Dendritic BC200 RNA in aging and in Alzheimer's disease (original) (raw)
Abstract
Small untranslated BC1 and BC200 RNAs are translational regulators that are selectively targeted to somatodendritic domains of neurons. They are thought to operate as modulators of local protein synthesis in postsynaptic dendritic microdomains, in a capacity in which they would contribute to the maintenance of long-term synaptic plasticity. Because plasticity failure has been proposed to be a starting point for the neurodegenerative changes that are seen in Alzheimer's disease (AD), we asked whether somatodendritic levels of human BC200 RNA are deregulated in AD brains. We found that in normal aging, BC200 levels in cortical areas were reduced by >60% between the ages of 49 and 86. In contrast, BC200 RNA was significantly up-regulated in AD brains, in comparison with age-matched normal brains. This up-regulation in AD was specific to brain areas that are involved in the disease. Relative BC200 levels in those areas increased in parallel with the progression of AD, as reflected by Clinical Dementia Rating scores. In more advanced stages of the disease, BC200 RNA often assumed a clustered perikaryal localization, indicating that dendritic loss is accompanied by somatic overexpression. Mislocalization and overexpression of BC200 RNA may be reactive–compensatory to, or causative of, synaptodendritic deterioration in AD neurons.
Keywords: dementia, non-protein-coding RNA, RNA transport
BC1 and BC200 RNAs (1, 2), collectively called BC RNAs, are small untranslated RNAs that modulate gene expression at the level of translation (3–6). BC RNAs are selectively expressed in neurons and are delivered to synaptodendritic compartments (1, 2, 7–9), where they are thought to contribute to the regulation of local protein synthesis (3, 4, 6).
Increasing evidence suggests that dendritic protein synthesis is instrumental in the implementation of long-term plastic changes at the synapse (10–14) and that small untranslated RNAs are intimately involved in such local translational control (6, 15). On-site translation of locally available mRNAs could provide a means for the input-specific modulation of synaptic strength, thus allowing for adaptive responses to external stimuli. This model relies on the targeted transport of select mRNAs to synaptic sites of translational competence. A further prerequisite is the dendritic delivery of protein synthetic machinery, including translational modulators such as BC RNAs (15).
Mechanisms and infrastructure of neuronal molecular transport have been suggested to be impaired in AD (16–20). Would transport or expression of dendritic RNAs be compromised under such conditions, possibly resulting in inadequate RNA delivery to synapses and thus, potentially, in “plasticity failure”? In an effort to address this question, we examined expression and localization of human BC200 RNA, a dendritic translational repressor (3, 5), in normal aging and in AD.
Results
Neocortical Expression of BC200 RNA Is Differentially Regulated in Normal Aging and in AD.
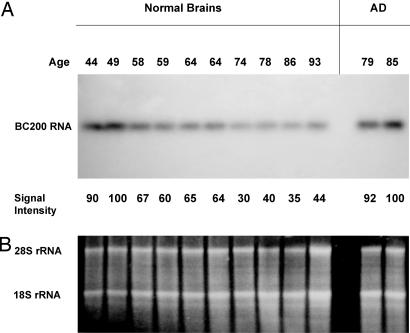
We analyzed BC200 expression in Brodmann's area 9, a prefrontal association region located in the superior frontal gyrus (21). This neocortical area was chosen because it is severely affected in AD (22, 23). BC200 expression was first examined by Northern hybridization in 10 normal cases, ages 44–93, with no history of AD or other neurological disease. We noted a steady decline of BC200 expression in area 9 (a decrease of 65% between the ages of 49 and 86; Fig. 1A). To control and compensate for any possible cell loss in analyzed brain tissues, care was taken to load equal amounts of RNA in all cases. To ascertain equal loading and RNA integrity, 18S and 28S rRNAs were monitored on the same gels by ethidium bromide staining (Fig. 1B). We performed such calibration to ensure that samples contained equivalent amounts of RNA (24, 25) and that loaded RNAs were intact and thus represented viable cells (note that 28S rRNA is exquisitely sensitive to degradation in dying cells; see ref. 26).
