Previously undetected human hematopoietic cell populations with short-term repopulating activity selectively engraft NOD/SCID-β2 microglobulin–null mice (original) (raw)
Abstract
Increasing use of purified or cultured human hematopoietic cells as transplants has revealed an urgent need for better methods to predict the speed and durability of their engraftment potential. We now show that NOD/SCID-β2 microglobulin–null (NOD/SCID-β2m–/–) mice are sequentially engrafted by two distinct and previously unrecognized populations of transplantable human short-term repopulating hematopoietic cells (STRCs), neither of which efficiently engraft NOD/SCID mice. One is predominantly CD34+CD38+ and is myeloid-restricted; the other is predominantly CD34+CD38– and has broader lymphomyeloid differentiation potential. In contrast, the long-term repopulating human cells that generate lymphoid and myeloid progeny in NOD/SCID mice engraft and self-renew in NOD/SCID-β2m–/– mice equally efficiently. In short-term expansion cultures of adult bone marrow cells, myeloid-restricted STRCs were preferentially amplified (greater than tenfold) and, interestingly, both types of STRC were found to be selectively elevated in mobilized peripheral blood harvests. These results suggest an enhanced sensitivity of STRCs to natural killer cell–mediated rejection. They also provide new in vivo assays for different types of human STRC that may help to predict the engraftment potential of clinical transplants and facilitate future investigation of early stages of human hematopoietic stem cell differentiation.
Introduction
Blood cells are generated throughout adult life from a tiny subpopulation of undifferentiated stem cells. In adults, these stem cells are concentrated in the bone marrow (BM), although at birth they are also present in the blood in relatively high numbers (1–4). Because hematopoietic stem cells can enter the BM from the circulation at high efficiency (5, 6) the intravenous transplantation of adult BM cells and, more recently, of mobilized peripheral blood (mPB) and cord blood (CB) cell harvests have become an important therapeutic modality for patients with a broad spectrum of malignant and genetic disorders. Nevertheless, in many instances of either allogeneic or autologous stem cell therapy, undesirable patterns of hematologic recovery are obtained (7). It is therefore critical to identify the various types of human hematopoietic cells that are transplantable and to determine how changes in a given inoculum will affect the kinetics and durability of engraftment it will give so that these parameters can be predicted with confidence, particularly for transplants that have been manipulated previously ex vivo to expand, purge, or genetically modify the cells originally present.
Previous studies in mice have distinguished several distinct classes of hematopoietic cells with different engraftment properties. Long-term repopulating cells (LTRCs) have lifelong ability to produce all blood cell types and generate progeny that display similar potentialities upon transfer to secondary and even tertiary recipients (8–11). Other cells with similar differentiation potentialities may reconstitute both myeloid (M) and lymphoid (L) compartments, but typically for less than 4 months (9, 12). Additional types of short-term repopulating cells (STRCs) that are either myeloid or lymphoid restricted have also been identified in the mouse (13–15).
In vitro studies have provided considerable evidence of parallel hierarchical structures in the human and murine hematopoietic systems (16), and recent studies of sheep transplanted in utero with human hematopoietic cells have suggested the existence of distinct populations of transplantable human cells (17). However, both in vitro approaches and human-sheep xenografts have limitations, and neither has yet proven to be useful clinically. The ability of human hematopoietic cells from multiple sources to engraft the BM of sublethally irradiated NOD/LtSz-Prkdc scid/Prkdc_scid_ mice (hereafter abbreviated as NOD/SCID mice) with both myeloid and lymphoid progeny within 6 weeks (2, 18–20) and at high efficiency (5, 6) has made this murine xenograft model a popular alternative for investigating the phenotype of human hematopoietic stem cells (3, 21, 22). Limiting dilution analyses using this model have shown that the human cell engraftment is quantitative (23), independent of exogenous cytokine administration if sufficient cells are coinjected (18, 19, 24), and attributable almost exclusively to the CD38– subset of CD34+ cells (3, 21). However, CD34– human hematopoietic stem cells capable of repopulating NOD/SCID mice (22, 25) and fetal sheep (26) have also been reported.
Recently, we developed a new strain of immunodeficient mice in which the residual low natural-killer activity (NK activity) present in the NOD/SCID mouse was essentially eliminated by backcrossing the β2 microglobulin-null (_β2m_–/–) allele onto the NOD/SCID background (27). Initial studies showed that higher levels of human lymphomyeloid engraftment could be consistently obtained 6–8 weeks after transplant in these recipients when human cells were injected compared with results obtained in NOD/SCID hosts (28). However, as detailed below, more extensive analyses have now revealed that NOD/SCID-_β2m_–/– mice are, in fact, repopulated to different degrees by three types of human hematopoietic cells. Most of the human cells regenerated in NOD/SCID-_β2m_–/– mice in the first 3 months after transplant are derived sequentially from two biologically distinct types of human STRC, first from one that is myeloid restricted (STRC-M) and then from one that has dual myeloid- and lymphoid-repopulating ability (STRC-ML), neither of which efficiently engraft NOD/SCID mice. In addition, NOD/SCID-_β2m_–/– mice are engrafted after 6 to 8 weeks by a more primitive class of LTRC-ML that engrafts NOD/SCID mice equally efficiently.
Methods
Hematopoietic cells.
