GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo (original) (raw)
. Author manuscript; available in PMC: 2007 Oct 1.
Published in final edited form as: Bioessays. 2007 Apr;29(4):356–370. doi: 10.1002/bies.20558
Summary
Rho/Rac proteins constitute a subgroup of the Ras superfamily of GTP hydrolases. Although originally implicated in the control of cytoskeletal events, it is currently known that these GTPases coordinate diverse cellular functions, including cell polarity, vesicular trafficking, the cell cycle and transcriptomal dynamics. In this review, we will provide an overview on the recent advances in this field regarding the mechanism of regulation and signaling, and the roles in vivo of this important GTPase family.
Introduction
The isolation of rhoA,(1) the first member of the Rho/Rac family ever identified, was achieved by Richard Axel’s group in 1985 during the search for ras -related genes in Aplysia.(1) The subsequent use of conventional cloning techniques and the more-recent characterization of genomes revealed that the original gene is not alone, having numerous family counterparts in other species including, among many others, S. cerevisiae(7 genes), A. taliana(11 genes), C. elegans(9 genes), D. melanogaster(9 genes) and H. sapiens (23 genes). In humans, these twenty-three different loci can generate at least twenty-six different proteins due to alternative splicing events. In accordance with their homology at the amino acid sequence level, these proteins are classified into six subfamilies: Rho, Rac, Cdc42, Rnd, RhoBTB and RhoT/Miro (Fig. 1). RhoBTB and RhoT proteins are also referred to as ‘‘atypical’’ Rho/Rac GTPases because they are very different from the other GTPase subfamilies according to structural, regulatory and functional criteria.
Figure 1.
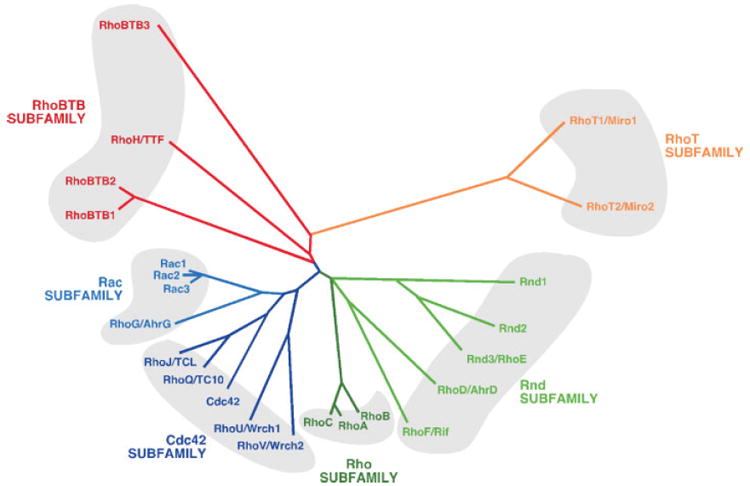
Dendrogram showing the classification of Rho/Rac subfamily members according to structural similarity criteria. Members of each subfamily are highlighted using the same color code and grouped by shaded areas. The first symbol used for each GTPase corresponds to that approved by the Human Genome Organization Gene Nomenclature Committee. When appropriate, other commonly used names are also included. The same criterium has been followed in the rest of this review article.
Like the majority of Ras superfamily proteins, most Rho/ Rac GTPases behave as ‘‘molecular switches’’ that fluctuate between inactive and active states, two conformations that depend on the binding of either GDP or GTP to the GTPases, respectively (Fig. 2). Two types of regulatory proteins control this cycling: GEFs and GAPs (Fig. 2). GEFs promote the exchange of GDP for GTP molecules, thereby producing the activation of these proteins during signal transduction. GAPs promote the hydrolysis of the bound GTP molecules, thus allowing the transfer of the GTPase back to the inactive state at the end of the stimulation cycle. In the GTP-bound state, these GTPases bind to a large collection of effector molecules that, in turn, lead to the stimulation of signaling cascades that promote general cellular responses such as cytoskeletal change, microtubule dynamics, vesicle trafficking, cell polarity and cell cycle progression.(2) The plasticity of Rho/ Rac proteins both in terms of subcellular localization, regulation, binding to effectors and crosstalk with other cellular pathways has put them in a central regulatory point for a quite large number of cellular processes. Unfortunately, the toll that we have to pay for this is the development of diseases when these routes become dysfunctional.(3,4) This crucial role has led to a comprehensive study on their mechanism of regulation, to the identification of additional elements of their signal transduction pathways, and to the characterization of their roles in vivo. In the present work, we will give an overall view of the recent developments in those areas, placing special emphasis on their regulatory and biological properties in vivo. Given that Rho, Rac and Cdc42 are the best-characterized Rho/Rac subfamilies, we will limit our review to these molecules. Readers can find additional information on other aspects of Rho/Rac biology in recent publications.(2,5)
Figure 2.
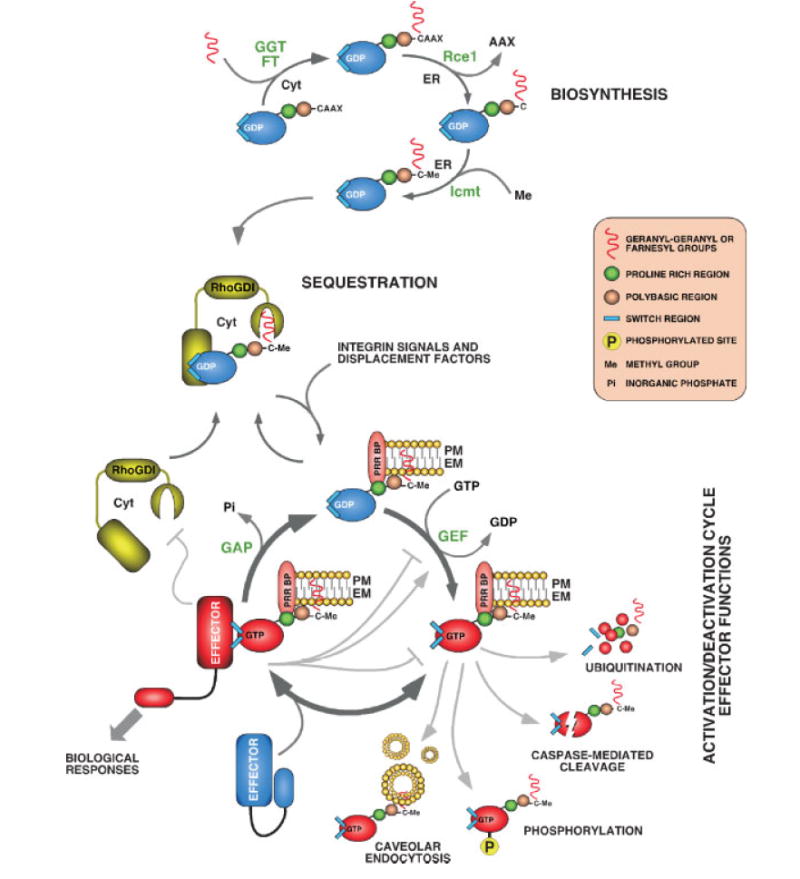
Schematic representation of the biosynthesis (top), sequestration (middle) and regulatory (bottom) cycles of Rho/Rac proteins. In the latter case, we have included the prototypical GDP/GTP cycle as well as other regulatory steps mediated by the action of either effectors or other biological pathways (ubiquitination, protease cleavage, internalization). The main steps in each cycle are highlighted using dark-gray arrows. Other less common regulatory interactions are indicated in light-gray arrows (when resulting in an activation signal) or blunted lanes (when resulting in a downmodulation signal). The enzymes catalyzing those steps are shown in green. For the sake of simplicity, we have not included here other post-translational events of Rho/Rac proteins that have been described in the main text such as palmitoylation. It is also still unclear whether the insertion of the GTPase into the docking membrane is achieved when in the GDP or GTP-bound state. The latter case is not contemplated in the scheme and would require the activation of the GTPase by GEFs, the re-association of the GTP-bound GTPase with either RhoGDI or other carrier proteins, and the subsequent delivery of the GTPase to the target membrane. Abbreviations used are: CAAX, an acronym derived from the combination of C=cysteine, A=aliphatic amino acids and X=Met, Ser, Ala or Gln; Cyt, cytosol; EM, endomembranes; ER, endoplasmic reticulum; FT, farnesyl transferase; GGT, geranyl-geranyl transferase; PM, plasma membrane; PRR BP, proline rich region binding protein. Consult main text for further details.
Regulation of Rho/Rac protein activity
In order to ensure proper signaling responses to extracellular stimuli, cells control the activity of Rho/Rac proteins through a number of regulatory steps. These include: (1) the control of nucleotide binding and hydrolysis by GEFs and GAPs, a process that has been already the object of recent reviews,(6,7) (2) the regulation of their subcellular localization, (3) the modulation of their protein expression levels, and (4) other regulatory events. We summarize below the advances in the understanding of these additional regulatory layers.
