Sequence-specific RNA binding by an SR protein requires RS domain phosphorylation: Creation of an SRp40-specific splicing enhancer (original) (raw)
Abstract
We showed previously that ASF/SF2, a member of the SR protein family of splicing factors, can activate a splicing enhancer element composed of high-affinity ASF/SF2 binding sites. To determine whether other SR proteins can behave similarly, we selected a high-affinity RNA-binding site (B1) for the SR protein SRp40. Strikingly, the success of this selection was completely dependent on phosphorylation of the RS domain, as unphosphorylated SRp40 failed to select specific sequences. We show that three copies of B1 function as a strong splicing enhancer, activating an intron with suboptimal splicing signals in nuclear extracts. Enhancer activity in S100 extracts (which lack SR proteins) required SRp40 and a nuclear fraction previously found to be required for ASF/SF2-dependent splicing. Importantly, enhancer activity was lost when SRp40 was replaced by ASF/SF2 or SC35, and SRp40 was the only classical SR protein found to be associated with the enhancer. Together, our results indicate that phosphorylation-dependent, sequence-specific RNA binding can impart unique activities to individual SR proteins.
Regulation of alternative splicing is a powerful mechanism by which higher eukaryotes control gene expression at the post-transcriptional level. Recently, progress has been made in the identification both of cis-acting elements required for regulation and of trans-acting factors that interact with such elements. Some of the best understood examples of regulated alternative splicing feature genes that control sexual somatic differentiation in Drosophila (for review, see ref. 1). Control of sex-specific splicing of the transformer pre-mRNA by the Sex-lethal gene product illustrates that some regulatory mechanisms may not necessarily use cis-regulatory elements other than the splice sites themselves (2). In contrast, activation of the female-specific 3′ splice site in the doublesex primary transcript requires a complex exonic-sequence element that interacts with both cell-specific and constitutive trans-acting factors (3–6). Recently, positively acting exonic sequence elements also have been discovered in mammalian genes downstream of weak 5′ or 3′ splice sites (7–12). Although most of these so-called exonic splicing enhancers (ESEs) identified to date have been purine-rich, sequences that are not purine-rich also have been shown to function as splicing enhancers (13, 14).
Members of the SR protein family of splicing factors play important roles in both ESE-independent and ESE-dependent splicing (15, 16). SR proteins typically contain one or two N-terminal ribonucleoprotein-type RNA-binding domains (RBDs) and a C-terminal domain rich in arginine-serine dipeptide repeats (RS domain). At least eight members of the family, including ASF/SF2, SC35, and SRp40, contain phosphoepitopes that are recognized by the monoclonal antibody mAb104 (17), consistent with the observation that the RS domains are highly phosphorylated in vivo (18, 19). The function of such phosphorylation is, however, unknown. Each SR protein is capable of complementing splicing-deficient cytoplasmic S100 extracts (which lack SR proteins) for splicing of test RNAs containing strong splicing signals (20–24). This suggests that SR proteins are essential splicing factors with partially redundant activities. However, the finding that ASF/SF2 is essential for cell viability in chicken DT40 cells and that viability cannot be restored by overexpression of SC35 or SRp40 (25) is compelling evidence for unique cellular functions of individual family members. In addition to their activities as essential splicing factors, SR proteins have been shown to influence selection of alternative splice sites both in vitro (20–22, 26, 27) and in transfected cells (28–30). At least part of these activities likely are related to the ability of SR proteins to interact with each other and with other essential splicing components such as the U1 small nuclear ribonucleoprotein particle (snRNP)-associated 70-kDa protein and the splicing factor U2AF (31, 32). Intriguingly, high levels of SR proteins have been reported to circumvent the need of U1 snRNP, consistent with the idea that the proteins function in 5′ splice site recognition (33, 34). In the case of ESE-dependent splicing, both SR proteins (5, 7, 8, 13, 35) and U1 snRNP (9, 13) have been shown to bind to enhancer sequences or to be associated with enhancer complexes. At least where introns with weak 3′ splice sites are involved, binding of SR proteins to an ESE may facilitate 3′ splice site complex formation through interaction with U2AF (14, 36).