Fig. 1.
Differential expression of BC200 RNA in normal aging (10 left lanes) and in AD (two right lanes). Total RNA from Brodmann's area 9 was probed by using Northern hybridization. (A) A substantial relative decrease in BC200 levels is observed after age 49. In contrast, no such decrease is apparent in AD brains. Relative signal intensities are indicated for each lane (intensity at age 49 set to 100 in the left lanes). (B) 18S and 28S rRNAs were monitored to ensure uniform sample loading.
In AD brains, we observed, to our initial surprise, that BC200 levels in Brodmann's area 9 were substantially higher than in normal brains of comparable age (Fig. 1A). Ribosomal RNA was again visualized for calibration (Fig. 1B). The data showed that BC200 expression is differentially regulated in AD brains and in normal aging brains and prompted us to expand our analysis and address, on a quantitative basis, the following two questions. (i) Can we associate higher regional BC200 expression specifically with AD involvement? (ii) Do BC200 expression levels in affected regions of AD brains reflect severity or progression of the disease?
BC200 Expression Is Increased in Brain Areas That Are Involved in AD.
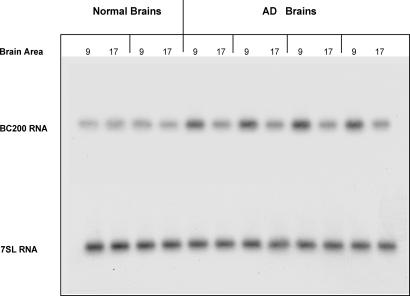
To address the first question, we examined Brodmann's area 9 in comparison with Brodmann's area 17 (primary visual cortex), a region that is typically spared in AD (22, 27). In all AD cases analyzed, noninvolvement of area 17 in the disease was verified by neuropathological examination. We initially analyzed four AD cases that showed Clinical Dementia Rating (CDR) scores of 4/5 in comparison with two normal cases (CDR 0). We found that in AD brains, BC200 levels were considerably higher in area 9 than in area 17 (Fig. 2). BC200 levels in area 9 of AD cases were also notably higher than in area 9 or 17 of normal cases. BC200 levels were quantified by using phosphorimaging (PI), and statistical analysis revealed that the observed differences were highly significant (Fig. 2). In contrast, BC200 levels in area 17 of AD cases did not significantly differ from those in area 9 or 17 of normal cases.
Fig. 2.
BC200 expression in Brodmann's areas 9 and 17 in normal and AD brains. Quantitative analysis showed that in AD, BC200 levels were significantly elevated in area 9, but not in area 17, compared with either area in normal brains. [One-way ANOVA, P < 0.001, n = 12; Scheffé's multiple comparison post hoc analysis (comparison with normal area 9), P < 0.001 for AD area 9]. Levels of 7SL RNA showed no significant differences. Ages (left to right): 77 and 78 (normal); 90, 88, 62, and 79 (AD).
In addition, expression levels of 7SL RNA, the RNA component of the ubiquitous signal recognition particle (28), were quantified by PI after reprobing the same blot. Expression levels of 7SL did not significantly differ between brain areas and between AD and normal brains (Fig. 2). In subsequent experiments, we therefore used 7SL levels in each analyzed area to normalize BC200 PI data from the same area.
In summary, the combined data thus indicate that in AD, BC200 levels are specifically elevated in area 9, which is involved in the disease, but not in area 17, which is generally not.
Relative Levels of BC200 RNA in Affected Areas Increase in Parallel with the Severity of the Disease.
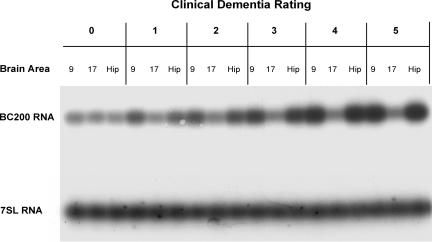
We next asked whether in AD, BC200 expression levels in affected areas reflected the severity of the disease. This analysis was designed to include a spectrum of cases ranging from normal, nondemented brains (CDR 0) to terminal AD brains (CDR 5). In addition to Brodmann's area 9, which is known to be progressively affected as dementia worsens (23), the hippocampus was included as a brain area with early and significant involvement in AD (22). Results from these experiments are presented in two formats: representative primary data from a set of Northern hybridization experiments (Fig. 3) and quantitative analysis of the entire data set, with 7SL expression used for calibration (Table 1 and Fig. 4).
