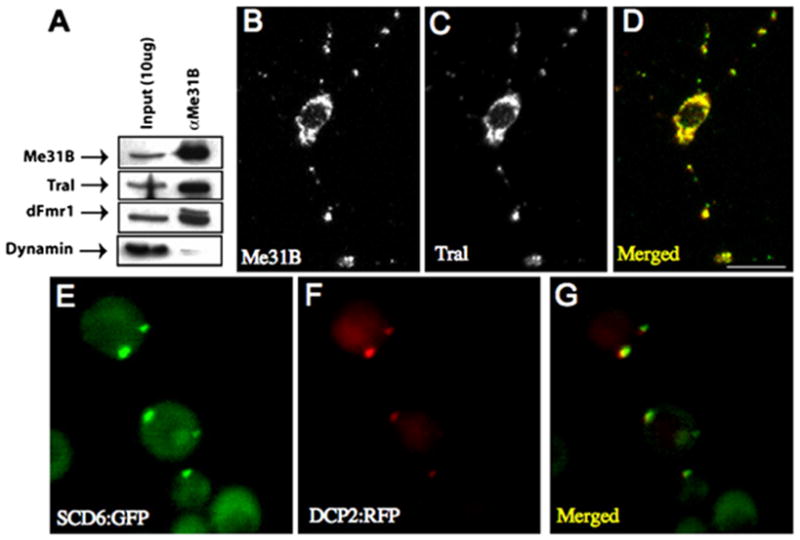
(A) Western blot of Me31B coimmunoprecipitates probed with antibodies against Me31B, Tral, dFMR1, and dynamin.
(B–D) Me31B (B) and Tral (C) colocalize in neuronal granules of dFMR1 expressing cultured motor neurons (similar results in w1118 cells are shown in Figures S5D–S5I).
(E–G) Yeast cells expressing Scd6p:GFP (E) and Dcp2p:RFP (F) showing colocalization of Scd6p:GFP to P bodies. Scale bar, 10 μm.
Me31B and Tral Are Required for _dFMR1_-Mediated Translational Repression
The compositional similarity of P bodies and staufen RNPs suggests that neuronal translational control is regulated through proteins and mechanisms associated with somatic P bodies. To test this prediction, we focused on the highly conserved DEAD-box RNA helicase Me31B, which functions in translational repression of maternal mRNAs and in the targeting of mRNAs to P bodies (Coller and Parker, 2005; Nakamura et al., 2001). The presence of Me31B and Tral with Ago-1 on dFMR1-containing complexes suggests that these proteins may function in neuronal translation control, potentially with dFMR1 in miRNA-mediated processes.
To test whether Me31B and Tral function in dFMR1- mediated translational repression, we asked if defects caused by dFMR1 overexpression in developing eyes were modified in genetic backgrounds deficient for Me31B or Tral. Ectopic overexpression of dFMR1 in the compound eye driven by the sevenless enhancer (sevdFMR1) results in a “rough-eye” phenotype through a pathway that requires dFMR1 domains essential for translational repression as well as Ago-1 function (Figure 5A; Jin et al., 2004; Wan et al., 2000).
Figure 5. Me31B and Tral Are Required for dFMR1-Induced Defects in the Drosophila Eye.
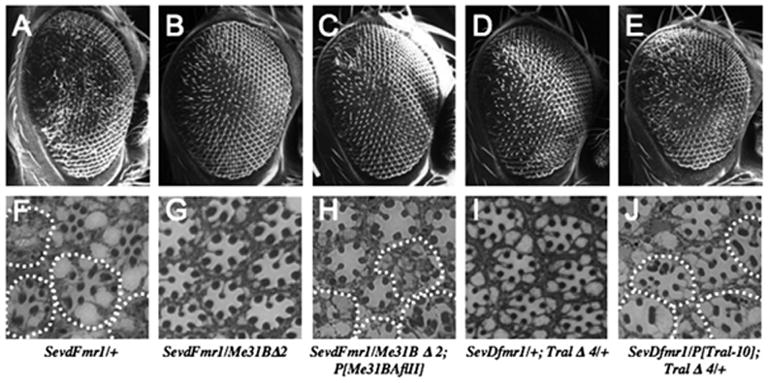
(A–E) SEMs of adult compound eyes with paired retinal sections (F–J). Magnification of SEMs is 150×. Tangential sections of each genotype are at approximately the same depth.
As shown in Figure 5B, loss of a single copy of me31B suppressed _sev-dFMR1_-induced rough eye phenotypes. Unambiguous suppression was observed with either me31BD1 or me31B_D_2 allele (Figure 5B; data not shown). Internally, the disruption of ommatidia caused by dFMR1 overexpression was also suppressed, as observed in tangential sections (Figures 5F and 5G). This suppression is a direct result of me31B deficiency, because a genomic me31B+ transgene, P[me31BAflII], which is capable of rescuing lethality of _me31B_Δmutants (Nakamura et al., 2001) rescues suppression of the sev-dFMR1 rough eye phenotype (Figures 5C and 5H).
Results with tral mutations were similar. We isolated deletion alleles for tral (see Supplementary Experimental Procedures) and found them to result in larval lethality. Both tral deletions dominantly suppressed _sev-dFMR1_- induced rough eyes (Figures 5D and 5I; data not shown). A tral+ genomic transgene (P[tral-10]) containing the entire tral locus was sufficient to rescue the lethality of tral mutants. This genomic transgene also “rescued” dominant suppression of the rough eye phenotype, thereby demonstrating that phenotypic suppression of _sev-dFMR1_occurs specifically due to loss of tral (Figures 5E and 5J).
Given that Me31B, Tral, and dFMR1 form a physical complex, the above results suggest that Me31B and Tral act, together with dFMR1, as translational regulators in neuronal cells. An alternative interpretation is that single-copy deletions of tral or me31B block apoptosis or other developmental errors induced by Sev-dFMR1. However, this is unlikely for three reasons: First, coimmunoprecipitation and colocalization of Me31B, Tral, and dFMR1 are more consistent with a direct mechanism. Second, all three proteins have RNA-binding domains that predict roles in translational control. Finally, ectopic expression of Me31B in the eye causes rough eyes via a mechanism requiring amino acid residues necessary for translational repression (Figures S6A–S6C; see below).
Me31B and Tral Regulate Dendrite Morphogenesis in Sensory Neurons
The observed effect of Me31B (and Tral) induction on dendritic development of sensory neurons (Figure 6 and Figures S6D–S6F) provides further evidence for function in neuronal translation regulation. Previous studies have established that translational control of gene expression regulates dendrite morphogenesis in vivo. For example, neurons of human fragile X patients and Drosophila dFMR1 mutants show an increase in dendritic spine number and length (Nimchinsky et al., 2001; Lee et al., 2003). Conversely, induction of dFMR1, Pum, or Nos in class IV Drosophila da sensory neurons greatly perturbs, and can dramatically reduce, higher-order dendritic branching (Lee et al., 2003; Ye et al., 2004). If Me31B and Tral act in dendritic translational control, we anticipated that their induction would also have specific effects on higher-order dendritic branching.