Regulation of Rho/Rac proteins by changes in the subcellular localization
In addition to GDP/GTP exchange, most Rho/Rac proteins require the docking onto cell membranes in order to perform their biological functions. However, unlike other Ras superfamily proteins, this anchoring step is not achieved by default during their biosynthesis and requires, instead, a combination of intrinsic tethering signals and cooperative signaling events.(8) The first and most crucial of the intrinsic tethering signals is the progressive post-translational modification of the so-called GTPase ‘‘CAAX box’’ (Fig. 2). The first stage of this modification is the incorporation of either a geranyl-geranyl or, less frequently, a farnesyl group to the cysteine residue of the CAAX box, a process catalyzed in the cytoplasm by either type I geranyl-geranyl or farnesyl transferases, respectively (Fig. 2).(8,9) The attachment of the isoprenoid group to the CAAX box promotes the translocation of the GTPases to the endoplasmic reticulum,(8,10) where the proteolytic cleavage of the AAX tripeptide tail ensues via the isoprenyl, CAAX-specific protease Rce1 (Fig. 2).(8,11) After this reaction, the newly exposed α-carboxyl group of the C-terminal cysteine residue becomes methylesterified by the carboxyl methyltransferase Icmt (Fig. 2).(8,12) In some cases (i.e. RhoB), Rho/Rac proteins are further modified in the endoplasmic reticulum by the attachment of palmitate groups on additional cysteine residues present nearby the CAAX motif.(8) The enzyme responsible for this step is still uncharacterized in mammals. Recent results have shown that the incorporation of farnesyl or geranyl-geranyl groups is a conditio sine qua non for proper membrane anchoring and biological activity of the majority of Ras superfamily members. Instead, the endoproteolytic and methylation steps are only essential for the subcellular localization and biological responses of farnesylated GTPases.(13)
The final destination of the post-translationally-modified GTPases depends on the computation by cells of other ancillary signals present in the GTPase C terminus (Fig. 2). In the case of palmitoylated GTPases, one of these additional signals is the nature of the isoprenyl group attached to the CAAX box. Perhaps the best example for this type of regulation is RhoB, since this protein is localized preferentially in endomembranes when geranyl-geranylated and at the plasma membrane when farnesylated.(14) In other cases, the signal mediating proper membrane localization is a polybasic amino acic sequence located just upstream of the CAAX box. This is the case of Rac subfamily proteins, where small differences in those regions are responsible for the differential localization of Rac1, Rac2 and RhoG in lipid rafts, endosomes, and caveolar vesicles, respectively.(15,16) Finally, in the case of Rac1, a proline-rich domain located near the CAAX box contributes to the translocation of this GTPase to focal adhesion complexes via its interaction with SH3 domain proteins such as β-Pix, a Rac1-specific GEF that is constitutively located in those subcellular regions.(17)
In addition to the presence of the above structural cues, Rho/Rac proteins need additional upstream signals in order to move from the cytosol to target membranes and, subsequently, to remain stably anchored in those structures. RhoGDIs play important roles in this regulatory context, because they hide the isoprenyl groups of the GTPases, an action that favors the sequestration of the inactive GTPases in the cytosol or organelles (Fig. 2). This property is also important for the removal of the GTPase from the plasma membrane at the end of the signaling process (Fig. 2). Due to the interaction of RhoGDIs with the GTPase switch regions, they also impede the release of GDP from the GTPase and, consequently, contribute to the maintenance of the GTPases in an inactive state in non-stimulated cells (Fig. 2).(18)
The dissociation of the RhoGDI from the GTPase, an essential requisite for the activation of GTPases by GEFs and for their subsequent association with membranes, is regulated at different levels during signal transduction. These regulatory steps have been mapped out extensively in the case of Rac1. Thus, it has been shown that integrins play an important role in this process, because they increase the affinity of Rac for lipid rafts, a process that in turn favors the displacement of the geranyl-geranyl motif of the GTPase from the hydrophobic pocket of the RhoGDI and its insertion into the phospholipid bilayer of the target membrane (Fig. 2).(19,20) Other factors cooperating in this dissociation step include RhoGDIs displacement factors (i.e. the cytoplasmic tail of the low-affinity nerve growth factor receptor)(21) and the decrease of the RhoGDI affinity towards Rac1 upon phosphorylation of RhoGDI molecules by protein kinase C(22) and Pak1,(23) a Rac1 downstream element(24) (Fig. 2). Some Rho/Rac GTPases also require the cooperation of additional pathways to remain anchored to membranes once they have been liberated from the RhoGDIs. In the case of Rac1, its residence at the plasma membrane requires in some instances integrin-dependent signals that block the co-internalization of the GTPase with lipid rafts(25) (Fig. 2). Taken together, these observations indicate that the localization of Rho/Rac GTPases is tightly modulated in time and space by a complex system of cell type-dependent regulatory pathways.
Transcriptional regulation and/or differential degradation
Many Rho/Rac GTPases show cell-type-specific and/or stimulus-dependent expression. For instance, Rac2 is mostly restricted to hematopoietic cells.(26) Rac3 is preferentially expressed in neurons of ganglia and the central nervous system.(27) Moreover, RhoG and RhoB proteins have been shown to have fluctuations during the cell cycle.(28,29) RhoB expression undergoes further regulation by extracellular stimuli such as UV irradiation, growth factors, cytokines and oncogenes,(30–34) a control that is facilitated by the relatively instability of its mRNA (t1/2=20 min).(32) Finally, rhoU (also known as wrch 1) is a Wnt-regulated gene.(35) Some Rho/Rac proteins are also controlled through degradation at specific sites in the cell. Thus, RhoA can be degraded by the ubiquitin ligase Smurf1 in a Rac1- and Cdc42-dependent manner (Fig. 2). This regulation contributes to inhibit the inappropriate formation of stress fibers in certain areas of the leading edge of the cell during the process of cell migration.(36) Partial proteolytic cleavage also plays regulatory roles on Rho/Rac proteins. This is the case for Cdc42, whose proteolytic degradation by caspases following the activation of the Fas death receptor contributes to the activation of Fas-dependent apoptotic events(37)(Fig. 2).
Other regulatory events
Rho/Rac GTPases can be also regulated by additional signaling mechanisms. Thus, RhoU has a putative autoinhi-bitory domain at its N-terminus that can be released by the binding of Grb2, an SH3–SH2 adaptor protein. This interaction is mediated by the recognition of an N-terminal proline-rich region of RhoU by one of the Grb2 SH3 domains. This interaction does not alter GDP/GTP exchange in RhoU but, instead, promotes a more-efficient binding of this GTPase to the downstream serine/threonine kinase Pak1.(38) Rho/Rac proteins can also undergo phosphorylation in specific residues, a post-translational event that may influence their interaction with RhoGDIs,(39) stability in the membrane(39,40) and effector functions(40) (Fig. 2).
The understanding of all these regulatory steps has allowed the development, for the first time, of drugs that can control the signaling output of Rho family GTPases in specific pathological states by interfering with their GDP/GTP exchange,(41) post-translational modification,(8) and sub-cellular localization.(42) Given the important contribution of Rho/Rac GTPases to the progression of some human diseases, it is likely that these current efforts will eventually crystallize into new therapeutic agents.
Effector molecules of Rho/Rac proteins
Once activated and translocated to their specific subcellular locations, Rho/Rac proteins interact with downstream effector molecules to engage specific signaling cascades.(5,24) To date, more than 70 potential effectors have been identified for members of the Rho/Rac family (Table 1). From a structural point of view, it is known that these effectors use distinct residues within the switch I and switch II regions as the major docking/recognition sites.(5,24) This structural property has made it possible to generate GTPase point mutants that can bind only to a subset of effectors and engage only a limited number of downstream effects (Fig. 3A). In some instances, the stable association of effectors requires the participation of additional structural cues located in the polybasic C-terminal region, the b2 sheet and/or the helices α3, α3´ and α5 of the upstream GTPases(24) (Fig. 3A,B). In other cases, it requires the localization of the upstream GTPase in specific sites of the cell. For instance, the functional specificity found for Rac1 and Rac2 in neutrophils is mainly due to their differential subcellular localization within these hematopoietic cells.15)
Table 1.