Fig. 3.
Expression levels of BC200 RNA in association with CDR status in AD brains. Results are shown for six patients with CDRs ranging from 0 (no dementia) to 5 (terminal dementia). BC200 expression was analyzed in Brodmann's area 9 (prefrontal association cortex), area 17 (primary visual cortex), and the hippocampus for each patient (18 samples total). BC200 expression is significantly increased in area 9 and in the hippocampus, but not in area 17, and the degree of this increase correlates with CDR status. Ages (in order of ascending CDR scores): 96, 93, 94, 77, 85, and 67. See Table 1 for a quantitative analysis of the entire data set.
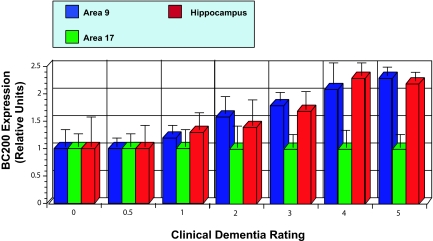
Table 1.
BC200 expression and CDR status
| Brain areas | CDR | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | 3 | 4 | 5 | |
| 9 | 1 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.3 | 1.9 ± 0.5 | 2.5 ± 0.4 | 3.5 ± 0.9 | 3.0 ± 0.3 |
| 17 | 1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.4 | 1.3 ± 0.5 | 1.4 ± 0.4 | 1.7 ± 0.7 | 1.3 ± 0.4 |
| Hip | 1 ± 0.2 | 1.2 ± 0.4 | 1.8 ± 0.4 | 1.6 ± 0.6 | 2.3 ± 0.5 | 4.0 ± 0.6 | 2.8 ± 0.3 |
Fig. 4.
Increase of BC200 expression levels relative with CDR status. The bar diagram is based on the same data set as Table 1, and it was derived from the data shown in the table by normalizing BC200 expression levels in area 17 to a relative value of 1.0 for each CDR data set.
The results show that BC200 expression was significantly elevated in the hippocampus and area 9 of AD brains but not in area 17 of the same brains. In contrast, no such elevation was detected in the hippocampus or area 9 of non-AD brains (CDR 0). In cases with CDRs ranging from 0 to 5, BC200 levels were characterized by continually increasing gradients (Fig. 4). In contrast, CDR score was not associated with expression levels of 7SL RNA (Fig. 3).
The data set presented in Fig. 4 was subjected to statistical analysis (see also Table 1). One-way ANOVA revealed significant differences as a function of CDR score, in BC200 expression in area 9 and hippocampus but not in area 17. Nonparametric Spearman's correlations showed a clear association of progressing CDR status with increasing BC200 expression in area 9 (rs = 0.855) and in the hippocampus (rs = 0.917) but not in area 17 (rs = 0.455) (see Table 1). The data indicate that BC200 expression is correlated with the severity of dementia and therefore with the progression of the disease. It is notable that both in the hippocampus and area 9, BC200 levels have already risen significantly at CDR 1 (Table 1), which suggests that up-regulation of BC200 expression is triggered early during the onset of the disease.
Somatodendritic Distribution of BC200 RNA Is Altered in Severe AD Cases.
How are the elevated BC200 levels in affected AD brain areas reflected in the RNA's somatodendritic distribution pattern? To address this question, we probed distribution of BC200 RNA in those areas by in situ hybridization. Analyzing BC200 levels in profound and terminal AD, we first confirmed that BC200 levels in area 9 were substantially higher than in area 9 of non-AD cases (nine AD cases and eight non-AD cases were analyzed) (Fig. 5). In contrast, we did not observe any significant elevation of BC200 levels in area 17 of AD brains, nor did we observe significantly changed levels of 7SL RNA in any area of AD brains, in comparison with normal brains (data not shown).