Normal adult BM cells from cadaveric sources were obtained from the Northwest Tissue Center (Seattle, Washington, USA). Samples of CB from normal, full-term infants delivered by caesarian section were collected in heparinized 50-ml tubes. Peripheral blood cells were collected by leukapheresis from cancer patients after mobilization by chemotherapy and daily subcutaneous injections of 5 μg/kg G-CSF. Single-cell suspensions of human fetal liver cells were obtained from 10- to 16-week-old abortus material after dispase digestion as described (29). The procurement and use of all human cells was undertaken according to approved protocols, including obtaining appropriate informed consent. Low-density cells (<1.077 g/ml) were cryopreserved (19) and the lin– fraction obtained from thawed cells using StemSep columns (StemCell Technologies, Vancouver, British Columbia, Canada). Cells expressing CD34 were isolated from mobilized peripheral blood samples before cryopreservation using the CliniMACS device (Miltenyi, Moenchen Gladbach, Germany). In some experiments, highly purified propidium iodide–negative (PI–) CD34+CD38+ and CD34+CD38– BM cells were isolated from the lin– fraction using a FACStar Plus cell sorter equipped with a 5-W argon laser and a 30-mW helium neon laser (Becton Dickinson, San Jose, California, USA) as described previously (3).
Short-term suspension cultures.
Purified CD34+CD38– and CD34+CD38+ BM cells were cultured for 5 days in serum-free medium (BIT; StemCell Technologies) supplemented with 10–4 M 2-mercaptoethanol (Sigma Chemical Co., St. Louis, Missouri, USA), 40 μg/ml LDLs (Sigma Chemical Co.), and the following five purified recombinant human growth factors: 20 ng/ml IL-3 (Novartis, Basel, Switzerland), 20 ng/ml IL-6 (Cangene Corp., Mississauga, Ontario, Canada), 20 ng/ml G-CSF (StemCell Technologies), 100 ng/ml Steel factor (SF) (purified from media conditioned by COS cells that had been transiently transfected with human SF cDNA), and 100 ng/ml Flt3-ligand (FL) (Immunex Corp., Seattle, Washington, USA). After 48 hours, the cultures were diluted twofold by adding an equal volume of fresh medium and the same five growth factors. CD34+ CB cells were cultured for 16 hours in serum-free medium supplemented with 50 ng/ml thrombopoietin (Genetech Inc., Palo Alto, California, USA), washed twice, and then cultured for an additional 4 days in serum-free growth factor–supplemented medium to reproduce conditions described previously (30).
Isolation of G0/G1 and S/G2/M cells from cultures of CB cells.
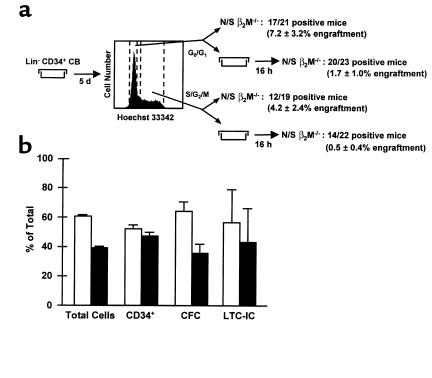
DNA staining of viable cells was performed as described previously (30). Briefly, after being washed in HBSS with 2% FCS (HF), cells were stained for 90 minutes at 37°C in HF containing 10 μmol/L Hoechst 33342 (Molecular Probes Inc., Eugene, Oregon, USA), washed twice again in HF plus 10 μmol/L Hoechst 33342 and 1 μg/ml PI, resuspended in HF plus 10 μmol/L Hoechst 33342, and the G0/G1 and S/G2/M cells were then sorted according to their DNA content (as illustrated in Figure 3).
Figure 3.
The distribution of 6-week repopulating activity in NOD/SCID-_β2m_–/– mice between the G0/G1 and S/G2/M fractions of 5-day human CB cell-expansion cultures is indistinguishable from that measured for total cells or other progenitors. CD34+ CB cells were cultured for 5 days in serum-free medium supplemented with SF, FL, IL-3, IL-6, and G-CSF. G0/G1 and S/G2/M cells were then isolated after DNA staining with Hoechst 33342. Approximately half of the fractionated cells were transplanted in NOD/SCID-_β2m_–/– mice immediately after their isolation. The other half of each fraction was first cultured for an additional 16 hours before being transplanted. (a) The proportion of engrafted mice (and the average level of engraftment) for the two populations compared (G0/G1 and S/G2/M). (b) The corresponding data for total cells, CD34+ cells, CFC, and LTC-IC in the G0/G1 (open bars) and S/G2/M (filled bars) fractions from the same experiments. All values shown are the mean ± SEM of data pooled from three experiments.
Xenotransplantation of hematopoietic cells.