A list of selected effector proteins for RhoA, Rac1 and Cdc42
| Effectora | Type of Proteinb | Upstream GTPasec | Main biological Action |
|---|---|---|---|
| Cnksr1 | Scaffold protein (Ksr) | RhoA | Interacts with RhoA (Rhophilin) and Ras effectors (RalGDS) |
| Rtkn1,2 | Scaffold protein | RhoA | Interaction with PDZ proteins, NFкB activation |
| Rhpn1,2 | Scaffold, PDZ containing protein | RhoA | Cytoskeletal regulation? |
| Ktn1 | Scaffold protein | RhoA, Rac1, Cdc42 | Kinesin binding, vesicular trafficking through microtubules |
| Diaph1,2 | Scaffold protein (Dia1,2) | RhoA, Rac1 | Cytoskeletal change via profilin and Baiap |
| Arfip2 | Scaffold protein (Por1) | Rac1 | Cytoskeletal regulation |
| Pard6 A,G | Scaffold protein (Par6α,γ) | Rac1, Cdc42 | Cell polarity. Links GTPases and atipical PKCs |
| Baiap2 | Scaffold protein (p53IRS) | Rac1, Cdc42 | Cytoskeletal organization via regulation of Wasf/Wave proteins |
| IQGAP1,2 | RhoGAP and scaffold protein | Rac1, Cdc42 | Regulator of the cytoskeleton, cell-cell contacts, and proliferation |
| Was | Scaffold protein (Wasp) | Rac1 | Cytoskeletal regulation via the Arp2/3 complex |
| Nck1 | Scaffold protein with SH2/SH3 domains | Rac1 | Complex formation with Wasp. Signal transduction |
| Nckap1 | Scaffold protein (Nap125, Nap1) | Rac1 | Regulation of the cytoskeleton via Wasf proteins |
| Cyfip2 | Scaffold protein (Pir121) | Rac1 | Regulation of the cytoskeleton via Wasf proteins |
| Cdc42SE1,2 | Scaffold protein (Spec1,2) | Rac1, Cdc42 | Modulation of GTPase signaling outputs |
| IL1Rap1 | Scaffold protein | Rac1 | Interleukin signalling |
| Hspc121 | Scaffold protein | Rac1, RhoA, Cdc42 | Regulation of kinase cascades and gene expression |
| WasL | Scaffold protein (N-Wasp) | Cdc42 | Cytoskeletal regulation via de Arp2/3 complex |
| Trip10 | Scaffold protein | Cdc42 | Binding of Was to microtubules |
| Cdc42EP1,3,5 | Scaffold protein (Borgs1,3,5) | Cdc42 | Regulation of septins |
| Mig-6 | Scaffold protein | Cdc42 | Activation of the Jnk route |
| Wasf1,2 | Scaffold protein (Wave1,2; Scar1,2) | Cdc42, Rac1 | Cytoskeletal regulation via the Arp2/3 complex |
| CopG2 | Coatomer protein (γ2-Cop) | Cdc42 | Vesicle trafficking (clathrin route) |
| Itpr1 | Inositol 1,4,5-triphosphate receptor | RhoA | Calcium entry in endothelial cells |
| PlcG1 | Phospholipase, C type (PLC- γ1) | RhoA | Production of second messengers |
| DgkQ | Diacylglycerol kinase θ | RhoA | Diacylglycerol depletion |
| PI-5-p5K | Lipid kinase | RhoA | Modulation of phosphatidylinositol biphosphate levels |
| SynJ2 | Polyphosphoinositide phosphatase | Rac1 | Inhibition of receptor endocytosis via the clathrin route |
| PIK3R1 | Regulatory p85 subunit of PIK3C | Rac1, Cdc42 | Regulation of PIK3C activity, signal transduction |
| Pld1 | Phospholipase, D type | RhoA, Rac, Cdc42 | Production of phosphatidic acid and choline (second messengers) |
| PlcB2 | Phospholipase, C type (PLC- β2) | Cdc42, Rac1 | Production of second messengers |
| Cit | Serine/threonine kinase (Citron) | RhoA | Citokinesis |
| Pkn1,2 | Serine/threonine kinase (Prk) | RhoA | Vesicle recycling, cell cycle regulation, Pld1 activation |
| Rock1,2 | Serine/threonine kinase (Rok α,β) | RhoA | Cytoskeleton, cytokinesis, blockage of cell contact inhibition |
| Pak1-7 | Serine/threonine kinases | Rac1, Cdc42 | Cytoskeletal organization, activation of kinase cascades |
| Map3K11 | Serine/threonine kinase (Mlk3) | Rac1, Cdc42 | Activation of kinase cascades |
| PrkcA | Serine/threonine kinase (PKCα) | RhoA, Rac1, Cdc42 | Signal transduction |
| Cdc42bpgA,B | Serine/threonine kinase (MRCKα,β) | Rac1, Cdc42 | Cytoskeletal regulation |
| Rps6kB1 | Serine/threonine kinase (p70S6K, S6K1) | Cdc42 | Regulation of translation, cell cycle |
| Map3K10 | Serine/threonine kinase (Mlk2) | Cdc42 | Activation of kinase cascades |
| Map3K4 | Serine/threonine kinase (Mekk4) | Cdc42 | Activation of the Jnk route |
| Tnk2 | Tyrosine kinase (Ack) | Cdc42 | Signal transduction, activation of GEFs |
| Ncf1,2 | NADPH oxidase complex subunit | Rac1, Cdc42 | Superoxide production |
| CybA | NADPH oxidase complex subunit | Rac1 | Superoxide production |
| Ppp1r12A | Regulatory subunit of phosphatase1 | RhoA | Myosin light chain inactivation, cytoskeletal regulation |
| Fml1 | Formin-like molecule | Rac1 | Cytoskeletal organization, cell polarity and cytokinesis |
| Fhod1 | Formin- like | Rac1 | Cytoskeletal and transcriptional regulation |
| Cyfip1 | Actin binding protein | Rac1 | Cytoskeletal organization |
| FlnA | Actin binding protein | RhoA, Rac1, Cdc42 | Cytoskeletal regulation, actin filament crosslinking |
| Tbc1d3 | Unknown, Tbc oncoprotein family | Cdc42 | Cytoskeletal regulation |
| TubA1 | Tubulin | Rac1 | Integral component of microtubules |
| KcnA2 | Potasium Channel subunit | RhoA | Potasium entry |
| Stat3 | Transcriptional factor | Rac1, Cdc42 | Transcription |
| Nos2A | Nitric oxide synthase | Rac1 | Nitric oxide production |
| Smurf2 | E3 ubiquitin protein ligase 2 | Rac1 | Smad and RhoA ubiquitination, TGFβreceptor signalling |
Figure 3.
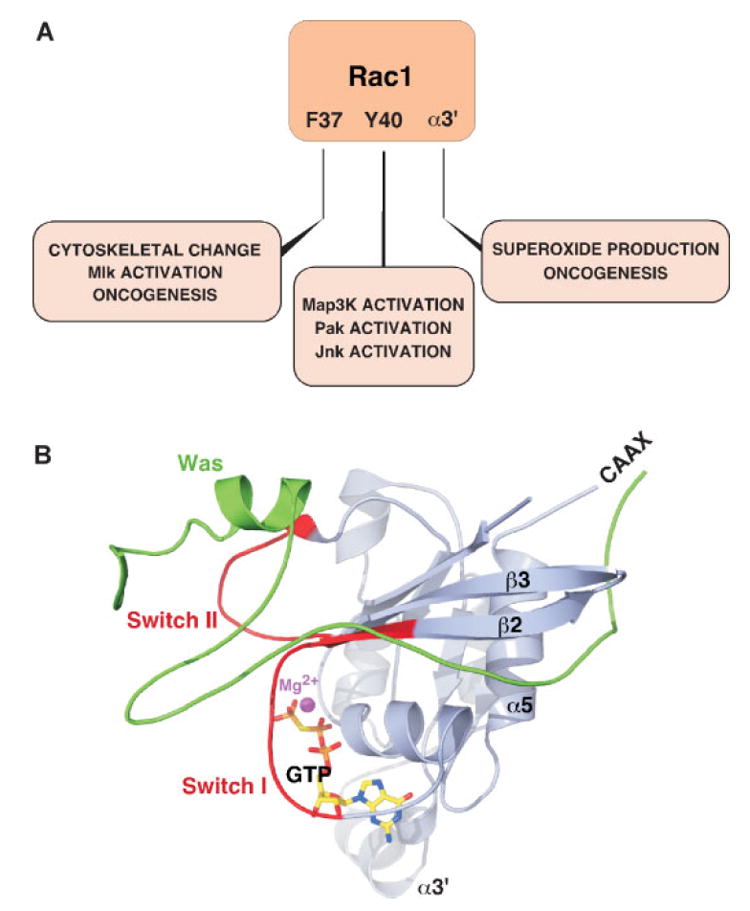
Structural determinants for the interaction of Rho/Rac proteins with downstream effectors. A: Scheme showing the residues of the switch (F37 and Y40) and α3′ regions involved in the selective interaction of GTP-bound Rac1 with effectors. Similar data have been obtained with other GTPases, including RhoA, RhoG and Cdc42. B: Crystal structure of GTP-bound Cdc42 associated to the Cdc42-binding region of Was. The reader can observe the extensive contacts established by Was with the switch I, switch II, β2 sheet and α5 helix of the GTPase.