Figure 6. Me31B Regulates Dendritic Growth in Sensory Neurons.
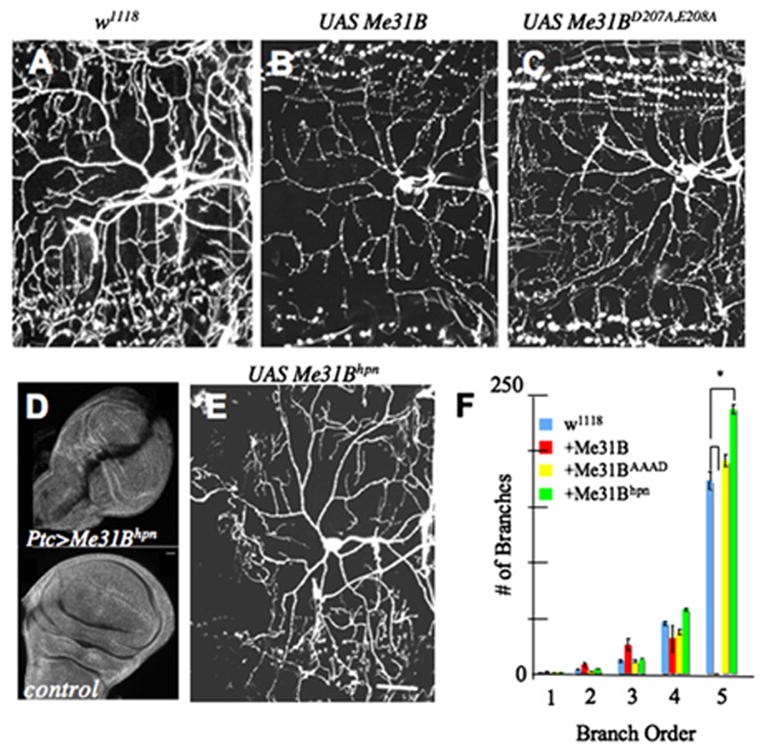
(A) Control class IV ddaC neuron expressing UAS-mCD8:GFP alone.
(B) Class IV ddaC neurons overexpressing Me31B and UAS-mCD8: GFP showing a reduction in higher-order dendrite arborization.
(C) The same neurons overexpressing a mutant Me31B incapable of translational repression (Me31BD207A, E208A) show normal dendritic branching.
(D) Transgenic RNAi dramatically reduces Me31B protein levels. Anti-Me31B staining of third-instar imaginal discs shows that UAS-Me31Bhpn expressed in the patched domain of wing imaginal discs reduces Me31B levels along the anterior-posterior border (top panel) compared to control wing imaginal discs (lower panel).
(E) Class IV ddaC neurons overexpressing a Me31B RNA hairpin (UAS-Me31Bhpn) exhibit abnormal dendrite morphology and increased high-order branching.
(F) Numbers of dendritic branches in each order, as revealed by reversed Strahler analysis (see Supplemental Data). Number of neurons analyzed for each genotype are: UAS-mCD8:GFP control (n = 15), UAS-Me31B (n = 10), UAS-Me31BD207A, E208A (n = 11), and UAS-Me31Bhpn (n = 13). Values are mean ± standard error. A star (*) indicates a significant reduction in fifth-order dendrite branching following Me31B overexpression compared to the control (p < 0.001) and a significant increase in fifth-order dendrite branching following Me31B RNAi (p < 0.001). Scale bar, 20 μm.
Overexpression of Me31B in class IV neurons substantially reduced high-order dendritic complexity (Figures 6A and 6B). In neurons overexpressing Me31B, the number of higher-order dendrites was significantly reduced compared with the control, in which only the reporter gene UAS-mCD8:GFP was overexpressed (p ≤0.001; Figures 6A, 6B, and 6F). To determine if this effect of Me31B induction reflected increased translational repression activity of Me31B, we asked whether similar effects would be shown by induction of an Me31B mutant protein (D207A, E208A) homologous to a yeast Dhh1p mutant incapable of translational repression (Coller and Parker, 2005). Expressed at comparable levels (data not shown), the mutant transgene had no effect on dendritic complexity (Figures 6C and 6F), consistent with the observed effect being dependent on Me31B-induced translational repression.
Overexpression of Tral in class IV neurons also substantially changed dendrite morphology compared to the control (Figures S6D–S6F). Interestingly, closer examination revealed a significant increase in the number of finer dendritic “tendrils” at terminal dendritic branches compared to control neurons. Differences between effects of Tral and Me31B induction on dendritic arborization are consistent with a relatively specific role for CAR-1, the C. elegans ortholog of Tral, in translational control compared to CGH-1 (the Me31B ortholog), suggested by phenotypic differences following RNAi-mediated inhibition of respective proteins in the C. elegans germline (Audhya et al., 2005; Navarro et al., 2001).
In class IV sensory neurons, loss of nanos or pumilio causes abnormal dendritic growth (Ye et al., 2004).This aberrant growth, visible in about 20% of mutant neurons, is most easily apparent as a loss of “tiling,” a term that refers to the complete, nonoverlapping coverage of the epidermis by dendrites of wild-type sensory neurons (Ye et al., 2004; Grueber et al., 2003). We therefore asked whether loss of me31B, achieved by expressing a transgenic RNAi construct that generates a hairpin Me31B RNA (UAS-Me31Bhpn) would cause similar defects. As shown in Figure 6E, UAS-Me31Bhpn sensory neurons showed frequent defects in terminal dendrite morphology and dendritic tiling highly reminiscent of nanos and pum phenotypes. Incomplete coverage of the epidermis was observed in at least 33% (n = 15 neurons) of neurons analyzed. Additionally, Me31Bhpn neurons show a modest increase (37%) in high-order dendritic complexity similar to that observed in dFmr1 mutants (Figure 6F; Lee et al., 2003). Parallel analyses of a hairpin construct for Lk6, which encodes the Drosophila homolog of the eIF4E-kinase MNK, showed no effect on dendritic branching of class IV sensory neurons (data not shown).
From these data, we conclude that Me31B (and Tral) regulates dendritic arborization of class IV da neurons. This observation, consistent with observations of other translational repressors such as dFMR1, Pum, and Nos provides a second line of evidence suggesting that Me31B and Tral function as neuronal translational regulators.