NOD/SCID and NOD/SCID-_β2m_–/– mice were bred and maintained in the animal facility of the British Columbia Cancer Research Centre (Vancouver, British Columbia, Canada) in microisolator cages and provided with autoclaved food and water. Mice were irradiated at 8–10 weeks of age with 350 cGy of 137Cs x-rays and thereafter received acidified water containing 100 mg/L ciprofloxacin (Bayer AG, Leverkusen, Germany). Test cells were injected intravenously with 106 irradiated (15 Gy) normal human BM cells as carrier cells within a few hours after the mice were irradiated. The presence of human cells in the BM of mice was determined using FACS analysis of cells harvested from the femurs and tibias after first blocking Fc receptors with human serum and an anti-mouse Fc receptor Ab (2.4G2), then by staining with mAb’s against human CD34 (8G12), CD71 (OKT9), glycophorin A (10F7; kindly provided by P.M. Lansdorp), CD15, CD19, CD20, CD45 (from Becton Dickinson), and CD41a and CD66b (from Pharmacia Biotech, Baie d-Urfe, Quebec, Canada), as described (23). Levels of nonspecific staining were established by parallel analyses of cells incubated with irrelevant isotype-matched control Ab’s labeled with the same fluorochromes. Positive events were counted using gates set to exclude more than 99.99% of events in the negative-control analyses. Poisson statistics and the method of maximum likelihood were used to calculate frequencies of human repopulating cells from proportions of negative mice using the L-calc software (StemCell Technologies).
Statistical analyses.
Comparisons were made using Student’s t test.
Results
Human lin– BM cells engraft NOD/SCID-β2m –/– and NOD/SCID mice with different kinetics.
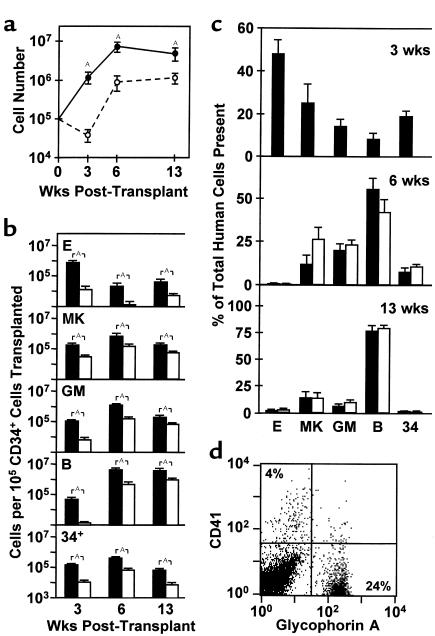
As shown in Figure 1a, when normal adult lin– BM cells were transplanted into sublethally irradiated NOD/SCID-_β2m_–/– and NOD/SCID mice, more human cells were present in the BM of the NOD/SCID-_β2m_–/– mice at all times, analyzed up to 13 weeks after transplant (P < 0.03). However, the difference between the levels of engraftment obtained was not constant and was most pronounced (approximately 30-fold; P < 0.01) at the earliest time point examined (3 weeks after transplant). By 6 weeks after transplant this difference had decreased to be eightfold and by 13 weeks was only fourfold. The large difference seen at 3 weeks after transplant was due primarily to the presence in the NOD/SCID-_β2m_–/– mice of a much larger population of human glycophorin A+ erythroid cells, CD41+ megakaryocytic cells, and CD15/66b+ granulopoietic cells (P < 0.01; Figure 1, b–d). However, similar differences were seen when the numbers of human CD34+ cells and CD19/20+ B-lymphoid cells were compared, although at this time the latter were detectable only in occasional mice of either genotype. Subsequently, human B-lymphoid cells became predominant, and maturing human erythroid cells were rarely detected in either type of recipient. These findings suggested an enhanced ability of human STRCs with myeloid-restricted differentiation potential to engraft NOD/SCID-β2m–/– mice.
Figure 1.
Different engraftment kinetics of human cells in NOD/SCID-_β2m_–/– and NOD/SCID mice. Groups of recipients were sacrificed 3, 6, and 13 weeks after transplantation, and the types and numbers of human cells present in the BM were determined by FACS analysis. (a) Total human CD45/71+ cells in NOD/SCID-_β2m_–/– mice (filled symbols, 13–14 mice/point) and NOD/SCID mice (open symbols, 15–16 mice/point) were calculated from data pooled from two independent experiments. (b) Absolute numbers of cells belonging to different human lineages present at different times. (c) Relative distribution of different types of human cells present after different periods in NOD/SCID-_β2m_–/– (filled bars) and NOD/SCID mice (open bars, same experiments as in b). Data for the 3-week NOD/SCID mice are not shown in this panel because of the low numbers of human cells present in these mice at this early time point. (d) Representative FACS profile of cells harvested from the BM of a NOD/SCID-_β2m_–/– mouse 3 weeks after transplantation of 2.5 × 105 human lin– BM cells. Note the high number of human erythroid (glycophorin A+) and megakaryocytic (CD41+) cells. Values for all figure parts are the mean ± SEM. ASignificant difference, P < 0.05.
Different types of human cells engraft NOD/SCID-β2m–/– mice and NOD/SCID mice.