Despite the large structural diversity of Rho/Rac effectors, we have learned a number of common regulatory themes that take place during the activation of the downstream effectors by Rho/Rac GTPases. Thus, the tethering of effector molecules to membranes is part of the mechanism by which they become activated (Fig. 4). Indeed, translocation of Pak, Pkn, citron, Rock and other effector proteins to signaling hot spots of cells has been shown recently.(43–47) Moreover, it has been shown that the activation of Pak1 only occurs when active Rac1 is at the plasma membrane but not when free in the cytosol.(19)Other results indicate that the interaction of effectors with Rho/ Rac GTPases provokes conformational changes that shift them from autoinhibitory conformations to fully active structures (Fig. 4). Such regulatory mechanism has been observed for a wide collection of both catalytic (i.e. Pak, Rock, Pkn) and non-catalytic, adaptor-like (i.e. Diaphanous, Was, and Baiap2) effectors.(24) These changes could be self-sufficient for activation or, alternatively, may cooperate with other signals to promote optimal effector activation (Fig. 4A). For example, Pkn needs RhoA binding, lipid association and autophosphorylation events to became fully active.(24,48) Some downstream signaling elements are also activated by the release of _trans_-inhibitory factors upon the binding of the GTPase (Fig. 4B). This is at least the case of Wasf/Wave/Scar proteins, which get released from an inhibitory complex formed with Nckap1–Nap125, Cyfip2–Pir121 and C3orf10–Hspc300 upon the binding of GTP-bound Rac1 to Nckap1 and Cyfip2.(49) It should be noted, however, that the binding of the activated GTPase results in the inhibition, not the stimulation, of the bound effector (Fig. 4C). This is the case, for instance, of the interaction of Cdc42 with Cdc42Eps (also referred to as Borgs).(50)
Figure 4.
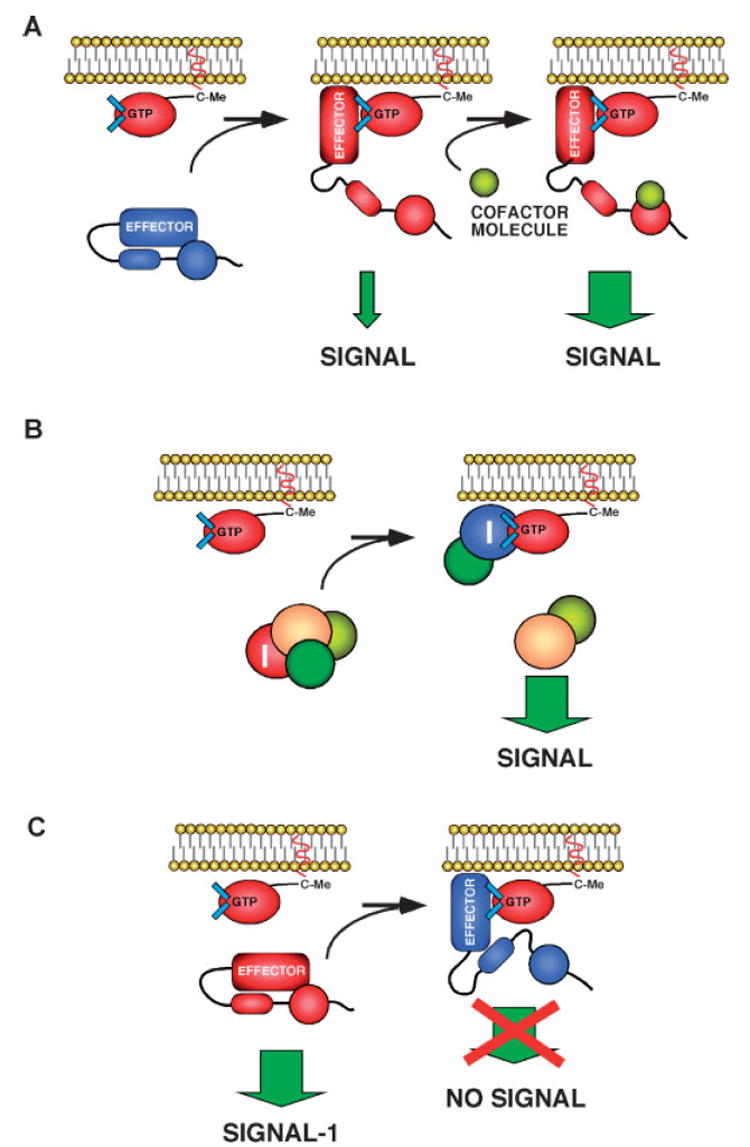
Examples of the types of signaling outputs derived from the interaction of Rho/Rac subfamily proteins and effectors. See main text for further details. I, inhibitor.
The final result of the modulation of the activity of these effectors is the generation of multibranched signals that promote, among other responses, cytoskeletal change, vesicle trafficking and cell cycle entry (Table 1). All these pathways have been extensively reviewed before and will not be re-mentioned here.(2,5) However, it should be noted that the activation of effectors might fire back on the GTPases themselves, thus contributing to the generation of balanced and time-restricted signals by Rho/Rac proteins. Pak family proteins are very active in this regulatory context, since they can modify the activity of both RhoGDIs and Rho/Rac GEFs(23,51) (Fig. 2). These results underscore the high level of plasticity and large number of feed-back loops taking place in the signal transduction pathways of these GTPases.
As in the case of the regulatory elements that mediate Rho/ Rac activation, the understanding of the mode of action of the downstream molecules has allowed the development of inhibitory molecules for Pak and Rock family proteins.(52–54) One of the Rock inhibitors, fasudil (also known as HA-1077 and AT877), is already being used for the treatment of patients with cardiovascular disorders.(55)
Genetic analysis of Rho/Rac GTPase functions in vivo
Most of the functional observations obtained with Rho/Rac proteins have been derived from cultured cells. While this approach has allowed the discovery of important aspects of their regulation and function, it has obvious limitations. For instance, it is rather difficult to verify whether the observations obtained using in vitro conditions can be extrapolated to more complex situations in which primary, non-immortalized cells are exposed to limited amounts of stimuli or to different tissue-and cell-type crosstalk. These approaches do not provide faithful information regarding the level of functional overlaps among closely related GTPases, either to verify the role of specific GTPases in the tissues where they are actually expressed, or to study their participation in complex physiological responses. To surmount some of these problems, different research groups have generated animal models in which specific members of the Rho/Rac subfamily and effectors have been disrupted using homologous recombination techniques. Below, we summarize the most-recent results achieved in this area. As a note of caution, please be adviced that the strict comparison of the phenotypes displayed by different genetically modified mouse strains cannot be interpreted in absolute terms, because the lack of effect of a null gene may not necessarily indicate less-important roles of its encoded protein but, rather, functional complementation events by related proteins or parallel signal transduction pathways. The reader can find additional information about phenotypes of transgenic mice expressing Rho/Rac proteins and knockout animals lacking genes for Rho/Rac GEFs in recent review articles.(6,56,57)
Genetic analysis of members of the Rac subgroup
The inactivation of the rac1, rac2, rac3 and rhoG loci has been already achieved in mice using standard homologous recombination techniques or, in some cases, via the use of either tissue-specific or inducible knockout strategies. In addition, some Rac1 effectors belonging to the Pak and Wasf families have also been knocked out to address their role in vivo.
The deletion of the _rac1_gene provokes embryonic lethality caused by both gastrulation defects and apoptosis of mesodermal cells.(58) In contrast, the inactivation of the other Rac subfamily members gives rise to more limited defects, which are usually found in the tissues where these GTPases are preferentially expressed. Thus, _rac2_–/– mice show hematopoietic defects (see below), _rac3_–/– animals present slight motor coordination problems and enhanced learning abilities,(59) and _rhoG_–/– mice have some hyperactivation of T-cell responses to antigens and minor defects in the super-oxide pathway of neutrophils,(60,61) the cells responsible for inflammatory responses.
The generation of inducible, cell-type-specific knockouts for the _rac1_gene and their side-by-side comparison with both _rac2_–/– and double _rac1_–/–; _rac2_–/– mutant animals has permited a comprehensive understanding of the physiological roles of these two GTPases and their level of signaling overlap/specificity (Fig. 5). In the case of HSCs, the role of Rac1 and Rac2 has been addressed using reconstitution experiments in sublethaly irradiated immunocompromised mice and inducible approaches of gene inactivation. These studies revealed important functional differences between these two family members. Rac1 has been shown to be important for the optimal reconstitution of the hematopoietic system, having roles both in the engraftment and retention of HSCs in the bone marrow. Instead, _rac2_–/– HSCs show normal behaviour in all these reponses. Rac1 and Rac2 also differ in the type of intracellular responses that they regulate in HSCs. Thus, Rac1 is essential for the entry of HSCs into the cell cycle upon extracellular stimulation as well as for their progression through S and G2/M phases whereas Rac2 is important for cytoskeletal responses, adhesion, spreading and Akt-dependent HSC survival(62,63) (Fig. 5).
Figure 5.