Fig. 5.
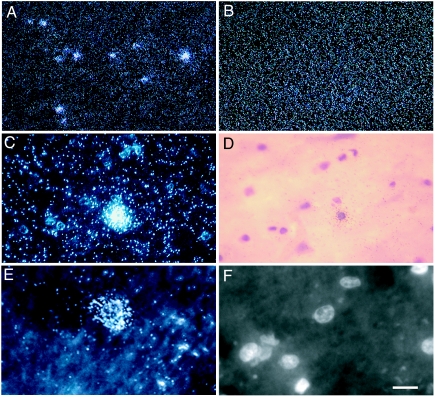
Altered distribution of BC200 RNA in terminal AD. In situ hybridization reveals differential expression of BC200 RNA in Brodmann's area 9 of terminal AD brain (A and C–F) and normal brain (B). (A–C and E) Autoradiographic signal appears as white-silver grains in darkfield photomicrographs. (B) In area 9 of normal brain, BC200 signal was found diffusely distributed, reflecting a somatodendritic distribution, as has been reported earlier (2). (A and C–F) In contrast, in area 9 of terminal AD brains, labeling often appeared in high intensity clusters. (A) A low-magnification overview. (C) An area 9 region at higher magnification. (D) A brightfield photomicrograph showing H&E counterstaining of the region shown in C. (E) A high-magnification darkfield photomicrograph. (F) An epifluorescence photomicrograph showing DAPI-counterstained nuclei of the region shown in E. Outside of high-intensity BC200 clusters, regions in Brodmann's area 9 frequently showed lower BC200 levels than average levels in non-AD cases. (Scale bars: A and B, 100 μm; C and D, 30 μm; E and F, 15 μm.)
In area 9 of a subset of the analyzed AD cases (44%), the distribution of BC200 RNA appeared atypically clustered in addition to being elevated (Fig. 5). In neocortical areas of normal, non-AD brains, BC200 RNA is known to be distributed in a characteristically diffuse manner, resulting from its localization to dendritic domains in neuropil (Fig. 5B) (2). In contrast, in area 9 of terminal AD brains, the BC200 signal often appeared in conspicuous high-intensity clusters. Are these clusters, which are particularly prominent in layer V, associated with neuronal somata? Counterstaining with H&E revealed a perikaryal distribution of such BC200 clusters (Fig. 5 C and D), a result that was further corroborated by using DAPI nuclear fluorescence labeling (Fig. 5 E and F). At the same time, levels in some neuropil areas appeared depressed compared with normal brains (Fig. 5). In the majority of neurons examined (74%), perikaryal accumulation was associated with nonpyknotic nuclei, suggesting that such accumulation preceded final-stage cell degeneration.
In summary, the results indicate that in these cases, elevated overall BC200 levels are primarily attributable to increased somatic expression.
Discussion
AD is a neurodegenerative disease of complex etiology (29, 30). The formation of neurofibrillary tangles (NFT), neuropil threads, and senile plaques have been implicated in the onset and development of the disease, but the relative causal weight of these and other factors in sporadic AD continues to be debated (30, 31). Although initiation and progression of AD may thus be multifactorial, it has been noted that incidence and regional distribution of NFT are most closely associated with the clinical manifestations of the disease (32, 33). NFT formation is the result of the accumulation of altered components of the neuronal cytoskeleton (34), and it has been suggested that “clogging” of neuronal processes and disruption of long-distance transport may underlie at least some of the cytopathological changes that characterize the disease (19).
We suggest that such cellular changes on one hand and deregulated expression and transport of dendritic RNAs on the other may be causally interrelated in AD. It has recently been speculated that non-protein-coding RNAs may be involved in AD (35), and we now show that expression of dendritic BC200 RNA, which is a translational repressor (3, 5), is differentially regulated in normal aging and in AD. In normal aging, BC200 levels in the neocortex decrease substantially after age 50. Because BC RNAs are prominently located throughout dendrites and at synapses (1, 2, 8), the substantial decline of BC levels may reflect the progressive atrophy of synaptodendritic structures that has previously been observed in normal aging (23, 36–39).