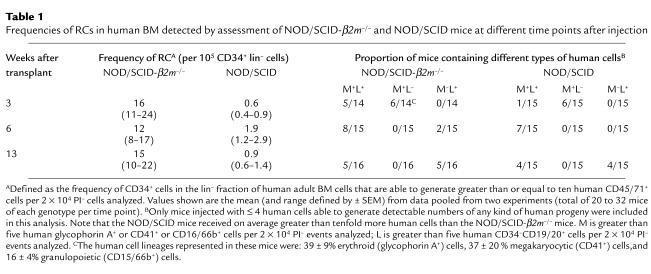
Two approaches were used to address this question. In the first, we compared the frequencies of cells responsible for the engraftment seen in the two different types of recipients 3, 6, and 13 weeks after transplant by using Poisson statistics to calculate the frequency of CD34+ cells in the injected lin– BM cells. As shown in Table 1, the frequency of cells able to repopulate NOD/SCID-_β2m_–/– hosts for 3 weeks was approximately 30-fold higher than the frequency of cells able to repopulate NOD/SCID mice assessed after the same period (P < 0.01), i.e., a factor similar to that seen when total engraftment levels in the two recipient genotypes were compared (Figure 1a). Moreover, approximately half of the 3-week engrafted mice that had been injected with low numbers of any type of human repopulating cell (RC) contained only human myeloid cells (erythroid, megakaryocytic, and granulopoietic, i.e., no human lymphoid cells), whereas the other half of the engrafted mice in these groups contained both. In contrast, at later times most of the mice in this analysis that were engrafted with human cells contained myeloid and lymphoid cells, although the frequency of human cells with this potential was much higher (P < 0.05) when NOD/SCID-_β2m_–/– mice were used as recipients, and NOD/SCID-β2m–/– mice were also more frequently engrafted only with B-lymphoid human cells.
Table 1.
Frequencies of RCs in human BM detected by assessment of NOD/SCID-_β2m_–/– and NOD/SCID mice at different time points after injection
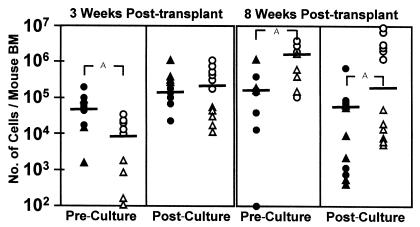
As a second approach we asked whether the cells that engraft NOD/SCID-β2m–/– mice after 3 and 8 weeks could be distinguished phenotypically. For this we analyzed the repopulating activity of the CD38+ and CD38– subsets of lin– CD34+ cells since NOD/SCID RCs are known to be CD34+CD38– primarily (3, 21). As shown in Figure 2, the CD38+ subset of lin– CD34+ adult human BM cells was responsible for 85% of the 3-week repopulating activity in NOD/SCID-β2m–/– mice. Conversely, 90% of the human cells present after 8 weeks was generated from CD34+CD38– cells. Limiting dilution analysis of the frequency of 3-week NOD/SCID-β2m–/– RCs yielded a value of 8 ± 2 per 106 CD34+CD38+ adult BM cells.
Figure 2.
Phenotype of STRC-M and STRC-ML and their expansion in short-term culture. CD34+CD38+ cells (filled symbols) in adult BM show an initially greater ability than CD34+CD38– cells (open symbols) to engraft NOD/SCID-_β2m_–/– mice for 3 weeks (P < 0.01), but CD34+CD38– cells have an equivalent ability to produce this activity in 5-day expansion cultures (P > 0.1). In contrast, CD34+CD38+ cells contribute much less than the CD34+CD38– cells to the 8-week engraftment of NOD/SCID-_β2m_–/– mice (P < 0.01) and show a parallel decline in this activity after 5 days in culture. Each symbol corresponds to the level of engraftment seen in an individual mouse originally injected with the yield of CD38+ or CD38– cells obtained from a starting equivalent of 105 CD34+ cells either directly (pre-culture) or after 5 days of culture with FL, SF, IL-3, IL-6, and G-CSF (post-culture). Different symbols show the results of two separate experiments. Bar, mean of each group. ASignificant differences, P < 0.05.
The seeding efficiency and subsequent expansion in vivo of cells that repopulate NOD/SCID mice for 6 weeks is similar in NOD/SCID-β2m–/– and NOD/SCID mice.
We next sought to determine whether NOD/SCID-β2m–/– mice might simply be more efficient in their ability to support the engraftment and proliferation of the same type of lymphomyeloid RCs that engraft NOD/SCID mice. For this we first compared the seeding efficiency in NOD/SCID-β2m–/– and NOD/SCID mice of human cells that repopulate NOD/SCID mice for 6–8 weeks. The very low frequency of these cells in adult human BM (Table 1 and refs. 4 and 23) precluded the use of adult BM. Because of the relatively higher numbers (and self-renewal activity) of NOD/SCID RC in human fetal liver (23), the latter source was used for these particular experiments. Accordingly, in each of three experiments 2 × 107 low-density human fetal liver cells were injected into groups of NOD/SCID-β2m–/– and NOD/SCID mice (six each). Twenty-four hours later, the BM cells from the femurs and tibias of all mice in each group were harvested and pooled. FACS analysis of a small aliquot consistently showed less than 0.01% human CD45/71+ cells to be present, as has been reported previously (20). The majority of the cells in each pool were injected into a total of six secondary NOD/SCID recipients (three with 30% each, three with 3% each). Six weeks later, four of nine (30% dose) and one of nine (3% dose) of the secondary recipients of cells from primary NOD/SCID mice contained both human lymphoid (CD34–CD19/20+) and myeloid (CD15/66b+) cells (greater than five each per 104 PI– events analyzed). The corresponding results for the NOD/SCID recipients of cells from primary NOD/SCID-β2m–/– mice were six of nine and one of nine positive mice. The number of NOD/SCID lymphomyeloid RC (3) that had seeded into the BM of each type of primary recipient within the first 24 hours was then calculated (6). In each experiment a second set of 24 primary NOD/SCID mice were transplanted with smaller aliquots of the same human fetal liver cells (106, 3 × 105, and 105 cells/mouse) and then similarly assessed for the presence of human lymphoid and myeloid cells in their marrow 6 weeks later to provide a measure of the number of NOD/SCID lymphomyeloid RC originally injected into each group of mice used for the seeding efficiency determinations. The pooled data from all three experiments showed the seeding efficiency values to be similar for the two different types of primary recipients (i.e., 1.4% and 2.5% in the primary NOD/SCID-β2m–/– mice and NOD/SCID mice, respectively).
