Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits (original) (raw)
Abstract
The innate immune system senses the invasion of pathogenic microorganisms and tissue injury through Toll-like receptors (TLR), a mechanism thought to be limited to immune cells. We now report that neurons express several TLRs, and that the levels of TLR2 and -4 are increased in neurons in response to IFN-γ stimulation and energy deprivation. Neurons from both TLR2 knockout and -4 mutant mice were protected against energy deprivation-induced cell death, which was associated with decreased activation of a proapoptotic signaling cascade involving jun N-terminal kinase and the transcription factor AP-1. TLR2 and -4 expression was increased in cerebral cortical neurons in response to ischemia/reperfusion injury, and the amount of brain damage and neurological deficits caused by a stroke were significantly less in mice deficient in TLR2 or -4 compared with WT control mice. Our findings establish a proapoptotic signaling pathway for TLR2 and -4 in neurons that may render them vulnerable to ischemic death.
Keywords: AP-1, apoptosis, innate immunity, ischemic stroke, microglia
Toll-like receptors (TLRs) are a family of at least 11 proteins that function as key mediators of innate immunity, responding to diverse microbial products and injury-induced endogenous ligands (1). Activation of TLRs initiates signal transduction cascades that involve kinases including atypical forms of protein kinase C and the transcription factors activator protein-1 (AP-1) and NF-κB, which induce the expression of genes encoding inflammation-associated molecules and cytokines (2–4). Although TLRs are expressed in leukocytes where their functions have been established, recent findings suggest they can also be expressed in nonimmune cells, including hepatocytes and muscle cells (5, 6). TLRs are present in the brain, where their expression is believed to be limited to glial cells (microglia, astrocytes, and oligodentrocytes) (7, 8). However, recent findings suggest that neurons may express at least some TLRs responsive to viral RNA or bacterial proteins (9, 10). Ischemic injury to the brain (stroke) is a major cause of morbidity and mortality for which effective treatments are lacking. Studies have shown that TLR2 and -4 are up-regulated in response to ischemia in the kidney and heart, suggesting these two TLRs may play important roles in ischemic tissue injury (11–13). However, neither the specific cells in which TLRs are activated in response to ischemia nor the consequences of TLR signaling for the clinical outcome of an ischemic event have been established. Here we use TLR2 and -4 mutant mice and primary neuronal cell culture and in vivo models of ischemic stroke to elucidate roles for neuronal TLR2 and -4 signaling in the pathogenesis of stroke.
Results
Neurons Express TLRs and Respond to IFNγ Stimulation.
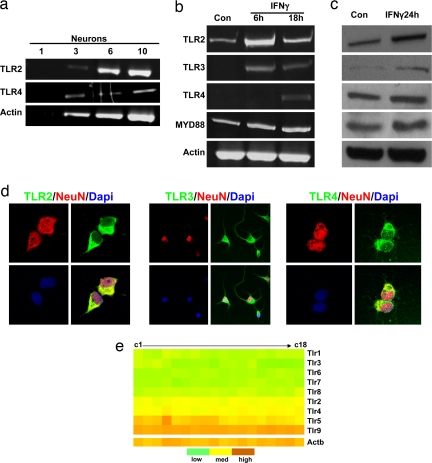
Previous studies suggested that neurons do not express TLRs (14), whereas others have suggested the presence of TLR3 and -8 in neurons (10). By using multiple technologies, we found that primary mouse cortical neurons express TLR2, -3, and -4. First, we used single-cell PCR analysis to reveal the presence of TLR2 and -4 mRNAs in neurons (Fig. 1a). Similar to immune cells (15), neurons respond to IFNγ with increases in their expression of TLR2, -3, and -4 at both the mRNA and protein levels (Fig. 1 b and c). Binding of ligand to TLRs results in the association of proteins of the MyD88 family with the intracellular Toll-interacting region of the TLR, which initiates downstream signaling (16). Consistent with the notion that IFNγ increases the sensitivity of neurons to TLR ligands, we observed increased levels of MyD88 mRNA and protein in neurons treated with