In AD, synapse loss and dendritic regression are substantial (40), but these degenerative changes are accompanied by significant dendritic sprouting and remodeling, often in the same neuron (30, 41, 42). Such reactive developments may be of a compensatory nature, directed at maintaining connectivity and plasticity. In one possible scenario, we therefore suggest that the substantially higher BC200 levels in AD, as compared with those in normal aging, may represent a molecular compensatory effort. If BC200 RNA is needed at the synapse for local translational control, its loss from synaptodendritic domains as the result of dendritic regression and clogging in AD may trigger compensatory mechanisms that result in the increased production of the RNA.
Increased synthesis of key synaptodendritic components may be an appropriate response in situations in which cargoes are not effectively delivered to postsynaptic sites. It may, at least initially or partially, be successful in overcoming moderate dendritic clogging that is caused by altered cytoskeletal components. Over time, however, such response may prove inadequate if further accumulation of cytoskeletal debris creates “roadblocks” that RNAs with dendritic destinations are no longer able to traverse. At this point, relative BC200 levels would begin to decrease in dendrites but increase in somata. In such cases, efforts to compensate would have failed because even increased production could no longer ensure that the RNA reaches its dendritic target sites. Transport deficits have previously been implicated in the progression of AD (18–20), and impaired microtubule-dependent transport, coupled with beginning axonal and dendritic blockage, may be an early event in AD that could eventually result in the local generation of amyloid-β peptides and thus in amyloid deposition (17).
Alternative scenarios are possible or even likely. Instead of, or in addition to, being reactive–compensatory to cytoskeletal degeneration, imbalances in the somatodendritic distribution of BC200 RNA could be causative because they may lead to aberrant local translational control. BC200 RNA contains a kink turn motif of the KT-58 subtype that has been implicated in dendritic transport of BC RNAs (9, 43). Because only slight perturbations of the kink turn motif architecture are sufficient to disrupt targeting (9), it is conceivable that single-nucleotide mutations in this region may prevent delivery of the RNA along the dendritic extent. Consequences would be twofold: compensatory elevation of BC200 transcription in an attempt to overcome dendritic delivery block and poor translational control in synaptodendritic domains. Consistent with this model, altered relative BC200 levels become manifest at a very early time point in the course of AD, possibly before clinical signs become detectable (44, 45).
A gradual worsening of somatodendritic BC200 imbalances may over time set off a self-reinforcing feed–forward cycle. Inadequate translational regulation in dendrites may lead to cytoskeletal overproduction and local dysfunction (16), which in turn would hinder the transport of mRNAs and protein synthetic machinery to postsynaptic target sites. In line with this concept, levels of somatodendritic RC3 mRNA have been shown to be significantly diminished in dendritic regions of AD brains (46). Having been disrupted in this manner, the system would find itself on a slow but accelerating course toward eventual catastrophe, manifesting as synaptic or plasticity failure (17, 30, 47–49). At the same time, increased perikaryal levels of BC200 RNA may inappropriately repress somatic protein synthesis and thus precipitate or exacerbate degenerative changes. We anticipate that future work, directed at the understanding of neuronal RNA transport and local translational control mechanisms, will be able to establish the respective contributions of the causative and reactive–compensatory scenarios.
Materials and Methods
Tissue Acquisition and Processing.
Brain material for autopsy was provided by the Alzheimer's Disease Research Center at Mount Sinai School of Medicine and by the Department of Psychiatry, University of Geneva (Geneva, Switzerland). Brains were processed within a postmortem interval of <10 h. Neuropathological evaluation was performed at the Department of Psychiatry, University of Geneva, and at the Department of Pathology, Division of Neuropathology, Mount Sinai School of Medicine. AD status was established on the basis of regional NFT density and amyloid deposits as described (50). CDR scores were ascertained as described previously (51, 52). Dementia was rated on a scale from 0 to 5 (CDR 0, no dementia; CDR 0.5, very mild; CDR 1, mild; CDR 2, moderate; CDR 3, severe; CDR 4, profound; and CDR 5, terminal dementia) (23). All procedures involving human subjects were reviewed and approved by the respective ethical committees and Institutional Review Boards.