Me31B Functions in MicroRNA-Mediated Translational Repression
Two previous findings led us to the hypothesis that the dFMR1-associated Me31B protein may be required for miRNA/RNAi function. First, FMRP/dFMR1, showing strong biochemical or genetic interactions with Ago-1 and Ago-2, is strongly implicated in microRNA-mediated translational repression (Kosik and Krichevsky, 2005). Second, miRNA-mediated repression has been proposed to occur in P bodies of somatic cells (Liu et al., 2005a; Pillai et al., 2005). Thus, we tested whether Me31B is required in vivo for the function of bantam, an endogenous miRNA that represses hid mRNA translation in wing imaginal discs (Brennecke et al., 2003).
We used two transgenically encoded GFP reporters to assay _bantam-_mediated translational repression (Brennecke et al., 2003). The “hid reporter,” which carries the 3′ UTR of hid fused to the 3′end of EGFP-coding sequence, closely reports bantam repression of a native target mRNA. This 3′UTR contains four repeats complementary to bantam target recognition sequences, with several mismatches typically associated with miRNAmediated translational repression. The “bantam reporter,” in which four synthetic repeats 100% complementary to the bantam target recognition element are fused 3′to EGFP coding module, also reports bantam function.
We used the heat-shock FLP/FRT system to generate _me31b_−/− clones in the wing disc and identified these clones by loss of β-galactosidase or Me31B staining with respective antibodies (Figure 7A–C). We then asked how a control protein (Dlg), hid reporter, or bantam reporter expression was affected by loss of Me31B (Figures 7F and 7G). While cells lacking me31B showed no detectable increase in a control protein (Dlg) expression (Figure 7G), they showed clear increases in both hid reporter (Figures 7C–7E) and bantam reporter (Figures 7H–7J) expression, indicating that _bantam-_mediated silencing does not function in the absence of me31B. These data, from in vivo analyses of an endogenous miRNA in cells carrying a null mutation for me31B, support a recent study showing a role for RCK (the human homolog of Me31B) in Let-7 miRNA-mediated translational repression in cultured mammalian cells (Chu and Rana, 2006). In addition, our observations extend this study by demonstrating a role for Me31B in repression mediated by perfectly base-paired miRNAs. It should be noted that the requirement for Me31B for efficient repression of the bantam reporter does not necessarily mean that Me31B is required for miRNA-mediated endonucleolytic cleavage, since it is likely that repression by perfectly base-paired miRNAs can be a combination of translation repression, decapping, and/or endonucleolytic cleavage of the mRNA (Valencia-Sanchez et al., 2006). Importantly, these data demonstrate that Me31B is required for repression mediated by an endogenous Drosophila miRNA. An obvious corollary of our analysis in wing imaginal discs is that Me31B plays a similar role in mediating functions of neuronal miRNAs, although assays to directly test this issue are not immediately available in Drosophila.
Figure 7. Me31B Is Required for the In Vivo Function of an Endogenous MicroRNA.
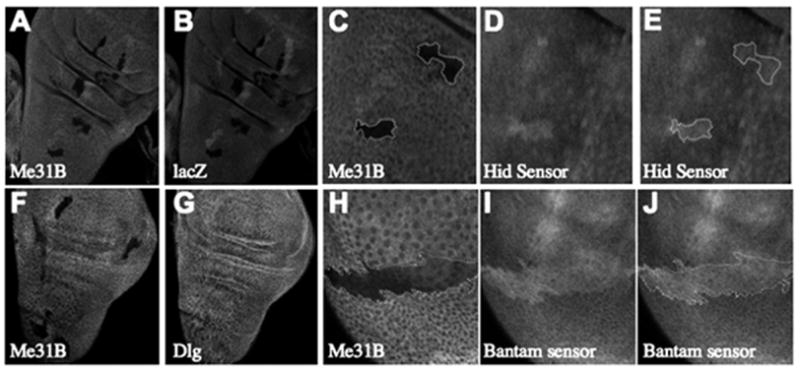
Heat-shock-induced clones in wing imaginal discs of hs-FLP1; FRT40A, arm-lacZ/FRT40A, _me31B_Δ1; reporter flies show dark me31B_−/me31B_− clones revealed either by staining for LacZ (B) or Me31B (A and C). Anti-GFP staining shows that the hid reporter (D and E) is upregulated in cell clones lacking Me31B (C). Similar analyses (F and G) show that a control protein Dlg (G) is not upregulated in me31B_−/me31B_− clones (F). However, consistent with a Me31B requirement in miRNA/RNAi, the bantam reporter expression is upregulated in me31B_−/me31B_−-lacking cells (H–J).
Discussion
Neuronal Staufen RNPs Are Related to Somatic P Bodies
Several observations now indicate that P bodies, maternal granules, and a major subclass of neuronal RNP are similar in underlying composition and represent a conserved system for the regulation of cytoplasmic mRNAs. As summarized in Table 2, known RNA transport and translational repressors shared between maternal and neuronal staufen granules now include, Stau, Btz, dFMR1, Pum, Nos, Yps, Me31B, Tral, Cup, eIF4E, Ago- 2, and Imp. Strikingly, in human cells, the Me31B homolog RCK/p54, the Tral homolog RAP55, the four human argonaute proteins, eIF4E, and a eIF4E-binding protein analogous to Cup, 4E-T, are all found in P bodies (Andrei et al., 2005; Cougot et al., 2004; Kedersha et al., 2005; Liu et al., 2005a; Pillai et al., 2005; Yang et al., 2006). In yeast, homologs of Me31B (Dhh1p) and Tral (Scd6p) are also known to be in P bodies (Sheth and Parker, 2003; Figures 4E–4G), and Dhh1p in particular plays a role in recruiting RNA-decapping proteins and exonucleases to these RNPs (Coller and Parker, 2005). Consistent with the above observations in yeast, the enzymes involved in mRNA hydrolysis including the 5′to 3′RNA exonuclease Xrn1p/Pcm and the RNA-decapping enzyme DCP1 are present on Drosophila neuronal staufen RNPs (Figure 2) and maternal RNA granules (Figure 3). Our data unequivocally demonstrate tight spatial proximity of components mediating various RNA regulatory processes in Drosophila neurons.