IFNγ (Fig. 1 b and c). To further confirm the expression of TLRs and elucidate their subcellular localization, we immunostained cultured cortical neurons with TLR2, -3, and -4 antibodies and visualized the immunoreactivity by fluorescence microscopy. Immunoreactivity for each TLR was present and localized to the cell surface and cytosolic compartments but not the nucleus (Fig. 1d). We also examined the expression of TLR1–9 in neuronal cultures by using microarray analysis and found that TLR1, -3, -6, -7, and -8 were expressed at relatively low levels, TLR2 and -4 at intermediate levels, and TLR5 and -9 at higher levels (Fig. 1e). Because the expression of TLR2 and -4 in neurons has not been reported, we performed a series of control experiments. We found that the specific TLR2 and -4 peptides used as immunogens were able to block TLR2 and -4 antibody binding on both immunoblots and immunostained cells [supporting information (SI) Fig. 6 a–c]. We also found that levels of the TLR2 immunoreactive band are lower in samples from neurons treated with TLR2 siRNAs (SI Fig. 6_d_), and TLR2 was immunoprecipitated from neuronal lysates using the TLR2 antibody (SI Fig. 6_e_).
Fig. 1.
Neurons express TLRs and respond to IFNγ stimulation. (a) TLR2 and -4 mRNA are present in cultured cortical neurons determined by single-cell RT-PCR analysis. The numbers indicate the number of neurons from which RNA was amplified; 3, 6, and 10 cortical neurons consistently yielded a positive PCR signal for the TLR2 and -4 with exactly predicted size. (b) Level of TLR mRNAs in neurons treated with IFNγ (500 μg/ml). Neurons expressed TLR2, -3, -4, and MYD88 mRNAs, and their expression levels increased in response to IFNγ. (c) Immunoblot showing relative levels of TLR2 (95 kDa), TLR3 (95 kDa), TLR4 (90 kDa), and MYD88 (33 kDa) in lysates of cultured neurons treated with vehicle (Con) or IFNγ. (d) TLR2, -3, and -4 immunoreactivities (green) in cultured neurons; cells were counterstained with DAPI (blue) to label all nuclei and with the neuron-specific marker NeuN (red). (e) Results of microarray analysis for the indicated TLR mRNAs in RNA samples isolated from cultured cortical neurons (six separate RNA samples run in triplicate; c1–c18). Relative expression levels are indicated by the color scale shown.
Neurons Deficient in TLR2 or -4 Receptors Are Resistant to Death Induced by Energy Deprivation.
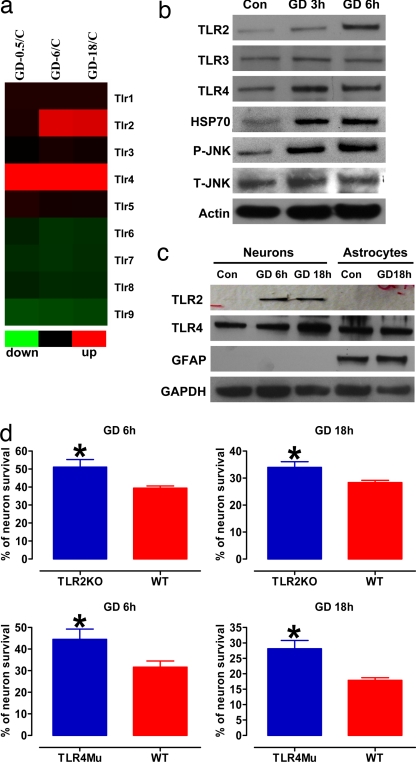
To determine whether TLR signaling is involved in neuronal responses to ischemic conditions, we first evaluated TLR expression levels in cultured neurons subjected to glucose deprivation (GD). Data from both gene microarray and immunoblot analysis showed that levels of TLR2 and -4 were increased within 0.5–6 h of the onset of GD (Fig. 2 a and b). In contrast, the expression of each of the other TLRs evaluated (TLR1, -3, and -5- to -9) were unchanged in neurons subjected to GD for up to 18 h. Consistent with GD inducing ischemia-like stress in the neurons, levels of heat-shock protein 70 were increased compared with neurons not deprived of glucose (Fig. 2b). Increased levels of TLR2 and -4 in response to GD were associated with increased levels of phosphorylated (activated) JNK (Fig. 2b), consistent with activation of downstream signaling by these TLRs (17). The TLR2 and -4 responses to metabolic stress were specific for neurons, because GD had no effect on levels of TLR2 and -4 in cultured astrocytes (Fig. 2c). We next performed a series of experiments in neuronal cultures established from TLR2 and -4 mutant mice to establish the role of each receptor in neuronal vulnerability to metabolic stress. Neurons from both TLR2 knockout and TLR4 mutant mice were significantly more resistant to GD-induced cell death compared with neurons from WT mice (Fig. 2d).