Evidence that SR proteins have different, functionally significant RNA-binding activities was provided by the findings that ASF/SF2 and SC35 displayed differences in their ability to commit different pre-mRNAs to the splicing pathway (37) and that ASF/SF2, but not SC35, was able to bind to an ESE within the bovine growth hormone gene (8). Consistent with this, the two proteins were found to have different RNA-binding specificities by employing the systematic evolution of ligands by expontential enrichment (SELEX) protocol to select high-affinity binding sites (38). ASF/SF2-selected sequences showed strong similarities with motifs in naturally occurring ESEs, and three copies of an ASF/SF2 binding site were shown to function as a splicing enhancer that could be activated by ASF/SF2 but not SC35 (38). In contrast, a SC35-selected sequence was not able to function as an ESE, and it thus is not clear whether the ability of high-affinity binding sites to function as ESEs is a property of SR proteins other than ASF/SF2.
In this study we demonstrate that three copies of a high-affinity SRp40 binding site identified by SELEX function in vitro as an ESE that can be activated by SRp40, but not by ASF/SF2 or SC35. We further show that SRp40 is the only SR protein efficiently selected from nuclear extracts (NE) by the ESE, providing evidence that specific binding of SRp40 is necessary for enhancer activation. Additionally, our data indicate that phosphorylation of the RS domain is necessary for sequence-specific binding by SRp40, as a lack of phosphorylation leads to strong, nonspecific RNA interactions.
MATERIALS AND METHODS
Expression of Recombinant Proteins.
To express human SRp40, a cDNA containing the complete coding sequence was isolated from a HeLa cell cDNA library, using as a probe a cDNA fragment obtained by reverse transcription/PCR of HeLa poly(A)+ RNA with degenerate oligonucleotide primers based on a published peptide sequence (24). The cDNA sequence is identical to the one published by Screaton et al. (29). A _Sal_I-_Sac_I fragment containing the coding sequence plus 5′ and 3′ noncoding sequences was subcloned into pGEM2 (Promega). After removal of 5′ noncoding sequences between the _Sal_I site and the _Bsp_HI site that encompasses the start codon, SRp40-related sequences were transferred into the baculovirus expression vector pVL1392. To express HSRp40 in bacteria, the coding sequence was subcloned as a PCR fragment into pET14b (Novagen). To express GSRp40ΔRS, a _Bsp_HI-_Bsp_MI cDNA fragment was inserted into pGEX-2T (Pharmacia). HSRp40 and GSRp40ΔRS were expressed, respectively, in BL21 and JM101, and purified, respectively, by Ni2+ and glutathione agarose chromatography. To phosphorylate HSRp40, 100 μg of the protein was incubated in 0.5 ml of HeLa S100 under splicing conditions for 30 min. After addition of urea to 7 M, the protein was repurified and renatured by dialysis against decreasing concentrations of urea. Untagged SR proteins were expressed in and purified from Sf9 cells infected with the corresponding recombinant baculoviruses as previously described (38), except that Phenyl-Superose chromatography was omitted.
In Vitro Assays.
All procedures required for SELEX were performed essentially as described previously (38). Selected sequences finally were subcloned as _Eco_RI-_Bam_HI fragments into pSP64polyA (Promega) and sequenced. Nuclear and cytoplasmic S100 extracts, NF20–40 fractions, and purified SR proteins were prepared from HeLa cells as previously described (38). In vitro splicing, UV cross-linking, and gel mobility-shift assays were performed as previously described (38). In vitro selection by biotinylated RNA was performed with RNA transcribed with 0.1 mM biotin-21 UTP and 0.5 mM UTP. Three to five micrograms of biotinylated RNA was bound to 15 μl of avidin-agarose beads and incubated in 250 μl of NE for 30 min under splicing conditions. After washing the beads three times with 0.5 ml of a buffer containing 20 mM Hepes (pH 7.9), 50 mM (NH4)2SO4, and 5% glycerol, selected proteins were eluted in SDS sample buffer, separated by SDS/9% PAGE, and analyzed by Western blotting. Polyclonal anti-SRp40 antibodies were raised against HSRp40 (Cocalico Biologicals, Reamstown, PA) and purified by affinity chromatography with GSRp40ΔRS.
RESULTS
Identification of a High-Affinity RNA-Binding Site for SRp40.
To identify high-affinity RNA-binding sites for SRp40, we chose to use the SELEX method (39) with His-tagged recombinant SRp40, expressed in and purified from Escherichia coli (HSRp40). However, SR proteins are phosphorylated poorly, if at all, in bacteria, and unphosphorylated RS domains are highly basic, which may affect protein–RNA interactions. Previously, we sought to overcome this problem by using truncated versions of SR proteins that lacked their RS domains in SELEX experiments (38). In this study, we evaluated the influence of RS domain phosphorylation on RNA-binding specificity by performing SELEX with both unphosphorylated and in vitro phosphorylated HSRp40. As shown in Fig. 1B, lane 2, HSRp40 is not recognized by mAb104, suggesting that the RS domain is not phosphorylated in E. coli. To phosphorylate HSRp40, the protein was incubated in S100 for 30 min under splicing conditions, repurified under denaturing conditions, and renatured. HSRp40 treated in this fashion could be detected by Western blotting with mAb104 (Fig. 1B, lane 3) and displayed a much lower electrophoretic mobility than the unphosphorylated protein (Fig. 1A, lanes 1 and 2).