We also compared the ability of NOD/SCID lymphomyeloid RC to expand their numbers in the two different host genotypes. In this case, low-density human fetal liver cells (containing approximately 105 CD34+ cells per recipient) were again injected into primary NOD/SCID-_β2m_–/– mice or NOD/SCID mice (three each in two experiments), but secondary transplants into NOD/SCID mice (only) were performed 4 weeks rather than 24 hours later (7%, 1.3%, and 2% of the contents of six femurs and six tibias per secondary mouse, nine secondary mice per group in total). Six weeks later the numbers of secondary mice that contained both human lymphoid and myeloid cells were six of nine versus five of nine, one of nine versus one of nine, and zero of eight versus zero of nine, respectively, for the different cell doses. From these data, the frequency and hence the number of 6-week NOD/SCID lymphomyeloid RC regenerated in the two types of primary hosts were again found to be similar (three and four per 105 CD34+ cells injected into NOD/SCID and NOD/SCID-_β2m_–/– primary recipients, respectively; P > 0.05). Thus both the seeding efficiency and the self-renewal behavior of human stem cells that engraft NOD/SCID mice is duplicated, but not enhanced, in NOD/SCID-_β2m_–/– mice. Therefore, the higher numbers of human lymphoid and myeloid cells seen in NOD/SCID_-β2m_–/– mice for up to 13 weeks after transplant are more likely to be indicative of a second type of human RC with lymphomyeloid differentiation potential and one that is poorly able to repopulate NOD/SCID mice.
Additional evidence to support this hypothesis was provided by experiments with human CB cells that had been stimulated to proliferate in vitro. Previous studies have shown that proliferating murine STRCs and LTRCs differ in their ability to engraft syngeneic hosts as they progress through the cell cycle, the engraftment ability of LTRCs being severely compromised during their passage through S/G2/M, whereas proliferating STRCs are little affected (31–33). We have shown recently that proliferating human CB stem cells also show a lack of repopulating activity in NOD/SCID mice as they transit S/G2/M (30). However, in the present studies we found no such deficiency when proliferating CB cells were assessed for their ability to engraft NOD/SCID-_β2m_–/– mice for 6 weeks. Thus, as shown in Figure 3, the 6-week NOD/SCID-_β2m_–/– lymphomyeloid repopulating activity (both in terms of the relative proportions of engrafted mice, 56% versus 44%, and in the levels of engraftment attained, 7.2% versus 4.2%) was distributed between the G0/G1 and S/G2/M cells exactly as expected based on the concomitantly measured distribution of other types of primitive cells between these two fractions. Moreover, further culture of the separated G0/G1 and S/G2/M cells did not differentially alter the NOD/SCID _β2m_–/– repopulating activity exhibited by their progeny assessed 1 day later.
The short- and long-term repopulating activities of different human hematopoietic tissues vary independently.
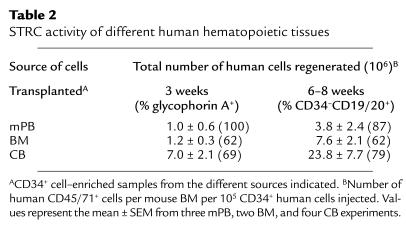
Transplants of human cells from different sources are known to be associated with different clinical engraftment kinetics. Moreover, these do not correlate with their content of cells that repopulate NOD/SCID mice for 6–8 weeks (4, 34). It was, therefore, of interest to compare the levels of engraftment obtained after 3 and 6–8 weeks in NOD/SCID-_β2m_–/– mice transplanted with CD34+ cell–enriched populations isolated from these three different sources of human cells. As shown in Table 2, in all groups the level of human engraftment was higher at the later time point, although the difference between 3 and 6–8 weeks was specific for each source of cells. In addition, in all groups most of the human cells seen after 3 weeks were again primarily erythroid (glycophorin A+), whereas after 6–8 weeks, all engrafted mice contained both lymphoid and myeloid human cells.
Table 2.
STRC activity of different human hematopoietic tissues
Selective expansion of human stem cells with short-term repopulating activity in short-term cultures of human BM.
Currently, much effort is focused on the identification of culture conditions that will allow the pace of hematologic recovery in recipients of cultured transplants to be accelerated. To determine the extent to which human cells with rapid repopulating activity may be expanded in vitro and to characterize the phenotype of their precursors, CD34+CD38– and CD34+CD38+ cells were isolated from adult human lin– BM cells using FACS, and then aliquots were transplanted into NOD/SCID-_β2m_–/– mice before and after being maintained in a serum-free expansion culture for 5 days (with FL, SF, IL-3, IL-6, and G-CSF). This resulted in an overall tenfold increase in early (3–week) NOD/SCID-_β2m_–/– mouse engrafting activity (P < 0.01), approximately half of which was generated from the CD34+CD38– subset (Figure 2). In contrast, the level of engraftment achieved after 8 weeks from the cultured human BM cells was maintained in one experiment and declined approximately 30-fold in the other. In both instances the expanded NOD/SCID-β2m–/– RC were generated primarily, albeit not exclusively, from the population of initially CD34+CD38– cells.