Fig. 2.
Expression of TLR2 and -4 increases after GD, and neurons deficient in TLR2 or -4 receptors are resistant to GD-induced death. (a) Results of a microarray analysis comparing levels of the indicated TLR mRNAs in neurons in control cultures with the level in cultures subjected to GD for 0.5, 6, or 18 h. Note that GD induced increased expression of TLR2 and -4 but not the other TLRs. (b) Immunoblot analysis of proteins in cell lysates of neurons in control cultures and cultures subjected to GD for 3 or 6 h. GD resulted in increased levels of TLR2, -4, HSP70, and _p_-JNK. (c) Immunoblot analysis of proteins in cell lysates of neurons or astrocytes in control cultures and cultures subjected to GD for the indicated time periods. (d) Cultured cortical neurons from WT mice, TLR2 knockout mice, and TLR4 mutant mice were subjected to GD for the indicated time periods, and cell survival was quantified. Values are the mean and SEM (n = 24–36 cultures). *, P < 0.05 compared with the WT value.
TLR2 and -4 Mediate Energy Deprivation-Induced Activation of the JNK-AP-1 Pathway, and Caspase-3 in Cortical Neurons.
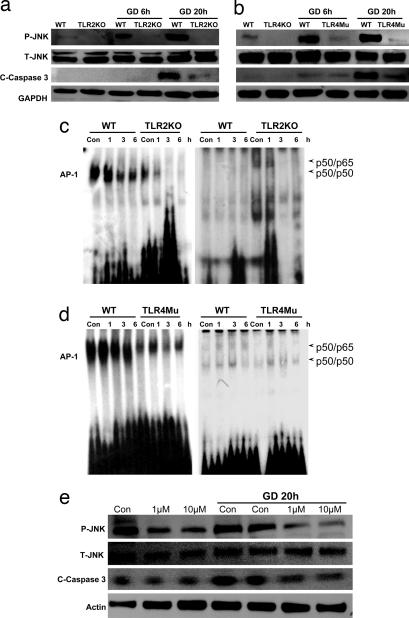
In contrast to the robust increase in JNK activation in WT neurons in response to GD, neurons deficient in TLR2 or -4 did not exhibit a JNK response to GD (Fig. 3 a and b). The latter results suggested a pivotal role for TLRs in neuronal JNK activation during energy deprivation. Because JNK activation and consequent mitochondrial alterations and caspase-3 activation are implicated in ischemic neuronal death (18), we measured levels of activated (cleaved) caspase-3 in the same cell lysates used to measure JNK levels. GD resulted in a much greater increase in activated caspase-3 in WT neurons compared with TLR2 knockout and TLR4 mutant neurons (Fig. 3 a and b), suggesting a proapoptotic role for these TLRs in neurons. Gel-shift analysis was performed on lysates of neurons from WT, TLR2 knockout, and TLR4 mutant mice to determine whether NF-κB and/or AP-1 is involved in the proapoptotic actions of TLR2 and -4 in neurons. We observed no difference in NF-κB DNA-binding activity, under normal or GD conditions, in neurons deficient in TLR2 and -4 compared with WT neurons (Fig. 3 c and d). In contrast, neurons deficient in either TLR2 or -4 exhibited reduced levels of AP-1 DNA-binding activity compared with WT neurons under both basal and GD conditions (Fig. 3 c and d). Neurons treated with the JNK inhibitor SP600125 exhibited reduced levels of activated JNK and were resistant to GD-induced apoptosis (Fig. 3e), demonstrating an essential role for JNK in GD-induced neuronal death. Collectively, the data from studies of cultured neurons suggest that, by increasing activation of JNK and its target transcription factor AP-1, TLR2 and -4 signaling renders neurons vulnerable to death under conditions of energy insufficiency.