Brains were dissected as described previously (23). Brain tissue was immediately quick-frozen in liquid nitrogen and stored at −80°C. Tissue samples were code-numbered to ensure that subsequent experimenters were blind to sample identity. Sample codes were kept at Mount Sinai School of Medicine and were disclosed only after completion of the respective set of experiments at State University of New York at Brooklyn.
RNA Filter (Northern) Hybridization.
Total RNA was isolated from brain tissues by using a modified guanidinium thiocyanate procedure as described in ref. 53. RNA was separated on 1% agarose formaldehyde gels, transferred to GeneScreen Plus membranes (DuPont, Wilmington, DE), and immobilized by using UV illumination. Equal loading and unbiased transfer were ascertained by using UV shadowing of 18S and 28S rRNA bands. BC200 RNA was probed by using oligonucleotide BC205 and 7SL RNA was probed by using oligonucleotide 7SL36B under hybridization conditions as described in ref. 53. We found it important to perform Northern hybridization experiments using only one type of filter membrane. Although other membrane types were, in our experience, not inferior in quality, quantitative data were more difficult to standardize and cross-correlate when more than one type of filter membrane was used.
Northern hybridization data were analyzed and quantified by using a Storm 860 PI system with ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Statistical analysis was performed in consultation with the State University of New York Scientific Computing Center. SPSS (Chicago, IL) software was used for statistical analyses, including one-way ANOVA and nonparametric Spearman's rho correlation.
In Situ Hybridization.
Brains from nine AD cases (of persons with profound or terminal AD) and eight normal cases were analyzed using in situ hybridization. These cases were in addition to those that were analyzed by using filter hybridization. Brain tissue was cryosectioned at a thickness of 10 μm and stored at −80°C until use. Sections were postfixed with 4% formaldehyde (made from paraformaldehyde) and immediately processed for hybridization (1). RNA probes specific for BC200 RNA were generated from plasmid pVL450-1 (2) or from plasmid pKK536-6 (54). The latter plasmid is analogous to pVL450-1 except that the insert corresponds to nucleotides 119–189 of BC200 RNA (2). It is important that BC200 probes be directed against the unique segment of the RNA (2) because probes directed against the Alu-homologous segment will cross-hybridize with a multitude of RNAs that contain repetitive Alu elements (see ref. 55).
Hybridization using probes derived from pVL450-1 was performed as described in ref. 2. Hybridization using probes derived from pKK536-6 was performed analogously except that the temperature of the final high-stringency wash was raised to 50°C. As described previously (53), 7SL RNA was detected by using RNA probes generated from plasmid pKK451-1. A small untranslated RNA that is part of the signal recognition particle (28), 7SL RNA was used as a reference RNA that undergoes little change in expression levels in aging or in AD. Established procedures were used for H&E counterstaining (56) and DAPI nuclear counterstaining (57).
Digital images of tissue sections were acquired using a Sony 3CCD DKC-5000 camera (Sony, New York, NY) or a CoolSnapHQ cooled CCD camera mounted on a Microphot-FXA microscope (Nikon, Melville, NY) as described previously (56).
Acknowledgments
We thank Drs. Vahram Haroutunian (Department of Psychiatry, Mount Sinai School of Medicine) and Constantin Bouras (Department of Psychiatry, University of Geneva School of Medicine, Geneva, Switzerland) for postmortem human tissue samples and Dr. Jürgen Brosius (Institute of Experimental Pathology, University of Münster School of Medicine, Münster, Germany) for plasmid pKK536-6. Statistical consultation was provided by Drs. Hans von Gizycki and Jeremy Weedon at the State University of New York Scientific Computing Center. This work was supported in part by the New York City Council Speaker's Fund for Biomedical Research (to E.M.), National Institutes of Health Grants AG02219 and AG05138 (to P.R.H.), the American Foundation for Aging Research (H.T.), and National Institutes of Health Grant NS046769 (to H.T.).