Table 2. Conserved Components of P Bodies, Maternal Granules, and Neuronal Granules.
| Protein Class | Mammalian and Yeast P Bodiesa | Drosophila Maternal Granulesb | Drosophila Neuronal Granulesc | Mammalian Neuronal Granulesd |
|---|---|---|---|---|
| RNA transport | ? | Stau, Btz | Stau, Btz | Stau, Btz |
| Fragile X-like | ? | dFMR1 | dFMR1 | FMRP, FXR1, FXR2 |
| Zip-code binding | ? | ? | Imp | ZBP1 |
| Pum domain | ? | Pum? | Pum | Puml |
| CCHC Zn-finger domain | ? | Nanos | Nanosalso f | ? |
| DEAD-box RNA helicase | RCK/Dhh1p | Me31B | Me31B | ? |
| Sm-like domain | RAP55/Scd6pj | Tral | Tral | ? |
| Cap-binding | eIF4Ee,k | eIF4E | eIF4Ealso g | under some conditions |
| Enhancers of decapping | Edc3p, Pat1pi | ? | ? | ? |
| eIF4E-binding | eIF4E-Tk | Cup | Cup | ? |
| PABP | PABPe | ? | PABPg | ? |
| Y-box | ? | Yps | Yps | ? |
| 5′ to 3′ RNA decay machinery | Lsm, Dcp1p, Dcp2p, Xrn1p | DCP1, Pacman | DCP1, Pacman (others not examined) | ? |
| NMD machineryh | Upf-1 | Upf-1 | Upf-1 | ? |
| miRNA, siRNA machinery | mAGO1 mAGO2 | ? | Ago-2 | ? |
The large collection of proteins and processes common to P bodies, staufen granules, and likely maternal RNA granules suggests that they share an underlying core biochemicalcompositionandfunction,which would then be elaborated in different biological contexts. For example, one anticipates that proteins involved inmRNA transport will be more prevalent in maternal and neuronal RNPs, which need to be transported for their biological function.
An interesting aspect of neuronal staufen RNPs described here is the diversity of translational repression systems that are present within them. First, in Me31B, they contain a protein that works in general translation repression of a wide variety of mRNAs and can also affect miRNA-based repression (Figure 7; Coller and Parker, 2005; Chu and Rana, 2006). Second, in Ago-2, they contain a component specific to miRNA/RNAidependent repression. Third, neuronal staufen granules also contain UPF1, which was originally thought to be solely involved in mRNA degradation. However, because UPF1 can act as a translation repressor (Muhlrad and Parker, 1999; Sheth and Parker, 2006) and physically interacts with Stau (Kim et al., 2005), a reasonable hypothesis is that UPF1 might work in neuronal granules, in conjunction with Stau, to repress the translation of a subset of mRNAs. The presence of multiple mechanisms for translation repression colocalizing in granules in Drosophila neurons may allow for differential translation control of subclasses of mRNA in response to different stimuli.
Neuronal Granule Diversity and Function
Evidence accumulating in the literature suggests that there is a potential diversity of RNA granule types in neurons. Our observations in Drosophila neurons are most consistent with a model in which a major subclass of neuronal RNP, in which various translational repressor and mRNA turnover proteins colocalize, is related to other compositionally distinct, diverse RNPs. A major subclass of staufen-containing RNP is indicated by our data showing substantial colocalization among various proteins we have analyzed (Table 1; data not shown). Diversity is indicated by the lack of 100% colocalization: for instance, 55% of staufen-positive particles in wildtype neurons do not contain detectable dFMR1.
Two types of observations suggest that the apparent subclasses of particles containing Stau or dFMR1, but not both, are related to the particles in which they colocalize. First, these two types of RNPs are clearly compositionally related to particles that contain both proteins. Second, this is supported by the observation that colocalization can be substantially increased under someconditions. Overexpression of either dFMR1 or Stau:GFP increases colocalization between Stau and dFMR1 from 45% in wild-type neurons to more than 80%. Concurrent with increased frequency of colocalization, Stau:GFP or dFMR1 induction increases apparent particle size (or brightness) and reduces the total number of particles. The increase in colocalization and brightness, as well as reduction in particle number, is most easily explained by growth and/or fusion of related RNPs. Significantly, similar effects on mammalian neuronal granule size and number have been reported following overexpression of Stau or another granule protein, RNG105 (Kiebler et al., 1999; Shiina et al., 2005). Thus, the underlying regulatory processes appear conserved between Drosophila and mammalian neurons.
While it remains unclear how FMRP, Stau, or RNG105 enhance granule growth or fusion, it is conceivable that individual mRNAs first form small RNPs whose compositions reflect specific requirements for translational repression of the mRNAs they contain. These small RNPs exist in dynamic equilibrium with larger RNPs in which multiple, diverse translational repression complexes are sequestered. Induction of factors that promote granule assembly could push the equilibrium toward mRNP sequestration within large granules. A requirement of this dynamic model, which postulates interactions among different types of RNP, is that the RNPs themselves can change in composition during transport to synaptic domains. This is supported by FRAP analyses showing rapid exchange of Stau:GFP between cytosol and granule (Figures 1L and 1M).
Additional types of RNPs have also been described in neurons. For example, polysomes apparently arrested in translation have been observed near dendritic spines, and these RNPs show no obvious similarity to large, ribosome-containing particles, termed neuronal RNA granules (Knowles et al., 1996; Greenough et al., 2001; Ostroff et al., 2002). In addition, a potentially distinctRNP containing Stau, kinesin, and translationally repressed RNAs, but not ribosomes, has been purified from the mammalian brain (Mallardo et al., 2003). More recently, it has been shown that RNPs containing stress-granule markers TIA-1 and TIA-R as well as pumilio2 are induced by arsenate-treatment of mammalian cultured neurons (Vessey et al., 2006). Interestingly, as previously shown for somatic cells, these large stress granules appear tightly apposed to domains containing DCP1 and Lsm1, markers of P bodies (Vessey et al., 2006). Determining the temporal and compositional relatedness of such varied RNPs, their pathways of assembly as well as their functions, is a broad area of future research not only in neuroscience but also in cell biology.
These diverse types of biochemical compartments for individual mRNAs suggest that neural activity or other developmental signaling events would influence translation in two steps: first, by desequestering mRNPs held within large granules and, then, by derepressing quiescent mRNAs in individual mRNPs. Thus, RNPs we describe here could have a complex precursor-product relationship with other RNPs, including polysomes discovered by now-classical studies at dendritic spines.
Two Functionally Important Translational Repressors in dFMR1-Containing Neuronal RNPs
Despite the complexity revealed by the diversity of neuronal RNPs, the importance and significance of the observed colocalization of Me31B, Tral, argonaute, and dFMR1 in staufen-positive neuronal RNPs is most clearly demonstrated by functional analyses revealing biological pathways in which these proteins function together.