Fig. 3.
TLR2 and -4 mediate energy deprivation-induced activation of the JNK-AP-1 pathway and caspase-3 in cultured cortical neurons. (a and b) Immunoblot analysis of proteins in cell lysates of neurons from WT, TLR2 knockout, and TLR4 mutant mice that had been subjected to GD for the indicated time periods. GD resulted in increased levels of _p_-JNK and the cleaved form of caspase-3 (19-kDa) in WT neurons but little or no increase in _p_-JNK or activated caspase-3 in neurons deficient in TLR2 or -4. (c and d) Gel-shift analysis showing NF-κB and AP-1 DNA-binding activities in lysates of neurons from WT, TLR2 knockout mice, or TLR4 mutant mice that had been subjected to GD for 1, 3, or 6 h. Levels of AP-1 DNA-binding activity were lower in samples from TLR2 and -4 mutant mice compared with WT mice. (e) Neuronal cultures were treated with 1 or 10 μM of the JNK inhibitor SP600125 and then subjected to GD (or not) for 20 h. Proteins in cell lysates were then subjected to immunoblot analysis by using the indicated antibodies. The JNK inhibitor attenuated GD-induced increases in levels of _p_-JNK and activated caspase-3.
To determine whether neurons respond to ligands that activate TLRs in immune cells, we treated neuronal cultures with peptidoglycan (TLR2 ligand) or LPS (TLR4 ligand) and measured levels of activated NF-κB and phospho-JNK in the cell lysates. We observed no effects of peptidoglycan or LPS on NF-κB or _p_-JNK in neurons (SI Fig. 7 a and b), despite robust NF-κB responses to each ligand in astrocytes.
TLR2 and -4 Mediate Ischemic Neuronal Death and Functional Deficits in a Mouse Stroke Model.