Abbreviations
AD
Alzheimer's disease
CDR
Clinical dementia rating
NFT
neurofibrillary tangles
PI
phosphorimaging.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tiedge H, Fremeau RT, Jr, Weinstock PH, Arancio O, Brosius J. Proc Natl Acad Sci USA. 1991;88:2093–2097. doi: 10.1073/pnas.88.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiedge H, Chen W, Brosius J. J Neurosci. 1993;13:2382–2390. doi: 10.1523/JNEUROSCI.13-06-02382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Iacoangeli A, Popp S, Muslimov IA, Imataka H, Sonenberg N, Lomakin IB, Tiedge H. J Neurosci. 2002;22:10232–10241. doi: 10.1523/JNEUROSCI.22-23-10232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Iacoangeli A, Lin D, Williams K, Denman RB, Hellen CUT, Tiedge H. J Cell Biol. 2005;171:811–821. doi: 10.1083/jcb.200506006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondrashov AV, Kiefmann M, Ebnet K, Khanam T, Muddashetty RS, Brosius J. J Mol Biol. 2005;353:88–103. doi: 10.1016/j.jmb.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Tiedge H. Neuroscientist. 2004;10:456–466. doi: 10.1177/1073858404265866. [DOI] [PubMed] [Google Scholar]
- 7.Muslimov IA, Santi E, Homel P, Perini S, Higgins D, Tiedge H. J Neurosci. 1997;17:4722–4733. doi: 10.1523/JNEUROSCI.17-12-04722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chicurel ME, Terrian DM, Potter H. J Neurosci. 1993;13:4054–4063. doi: 10.1523/JNEUROSCI.13-09-04054.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muslimov IA, Iacoangeli A, Brosius J, Tiedge H. J Cell Biol. 2006;175:427–439. doi: 10.1083/jcb.200607008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steward O, Schuman EM. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- 11.Job C, Eberwine J. Nat Rev Neurosci. 2001;2:889–898. doi: 10.1038/35104069. [DOI] [PubMed] [Google Scholar]
- 12.Kindler S, Wang H, Richter D, Tiedge H. Annu Rev Cell Dev Biol. 2005;21:223–245. doi: 10.1146/annurev.cellbio.21.122303.120653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klann E, Richter JD. In: Translational Control in Biology and Medicine. Mathews MB, Sonenberg N, Hershey JWB, editors. Woodbury, NY: Cold Spring Harbor Lab Press; 2007. pp. 485–506. [Google Scholar]
- 14.Bassell GJ, Twiss JL. EMBO Rep. 2006;7:31–35. doi: 10.1038/sj.embor.7400616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao X, Yeo G, Muotri AR, Kuwabara T, Gage FH. Annu Rev Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- 16.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. J Cell Biol. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, et al. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 18.Matsuyama SS, Jarvik LF. Proc Natl Acad Sci USA. 1989;86:8152–8156. doi: 10.1073/pnas.86.20.8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandelkow E, Mandelkow EM. Trends Cell Biol. 2002;12:585–591. doi: 10.1016/s0962-8924(02)02400-5. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein LS. Proc Natl Acad Sci USA. 2001;98:6999–7003. doi: 10.1073/pnas.111145298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig, Germany: Barth; 1909. [Google Scholar]
- 22.Braak H, Braak E. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 23.Bussière T, Giannakopoulos P, Bouras C, Perl DP, Morrison JH, Hof PR. J Comp Neurol. 2003;463:281–302. doi: 10.1002/cne.10760. [DOI] [PubMed] [Google Scholar]
- 24.De Leeuw WJ, Slagboom PE, Vijg J. Nucleic Acids Res. 1989;17:10137–10138. doi: 10.1093/nar/17.23.10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbu V, Dautry F. Nucleic Acids Res. 1989;17:7115. doi: 10.1093/nar/17.17.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houge G, Robaye B, Eikhom TS, Golstein J, Mellgren G, Gjertsen BT, Lanotte M, Doskeland SO. Mol Cell Biol. 1995;15:2051–2062. doi: 10.1128/mcb.15.4.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hof PR, Morrison JH. J Comp Neurol. 1990;301:55–64. doi: 10.1002/cne.903010106. [DOI] [PubMed] [Google Scholar]