Several independent lines of evidence are consistent with a function for Me31B in neuronal translational repression as part of a biochemical complex that includes dFMR1. First, subcellular localization studies indicate that Me31B and Tral localize to dFMR1-containing RNPs especially prominent at neurite branch points in cultured Drosophila neurons (Figures S5D–S5I; data not shown). Second, Me31B, Tral, and dFMR1 coimmunoprecipitate from Drosophila head extract, thus confirming the physical association of three proteins (Figure 4A). Third, lossof- function alleles of either Me31B or Tral suppress the rough eye phenotype seen when dFMR1 is overexpressed in the _sev-_positive photoreceptors (Figure 5). Fourth, overexpression of Me31B in sensory neurons leads to altered branching of terminal dendrites, a phenotype also seen with overexpression analyses of Nos, Pum, and dFMR1 (Figures 6B and 6F; Lee et al., 2003; Ye et al., 2004). Finally, reduction of Me31B expression in sensory neurons by RNAi results in abnormal dendrite morphogenesis and tiling defects, phenotypes similar to that observed following loss of nanos, pum, or dFmr1 function (Figures 6E and 6F; Ye et al., 2004; Lee et al., 2003). Significantly, the effect of Me31B on dendritic growth is correlated with its ability to function in translational repression (Figures 6C and 6F). These five independent lines of evidence provide considerable support for Me31B (and Tral) function in neuronal translation control processes. While the site of functional interaction between dFMR1, Me31B, and Tral (soma or neuronal processes) is not identified here, the importance of the physical interactions is clearly demonstrated.
Several observations also argue that Me31B acts, at least in part, within neurons to promote translation repression and/or mRNA degradation in response to miRNAs. This possibility was first suggested by the physical and genetic interactions of Me31B with dFMR1 (discussed above; Figure 5; Figures S5D–S5F), a protein that has previously been implicated in the miRNA-mediated repression (Ishizuka et al., 2002; Jin et al., 2004). Using direct assays for miRNA-mediated function in vivo (Brennecke et al., 2003), we show that Me31B is required for efficient repression by the bantam miRNA in developing wing imaginal discs (Figure 7). This identifies Me31B as a protein required for efficient miRNAbased repression.
Recently, miRNA-based regulation has been shown to be important for the control of spine growth in hippocampal neurons (Schratt et al., 2006) and to be a target of protein-degradative pathways involved in long-term memory formation in Drosophila (Ashraf et al., 2006). Thus, our data predict that Me31B will be important in modulating miRNA function pertinent to development of functional neuronal plasticity. More generally, because Me31B homologs in yeast and mammals have been shown to function in P body formation in somatic cells (Andrei et al., 2005; Coller and Parker, 2005), the requirement for Me31B in miRNA function provides evidence to support a model in which formation of P bodies is required for efficient miRNA-based repression in varied cell types and biological contexts.
Implications for Translational Control in Neurons
Our conclusion that staufen- and dFMR1-containing neuronal RNPs are similar in organization and function to P bodies has several implications for neuronal translational control. First, the presence of diverse translational repression systems on these RNPs suggests that, like in P bodies, different classes of mRNAs will be repressed by different mechanisms. This may allow specific RNA classes to be released for new translation in response to different stimuli. Such diversity of control may allow synapses to remodel themselves differently,depending on the frequency and strength of stimulation (e.g., LTD or LTP). Second, FRAP experiments indicate that both P bodies and staufen granules are dynamic structures (Andrei et al., 2005; Kedersha et al., 2005; Figures 1K and 1L). This argues that, like P bodies, staufen granules are in a state of dynamic flux, perhaps in activity- regulated equilibrium with the surrounding translational pool. Third, the presence of mRNA-degradative enzymes on staufen granules suggests regulation of mRNA turnover may play an important role in local synaptic events. For example, if synaptic signaling were to induce turnover of specific mRNAs at a synapse, then stimulated synapses could acquire properties different from unstimulated ones that retain a “naïve” pool of stored synaptic mRNAs. Finally, these observations imply that the proteins known to function in translation repression within P bodies will play important roles in modulating translation in neurons. Thus, we anticipate that proteins of mammalian or yeast P bodies such as Edc3p, Pat1p, the Lsm1-7p complex, GW182, and FAST will be present on and influence assembly and function of neuronal granules (Cougot et al., 2004; Eystathioy et al., 2003; Kedersha et al., 2005; Sheth and Parker, 2003).
Experimental Procedures
Drosophila Stocks
Fly stocks were raised at 25°C on standard cornmeal and agar media. Wild-type (Oregon-R and w1118) were from Ramaswami lab stocks. Other strains were obtained from D42-Gal4-chaGal80 (constructed by S. Sanyal with components from T. Kitamoto and G. Boulianne); C380 (V. Budnik); C155 (C. Goodman); UAS-dFMR1 (T. Jongens); sev-dFMR1 (P. Jin); UAS-Stau-GFP (A. Brand); UASMe31Bhpn (R. Ueda); and elav, UAS-GFP:MCP:nls and UAS-Cam- KII3′UTR - ms2 (S. Ashraf and S. Kunes). Gal4477; UASmCD8-GFP, UAS-flip Act < CD2 < Gal4 was constructed using strains from W. Grueber and S. Sanyal; hsFLP-1; FRT 40A, armadillo-lacZ (Bloomington); “hid-reporter” and “bantam reporter” lines are described in Brennecke et al. (2003). Me31B alleles were as previously described (Nakamura et al., 2001).
Drosophila Neuron Primary Cell Culture
Cells for culture were obtained from the thoracic-abdominal (ventral) region of the CNS of late third-instar larvae. Tissues were dissected and placed into a Liberase enzyme (combination of collagenase and dispase) solution and incubated at room temperature for 1 hr. Tissues were then rinsed in culture medium (Schneider’s- or IL15- based medium) and subjected to two mechanical trituration steps. Cells were plated onto coverslips coated with concanavalin A and laminin in tissue culture dishes and allowed to grow at 25°C for 3–4 days prior to immunostaining. We used a composite Gal4/Gal80 system (D42-Gal4; chaGal80) to drive expression of a functional Stau:GFP fusion protein (UAS-Stau:GFP) or dFMR1 (UASdFMR1) in a subset of motor neurons. Cells were identified using confocal microscopy by the presence of Stau:GFP (or dFMR1-positive) punctae allowing for the identification of a discrete population of neurons in an otherwise heterogeneous neuronal culture.