Figure 1.
In vitro phosphorylation of HSRp40 in HeLa S100. (A) Coomassie stain of 3 μg of purified HSRp40 (lane 1), and of in vitro phosphorylated HSRp40 (ph.HSRp40) after repurification from S100 (lane 2). Proteins were resolved by SDS/9% PAGE. Molecular mass markers are shown on the left. (B) Western blot analysis with mAb104 of 150 ng of purified rSRp40 (lane 1), HSRp40 (lane 2), and in vitro phosphorylated HSRp40 (lane 3).
We then performed SELEX with unphosphorylated and phosphorylated HSRp40 in parallel. Unphosphorylated HSRp40 bound extremely tightly to the starting pool of RNA and did not reveal a consensus recognition motif, even after nine cycles of SELEX (results not shown). In contrast, only very few different sequences were obtained with the phosphorylated protein after seven cycles. As shown in Fig. 2, 23 sequenced clones represented only six different sequences. Of the two most frequently found sequences, one (pH1) was obtained nine times and the other (pH23) six times. The sequence of pH7, which was found once, is identical with pH23 except at one position, while pH2 is very similar to pH1. Inspection of sequences from preceding cycles indicated that pH23 was selected predominantly after five cycles, thus identifying it as the winner sequence of the SELEX experiment with phosphorylated HSRp40. Together, these results suggest that SRp40 is a sequence-specific RNA-binding protein, and that RS domain phosphorylation is required for recognition of specific sequences.
Figure 2.
Identification of a high-affinity RNA-binding site for SRp40 by SELEX with in vitro phosphorylated HSRp40. The sequences of individual clones selected after the indicated cycles are presented. Nucleotides shown in lowercase characters belong to the flanking constant region. Numbers on the right indicate the number of times each sequence was recovered. Nucleotides that contribute to the consensus sequence (bottom) are represented in boldface.
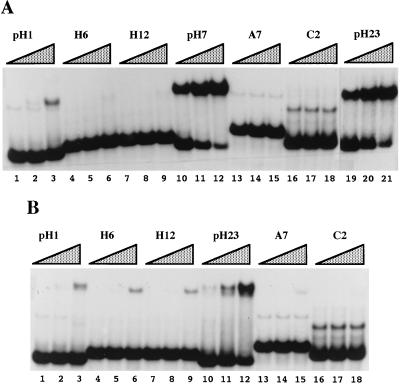
The requirement of RS domain phosphorylation for proper sequence-specific binding may reflect the possibility that the highly positive charge of the unphosphorylated domain obscures sequence-specific binding mediated by the two RNA-binding domains. Alternatively, the phosphorylated RS domain itself may contribute to sequence-specific RNA binding. To distinguish between these two possibilities and to determine whether the SELEX sequences are high-affinity SRp40 binding sites, we performed gel mobility-shift experiments both with SRp40 purified from baculovirus-infected Sf9 cells (referred to as recombinant or rSRp40) and with a bacterially expressed, truncated glutathione _S_-transferase-fusion protein lacking the RS domain (GSRp40ΔRS). Fig. 3 shows gel mobility-shift assays in which increasing concentrations of both proteins were incubated with constant amounts of various radiolabeled RNA sequences. Even at a concentration of 3 nM, rSRp40 bound pH7 (Fig. 3A, lane 10) and pH23 RNAs (Fig. 3A, lane 19) with high and equal efficiencies. Binding to pH1 also could be detected, but was considerably weaker (Fig. 3A, lanes 1–3). No significant binding was observed with various other sequences, including two sequences recovered with unphosphorylated HSRp40, H6 and H12 (Fig. 3A, lanes 4–9), a sequence containing a purine-rich high-affinity binding site for ASF/SF2 (38), A7 (Fig. 3A, lanes 13–15), and an arbitrary sequence, C2 (Fig. 3A, lanes 16–18). Gel-shift assays with GSRp40ΔRS yielded results very similar to those obtained with rSRp40. Most importantly, the winner sequence pH23 bound GSRp40ΔRS with higher affinity than any other sequence tested (Fig. 3B, lanes 10–12), albeit with lower affinity than did rSRp40. It is possible that the lower affinity is due to the absence of the RS domain, but it also could reflect a property of the glutathione _S_-transferase-fusion protein. We infer from our results that the RS domain of SRp40 does not play a significant role in determining the protein’s RNA-binding specificity.