Discussion
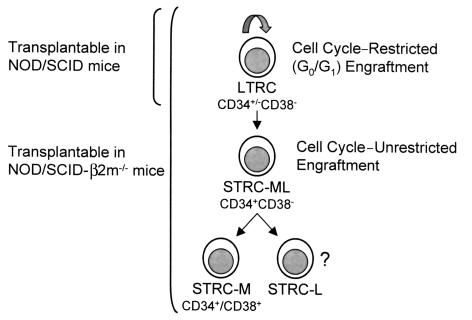
The use of xenogeneic hosts for analyzing the transplantable compartment of human hematopoietic cells is of pivotal importance to future clinical and experimental studies. Here we show that sublethally irradiated NOD/SCID-_β2m_–/– mice allow the efficient engraftment of two previously undescribed populations of human STRCs that engraft very poorly in the more widely used NOD/SCID mouse. We have designated these as STRC-M and STRC-ML to reflect the different lineage potentials these cells display in addition to their transient engrafting activity in NOD/SCID-_β2m_–/– mice (Figure 4). It is important to note that because of the reduced terminal differentiation and poor peripheralization of all human hematopoietic cells that mature in the BM of either of these mouse strains, the characterization of their precursors requires analysis of the progeny they generate in the murine BM microenvironment.
Figure 4.
Model indicating a hierarchy of transplantable human hematopoietic cells with distinct biological properties. LTRCs include CD34–CD38– cells (22, 26) as well as cells expressing CD34 but not CD38 (3, 21). The engraftment ability of LTRCs is restricted to the G0/G1 phases of the cell cycle (30) and are the only human cells that efficiently engraft NOD/SCID mice. STRC-ML are CD34+CD38–, and their ability to engraft NOD/SCID-_β2m_–/– mice is not cell cycle–restricted. Most freshly isolated STRC-M express both CD34 and CD38.
Evidence for multiple classes of human stem cells with engrafting potential detectable in NOD/SCID-β2m–/– mice.
Time-course studies of a large series of NOD/SCID-_β2m_–/– mice transplanted with multiple sources of human hematopoietic cells, including both fresh and cultured cells, provided the first indication that these mice support a broader range of transplantable human cells than those that reconstitute the closely related NOD/SCID mouse. A hallmark of human STRC-Ms is the large and rapid but transient burst of erythroid cells they produce in the first 3 weeks after transplant, although analyses of oligoclonally repopulated mice showed that these cells also consistently produce detectable numbers of granulocytes and megakaryocytes (but not lymphoid cells). Most STRC-Ms isolated directly from normal adult human BM were shown to express CD38 and could be rapidly amplified in short-term (5–day) cultures from CD34+CD38+ as well as CD34+CD38– precursors. These features indicate a stage of human hematopoietic stem cell differentiation characterized by a lack of self-renewal activity and lymphopoietic potential, most likely analogous to murine day 9–12 CFU-S (13, 35) and the common myeloid progenitor in mice recently described (15).
The evidence for a second type of human STRC (with lymphoid as well as myeloid differentiation potential, but distinct from the human cells that regenerate the lymphoid and myeloid progeny seen after 6 weeks in NOD/SCID mice) is derived from a different set of observations. The first of these indicated a difference in the kinetics of human lymphomyeloid engraftment of NOD/SCID-_β2m_–/– and NOD/SCID mice. This reached a much higher peak after 6–8 weeks in the NOD/SCID-_β2m_–/– hosts, which then declined more rapidly so that the total level of engraftment in the two strains was increasingly similar by 13 weeks. In addition, the frequency of 8- to 13-week human RC was different in the two types of recipients despite the fact that cells able to engraft NOD/SCID mice were found to home to the marrow of NOD/SCID-_β2m_–/– mice with similar efficiency. We also found that the amplification of NOD/SCID RC is not enhanced in NOD/SCID-_β2m_–/– mice. Finally, we showed that the ability of STRC-MLs to engraft NOD/SCID-_β2m_–/– mice is not altered as these cells progress through the different phases of the cell cycle. This latter finding contrasts dramatically with the transient loss of engrafting ability that accompanies the S/G2/M transit of lymphomyeloid cells that repopulate NOD/SCID mice (30, 36). Parallel differences between short and prolonged durability of engraftment and low and high sensitivity to cell cycle progression have been reported for murine RCs (31–33). It is also interesting to note that clonal analyses of gene-marked autografts in nonhuman primates have recently revealed exclusively myeloid clones (containing both erythroid and granulopoietic cells) during the first 24 weeks after transplant with lymphomyeloid clones first becoming detectable after that time (37). However, since most STRC-MLs (Figure 2) and LTRCs (3, 21) appear to be CD34+CD38–, further studies will be required to determine whether these two populations can be phenotypically separated by other molecular markers that would facilitate their further characterization.
Implications of the differential ability of human STRCs and LTRCs to engraft NOD/SCID-β2m–/– and NOD/SCID mice.