Immunohistochemistry
Primary antibodies used for neuronal granule staining are listed in Table 1. Additional primary antibodies used were mouse anti-βgalactosidase (Molecular Probes), rabbit anti-GFP (Molecular Probes), mouse anti-DLG 4F3 (Developmental Studies Hybridoma Bank), and goat anti-HRP-TRITC (Sigma). Secondary antibodies used were FITC (Sigma), Alexa 488-, Alexa 555-, Alexa 568-, and Alexa 647-conjugated anti-rat, mouse and -rabbit IgG (Molecular Probes). Cultured cells were fixed and stained as follows. Briefly, 3-day old ventral ganglion cell cultures were rinsed in prewarmed PBS buffer (pH = 7.2) and fixed for 10 min in 3.5% paraformaldehyde in PBS. Cells were blocked for 30 min in Block solution (PBS containing 0.1% Triton X-100, 2% BSA, and 5% normal goat serum). Primary and secondary antibodies were diluted in Block solution and incubated with cells for 2 hr and 1 hr, respectively, at RT. After rinsing, preparations were mounted in Vectashield Mounting Medium (Vector Labs) and imaged on a Nikon PCM2000 laser confocal microscope using Simple PCI software. Further discussion of methods used to examine colocalization of neuronal granule components can be found in the Supplemental Data.
For larval CNS preparations, wandering third-instar larvae were processed according to the method of Sanyal et al. (2003), with the following modification. To permeabilize the sheath surrounding the ventral ganglion, CNS preps were treated with 50 μg/ml collagenase diluted in HL-3 saline (+Ca2+) for 3 min prior to fixation.
Immunostaining of Drosophila oocytes was done essentially as described in Wilhelm et al. (2003), with the following alterations. Ovaries were dissected in room temperature PBS + 0.1% Triton X-100 and fixed for 10 min in one part 3.7% paraformaldehyde in PBS to six parts heptane.
Immunoprecipitation of Me31B
Immunoprecipitation from head extracts with rat anti-Me31B was carried out essentially as described (Nakamura et al., 2001). Samples were separated by SDS-PAGE, transferred to PVDF membrane (Millipore), and analyzed by Western blotting. Proteins were detected by ECL (Amersham).
Analysis of Drosophila Rough Eye Phenotypes
Drosophila genotypes used for SEM analysis and tangential eye sectioning were as follows: dFMR1 overexpression, +/SevdFMR1; Me31B suppression, Me31B_Δ1_FRT40A/SevdFMR1 and Me31B_Δ2 FRT40A/SevdFMR1; Me31B “rescue”, Me31B_Δ2_FRT40A/SevdFMR1; +/P[w+MeAflII]; Tral suppression, +/SevdFMR1; +/Tral Δ3- FRT2A and +/SevdFMR1; +/Tral_Δ4_FRT2A; Tral “rescue”, P[Tral-10]/SevdFMR1; +/Tral Δ4_FRT2A. All indicated stocks (above) were crossed to w1118 to generate heterozygotes for subsequent analysis.
Further analysis of the rough eye phenotype using scanning electron microscopy (SEM) and in tangential eye sections is described in the Supplemental Experimental Procedures.
Analysis of Dendritic Processes
Experiments were done in a Gal4477, UAS-mCD8-GFP; UAS-flp, Act < CD2 < Gal4 background in which flp-recombinase target sequences (“<”) flanking CD2 stuffer sequence is often excised thorough the activity of _Gal4477_-driven Flp recombinase. Thus, in this background, individual _Gal4477_-positive da sensory neurons are occasionally very brightly labeled by Actin-Gal4 mCD8:GFP. In genetic backgrounds carrying a Gal4-responsive transgene, the transgene is also strongly expressed. Further analysis of sensory neuron dendritic complexity is described in the Supplemental Experimental Procedures.
Generation and Characterization of _me31B-_Mitotic Clones
Mitotic recombination clones were induced 48±2 hr after egg laying (AEL) in staged larvae by heat shock at 37°C for 90 min. Larval genotypes used were hs-FLP1; FRT 40A, arm-lacZ/Me31B Δ1_, FRT 40A; bantam-reporter (or hid-reporter)_. Discs were dissected at 120 ± 2 hr AEL, fixed with 4% formaldehyde, and stained with different antibodies. The discs were mounted in Vectashield (Vector Labs) and analyzed by confocal microscopy (Zeiss LSM 510) with a 20×objective. Clone areas were measured and analyzed using Adobe Photoshop.
Supplementary Material
Supplementary Data
Movie - Cell6 moving granule
Acknowledgments
We thank T. Kitamoto, T. Aigaki, W. Grueber, Y.N. Jan, S. Warren, P. Jin, V. Budnik, G. Boulianne, D. St. Johnston, S. Sanyal, J. Brennecke, S. Cohen, the National Institute of Genetics (Japan) fly stock center, and the Bloomington Drosophila stock center for Drosophila stocks; W. Grueber and S. Sanyal for advice; J. Brennecke, E. Holohan, D. Zarnescu, P. Macchi, V. Rodrigues, and M. Kiebler for useful discussions; G. Hannon, T. Jongens, P. Lasko, D. St. Johnston, C. Temme, J. Raff, and the Developmental Studies Hybridoma Bank for antibodies; B. Suter for a Drosophila genomic library; S. Zusman (Genetic Services Inc.) for germline transformation services; C. Boswell of the MCB Imaging Facility for help with microscopy; D. Bentley, P. Jansma, N. Ingraham, and C. Hedgcock of the Arizona Research Labs Divisions of Neurobiology and Biotechnology for access to and instruction in the SEM, Microscopy (tissue sectioning), Tissue Culture Core, and Photo/Graphics Facilities (respectively), as well as their imaging and computing facilities; and G. Bosco for use of the Nikon Eclipse E800 microscope, camera, and software. This work was funded by grants from the NIH/NIDA (DA15495; DA17749) and the Science Foundation of Ireland to M.R., from the Howard Hughes Medical Institute to R.P., from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to A.N.), the UK Biotechnology and Biological Sciences Research Council (S.F.N.), NIH grant GM54409 to Paul MacDonald and an NIH grant to R.B.L.