Figure 3.
Gel mobility-shift assays of rSRp40 and GSRp40ΔRS. Radiolabeled RNA probes were incubated with increasing concentrations of rSRp40 (3, 15, and 75 nM) (A) or GSRp40ΔRS (8, 40, and 200 nM) (B). Free RNA and RNA-protein complexes were resolved by a 5% polyacrylamide nondenaturing gel. The sequences of H6 and H12 are GGGAGGAATGTCGCGTTCGGA and CGGAATGTCTTTACACTAC, respectively. For identification of other sequences, see Fig. 2 and ref. 38 (A7, C2).
SRp40 in HeLa Nuclear Extracts Binds Selectively and Efficiently to the SELEX Winner Sequence.
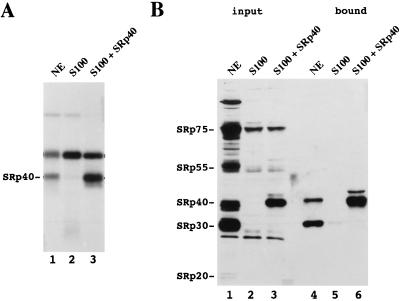
To extend the above results, we next determined whether authentic SRp40 present in nuclear extracts (NE) could bind selectively to the SELEX winner sequence (pH23, designated as B1). To this end, radiolabeled B1 was incubated for 30 min under splicing conditions in S100, S100 supplemented with rSRp40 or NE, and then irradiated with UV light. As shown in Fig. 4A, lane 3, UV cross-linking of B1 in S100 supplemented with rSRp40 yielded a strong band that was absent when S100 was used alone (Fig. 4A, lane 2). UV cross-linking of B1 in NE revealed a protein that comigrated with SRp40 (Fig. 4A, lane 1), strongly suggesting that authentic SRp40 in NE binds selectively to B1. To confirm this result, we used an in vitro selection assay in which proteins were bound to biotinylated B1 RNA, removed from the extracts using avidin agarose, and visualized by Western blot analysis with mAb104. A 40-kDa SR protein was strongly selected by B1 when rSRp40 was added to S100 (Fig. 4B, lane 6) but not with S100 alone (Fig. 4B, lane 5). When NE was used, a selected protein comigrating with rSRp40 was recognized by mAb104, strongly suggesting that the protein selected from NE was SRp40 (Fig. 4B, lane 4). The identity of the protein was confirmed by Western blotting with affinity-purified polyclonal anti-SRp40 antibodies (results not shown). In addition to SRp40, an SRp30 protein also was selected. The reason this protein was not detected by UV cross-linking may reside in the fact that this assay, in contrast to the biotin assay, uses limiting amounts of RNA. The latter assay thus may detect proteins that bind the RNA with lesser affinity but are more abundant. We conclude that B1 is efficiently recognized by authentic SRp40 present in NE.
Figure 4.
Authentic SRp40 present in NE binds efficiently to B1 RNA. (A) UV cross-linking of B1 RNA in NE (lane 1), S100 (lane 2), and S100 supplemented with 50 nM SRp40 (lane 3). Before irradiation with UV, radiolabeled B1 RNA was incubated for 30 min under splicing conditions. Cross-linked proteins were resolved by SDS/10% PAGE. (B) Western blot analysis with mAb104 of proteins selected by biotinylated B1 RNA, from NE (lane 4), S100 (lane 5), and S100 supplemented with 50 nM rSRp40 (lane 6). A fraction of the corresponding input material is shown in lanes 1–3.
High-Affinity SRp40 Binding Sites Function as an SRp40-Specific Splicing Enhancer in Vitro.