The poor ability of human STRCs to engraft NOD/SCID mice, despite equivalent engraftment and self-renewal in NOD/SCID-_β2m_–/– mice of LTRCs, suggests an early change in differentiating human stem cell populations that renders them sensitive to rejection mechanisms that are eliminated by introduction of the _β2m_–/– mutation onto the NOD/SCID background. Both mice lack B and T cells and hemolytic complement, but differ in the extent of NK-cell activity they possess (27). In NOD/SCID mice NK cells are reduced, but not absent, whereas in NOD/SCID-_β2m_–/– mice NK-cell activity is undetectable. It is, therefore, inviting to speculate that the mechanism underlying the differential engraftment of human STRCs in these two mouse strains may involve parameters that increase their ability to be recognized or killed by foreign NK cells. Such an explanation, if validated, would predict that certain allogeneic clinical transplants might also result in delayed hematologic recovery due to impaired engraftment of the STRCs they contain.
On the other hand, the relative inability of human STRCs to engraft NOD/SCID mice enables human LTRCs to be detected and quantified in these recipients with greater specificity at early times after transplant without the need to undertake a pre-enrichment or serial transplant step. This feature has obvious practical advantages and should establish greater confidence in the continued use of the NOD/SCID host for analyzing the most primitive types of human stem cell populations. For example, it would be anticipated from the findings reported here that human CD34– hematopoietic stem cells, which have been postulated to be precursors of CD34+CD38– stem cells (22, 26), would also not engraft NOD/SCID-_β2m_–/– mice any more efficiently than NOD/SCID hosts.
Clinical relevance of human STRCs.
Recovery rates of peripheral blood neutrophil and platelet counts in patients can be highly variable, and, in some cases, recovery of both can be very protracted. There is therefore an important need for assays that might allow the reasons for these differences to be understood. Interestingly, differences in the average rate of blood-count recovery seen in patients given different types of transplants do not correlate with the NOD/SCID RC (LTRC) frequencies reported for these different cell sources. In particular, the frequency of NOD/SCID RCs in mPB CD34+ cells has been found to be ten- and 100-fold lower than in BM or CB (34). Nevertheless, recipients of mPB show typically more rapid recovery rates than patients transplanted with BM (7). Here we have shown that relative to a fixed number of LTRCs, both STRC-M and STRC-ML activities are markedly elevated (7–to 15–fold) in mPB as compared with BM and CB. This relatively elevated STRC content of mPB could explain the apparently faster rates of hematologic recovery they typically achieve clinically since much higher numbers of CD34+ mPB cells are usually transplanted. This would not only compensate for the lower frequency of LTRCs in mPB, but would also mean that the total absolute number of STRCs transplanted in a mPB graft frequently might be more than tenfold higher than with BM or CB.
The principles described here for distinguishing and measuring different categories of human stem cell populations may now make it possible to more accurately predict clinical transplant recovery times. Similarly, these assays should be valuable for future investigations of genetically manipulated or naturally occurring leukemic populations.
Acknowledgments
The authors thank Maya Sinclaire for assistance with the animal work, Gayle Thornbury, Giovanna Cameron, and Rick Zapf for assistance in cell sorting, the staff of the Stem Cell Assay Service for initial hematopoietic cell processing, and Amy Ahamed for manuscript preparation. This study was supported by grants from the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Run, from the NIH (P01 HL-55435, R01 A130389, DKS7199), from the Deutsche Forschungsgemeinschaft (SFB364 C7), and from the German Minister for Education and Research (BMBF 01 KV 9907). H. Glimm received grants from the Dr. Mildred Scheel Stiftung für Krebsforschung, Bonn, Germany, and the Verein zur Foerderung der Leukaemie- und Tumorforschung, Freiburg, Germany. C.J. Eaves was a Terry Fox Cancer Research Scientist of the NCIC.
References
- 1.Gluckman E, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 2.Pflumio F, et al. Phenotype and function of human hematopoietic cells engrafting immune-deficient CB17-severe combined immunodeficiency mice and nonobese diabetic-severe combined immunodeficiency mice after transplantation of human cord blood mononuclear cells. Blood. 1996;88:3731–3740. [PubMed] [Google Scholar]
- 3.Conneally E, Cashman J, Petzer A, Eaves C. Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lympho-myeloid repopulating activity in nonobese diabetic-scid/scid mice. Proc Natl Acad Sci USA. 1997;94:9836–9841. doi: 10.1073/pnas.94.18.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JCY, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–3924. [PubMed] [Google Scholar]
- 5.van Hennik PB, de Koning AE, Ploemacher RE. Seeding efficiency of primitive human hematopoietic cells in nonobese diabetic/severe combined immune deficiency mice: implications for stem cell frequency assessment. Blood. 1999;94:3055–3061. [PubMed] [Google Scholar]
- 6.Cashman JD, Eaves CJ. High marrow seeding efficiency of human lympho-myeloid repopulating cells in irradiated NOD/SCID mice. Blood. 2000;96:3979–3981. [PubMed] [Google Scholar]
- 7.To LB, Haylock DN, Simmons PJ, Juttner CA. The biology and clinical uses of blood stem cells. Blood. 1997;89:2233–2258. [PubMed] [Google Scholar]
- 8.Wu AM, Till JE, Siminovitch L, McCulloch EA. Cytological evidence for a relationship between normal hematopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968;127:455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan CT, Lemischka IR. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990;4:220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- 10.Keller G, Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990;171:1407–1418. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iscove NN, Nawa K. Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Curr Biol. 1997;7:805–808. doi: 10.1016/s0960-9822(06)00341-1. [DOI] [PubMed] [Google Scholar]
- 12.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 13.Magli MC, Iscove NN, Odartchenko N. Transient nature of early haematopoietic spleen colonies. Nature. 1982;295:527–529. doi: 10.1038/295527a0. [DOI] [PubMed] [Google Scholar]
- 14.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 15.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 16.Eaves, C.J., and Eaves, A.C. 1999. Anatomy and physiology of hematopoiesis. In Childhood leukemias. C.-H. Pui, editor. Cambridge University Press. New York, New York, USA. 53–71.