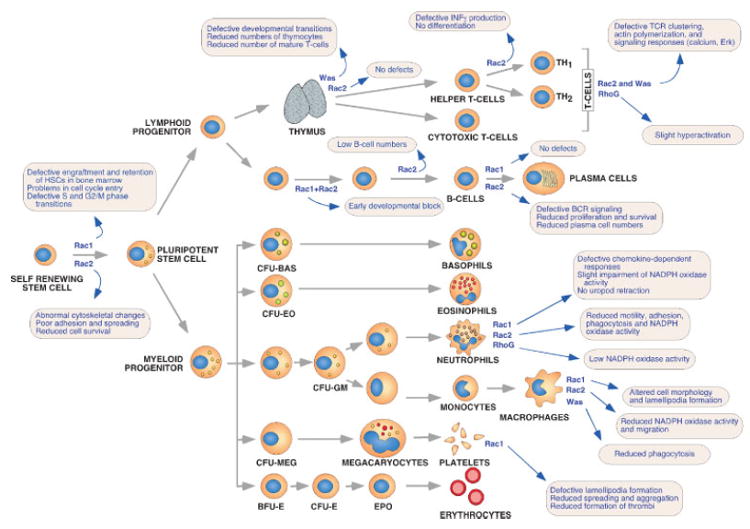
Representation of the main developmental routes for hematopoietic cells and the steps that are dependent on either Rho/Rac subfamily proteins or Rho/Rac effectors. The GTPases and/or effectors involved in those steps are highlighted in blue. The processes impaired by the gene inactivations in each hematopoietic lineage are summarized into light-brown boxes.
In the case of T-cells, _rac2_–/– mice show no apparent problems in the differentiation of those cells in the thymus. In contrast, mature _rac2_–/– T-cells display defects in T-cell receptor clustering, actin polymerization, generation of Ca2+ fluxes and Erk activation upon engagement of the T-cell receptor(64) (Fig. 5). These defects are rather marginal, probably due to the compensation exerted by the endogenous Rac1 protein present at high levels in those cells. Rac2 is also important for the differentiation of helper T-cells to the TH1 subtype because it regulates the p38- and NFkB-dependent induction of interferon-γ, an important mediator of this maturation step(65) (Fig. 5). The effects of the Rac1 deficiency in T-cells have not been addressed as yet. However, as inferred from the results obtained by Rac1 GEFs,(57) we can predict that this GTPase will have roles in T-cell differentiation, positive and negative selection, stimulation of phosphatidylinositol-3 kinase/Akt and the Ras/Erk routes, and overall responses to antigens.
In the case of B-cells, _rac2_-deficient animals show defects in the B-cell compartment, displaying reduced numbers of peripheral B-cells, peritoneal B1 cells and IgM-secreting plasma cells. Mature _rac2_–/– B-cells respond poorly to stimulation of the B-cell receptor, showing reduced levels of Ca2+ fluxes and of cell proliferation.(66) Rac1 seems to have only a marginal and overlapping role with Rac2 in these cells.(67) In agreement to this, the simultaneous elimination of rac1 and rac2 genes induces an aggravation of the _rac2_–/– phenotype, leading to a developmental block of B-cell development at very immature stages.(67) This is caused by low survival rates derived from the improper activation of the Akt route and the inefficient expression of two anti-apoptotic molecules, Bcl2L1 (most commonly known as Bcl-xL) and the BAFF receptor.(67) Instead, the single rac1 gene knockout has no detectable effects per se in this lymphoid lineage(67) (Fig. 5).
Unlike the case of B-cells, Rac1 and Rac2 exert non-overlapping functions in the neutrophil lineage. rac2 null neurophils show a severe impairment of motility, adhesion, chemotaxis and phagocytosis as well as a drastic reduction (≈60%) in the activity of the NADPH oxidase, the enzyme complex responsible for the generation of anti-bacterial superoxide molecules.(68,69)Recent results have shown that the residual level of NADPH oxidase activity found in these animals is due to the action of RhoG and, to a minor extent, of Rac1(61,70) (Fig. 5). _rac1_–/– neutrophils have milder problems, with defects detectable only in chemokine-dependent responses.(70,71) Unlike _rac2_–/– neutrophils, these cells do not show significant problems in the cytoskeleton in the absence of chemokines with the exception of defects in the RhoA-dependent retraction of the uropod during stochastic migration(70,72) (Fig. 5).
The role of rac genes in macrophages has only begun to be elucidated. Available reports indicate that Rac2 is important for superoxide production and phagocytosis to some (i.e. Fc γR stimulation, IgG-sensitized sheep red blood cells) but not all (i.e. serum-opsonized zymosan) stimuli.(73) In addition, it is important for the migration of these cells, as evidenced by the lack of the accumulation of exudate macrophages during the peritoneal inflammation of _rac2_–/– mice(73) (Fig. 5). Contrary to Rac2, Rac1 seems to be important for regulating macrophage cell morphology and proper lamellipodia formation(74) (Fig. 5). However, these defects do not induce any significant defect on the migration and chemotactic responses of this cell type.(74)
In agreement with the high levels of expression in platelets, Rac1 seems to be the major player of the Rac subfamily in this cell type. Its functions include the generation of lamellipodia upon the stimulation of platelets with ADP, the induction of proper spreading and aggregation of platelets, and the formation of thrombi in vivo (Fig. 5). These defects are not very severe, because Rac1-deficient animals do not experience hemorrhages.(75)
More recently, other tissue-specific rac1 gene knockouts have begun to shed light on its function in non-hematopoietic tissues. Thus, it has been shown that Rac1 is important for the formation of myelin sheaths in the central nervous system.(76) In the case of the skin, Rac1 has been shown to be important for the integrity of hair follicles and, as consequence, mice with a keratinocyte-specific inactivation of the rac1 locus develop a hairless phenotype.(77) Finally, it has been shown that Rac1 and Rac2 proteins play important roles in the dendritic cells that present antigens to T-cells.(78) Due to this, dendritic cells lacking expression of both Rac1 and Rac2 show defective cytoskeletal change, migration and antigen presentation that, as a result, preclude adequate cell contacts with T-cells. The development of this defect requires the simultaneous deletion of both rac1 and rac2 genes, indicating that these two proteins exert similar and additive roles in dendritic cells.(78)
Consistent with the important role of Pak and Wasf family proteins in Rac1 signaling, the deletion of some of those cytoskeletal regulators has dire consequences during embryonic development. Thus, the elimination of the pak4 gene leads to embryonic lethality due to heart development problems. This protein is also important for the migration, differentiation and axogenesis of spinal cord neurons (both motorneurons and interneurons) and for the proper folding of the caudal region of the neural tube.(79) The disruption of the wasf2 gene also leads to embryonic lethality at later stages (E12.5). These embryos show growth retardation, brain ventricle malformations and vascularization deficiencies when compared to wild-type embryos.(80,81) As expected from the previous functional characterization of Wasf proteins, the analysis of _wasf2_–/– mouse embryonic fibroblasts (MEFs) indicates that this cytoskeletal regulator is important for the generation of lamellipodia, Rac1-dependent actin polymeriza-tion, and cell migration events.(81)Despite these examples, the disruption of other Rac effectors in mice induces milder phenotypes. Thus, _wasf1_–/– adult mice develop normaly but have reduced size and experience anxiety, sensorimotor retardation and deficits in hippocampal-dependent learning and memory.(82)_pak5_–/– mice are fully viable and display no obvious abnormalities.(83)
Genetic analysis of members of the Rho subgroup
The phenotypes of mice lacking functional rhoB and rhoC genes have been recently described. These two mouse strains are fully viable and fertile. When MEFs from these animals were studied in vitro, it was found that RhoB is important for proper cell motility but not for adhesion or spreading.(84) However, these latter functions became diminished when MEFs were transformed by both E1A and ras oncogenes, suggesting that RhoB function is probably required for oncogenic-dependent cytoskeletal responses.(84)_rhoC_–/– MEFs show only cytoskeletal defects under serum-starved conditions.(84) In contrast to these apparently mild phenotypes, it has been observed that rhoB and rhoC have important, although antagonistic, roles in tumor progression. RhoB-deficient animals are more susceptible to developing tumors when tested in skin carcinogenesis assays, indicating that this GTPase may have tumor-suppressor properties, at least in the case of skin.(84) Using crosses with transgenic mice expressing the oncogenic polyomavirus middle T-antigen, it was observed that the absence of RhoC is not important for tumor development.(85) Despite this, _rhoC_–/–tumor cells are less metastatic than the wild-type counterparts, a phenotype attributed to the reduced migration, lower invasiveness and poor survival of RhoC-deficient cells.(85) Despite these advances, more work will be required to assess the relative contributions of RhoB and RhoC to the life and pathogenesis of animals. An important step in that direction will be the side-by-side comparison of these two strains using identical genetic backgrounds and conditions. In addition, it will be interesting to generate the double RhoB/RhoC knockout to corroborate that their functions are not overlapping in vivo.