We showed previously that three copies of a high-affinity binding site for ASF/SF2 (A3) function as a splicing enhancer in vitro (38). The splicing enhancer test substrate (GN-A3) used in that study contained the enhancer sequence A3 80 bases downstream of the second intron of an α-globin gene with a modified, weak 5′ splice site. To test whether the high-affinity binding site for SRp40 also might possess enhancer function, A3 was replaced in GN-A3 by three copies of the selected sequence plus six 5′ nucleotides of B1 (B3, Fig. 5A). The resulting pre-mRNA, GN-B3, was spliced in NE almost as efficiently as GN-A3 (Fig. 5B, compare lanes 1–4 and 9–12). In contrast, only poor splicing was obtained with a substrate containing the B3 sequence in the antisense orientation (GN-B3as; Fig. 5B, lanes 5–8). Two copies of the multimerized sequence also displayed significant activity, although a single copy of B1 was inactive (results not shown). We next tested whether SRp40 was specifically required for enhancer function of B3. GN-B3 was efficiently spliced with a combination of S100, the nuclear fraction NF20–40 (38), and rSRp40 (Fig. 5C, lane 5). As previously reported for GN-A3, splicing of GN-B3 was absolutely dependent on the presence of NF20–40 (results not shown), although it was not required for splicing of PIP7A, a substrate with consensus splice sites (Fig. 5D). Splicing of GN-B3 was also observed when SRp40 was replaced by a preparation of classical SR proteins from NE, but was barely, if at all, detected with recombinant ASF/SF2 or SC35, which were active on PIP7A (compare Fig. 5 C and D). Together, our results indicate that three copies of a high-affinity SRp40 binding site function as a splicing enhancer in vitro, and that SRp40 is specifically required for enhancer activation.
Figure 5.
Three copies of a high-affinity SRp40 binding site (B3) function as a splicing enhancer in vitro. (A) B3 contains three copies of the variable sequence of B1 (boldface) plus six nucleotides of the 5′ flanking region (underlined). (B) In vitro splicing of enhancer test substrates GN-A3, GN-B3as, and GN-B3 (see text). In vitro splicing was performed for the indicated times. Splicing products and intermediates are indicated schematically. (C) In vitro splicing of GN-B3 for 2 h in NE (lane 1), S100 (lane 2), NF20–40 (lane 3), as a combination of S100 and NF20–40, supplemented with no protein (lane 4), recombinant SRp40 (lane 5), ASF/SF2 (lanes 6), SC35 (lane 7), or SR proteins purified from NE (lane 8). (D) In vitro splicing of Pip7A (see ref. 32) for 90 min in NE (lane 1) and S100 supplemented with the indicated SR proteins (lanes 2–5). The concentrations of recombinant SR proteins used were 50 nM in all experiments.
The activities of several previously characterized splicing enhancers appear to require complexes of SR proteins, as opposed to a single SR protein (13, 35, 40). To determine whether SR proteins in addition to SRp40 assemble on B3, we performed in vitro selection experiments with biotinylated B3 RNA and Western blot analysis of the selected proteins with mAb104. Strikingly, SRp40 was the only SR protein efficiently selected from NE by B3 (Fig. 6, lane 1). The identity of the selected SR protein as SRp40 again was confirmed by Western blots with anti-SRp40 antibodies (data not shown). SRp40 was not selected by B3 from NF20–40 (Fig. 6, lane 2) or S100 (Fig. 6, lane 3). However, two proteins of approximately 65 and 100 kDa, recognized by mAb104, were selected from NF20–40. While their role in enhancer activation, if any, is unknown, our results show that SRp40 is the only classical SR protein that binds the B3 enhancer efficiently. Thus, a high-affinity SRp40 binding site can form a splicing enhancer that is activated specifically by SRp40.
Figure 6.

SRp40 is the only SR protein selected efficiently from NE by B3 RNA. Western blot analysis of proteins selected by biotinylated B3 RNA using mAb104. Proteins were selected from NE (lane 1), NF20–40 (lane 2), S100 (lane 3), or S100 supplemented with 50 nM rSRp40 (lane 4). The migration of SR proteins present in NE (not shown) is indicated at right.
DISCUSSION
We have identified a high-affinity RNA-binding site for SRp40 by employing SELEX with an in vitro phosphorylated version of the protein. SRp40 produced from recombinant baculovirus bound B1 with high affinity in gel mobility-shift assays, with an apparent _K_d in this assay of ≈1 nM. Overall, the SELEX data suggest that SRp40 recognizes an extended, possibly bipartite, sequence of approximately 18 nt (Fig. 2). It is conceivable that each “half-site” in the consensus sequence is recognized by one of the two SRp40 RBDs. This would contrast with findings for two other RBD-containing proteins, such as ASF/SF2 (38) and heterogeneous nuclear ribonucleoprotein A1 (41), which recognize short elements of 6–10 nt with no evidence for half-sites. In fact, the high-affinity binding site selected by a single ASF/SF2 RBD was found to bear no resemblance to the sequence selected by the two-RBD protein (38). The SRp40 consensus sequence also contains an inverted repeat with the potential to form a stem-loop (GAGCAGTCGGCTC). This contrasts with high-affinity sites identified for ASF/SF2 and SC35 (38), which do not display any predicted secondary structure. However, several other RBD-containing proteins may recognize structured elements. For example, the U1 snRNP-associated 70-kDa and A proteins recognize stem-loops 1 and 2, respectively, of U1 small nuclear RNA. Although the actual sequence recognized by U1A resides within the loop (42), efficient binding of the 70-kDa protein seems to require the intact stem-loop (43). The significance of the possible RNA secondary structure for SRp40 binding, and the bipartite nature of the site, remain to be elucidated.