- 17.Civin CI, et al. Sustained, retransplantable, multilineage engraftment of highly purified adult human bone marrow stem cells in vivo. Blood. 1996;88:4102–4109. [PubMed] [Google Scholar]
- 18.Cashman JD, et al. Kinetic evidence of the regeneration of multilineage hematopoiesis from primitive cells in normal human bone marrow transplanted into immunodeficient mice. Blood. 1997;89:4307–4316. [PubMed] [Google Scholar]
- 19.Cashman J, Bockhold K, Hogge DE, Eaves AC, Eaves CJ. Sustained proliferation, multi-lineage differentiation and maintenance of primitive human haematopoietic cells in NOD/SCID mice transplanted with human cord blood. Br J Haematol. 1997;98:1026–1036. doi: 10.1046/j.1365-2141.1997.3233140.x. [DOI] [PubMed] [Google Scholar]
- 20.Nicolini FE, et al. Unique differentiation programs of human fetal liver stem cells revealed both in vitro and in vivo in NOD/SCID mice. Blood. 1999;94:2686–2695. [PubMed] [Google Scholar]
- 21.Bhatia M, Wang JCY, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick J. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 23.Holyoake TL, Nicolini FE, Eaves CJ. Functional differences between transplantable human hematopoietic stem cells from fetal liver, cord blood, and adult marrow. Exp Hematol. 1999;27:1418–1427. doi: 10.1016/s0301-472x(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 24.Bonnet D, Bhatia M, Wang JCY, Kapp U, Dick JE. Cytokine treatment of accessory cells are required to initiate engraftment of purified primitive human hematopoietic cells transplanted at limiting doses into NOD/SCID mice. Bone Marrow Transplant. 1999;23:203–209. doi: 10.1038/sj.bmt.1701564. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, et al. Ex vivo generation of CD34+ cells from CD34- hematopoietic cells. Blood. 1999;94:4053–4059. [PubMed] [Google Scholar]
- 26.Zanjani ED, Almeida-Porada G, Livingston AG, Flake AW, Ogawa M. Human bone marrow CD34- cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+ cells. Exp Hematol. 1998;26:353–360. [PubMed] [Google Scholar]
- 27.Christianson SW, et al. Enhanced human CD4+ T cell engraftment in β2-microglobulin-deficient NOD-scid mice. J Immunol. 1997;158:3578–3586. [PubMed] [Google Scholar]
- 28.Kollet O, et al. β2 microglobulin-deficient (B2mnull) NOD/SCID mice are excellent recipients for studying human stem cell function. Blood. 2000;95:3102–3105. [PubMed] [Google Scholar]
- 29.Nishikawa S-I, et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 30.Glimm H, Oh I, Eaves C. Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G2/M transit and do not re-enter G0. Blood. 2000;96:4185–4193. [PubMed] [Google Scholar]
- 31.Monette FC, DeMello JB. The relationship between stem cell seeding efficiency and position in cell cycle. Cell Tissue Kinet. 1979;12:161–175. doi: 10.1111/j.1365-2184.1979.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 32.Szilvassy SJ, Meyerrose TE, Grimes B. Effects of cell cycle activation on the short-term engraftment properties of ex vivo expanded murine hematopoietic cells. Blood. 2000;95:2829–2837. [PubMed] [Google Scholar]
- 33.Habibian HK, et al. The fluctuating phenotype of the lymphohematopoietic stem cell with cell cycle transit. J Exp Med. 1998;188:393–398. doi: 10.1084/jem.188.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van der Loo JCM, et al. Nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse as a model system to study the engraftment and mobilization of human peripheral blood stem cells. Blood. 1998;92:2556–2570. [PubMed] [Google Scholar]
- 35.Ploemacher RE, Brons NHC. Isolation of hemopoietic stem cell subsets from murine bone marrow. I. Radioprotective ability of purified cell suspensions differing in the proportion of day-7 and day-12 CFU-S. Exp Hematol. 1988;16:21–26. [PubMed] [Google Scholar]
- 36.Gothot A, Van der Loo JCM, Clapp W, Srour EF. Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34+ cells in non-obese diabetic/severe combined immune-deficient mice. Blood. 1998;92:2641–2649. [PubMed] [Google Scholar]
- 37.Kim HJ, et al. Many multipotential gene-marked progenitor or stem cell clones contribute to hematopoiesis in nonhuman primates. Blood. 2000;96:1–8. [PubMed] [Google Scholar]