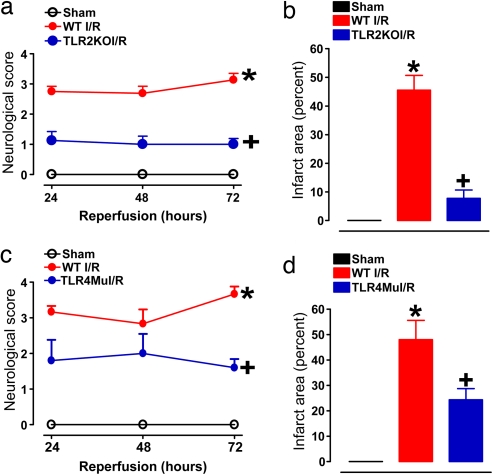
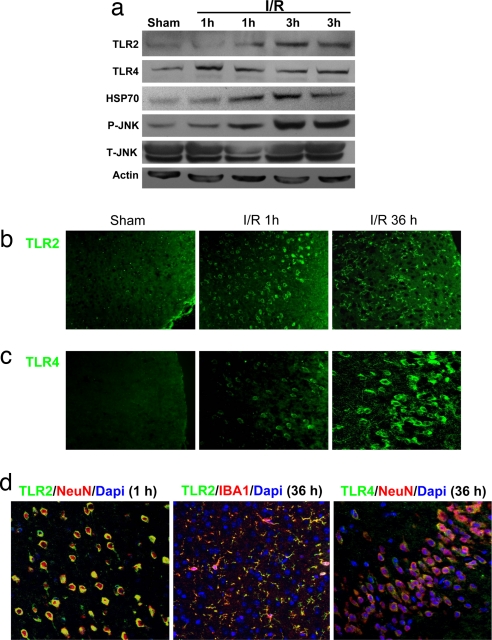
We next asked whether TLR2 and -4 are critically involved in neuronal damage and functional deficits after a stroke. By using a middle cerebral artery occlusion/reperfusion mouse model, we found that the amount of stroke-induced brain damage and neurological deficits, measured 3 d after the stroke, were significantly less in the TLR2 knockout mice and the TLR4 mutant mice compared with WT controls (Fig. 4). Immunoblots of tissue samples taken from the ischemic cortex demonstrated increased levels of TLR2, TLR4, HSP70, and _p_-JNK proteins within 1–3 h of the ischemic episode (Fig. 5a). In the cerebral cortex of sham-operated control mice, little or no immunoreactivity with TLR2 or -4 antibodies was observed. At 1 h after stroke, neurons in the ischemic cortex exhibited robust TLR2 immunoreactivity and weaker TLR4 immunoreactivity (Fig. 5 b–d). At 36 h after stroke, neuronal TLR2 immunoreactivity was diminished, and TLR2-positive microglia were present (Fig. 5 b and d), whereas neuronal TLR4 immunoreactivity remained elevated (Fig. 5c).
Fig. 4.
TLR2 and -4 mediate ischemic neuronal death and functional deficits in a mouse stroke model. Mice of the indicated genotypes were subjected to sham surgery or ischemia/reperfusion (I/R), neurological function was evaluated daily for 3 d, and brain damage was evaluated at 3 d. (a and b) Neurological scores (a) and infarct volumes (b) for WT mice subjected to sham surgery (n = 5), and WT (n = 12) and TLR2 knockout (n = 11) mice subjected to I/R. *, P < 0.001 compared with the sham value. +, P < 0.01 compared with the WT I/R value. (c and d) Neurological scores (c) and infarct volumes (d) for WT mice subjected to sham surgery (n = 5), and WT (n = 8) and TLR4 mutant (n = 7) mice subjected to I/R. *, P < 0.001 compared with the sham value. +, P < 0.01 compared with the WT I/R value.
Fig. 5.
Cerebral ischemia induces a rapid increase in TLR2 and -4 immunoreactivities in neurons and a delayed appearance of TLR2-positive microglia. (a) Immunoblot analysis of protein samples from the cerebral cortex of sham-operated control mice and mice killed either 1 or 3 h after I/R. (b–d) Images of brain sections showing TLR2 and -4 immunoreactivities (green), DAPI staining (blue), and NeuN (neuronal marker) or IBA1 (microglial marker) immunoreactivities (red) in WT mice subjected to sham surgery of I/R for the indicated time periods. Ischemia resulted in rapid increases in the levels of TLR2 and -4 immunoreactivities in neurons in the cerebral cortex and a delayed appearance of TLR2 immunoreactive IBA1-positive microglial cells.
Discussion
Our findings show that TLR2 and -4 are expressed in cerebral cortical neurons, where their levels and downstream signaling via JNK are increased in response to energy deprivation in cell culture and ischemia in vivo. Selective elimination of TLR2 or -4 function suppresses activation of proapoptotic JNK and protects cortical neurons against death induced by energy deprivation and stroke. TLR2 expression increased rapidly in neurons within the first few hours after a stroke, well before its up-regulation in microglia.