Although the rhoA locus has not been targeted as yet, several of the main RhoA effectors have been inactivated by homologous recombination. These studies have revealed that Rock1 and Rock2 are important for eyelid closure and fusion of the ventral body wall, because the disruption of any of those two genes give rise to neonates with omphalocele and open eyes.(86,87) In agreement with the described routes modulated by Rocks, keratinocytes derived from these tissues show defective stress fiber formation and low myosin light chain phosphorylation upon EGF stimulation.(87) This mild phenotype is highly dependent of the genetic background, because the inactivation of the rock2 gene in another mouse strain leads to placental defects and embryonic death.(88) _cit_–/– animals also develop normally but they succumb to lethal epileptic seizures during the first postnatal month.(89) This is due to a marked reduction in the number of GABAergic interneurons and of both dentate gyrus and cerebellar neurons, a phenotype caused by cytokinesis defects in neuroblast subsets.(89) More recently, it has been shown that cit–/– also have defects in both the survival and cytokinesis of spermatogenic precursors, leading to a severe impairment of testicular function.(90) The knockout of limk2, a locus encoding a serine/threonine kinase that is activated by Rock,(5,24) also induces defects in spermatogenesis, although they develop normally and show no major disturbances in the adult period.(91) Finally, _rhpn2_–/– animals show no detectable defects.(92) The relatively mild phenotype of Rock-, Cit- and Limk2-deficient animals is somewhat surprising, given the crucial role attributed to these three kinases in general cytoskeletal and cytokinesis events. At least in the case of Limk2, this mild phenotype cannot be attributed to compensation effects by other Rho/Rac effectors, because Limk2-deficient cells show a total impairment in the phosphorylation of the main substrate of this kinase family, the cytoskeletal regulator cofilin.(91) An intriguing possibility derived from these results is that, at least during embryonic development, the migration and adhesion of cells may follow different pathways to those described in immortalized cultured cells.
Genetic analysis of members of the Cdc42 subgroup
The knockout of the cdc42 locus leads to embryonic lethality prior to the E6.5 stage.(93) The isolation of embryonic stem cells from E3.5 _cdc42_–/– blastocysts has allowed a glimpse of the functional relevance of Cdc42 inside cells. Under these conditions, it has been shown that Cdc42 is essential for the phosphatidylinositol bisphosphate-mediated polymeriza-tion of actin and, due to this, its deletion induces a highly disorganized cytoskeleton, round-up morphologies, and smaller cell sizes.(94) In contrast to these results, the cell-specific inactivation of the cdc42 locus in fibroblasts does not induce any impairment on cytoskeletal structures or cell migration.(94) It has been argued that this result is due to functional redundancy with other Rho/Rac proteins, because the expression of a dominant negative mutant of Cdc42 in the _cdc42_–/– fibroblasts significantly impairs most of those biological processes.(95)_cdc42_–/– cells do show defects in polarity, including minor disturbances in establishment of the proper directionality and relocation of the Golgi apparatus in migrating fibroblasts.(95) These results seem to be however highly dependent on the fibroblast type, because a more recent study has shown that primary fibroblasts do show problems in filopodia formation, migration and proliferation in the absence of Cdc42 expression.(94) More recently, the specific inactivation of the cdc42 gene in oligodendrocytes and neuronal precursors has revealed a role for Cdc42 in the central nervous system.(76) In the case of oligodendrocytes, Cdc42 is important for the correct formation of myelin sheaths.(76) In the case of neuronal precursors, Cdc42 plays crucial roles in the establishment of Par6-dependent apico-basal polarity processes of stem cells.(96) In contrast, it does not seem important for the adhesion, cell-cycle regulation or cytokinesis of this stem cell population.(96)
Several Cdc42 effectors have been also targeted by homologous recombination. Was-deficient mice show reduced numbers of thymocytes, mature lymphocytes and platelets. The reduced production of thymocytes is due to impaired progression from the CD44–/CD25+ to the CD44–/ CD25– stage of differentiation. Was–/– thymocytes and mature T cells show impaired T-cell receptor capping and endocytosis, generation of Ca2+ fluxes and actin polymeriza-tion. As a consequence, they proliferate poorly upon engagement of the T-cell receptor(97) (Fig. 5). Probably due to all these immunological disturbances, was–/– mice develop colitis as they age.(97) These animals have also neurophils with reduced phagocytic activity and osteoclasts with severe cytoskeletal defects that generate abnormal patterns of bone resorption.(98,99) Iqgap1–/– animals show no detectable phenotypic defects with the exception of the development of gastric hyperplasia,(100) a result that suggests that this Cdc42 effector may exert inhibitory properties for the proliferation of intestinal epithelial cells.
Taken together, these studies confirm the important role of specific Rho/Rac family members in the biological pathways related to cytoskeletal dynamics, polarity, cell survival/apoptosis, cell proliferation, immune system responses and oncogenesis. In addition, they show that despite the high structural homology, these proteins exert related, but not identical, functions in vivo at least in certain cell types.
Conclusion
Since the isolation of the first Rho/Rac family more than 20 years ago, substantial information has been gained regarding the number of family members, the type of effectors they engage, the main regulatory layers controling their activities and the biological processes that they are implicated on. Despite these advances, more information remains to be gathered in the near future. For instance, we have to delineate the dynamics and kinetics of engagement of the different interactive Rho/Rac-dependent networks during cell signaling. Likewise, we need to get additional information regarding the type of signaling networks engaged and signaling outputs generated in function of the type, concentration, and/or combination of the extracellular stimuli received by cells. Given the complex array of signaling molecules involved and, in some instances, the multifunctional nature of them, the execution of this aim will not be an easy task. Fortunately, the high-throughput techniques that are being developed in the cellomics field to monitor the behavior of molecules in real time will probably help tackling these issues. Likewise, proteomic and genomic techniques will be also useful for isolating all the signaling complexes and regulatory molecules involved in these pathways. Given that most of the studies done up to now have focused on few GTPases, more work remains be done to elucidate the functions of the less-studied family counterparts. In this context, the generation of new animal models will help assigning specific functional tasks to these GTPases and, in addition, provide information about the level of signaling overlap and/or cooperativity existing among them. Given the important roles that these GTPases play in different pathologies, it is likely that the progress in these areas of research will contribute to a better understanding and treatment of human disease.
Acknowledgments
The authors wish to apologize to the scientists not cited in this work due to space constrains. We also like to thank M. Dosil and P. Crespo for helpful comments on the manuscript.
Funding agencies: XRB’s work is supported by grants from the US National Cancer Institute (5R01-CA73735-10), the Spanish Ministry of Education and Science (SAF2003-00028), the Castilla-León Autonomous Government (SA053A05), and the Red Temática de Investigación Cooperativa en Cáncer (RD06/0020/0001, Spanish Ministry of Health). VS was supported by an EMBO long term postdoctoral fellowship. IMB was partially supported by a Spanish Ministry of Education and Science FPU fellowship (FP2000-6489). The Centro de Investigación del Cáncer is supported by endowments from the CSIC, University of Salamanca, Castilla-León Autonomous Government, the Red Temática de Investigacián Cooperativa de Centros de Cáncer (C03/10, Spanish Ministry of Health), and the Foundation for Cancer Research of Salamanca.