Specific RNA Binding by SRp40 Requires RS Domain Phosphorylation.
Using two different methods, UV cross-linking and selection by biotinylated RNA, we demonstrated efficient and selective binding of SRp40 from HeLa NE to B1. This illustrates that an in vitro phosphorylated, recombinant SR protein can be used successfully to determine the accurate RNA-binding specificity of the authentic protein. In contrast, unphosphorylated HSRp40 failed to display any binding specificity in the SELEX experiment, and individual sequences recovered after seven cycles were not bound by rSRp40 in gel-shift assays. Because the C-terminal 92 amino acid residues constituting the RS domain of SRp40 comprise 29 arginine, 6 lysine, and 38 serine residues, it is highly probable that the positive charges of the basic amino acids promote nonspecific interaction of this domain with RNA unless adequately compensated by negative charges provided by phosphorylation on neighboring serine residues.
Studies with protein phosphatases and their inhibitors have suggested that SR proteins may be subject to a cycle of phosphorylation and dephosphorylation during splicing, which would facilitate assembly and resolution of the spliceosome (44, 45). It is possible that this could reflect, at least in part, changes in RNA affinity that would result from changes in RS domain phosphorylation. Moreover, two different kinases, SRPK1 (18) and Clk/Sty (19), which are able to phosphorylate SR proteins in vitro, have been shown to be capable of affecting the nuclear localization of splicing factors, including SR proteins. For example, expression of a catalytically inactive version of Clk/Sty, which may have a dominant-negative effect, has been shown to disrupt proper nuclear localization (19). It is likely that this reflects hypophosphorylation of RS domains (J. Prasad, K. Colwill, T. Pawson, and J.L.M., unpublished results), perhaps leading to nonspecific interaction with RNA. RS domains also are required for protein–protein interactions (31, 32) and phosphorylation can influence such interactions (46).
High-Affinity RNA-Binding Sites of SR Proteins as ESEs.
We have created an ESE using three copies of a high-affinity binding site for SRp40. Unlike previously characterized purine-rich splicing enhancers, the SRp40-specific ESE (B3) exhibited an exclusive binding preference for SRp40. The fact that the 30-kDa SR protein in NE that bound to B1 was not selected by B3 indicates that binding of this protein is not necessary for enhancer activity. It is possible that sequences present in B1 but absent from B3 (i.e., the flanking sequences required for the SELEX procedure) account for the difference in binding. Alternatively, cooperative interactions of SRp40 on the multimerized sequence might prevent binding of the 30-kDa protein to B3. Our findings strongly suggest that a defined sequence with specificity for a single SR protein can be sufficient to function as an ESE, and that cooperative interactions between different SR proteins may not be essential for the formation of competent splicing enhancer complexes. Purine-rich ESEs have been shown in some cases to bind ASF/SF2 preferentially (8, 38), but in general they seem to be recognized by combinations of different SR proteins (7, 13, 35, 47). This could indicate that at least some purine-rich ESEs require cooperative binding of several SR proteins to function. Extensive analysis of the Drosophila double-sex enhancer, which consists of six 13-nt repeats and a purine-rich element, has provided support for this idea (5, 6, 40, 48). In particular, the regulators Tra and Tra2 were shown to associate with two different sequences, the repeats and the purine-rich element, by cooperative interaction with two different SR proteins. Conversely, binding of the SR proteins to the repeats and the purine-rich element, respectively, was strongly enhanced by Tra/Tra2 (40).