When taken together with the involvement of TLR2 and -4 in GD-induced death of cultured cortical neurons, our findings suggest a pivotal role for neuronal TLR signaling in the triggering of neuronal death during the first few hours after a stroke. Subsequent activation of TLRs in microglia likely contributes to the delayed inflammatory processes that occur within the infarct region (8). Although TLR4 and, to a lesser extent, TLR2 have been reported to be expressed in vascular endothelial cells (19), we observed little or no TLR2 or -4 immunoreactivity in cerebral vessels and found no difference in cerebral blood flow among WT, TLR knockout, and TLR4 mutant mice (data not shown).
TLRs are widely conserved in organisms ranging from sponges to humans and are believed to have evolved as a mechanism to protect organisms from invading pathogens, including bacteria, viruses, and parasites (20–22). However, recent evidence suggests that TLRs are activated in immune cells (T cells, dendritic cells, and macrophages) in response to tissue injury, including trauma, hemorrhagic shock, and ischemia (23, 24). Our data demonstrate that neurons express TLR2 and -4, and that these receptors are up-regulated and activated in neurons in response to ischemic conditions. Neurons have previously been shown to express other molecules involved in innate immunity, including components of the complement cascade (25, 26). The complement cascade is activated in response to focal cerebral ischemia and may contribute to the neuronal death process (26). Our findings suggest that TLR signaling, another major system involved in innate immunity, is engaged in neurons in response to brain injury and contributes to their death by activating the JNK-AP-1 pathway. Gene targets of the JNK-AP-1 pathway that mediate neuronal apoptosis in stroke may include p53 and cyclins (27, 28). Activation of TLRs in immune cells by ligands derived from infectious agents (lipoteichoic acid, bacterial LPS, viral RNA, CpG-containing DNA, and flagellin) initiates signaling cascades that result in the activation of transcription factors such as NF-κB or AP-1 leading to the induction of cytokines and other inflammatory mediators (1–4). However, it is not known whether neurons respond to these classic ligands. We did not observe responses (NF-κB or _p_-JNK) of neurons to either a TLR2 ligand (peptidoglycan) or a TLR4 ligand (LPS), despite a robust response of astrocytes to these ligands. These findings suggest the possibility that neurons may not respond to the same infectious agent-derived molecules to which immune and glial cells respond.
The ligands that activate TLRs in neurons under ischemic conditions are unknown. IFNγ is a major inducer of TLR expression and enhancer of TLR signaling in immune cells during responses to infection and inflammation (29), and our evidence suggests that IFNγ can also induce TLR2 and -4 expression in neurons. We also observed that levels of HSP70, a putative TLR ligand (30), were increased in association with TLR2 and -4 up-regulation and JNK activation in neurons subjected to energy deprivation. Another candidate for the endogenous ligand that activates TLR2 and -4 in neurons in response to cerebral ischemia is hyaluronan. Indeed, hyaluronidases are activated in injured neural tissue, resulting in the generation of hyaluronan fragments (31), and hyaluronan fragments have recently been shown to activate TLR2 and -4 in lymphocytes and macrophages (32, 33). Our findings suggest that agents that target TLR expression, endogenous TLR ligands or TLR signal transduction molecules may prove effective in reducing brain damage and improving the clinical outcome following a stroke.
Methods
Animals and Reagents.
TLR2 knockout mice (B6.129-Tlr2tm1Kir), TLR4 mutant mice (C.C3H-Tlr4lps-d), and WT mice of the same genetic backgrounds were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in our animal facility under pathogen-free conditions on a 12-h light/12-h dark cycle with continuous access to food and water. All procedures described below were approved by the National Institute on Aging Animal Care and Use Committee. A description of reagents used in this study can be found in SI Text.
Cell Cultures and GD.