Abbreviations
Akt
v-akt murine thymoma viral oncogene homolog
BAFF
B-cell activating factor
Baiap
brain-specific angiogenesis inhibitor 1-associated protein
Bcl
B-cell chronic lymphocytic leukemia/lymphoma 2 (Bcl2) like
Bcl2L
Bcl2 like
Borg
binder of rho GTPases
Cdsc42Ep
Cdc42 effector protein
C3orf10
chromosome 3 open reading frame 10
Cyfip
cytoplasmic fragile X mental retardation 1 (FMR1) interacting protein
Erk
extracellular-regulated MAP kinase
GAP
GTPase activating protein
GEF
Guanosine nucleotide exchange factor
GTPase
GTP hydrolase
HSC
Hematopoietic stem cell
Hspc
Hematopoietic stem progenitor cell
Ictm
Isoprenylcysteine carboxyl methyltransferase
Ig
immunoglobulin
IQGAP
IQ motif containing GTPase activating protein
Limk
LIM (Lin-11, Isl-1 and Mec-3) domain kinase 2
NADPH
nicotinamide adenine dinucleotide phosphate
Nap
non-catalytic region of tyrosine kinase (Nck)-associated protein
Nckap
Nck-activated protein
Pak
p21-activated kinase
Pir
p53-inducible mRNA
Pix
Pak-interacting exchange factor
Pkn
protein kinase N
Rce
Ras and a factor converting enzyme
RhoGDI
Rho GDP dissociation inhibitor
Rhpn
Rhophilin
Rock
Rho-associated, coiled-coil containing protein kinase
Smurf
Smad ubiquitination regulatory factor
Was
Wiskott-Aldrich syndrome protein
Wasf
Was protein family
Wave
Was protein family verprolin-homologous protein
Wrch
Wint-1 responsive Cdc42 homolog
References
- 1.Madaule P, Axel R. A novel ras-related gene family. Cell. 1985;41:31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- 2.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 3.Boettner B, Van Aelst L. The role of Rho GTPases in disease development. Gene. 2002;286:155–174. doi: 10.1016/s0378-1119(02)00426-2. [DOI] [PubMed] [Google Scholar]
- 4.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 6.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHOGTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 7.Peck J, Douglas Gt, Wu CH, Burbelo PD. Human RhoGAP domain-containing proteins: structure, function and evolutionary relationships. FEBS Lett. 2002;528:27–34. doi: 10.1016/s0014-5793(02)03331-8. [DOI] [PubMed] [Google Scholar]
- 8.Winter-Vann AM, Casey PJ. Post-prenylation-processing enzymes as new targets in oncogenesis. Nat Rev Cancer. 2005;5:405–412. doi: 10.1038/nrc1612. [DOI] [PubMed] [Google Scholar]
- 9.Casey PJ, Seabra MC. Protein prenyltransferases. J Biol Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 10.Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, et al. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 11.Boyartchuk VL, Ashby MN, Rine J. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science. 1997;275:1796–1800. doi: 10.1126/science.275.5307.1796. [DOI] [PubMed] [Google Scholar]
- 12.Dai Q, Choy E, Chiu V, Romano J, Slivka SR, et al. Mammalian prenylcysteine carboxyl methyltransferase is in the endoplasmic reticulum. J Biol Chem. 1998;273:15030–15034. doi: 10.1074/jbc.273.24.15030. [DOI] [PubMed] [Google Scholar]
- 13.Michaelson D, Ali W, Chiu VK, Bergo M, Silletti J, et al. Postprenylation CAAX processing is required for proper localization of Ras but not Rho GTPases. Mol Biol Cell. 2005;16:1606–1616. doi: 10.1091/mbc.E04-11-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebowitz PF, Davide JP, Prendergast GC. Evidence that farnesyltransferase inhibitors suppress Ras transformation by interfering with Rho activity. Mol Cell Biol. 1995;15:6613–6622. doi: 10.1128/mcb.15.12.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filippi MD, Harris CE, Meller J, Gu Y, Zheng Y, et al. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat Immunol. 2004;5:744–751. doi: 10.1038/ni1081. [DOI] [PubMed] [Google Scholar]
- 16.Prieto-Sanchez RM, Bustelo XR. Structural basis for the signaling specificity of RhoG and Rac1 GTPases. J Biol Chem. 2003;278:37916–37925. doi: 10.1074/jbc.M301437200. [DOI] [PubMed] [Google Scholar]
- 17.ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol. 2006;172:759–769. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.del Pozo MA, Price LS, Alderson NB, Ren XD, Schwartz MA. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. Embo J. 2000;19:2008–2014. doi: 10.1093/emboj/19.9.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, et al. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol. 2002;4:232–239. doi: 10.1038/ncb759. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- 22.Price LS, Langeslag M, ten Klooster JP, Hordijk PL, Jalink K, et al. Calcium signaling regulates translocation and activation of Rac. J Biol Chem. 2003;278:39413–39421. doi: 10.1074/jbc.M302083200. [DOI] [PubMed] [Google Scholar]
- 23.DerMardirossian C, Schnelzer A, Bokoch GM. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell. 2004;15:117–127. doi: 10.1016/j.molcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 348Pt. 2000;2:241–255. [PMC free article] [PubMed] [Google Scholar]
- 25.del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, et al. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 26.Didsbury J, Weber RF, Bokoch GM, Evans T, Snyderman R. rac, a novel ras-related family of proteins that are botulinum toxin substrates. JBiol Chem. 1989;264:16378–16382. [PubMed] [Google Scholar]
- 27.Bolis A, Corbetta S, Cioce A, de Curtis I. Differential distribution of Rac1 and Rac3 GTPases in the developing mouse brain: implications for a role of Rac3 in Purkinje cell differentiation. Eur J Neurosci. 2003;18:2417–2424. doi: 10.1046/j.1460-9568.2003.02938.x. [DOI] [PubMed] [Google Scholar]
- 28.Zalcman G, Closson V, Linares-Cruz G, Lerebours F, Honore N, et al. Regulation of Ras-related RhoB protein expression during the cell cycle. Oncogene. 1995;10:1935–1945. [PubMed] [Google Scholar]
- 29.Vincent S, Jeanteur P, Fort P. Growth-regulated expression of rhoG, a new member of the ras homolog gene family. Mol Cell Biol. 1992;12:3138–3148. doi: 10.1128/mcb.12.7.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jahner D, Hunter T. The ras-related gene rhoB is an immediate-early gene inducible by v-Fps, epidermal growth factor, and platelet-derived growth factor in rat fibroblasts. Mol Cell Biol. 1991;11:3682–3690. doi: 10.1128/mcb.11.7.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fritz G, Kaina B. rhoB encoding a UV-inducible Ras-related small GTP-binding protein is regulated by GTPases of the Rho family and independent of JNK, ERK, and p38 MAP kinase. J Biol Chem. 1997;272:30637–30644. doi: 10.1074/jbc.272.49.30637. [DOI] [PubMed] [Google Scholar]
- 32.Fritz G, Kaina B, Aktories K. The ras-related small GTP-binding protein RhoB is immediate-early inducible by DNA damaging treatments. J Biol Chem. 1995;270:25172–25177. doi: 10.1074/jbc.270.42.25172. [DOI] [PubMed] [Google Scholar]
- 33.Fritz G, Kaina B. Transcriptional activation of the small GTPase gene rhoB by genotoxic stress is regulated via a CCAAT element. Nucleic Acids Res. 2001;29:792–798. doi: 10.1093/nar/29.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang K, Sun J, Cheng J, Djeu JY, Wei S, et al. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol Cell Biol. 2004;24:5565–5576. doi: 10.1128/MCB.24.12.5565-5576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao W, Pennica D, Xu L, Kalejta RF, Levine AJ. Wrch-1, a novel member of the Rho gene family that is regulated by Wnt-1. Genes Dev. 2001;15:1796–1807. doi: 10.1101/gad.894301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- 37.Tu S, Cerione RA. Cdc42 is a substrate for caspases and influences Fas-induced apoptosis. J Biol Chem. 2001;276:19656–19663. doi: 10.1074/jbc.M009838200. [DOI] [PubMed] [Google Scholar]
- 38.Shutes A, Berzat AC, Cox AD, Der CJ. Atypical mechanism of regulation of the Wrch-1 Rho family small GTPase. Curr Biol. 2004;14:2052–2056. doi: 10.1016/j.cub.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Forget MA, Desrosiers RR, Gingras D, Beliveau R. Phosphoryla-tion states of Cdc42 and RhoA regulate their interactions with Rho GDP dissociation inhibitor and their extraction from biological membranes. Biochem J. 2002;361:243–254. doi: 10.1042/0264-6021:3610243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, et al. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. Embo J. 1996;15:510–519. [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelish HE, Peterson JR, Salvarezza SB, Rodriguez-Boulan E, Chen JL, et al. Secramine inhibits Cdc42-dependent functions in cells and Cdc42 activation in vitro. Nat Chem Biol. 2006;2:39–46. doi: 10.1038/nchembio751. [DOI] [PubMed] [Google Scholar]
- 43.Phee H, Abraham RT, Weiss A. Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nat Immunol. 2005;6:608–617. doi: 10.1038/ni1199. [DOI] [PubMed] [Google Scholar]
- 44.Brown MC, West KA, Turner CE. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell. 2002;13:1550–1565. doi: 10.1091/mbc.02-02-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mellor H, Flynn P, Nobes CD, Hall A, Parker PJ. PRK1 is targeted to endosomes by the small GTPase, RhoB. J Biol Chem. 1998;273:4811–4814. doi: 10.1074/jbc.273.9.4811. [DOI] [PubMed] [Google Scholar]
- 47.Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, et al. Rho-kinase/ROCK is involved in cytokinesis through the phosphoryla-tion of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000;19:6059–6064. doi: 10.1038/sj.onc.1203987. [DOI] [PubMed] [Google Scholar]
- 48.Mukai H, Kitagawa M, Shibata H, Takanaga H, Mori K, et al. Activation of PKN, a novel 120-kDa protein kinase with leucine zipper-like sequences, by unsaturated fatty acids and by limited proteolysis. Biochem Biophys Res Commun. 1994;204:348–356. doi: 10.1006/bbrc.1994.2466. [DOI] [PubMed] [Google Scholar]