A cellular scenario that relies on overlapping RNA-binding specificities and cooperative interactions between SR proteins to modulate them is expected to display a high degree of flexibility in the exploitation of sequences that also must serve as coding sequences, as is the case with ESEs. However, the activities of such ESEs, as compared with ESEs based on high-affinity binding sites for single SR proteins, may be less able to respond to changes in the expression levels of specific factors and may thus require more complex mechanisms for regulation. An interesting possibility is that a spectrum of SR protein-dependent enhancers exists in vivo, ranging from low-affinity sites requiring cooperative interactions of multiple SR proteins to high-affinity sites recognized by single SR proteins. In this respect it is noteworthy that SRp40 mRNA levels have been shown to be highly and rapidly induced by insulin in regenerating liver (49). It will be interesting to learn whether SRp40-specific regulation of splicing plays an important role in liver regeneration, and if so, whether high-affinity binding sites similar to those described here are involved.
Acknowledgments
We are grateful to K. G. K. Murthy for providing HeLa poly(A)+ RNA and for helpful discussions. We also thank D. Foukal for advice with the baculovirus technology. This work was supported by a grant from the National Institutes of Health (GM 48259).
Footnotes
Abbreviations: NE, nuclear extract; RBD, RNA-binding domain; SELEX, systematic evolution of ligands by exponential enrichment; ESE, exonic splicing enhancer; snRNP, small nuclear ribonucleoprotein particle.
References
- 1.Baker B. Nature (London) 1989;340:521–524. doi: 10.1038/340521a0. [DOI] [PubMed] [Google Scholar]
- 2.Valcárcel J, Singh R, Zamore P D, Green M. Nature (London) 1993;362:171–175. doi: 10.1038/362171a0. [DOI] [PubMed] [Google Scholar]
- 3.Inoue K, Hoshijima K, Higuchi I, Sakamoto H, Shimura Y. Proc Natl Acad Sci USA. 1992;89:8092–8096. doi: 10.1073/pnas.89.17.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian M, Maniatis T. Science. 1992;256:237–240. doi: 10.1126/science.1566072. [DOI] [PubMed] [Google Scholar]
- 5.Tian M, Maniatis T. Cell. 1993;74:105–114. doi: 10.1016/0092-8674(93)90298-5. [DOI] [PubMed] [Google Scholar]
- 6.Heinrichs V, Baker B S. EMBO J. 1995;14:3987–4000. doi: 10.1002/j.1460-2075.1995.tb00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavigueur A, La Branche H, Kornblihtt A R, Chabot B. Genes Dev. 1993;7:2405–2417. doi: 10.1101/gad.7.12a.2405. [DOI] [PubMed] [Google Scholar]
- 8.Sun Q, Mayeda A, Hampson R K, Krainer A R, Rottman F M. Genes Dev. 1993;7:2598–2608. doi: 10.1101/gad.7.12b.2598. [DOI] [PubMed] [Google Scholar]
- 9.Watakabe A, Tanaka K, Shimura Y. Genes Dev. 1993;7:407–418. doi: 10.1101/gad.7.3.407. [DOI] [PubMed] [Google Scholar]
- 10.Xu R, Teng J, Cooper T A. Mol Cell Biol. 1993;13:3660–3674. doi: 10.1128/mcb.13.6.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeakley J M, Hedjran F, Morfin J-P, Merillat N, Rosenfeld M G, Emeson R B. Mol Cell Biol. 1993;13:5999–6011. doi: 10.1128/mcb.13.10.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphrey M B, Bryan J, Cooper T A, Berget S M. Mol Cell Biol. 1995;15:3979–3988. doi: 10.1128/mcb.15.8.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staknis D, Reed R. Mol Cell Biol. 1994;14:7670–7682. doi: 10.1128/mcb.14.11.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Hoffman H M, Grabowski P J. RNA. 1995;1:21–35. [PMC free article] [PubMed] [Google Scholar]
- 15.Fu X D. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 16.Manley J L, Tacke R. Genes Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 17.Roth M B, Zahler A M, Stolk J A. J Cell Biol. 1991;115:587–596. doi: 10.1083/jcb.115.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gui J F, Lane W S, Fu X D. Nature (London) 1994;369:678–682. doi: 10.1038/369678a0. [DOI] [PubMed] [Google Scholar]
- 19.Colwill K, Pawson T, Andrews B, Prasad J, Manley J L, Bell J C, Duncan P I. EMBO J. 1996;15:265–275. [PMC free article] [PubMed] [Google Scholar]