Details of methods for preparing and maintaining primary cultures of mouse cortical neurons and astrocytes (34) are provided in SI Text. For glucose-deprivation studies, glucose-free Locke's buffer (Locke's without glucose) containing 154 mM NaCl/5.6 mM KCl/2.3 mM CaCl2/1 mM MgCl2/3.6 mM NaHCO3/5 mM Hepes, pH 7.2, supplemented with gentamicin (5 mg/liter) was used. The cultured neurons were incubated with glucose-free Locke's buffer and incubated for 3–24 h. Control cultures were incubated in Locke's buffer containing 10 mM glucose.
RT-PCR and Microarray Analysis.
For RT-PCR and microarray analysis, total RNA from the untreated and treated neurons was analyzed as described in SI Text.
Immunoblot Analysis.
Total cellular lysates were obtained by washing the cells in ice-cold PBS and resuspending the cell pellets in cell lysis buffer. Brain tissue protein was extracted by using T-PER tissue protein extraction buffer containing a protease inhibitor mixture (Sigma, St. Louis, MO). Protein concentration was determined by using a BCA protein assay kit (Pierce, Rockford, IL). Protein in samples (40 μg) was separated by SDS/PAGE (8–12%) and transferred to a nitrocellulose membrane. The membrane was blocked in 5% nonfat milk for 1 h at room temperature, followed by an overnight incubation at 4°C with primary antibodies. The membrane was then washed and incubated with a secondary antibody for 1 h at room temperature. Protein bands were visualized by using a chemiluminescence detection kit (Amersham Biosciences, Buckinghamshire, U.K.).
Immunocytochemistry.
Neurons were cultured on 24-chamber microscope slides. Cells were fixed in 4% paraformaldehyde and incubated at 4°C with primary antibody overnight. Primary antibody was detected with FITC-conjugated secondary antibody. The cells were counterstained with DAPI. Coronal brain sections (5 μm) were cut on a freezing microtome. Retrieval of antigen sites, blocking of endogenous peroxidase activity, and blocking of nonspecific binding sites was performed according to standard protocols. Images of cells and tissue sections were acquired by using a Zeiss Axiophot microscope (Zeiss, Oberkochen, Germany).
Cell Viability Assay.
Cell survival was evaluated with the dye Alamar blue by using methods similar to those described previously (35). Briefly, dissociated cells were counted and plated in 24-well plates and exposed to treatments for 24 h. The culture medium was removed and replaced with 300 μl per well of 0.5% Alamar blue diluted in Locke's solution and incubated for 1–2 h at 37°C in a 5% CO2 incubator. Levels of the Alamar blue reaction product were measured by using a HTS 7000 Plus BioAssay Reader (540-nm excitation and 590-nm emission wavelengths). Values for cultures exposed to experimental treatments were expressed as a percentage of the mean value for untreated control cultures.
Focal Cerebral Ischemia Model.
These methods for transient occlusion of the middle cerebral artery have been described (36); details are available in SI Text.
Statistical Analysis.
Experimental results are expressed as mean ± SEM. Statistical comparisons were made with ANOVA, followed by Newman–Keuls post hoc analysis. Neurological behavior scores were analyzed by using a nonparametric Kruskal–Wallis test and Dunn's Multiple Comparison Test.
Supplementary Material
Supporting Information
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging.