- 49.Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- 50.Joberty G, Perlungher RR, Sheffield PJ, Kinoshita M, Noda M, et al. Borg proteins control septin organization and are negatively regulated by Cdc42. Nat Cell Biol. 2001;3:861–866. doi: 10.1038/ncb1001-861. [DOI] [PubMed] [Google Scholar]
- 51.Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF- H1. JCell Sci. 2005;118:1861–1872. doi: 10.1242/jcs.02313. [DOI] [PubMed] [Google Scholar]
- 52.Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- 53.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 54.Nagumo H, Sasaki Y, Ono Y, Okamoto H, Seto M, et al. Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am J Physiol Cell Physiol. 2000;278:C57–C65. doi: 10.1152/ajpcell.2000.278.1.C57. [DOI] [PubMed] [Google Scholar]
- 55.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- 56.Bustelo XR. Understanding Rho/Rac biology in T-cells using animal models. Bioessays. 2002;24:602–612. doi: 10.1002/bies.10107. [DOI] [PubMed] [Google Scholar]
- 57.Turner M, Billadeau DD. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat Rev Immunol. 2002;2:476–486. doi: 10.1038/nri840. [DOI] [PubMed] [Google Scholar]
- 58.Sugihara K, Nakatsuji N, Nakamura K, Nakao K, Hashimoto R, et al. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene. 1998;17:3427–3433. doi: 10.1038/sj.onc.1202595. [DOI] [PubMed] [Google Scholar]
- 59.Corbetta S, Gualdoni S, Albertinazzi C, Paris S, Croci L, et al. Generation and characterization of Rac3 knockout mice. Mol Cell Biol. 2005;25:5763–5776. doi: 10.1128/MCB.25.13.5763-5776.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vigorito E, Bell S, Hebeis BJ, Reynolds H, McAdam S, et al. Immunological function in mice lacking the Rac-related GTPase RhoG. Mol Cell Biol. 2004;24:719–729. doi: 10.1128/MCB.24.2.719-729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Condliffe AM, Webb LM, Ferguson GJ, Davidson K, Turner M, et al. RhoG regulates the neutrophil NADPH oxidase. J Immunol. 2006;176:5314–5320. doi: 10.4049/jimmunol.176.9.5314. [DOI] [PubMed] [Google Scholar]
- 62.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine tripho-sphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 63.Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, et al. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 64.Yu H, Leitenberg D, Li B, Flavell RA. Deficiency of small GTPase Rac2 affects T cell activation. J Exp Med. 2001;194:915–926. doi: 10.1084/jem.194.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li B, Yu H, Zheng W, Voll R, Na S, et al. Role of the guanosine triphosphatase Rac2 in T helper 1 cell differentiation. Science. 2000;288:2219–2222. doi: 10.1126/science.288.5474.2219. [DOI] [PubMed] [Google Scholar]
- 66.Croker BA, Tarlinton DM, Cluse LA, Tuxen AJ, Light A, et al. The Rac2 guanosine triphosphatase regulates B lymphocyte antigen receptor responses and chemotaxis and is required for establishment of B-1a and marginal zone B lymphocytes. J Immunol. 2002;168:3376–3386. doi: 10.4049/jimmunol.168.7.3376. [DOI] [PubMed] [Google Scholar]
- 67.Walmsley MJ, Ooi SK, Reynolds LF, Smith SH, Ruf S, et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 68.Kim C, Dinauer MC. Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J Immunol. 2001;166:1223–1232. doi: 10.4049/jimmunol.166.2.1223. [DOI] [PubMed] [Google Scholar]
- 69.Li S, Yamauchi A, Marchal CC, Molitoris JK, Quilliam LA, et al. Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J Immunol. 2002;169:5043–5051. doi: 10.4049/jimmunol.169.9.5043. [DOI] [PubMed] [Google Scholar]
- 70.Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, et al. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol. 2003;170:5652–5657. doi: 10.4049/jimmunol.170.11.5652. [DOI] [PubMed] [Google Scholar]
- 71.Sun CX, Downey GP, Zhu F, Koh AL, Thang H, et al. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood. 2004;104:3758–3765. doi: 10.1182/blood-2004-03-0781. [DOI] [PubMed] [Google Scholar]
- 72.Pestonjamasp KN, Forster C, Sun C, Gardiner EM, Bohl B, et al. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814–2820. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamauchi A, Kim C, Li S, Marchal CC, Towe J, et al. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol. 2004;173:5971–5979. doi: 10.4049/jimmunol.173.10.5971. [DOI] [PubMed] [Google Scholar]
- 74.Wells CM, Walmsley M, Ooi S, Tybulewicz V, Ridley AJ. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. J Cell Sci. 2004;117:1259–1268. doi: 10.1242/jcs.00997. [DOI] [PubMed] [Google Scholar]
- 75.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, et al. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem. 2005;280:39474–39484. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thurnherr T, Benninger Y, Wu X, Chrostek A, Krause SM, et al. Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. J Neurosci. 2006;26:10110–10119. doi: 10.1523/JNEUROSCI.2158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chrostek A, Wu X, Quondamatteo F, Hu R, Sanecka A, et al. Rac1 is crucial for hair follicle integrity but is not essential for maintenance of the epidermis. Mol Cell Biol. 2006;26:6957–6970. doi: 10.1128/MCB.00075-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, et al. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 79.Qu J, Li X, Novitch BG, Zheng Y, Kohn M, et al. PAK4 kinase is essential for embryonic viability and for proper neuronal development. Mol Cell Biol. 2003;23:7122–7133. doi: 10.1128/MCB.23.20.7122-7133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamazaki D, Suetsugu S, Miki H, Kataoka Y, Nishikawa S, et al. WAVE2 is required for directed cell migration and cardiovascular development. Nature. 2003;424:452–456. doi: 10.1038/nature01770. [DOI] [PubMed] [Google Scholar]
- 81.Yan C, Martinez-Quiles N, Eden S, Shibata T, Takeshima F, et al. WAVE2 deficiency reveals distinct roles in embryogenesis and Rac-mediated actin-based motility. Embo J. 2003;22:3602–3612. doi: 10.1093/emboj/cdg350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soderling SH, Langeberg LK, Soderling JA, Davee SM, Simerly R, et al. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc Natl Acad Sci USA. 2003;100:1723–1728. doi: 10.1073/pnas.0438033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li X, Minden A. Targeted disruption of the gene for the PAK5 kinase in mice. Mol Cell Biol. 2003;23:7134–7142. doi: 10.1128/MCB.23.20.7134-7142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu AX, Rane N, Liu JP, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol Cell Biol. 2001;21:6906–6912. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hakem A, Sanchez-Sweatman O, You-Ten A, Duncan G, Wakeham A, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–1979. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thumkeo D, Shimizu Y, Sakamoto S, Yamada S, Narumiya S. ROCK-I and ROCK-II cooperatively regulate closure of eyelid and ventral body wall in mouse embryo. Genes Cells. 2005;10:825–834. doi: 10.1111/j.1365-2443.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- 87.Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, et al. ROCKI regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, et al. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Di Cunto F, Imarisio S, Hirsch E, Broccoli V, Bulfone A, et al. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron. 2000;28:115–127. doi: 10.1016/s0896-6273(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 90.Cunto FD, Imarisio S, Camera P, Boitani C, Altruda F, et al. Essential role of citron kinase in cytokinesis of spermatogenic precursors. J Cell Sci. 2002;115:4819–4826. doi: 10.1242/jcs.00163. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi H, Koshimizu U, Miyazaki J, Nakamura T. Impaired spermatogenic ability of testicular germ cells in mice deficient in the LIM-kinase 2 gene. Dev Biol. 2002;241:259–272. doi: 10.1006/dbio.2001.0512. [DOI] [PubMed] [Google Scholar]
- 92.Behrends J, Clement S, Pajak B, Pohl V, Maenhaut C, et al. Normal thyroid structure and function in rhophilin 2-deficient mice. Mol Cell Biol. 2005;25:2846–2852. doi: 10.1128/MCB.25.7.2846-2852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen F, Ma L, Parrini MC, Mao X, Lopez M, et al. Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr Biol. 2000;10:758–765. doi: 10.1016/s0960-9822(00)00571-6. [DOI] [PubMed] [Google Scholar]
- 94.Yang L, Wang L, Zheng Y. Gene Targeting of Cdc42 and Cdc42GAP Affirms the Critical Involvement of Cdc42 in Filopodia Induction, Directed Migration, and Proliferation in Primary Mouse Embryonic Fibroblasts. Mol Biol Cell. 2006;17:4675–4685. doi: 10.1091/mbc.E06-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Czuchra A, Wu X, Meyer H, van Hengel J, Schroeder T, et al. Cdc42 is not essential for filopodium formation, directed migration, cell polarization, and mitosis in fibroblastoid cells. Mol Biol Cell. 2005;16:4473–4484. doi: 10.1091/mbc.E05-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–1107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- 97.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H, Schaff UY, Green CE, Chen H, Sarantos MR, et al. Impaired integrin-dependent function in Wiskott-Aldrich syndrome protein-deficient murine and human neutrophils. Immunity. 2006;25:285–295. doi: 10.1016/j.immuni.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calle Y, Jones GE, Jagger C, Fuller K, Blundell MP, et al. WASp deficiency in mice results in failure to form osteoclast sealing zones and defects in bone resorption. Blood. 2004;103:3552–3561. doi: 10.1182/blood-2003-04-1259. [DOI] [PubMed] [Google Scholar]
- 100.Li S, Wang Q, Chakladar A, Bronson RT, Bernards A. Gastric hyperplasia in mice lacking the putative Cdc42 effector IQG AP1. Mol Cell Biol. 2000;20:697–701. doi: 10.1128/mcb.20.2.697-701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]