- 28.Walter P, Blobel G. Nature. 1982;299:691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- 29.Morrison JH, Hof PR. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 30.Mesulam MM. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 31.Mesulam M. Learn Mem. 2004;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- 32.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 33.Giannakopoulos P, Herrmann FR, Bussière T, Bouras C, Kövari E, Perl DP, Morrison JH, Gold G, Hof PR. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Sternberger NH, Rubinstein LJ, Herman MM, Binder LI, Sternberger LA. Proc Natl Acad Sci USA. 1989;86:8045–8049. doi: 10.1073/pnas.86.20.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paquet C, Hugon J. Med Hypotheses. 2007 in press. [Google Scholar]
- 36.Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Exp Neurol. 1975;47:392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- 37.Rafols JA, Cheng HW, McNeill TH. J Comp Neurol. 1989;279:212–227. doi: 10.1002/cne.902790205. [DOI] [PubMed] [Google Scholar]
- 38.Vaughan DW. J Comp Neurol. 1977;171:501–516. doi: 10.1002/cne.901710406. [DOI] [PubMed] [Google Scholar]
- 39.Hof PR, Morrison JH. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Terry RD, Masliah E, Hansen LA. In: Alzheimer Disease. Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Philadelphia: Lippincott; 1999. pp. 187–206. [Google Scholar]
- 41.Ihara Y. Brain Res. 1988;459:138–144. doi: 10.1016/0006-8993(88)90293-4. [DOI] [PubMed] [Google Scholar]
- 42.Gertz HJ, Krüger H, Patt S, Cervos-Navarro J. Brain Res. 1991;548:260–266. doi: 10.1016/0006-8993(91)91130-s. [DOI] [PubMed] [Google Scholar]
- 43.Tiedge H. RNA Biol. 2006;3:133–139. doi: 10.4161/rna.3.4.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albert MS. Proc Natl Acad Sci USA. 1996;93:13547–13551. doi: 10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, Newman AB, Kuller L. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- 46.Chang JW, Schumacher E, Coulter PM, II, Vinters HV, Watson JB. J Neuropathol Exp Neurol. 1997;56:1105–1118. doi: 10.1097/00005072-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Selkoe DJ. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 48.Rekart JL, Quinn B, Mesulam MM, Routtenberg A. Neuroscience. 2004;126:579–584. doi: 10.1016/j.neuroscience.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 49.Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, et al. Proc Natl Acad Sci USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirra SS, Hart MN, Terry RD. Arch Pathol Lab Med. 1993;117:132–144. [PubMed] [Google Scholar]
- 51.Morris JC. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 52.Dooneief G, Marder K, Tang MX, Stern Y. Neurology. 1996;46:1746–1749. doi: 10.1212/wnl.46.6.1746. [DOI] [PubMed] [Google Scholar]
- 53.Chen W, Böcker W, Brosius J, Tiedge H. J Pathol. 1997;183:345–351. doi: 10.1002/(SICI)1096-9896(199711)183:3<345::AID-PATH930>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 54.Khanam T, Rozhdestvensky TS, Bundman M, Galiveti CR, Handel S, Sukonina V, Jordan U, Brosius J, Skryabin BV. Nucleic Acids Res. 2007;35:529–539. doi: 10.1093/nar/gkl1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukiw WJ, Handley P, Wong L, Crapper McLachlan DR. Neurochem Res. 1992;17:591–597. doi: 10.1007/BF00968788. [DOI] [PubMed] [Google Scholar]
- 56.Iacoangeli A, Lin Y, Morley EJ, Muslimov IA, Bianchi R, Reilly J, Weedon J, Diallo R, Böcker W, Tiedge H. Carcinogenesis. 2004;25:2125–2133. doi: 10.1093/carcin/bgh228. [DOI] [PubMed] [Google Scholar]
- 57.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]