Footnotes
References
- Andrei MA, Ingelfinger D, Heintzmann R, Achsel T, Rivera-Pomar R, Luhrmann R. A role for eIF4E and eIF4Etransporter in targeting mRNPs to mammalian processing bodies. RNA. 2005;11:717–727. doi: 10.1261/rna.2340405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4:350–359. doi: 10.1111/j.1601-183X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Audhya A, Hyndman F, McLeod IX, Maddox AS, Yates JR, 3rd, Desai A, Oegema K. A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. J Cell Biol. 2005;171:267–279. doi: 10.1083/jcb.200506124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Fillipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Boag PR, Nakamura A, Blackwell TK. A conserved RNA-protein complex component involved in physiological germline apoptosis regulation in C. elegans. Development. 2005;132:4975– 4986. doi: 10.1242/dev.02060. [DOI] [PubMed] [Google Scholar]
- Brengues M, Teixeira D, Parker R. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science. 2005;310:486–489. doi: 10.1126/science.1115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated micro- RNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- Chu CY, Rana TM. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 2006;4:e210. doi: 10.1371/journal.pbio.0040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller J, Parker R. General translational repression by activators of mRNA decapping. Cell. 2005;122:875–886. doi: 10.1016/j.cell.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller JM, Tucker M, Sheth U, Valencia-Sanchez MA, Parker R. The DEAD box helicase, Dhh1p, functions in mRNA decapping and interacts with both the decapping and deadenylase complexes. RNA. 2001;7:1717–1727. doi: 10.1017/s135583820101994x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Wang Y, Dockendorff TC, Erdjument-Bromage H, Tempst P, Schedl P, Jongens TA. The Drosophila fragile X protein functions as a negative regulator in the orb autoregulatory pathway. Dev Cell. 2005;8:331–342. doi: 10.1016/j.devcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cougot N, Babajko S, Seraphin B. Cytoplasmic foci are sites of mRNA decay in human cells. J Cell Biol. 2004;165:31–40. doi: 10.1083/jcb.200309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvira G, Wasiak S, Blandford V, Tong XK, Serrano A, Fan X, Del Rayo Sanchez-Carbente M, Servant F, Bell AW, Boismenu D, et al. Characterization of an RNA granule from developing brain. Mol Cell Proteomics. 2006;5:635–651. doi: 10.1074/mcp.M500255-MCP200. [DOI] [PubMed] [Google Scholar]
- Eystathioy T, Jakymiw A, Chan EK, Seraphin B, Cougot N, Fritzler MJ. The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA. 2003;9:1171–1173. doi: 10.1261/rna.5810203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Elphick L, Nusslein-Volhard C, St Johnston D. Staufen protein associates with the 3′UTR of bicoid mRNA to form particles that move in a microtubule-dependent manner. Cell. 1994;79:1221–1232. doi: 10.1016/0092-8674(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Forbes A, Lehmann R. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development. 1998;125:679–690. doi: 10.1242/dev.125.4.679. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Klintsova AY, Irwin SA, Galvez R, Bates KE, Weiler IJ. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci USA. 2001;98:7101–7106. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Ye B, Moore AW, Jan LY, Jan YN. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Curr Biol. 2003;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Ishizuka A, Siomi MC, Siomi H. A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 2002;16:2497–2508. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakymiw A, Lian S, Eystathioy T, Li S, Satoh M, Hamel JC, Fritzler MJ, Chan EK. Disruption ofGWbodies impairs mammalian RNA interference. Nat Cell Biol. 2005;7:1267–1274. doi: 10.1038/ncb1334. [DOI] [PubMed] [Google Scholar]
- Jin P, Alisch RS, Warren ST. RNA and microRNAs in fragile X mental retardation. Nat Cell Biol. 2004;6:1048–1053. doi: 10.1038/ncb1104-1048. [DOI] [PubMed] [Google Scholar]
- Johnstone O, Lasko P. Translational regulation and RNA localization in Drosophila oocytes and embryos. Annu Rev Genet. 2001;35:365–406. doi: 10.1146/annurev.genet.35.102401.090756. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fitzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebler MA, Bassell GJ. Neuronal RNA granules: movers and makers. Neuron. 2006;51:685–690. doi: 10.1016/j.neuron.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Kiebler MA, Hemraj I, Verkade P, Kohrmann M, Fortes P, Marion RM, Ortin J, Dotti CG. The mammalian staufen protein localizes to the somatodendritic domain of cultured hippocampal neurons: implications for its involvement in mRNA transport. J Neurosci. 1999;19:288–297. doi: 10.1523/JNEUROSCI.19-01-00288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Furic L, Desgroseillers L, Maquat LE. Mammalian Staufen1 recruits Upf1 to specific mRNA 3′UTRs so as to elicit mRNA decay. Cell. 2005;120:195–208. doi: 10.1016/j.cell.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Knowles RB, Sabry JH, Martone ME, Deerinck TJ, Ellisman MH, Bassell GJ, Kosik KS. Translocation of RNA granules in living neurons. J Neurosci. 1996;16:7812–7820. doi: 10.1523/JNEUROSCI.16-24-07812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrmann M, Luo M, Kaether C, DesGroseillers L, Dotti CG, Kiebler MA. Microtubule-dependent recruitment of Staufen-green fluorescent protein into large RNA-containing granules and subsequent dendritic transport in living hippocampal neurons. Mol Biol Cell. 1999;10:2945–2953. doi: 10.1091/mbc.10.9.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Krichevsky AM. The elegance of the micro- RNAs: a neuronal perspective. Neuron. 2005;47:779–782. doi: 10.1016/j.neuron.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Kraft R, Levine RB, Restifo LL. The steroid hormone 20-hydroxyecdysone enhances neurite growth of Drosophila mushroom body neurons isolated during metamorphosis. J Neurosci. 1998;18:8886–8899. doi: 10.1523/JNEUROSCI.18-21-08886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001;32:683–696. doi: 10.1016/s0896-6273(01)00508-6. [DOI] [PubMed] [Google Scholar]
- Ladomery M, Wade E, Sommerville J. Xp54, the Xenopus homologue of human RNA helicase p54, is an integral component of stored mRNP particles in oocytes. Nucleic Acids Res. 1997;25:965–973. doi: 10.1093/nar/25.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGrandeur TE, Parker R. Isolation and characterization of Dcp1p, the yeast mRNA decapping enzyme. EMBO J. 1998;17:1487–1496. doi: 10.1093/emboj/17.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall S, Piano F, Davis RE. Caenorhabditis elegans decapping proteins: localization and functional analysis of Dcp1, Dcp2, and DcpS during embryogenesis. Mol Biol Cell. 2005;16:5880– 5890. doi: 10.1091/mbc.E05-07-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko P, Cho P, Poulin F, Sonenberg N. Contrasting mechanisms of regulating translation of specific Drosophila germline mRNAs at the level of 5′-cap structure binding. Biochem Soc Trans. 2005;33:1544–1546. doi: 10.1042/BST0331544. [DOI] [PubMed] [Google Scholar]
- Lee A, Li W, Xu K, Bogert BA, Su K, Gao FB. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development. 2003;130:5543– 5552. doi: 10.1242/dev.00792. [DOI] [PubMed] [Google Scholar]
- Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol. 2005a;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Rivas FV, Wohlschlegel J, Yates JR, Parker R, Hannon GJ. A role for the P-body component GW182 in microRNA function. Nat Cell Biol. 2005b;2005:1261–1266. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi P, Kroening S, Palacios IM, Baldassa S, Grunewald B, Ambrosino C, Goetze B, Lupas A, St Johnston D, Kiebler M. Barentsz, a new component of the Staufen-containing ribonucleoprotein particles in mammalian cells, interacts with Staufen in an RNA-dependent manner. J Neurosci. 2003;23:5778–5788. doi: 10.1523/JNEUROSCI.23-13-05778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallardo M, Deitinghoff A, Muller J, Goetze B, Macchi P, Peters C, Kiebler MA. Isolation and characterization of Staufen-containing ribonucleoprotein particles from rat brain. Proc Natl Acad Sci USA. 2003;100:2100–2105. doi: 10.1073/pnas.0334355100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield JH, Wilhelm JE, Hazelrigg T. Ypsilon Schachtel, a Drosophila Y-box protein, acts antagonistically to Orb in the oskar mRNA localization and translation pathway. Development. 2002;129:197–209. doi: 10.1242/dev.129.1.197. [DOI] [PubMed] [Google Scholar]
- Martin KC. Local protein synthesis during axon guidance and synaptic plasticity. Curr Opin Neurobiol. 2004;14:305–310. doi: 10.1016/j.conb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Muhlrad D, Parker R. Recognition of yeast mRNAs as “nonsense containing” leads to both inhibition of mRNA translation and mRNA degradation: implications for the control of mRNA decapping. Mol Biol Cell. 1999;10:3971–3978. doi: 10.1091/mbc.10.11.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro TP, Kwon S, Schnapp BJ, St Johnston D. A repeated IMP-binding motif controls oskar mRNA translation and anchoring independently of Drosophila melanogaster IMP. J Cell Biol. 2006;172:577–588. doi: 10.1083/jcb.200510044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Amikura R, Hanyu K, Kobayashi S. Me31B silences translation of oocyte-localizing RNAs through the formation of cytoplasmic RNP complex during Drosophila oogenesis. Development. 2001;128:3233–3242. doi: 10.1242/dev.128.17.3233. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Sato K, Hanyu-Nakamura K. Drosophila cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev Cell. 2004;6:69–78. doi: 10.1016/s1534-5807(03)00400-3. [DOI] [PubMed] [Google Scholar]
- Navarro RE, Shim EY, Kohara Y, Singson A, Blackwell TK. cgh-1, a conserved predicted RNA helicase required for gametogenesis and protection from physiological germline apoptosis in C. elegans. Development. 2001;128:3221–3232. doi: 10.1242/dev.128.17.3221. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff LE, Fiala JC, Allwardt B, Harris KM. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35:535–545. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- Parker R, Song H. The enzymes and control of eukaryotic mRNA turnover. Nat Struct Mol Biol. 2004;11:121–127. doi: 10.1038/nsmb724. [DOI] [PubMed] [Google Scholar]
- Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot N, Basyuk E, Bertrand E, Filipowicz W. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- Richter JD, Lorenz LJ. Selective translation of mRNAs at synapses. Curr Opin Neurobiol. 2002;12:300–304. doi: 10.1016/s0959-4388(02)00318-5. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Narayanan R, Consoulas C, Ramaswami M. Evidence for cell autonomous AP1 function in regulation of Drosophila motor-neuron plasticity. BMC Neurosci. 2003;4:20. doi: 10.1186/1471-2202-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Sheth U, Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808. doi: 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U, Parker R. Nonsense-mediated decay in yeast involves targeting of aberrant mRNAs to cytoplasmic processing bodies. Cell. 2006;125:1095–1109. doi: 10.1016/j.cell.2006.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina N, Shinkura K, Tokunaga M. A novel RNA-binding protein in neuronal RNA granules: regulatory machinery for local translation. J Neurosci. 2005;25:4420–4434. doi: 10.1523/JNEUROSCI.0382-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Thiel PR, Reiff DF, Lachance PE, Lasko P, Schuster CM. Postsynaptic translation affects the efficacy and morphology of neuromuscular junctions. Nature. 2000;405:1062– 1065. doi: 10.1038/35016598. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Gingras AC. The mRNA 5′cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Beuchle D, Nusslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991;66:51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Meulemans D, Vazquez L, Colaco N, Schuman E. A role for a rat homolog of staufen in the transport of RNA to neuronal dendrites. Neuron. 2001;32:463–475. doi: 10.1016/s0896-6273(01)00493-7. [DOI] [PubMed] [Google Scholar]
- Teixeira D, Sheth U, Valencia-Sanchez MA, Brengues M, Parker R. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA. 2005;11:371–382. doi: 10.1261/rna.7258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Palacios IM, Petronczki M, Weston MJ, St Johnston D. Barentsz is essential for the posterior localization of oskar mRNA and colocalizes with it to the posterior pole. J Cell Biol. 2001;154:511–523. doi: 10.1083/jcb.200105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JP, Vaccani A, Xie Y, Dahm R, Karra D, Kiebler M, Macchi P. Dendritic localization of the translational repressor Pumilio 2 and its contribution to dendritic stress granules. J Neurosci. 2006;26:6496–6508. doi: 10.1523/JNEUROSCI.0649-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Dockendorff TC, Jongens TA, Dreyfuss G. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol. 2000;20:8536–8547. doi: 10.1128/mcb.20.22.8536-8547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Dickinson LK, Lehmann R. Genetics of nanos localization in Drosophila. Dev Dyn. 1994;199:103–115. doi: 10.1002/aja.1001990204. [DOI] [PubMed] [Google Scholar]
- Wilhelm JE, Mansfield J, Hom-Booher N, Wang S, Turck CW, Hazelrigg T, Vale RD. Isolation of a ribonucleoprotein complex involved in mRNA localization in Drosophila oocytes. J Cell Biol. 2000;148:427–440. doi: 10.1083/jcb.148.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JE, Hilton M, Amos Q, Henzel WJ. Cup is an eIF4E binding protein required for both the translational repression of oskar and the recruitment of Barentsz. J Cell Biol. 2003;163:1197–1204. doi: 10.1083/jcb.200309088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Yu JH, Gulick T, Bloch KD, Bloch DB. RNA-associated protein 55 (RAP55) localizes to mRNA processing bodies and stress granules. RNA. 2006;12:547–554. doi: 10.1261/rna.2302706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Petritsch C, Clark IE, Gavis ER, Jan LY, Jan YN. Nanos and Pumilio are essential for dendrite morphogenesis in Drosophila peripheral neurons. Curr Biol. 2004;14:314–321. doi: 10.1016/j.cub.2004.01.052. [DOI] [PubMed] [Google Scholar]
- Zhang HL, Eom T, Oleynikov Y, Shenoy SM, Liebelt DA, Dictenberg JB, Singer RH, Bassell GJ. Neurotrophin- induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron. 2001;31:261–275. doi: 10.1016/s0896-6273(01)00357-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data
Movie - Cell6 moving granule