- 20.Ge H, Zuo P, Manley J L. Cell. 1991;66:373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- 21.Krainer A R, Mayeda A, Kozak D, Binns G. Cell. 1991;66:383–394. doi: 10.1016/0092-8674(91)90627-b. [DOI] [PubMed] [Google Scholar]
- 22.Fu X D, Mayeda A, Maniatis T, Krainer A. Proc Natl Acad Sci USA. 1992;89:11224–11228. doi: 10.1073/pnas.89.23.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayeda A, Zahler A M, Krainer A R, Roth M B. Proc Natl Acad Sci USA. 1992;89:1301–1304. doi: 10.1073/pnas.89.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahler A M, Lane W S, Stolk J A, Roth M B. Genes Dev. 1992;6:837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Takagaki Y, Manley J L. Genes Dev. 1996;10:2588–2599. doi: 10.1101/gad.10.20.2588. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y J, Zou P, Manley J L, Baker B S. Genes Dev. 1992;6:2569–2579. doi: 10.1101/gad.6.12b.2569. [DOI] [PubMed] [Google Scholar]
- 27.Zahler A M, Neugebauer K M, Lane W S, Roth M B. Science. 1993;260:219–222. doi: 10.1126/science.8385799. [DOI] [PubMed] [Google Scholar]
- 28.Cáceres J F, Stamm S, Helfman D M, Krainer A R. Science. 1994;265:1706–1709. doi: 10.1126/science.8085156. [DOI] [PubMed] [Google Scholar]
- 29.Screaton G R, Cáceres J F, Mayeda A, Bell M V, Plebanski M, Jackson D G, Bell J I, Krainer A R. EMBO J. 1995;14:4336–4349. doi: 10.1002/j.1460-2075.1995.tb00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Manley J L. RNA. 1995;1:335–346. [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J Y, Maniatis T. Cell. 1993;75:1061–1070. doi: 10.1016/0092-8674(93)90316-i. [DOI] [PubMed] [Google Scholar]
- 32.Kohtz J D, Jamison S F, Will C L, Zuo P, Lührmann R, Garcia-Blanco M A, Manley J L. Nature (London) 1994;368:119–124. doi: 10.1038/368119a0. [DOI] [PubMed] [Google Scholar]
- 33.Crispino J D, Blencowe B J, Sharp P A. Science. 1994;265:1866–1869. doi: 10.1126/science.8091213. [DOI] [PubMed] [Google Scholar]
- 34.Tarn W Y, Steitz J A. Proc Natl Acad Sci USA. 1995;92:2504–2508. doi: 10.1073/pnas.92.7.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramchatesingh J, Zahler A M, Neugebauer K M, Roth M B, Cooper T A. Mol Cell Biol. 1995;15:4898–4907. doi: 10.1128/mcb.15.9.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuo P, Maniatis T. Genes Dev. 1996;10:1356–1368. doi: 10.1101/gad.10.11.1356. [DOI] [PubMed] [Google Scholar]
- 37.Fu X D. Nature (London) 1993;365:82–85. doi: 10.1038/365082a0. [DOI] [PubMed] [Google Scholar]
- 38.Tacke R, Manley J L. EMBO J. 1995;14:3540–3551. doi: 10.1002/j.1460-2075.1995.tb07360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuerk C, Gold L. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 40.Lynch K W, Maniatis T. Genes Dev. 1996;10:2089–2101. doi: 10.1101/gad.10.16.2089. [DOI] [PubMed] [Google Scholar]
- 41.Burd C G, Dreyfuss G. EMBO J. 1994;13:1197–1204. doi: 10.1002/j.1460-2075.1994.tb06369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scherly D, Boelens W, van Venroij W J, Dathan N A, Hamm J, Mattaj I W. EMBO J. 1989;8:4163–4170. doi: 10.1002/j.1460-2075.1989.tb08601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Query C C, Bentley R C, Keene J D. Mol Cell Biol. 1989;9:4872–4881. doi: 10.1128/mcb.9.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mermoud J E, Cohen P, Lamond A I. Nucleic Acids Res. 1992;20:5263–5269. doi: 10.1093/nar/20.20.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mermoud J E, Cohen P T W, Lamond A I. EMBO J. 1994;13:5679–5688. doi: 10.1002/j.1460-2075.1994.tb06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao, S. & Manley, J. L. (1997) Genes Dev.11, in press. [DOI] [PubMed]
- 47.Yeakley J M, Morfin J-P, Rosenfeld M G, Fu X-D. Proc Natl Acad Sci USA. 1996;93:7582–7587. doi: 10.1073/pnas.93.15.7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch K W, Maniatis T. Genes Dev. 1995;9:284–293. doi: 10.1101/gad.9.3.284. [DOI] [PubMed] [Google Scholar]
- 49.Diamond R H, Du K, Lee V M, Mohn K L, Haber B A, Tewar D S, Taub R. J Biol Chem. 1993;268:15185–15192. [PubMed] [Google Scholar]