Abbreviations
TLR
Toll-like receptors
GD
glucose deprivation
I/R
ischemia/reperfusion.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Akira S. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 2.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. J Exp Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, et al. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 4.Janssens S, Burns K, Vercammen E, Tschopp J, Beyaert R. FEBS Lett. 2003;548:103–107. doi: 10.1016/s0014-5793(03)00747-6. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Gallo DJ, Green AM, Williams DL, Gong X, Shapiro RA, Gambotto AA, Humphris EL, Vodovotz Y, Billiar TR. Infect Immunol. 2002;70:3433–3442. doi: 10.1128/IAI.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Coriolan D, Murthy V, Schultz K, Golenbock DT, Beasley D. Am J Physiol. 2005;289:H1069–H1076. doi: 10.1152/ajpheart.00143.2005. [DOI] [PubMed] [Google Scholar]
- 7.Olson JK, Miller SD. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 8.Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 9.Lafon M, Megret F, Lafage M, Prehaud C. J Mol Neurosci. 2006;29:185–194. doi: 10.1385/JMN:29:3:185. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. Circulation. 2002;105:685–690. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- 12.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M, et al. Circulation. 2003;108:2905–2910. doi: 10.1161/01.CIR.0000101921.93016.1C. [DOI] [PubMed] [Google Scholar]
- 13.Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW. Transplantation. 2005;79:1370–1377. doi: 10.1097/01.tp.0000158355.83327.62. [DOI] [PubMed] [Google Scholar]
- 14.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Proc Natl Acad Sci USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi S, Nathan C, Schnappinger D, Drenkow J, Fuortes M, Block E, Ding A, Gingeras TR, Schoolnik G, Akira S, et al. J Exp Med. 2003;198:987–997. doi: 10.1084/jem.20030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tauszig-Delamasure S, Bilak H, Capovilla M, Hoffmann JA, Imler JL. Nat Immunol. 2002;3:91–97. doi: 10.1038/ni747. [DOI] [PubMed] [Google Scholar]
- 17.Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. J Immunol. 2006;176:7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- 18.Okuno S, Saito A, Hayashi T, Chan PH. J Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, Polentarutti N, Muzio M, Arditi M. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 20.Beutler B. Immunogenetics. 2005;57:385–392. doi: 10.1007/s00251-005-0011-3. [DOI] [PubMed] [Google Scholar]
- 21.Barton GM. Semin Immunol. 2007;19:33–40. doi: 10.1016/j.smim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Wiens M, Korzhev M, Perovic-Ottstadt S, Luthringer B, Brandt D, Klein S, Muller WE. Mol Biol Evol. 2007;24:792–804. doi: 10.1093/molbev/msl208. [DOI] [PubMed] [Google Scholar]
- 23.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 24.Frink M, Hsieh YC, Thobe BM, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mol Immunol. 2007;44:2625–2630. doi: 10.1016/j.molimm.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Nataf S, Stahel PF, Davoust N, Barnum SR. Trends Neurosci. 1999;22:397–402. doi: 10.1016/s0166-2236(98)01390-3. [DOI] [PubMed] [Google Scholar]
- 26.Mack WJ, Sughrue ME, Ducruet AF, Mocco J, Sosunov SA, Hassid BG, Silverberg JZ, Ten VS, Pinsky DJ, Connolly ES., Jr J Neurosci Res. 2006;83:883–889. doi: 10.1002/jnr.20775. [DOI] [PubMed] [Google Scholar]
- 27.Herdegen T, Waetzig V. Oncogene. 2001;20:2424–2437. doi: 10.1038/sj.onc.1204387. [DOI] [PubMed] [Google Scholar]
- 28.Culmsee C, Mattson MP. Biochem Biophys Res Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- 29.Schroder K, Sweet MJ, Hume DA. Immunobiology. 2006;211:511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 31.Al'Qteishat A, Gaffney J, Krupinski J, Rubio F, West D, Kumar S, Kumar P, Mitsios N, Slevin M. Brain. 2006;129:2158–2176. doi: 10.1093/brain/awl139. [DOI] [PubMed] [Google Scholar]
- 32.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, et al. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 33.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 34.Blanc EM, Bruce-Keller AJ, Mattson MP. J Neurochem. 1998;70:958–970. doi: 10.1046/j.1471-4159.1998.70030958.x. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien J, Wilson I, Orton T, Pognan F. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 36.Arumugam TV, Chan SL, Jo DG, Yilmaz G, Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, et al. Nat Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information