Cytokine-induced survival of activated T cells in vitro and in vivo (original) (raw)
Abstract
Many antigen-specific T cells die after exposure to antigen in animals. These cells also die if they are isolated from animals shortly after activation and cultured. Various cytokines were tested for their ability to interfere with this in vitro death. Surprisingly, tumor necrosis factor α and other inflammatory cytokines did not prevent the in vitro death of activated T cells, even though these cytokines do prevent activated T cell death in animals. Therefore, the inflammatory cytokines probably act on T cells in vivo via an intermediary factor. Four cytokines, interleukin (IL)-2, IL-4, IL-7, and IL-15, did prevent activated T cell death in vitro, with IL-4 and IL-15 more effective than IL-2 or IL-7. These cytokines share a component of their receptors, the common γ chain, γc. Therefore, their collective ability to protect activated T cells from death may be mediated by signals involving γc. To assess their activity in vivo, two of the cytokines, IL-2 and IL-4, were expressed in animals at local sites of superantigen responses. Both cytokines increased the numbers of T cells found at the local sites 14 days later. Interleukin 4 was more effective than IL-2, even though IL-2 stimulates T cell proliferation better than IL-4. This result suggested that IL-4 and related cytokines can promote T cell survival in vivo as well as in vitro. The ability of these cytokines to prevent the death of activated T cells may be important at certain stages of immune responses in animals.
Keywords: interleukin 4, interleukin 2, interleukin 7, interleukin 15
In animals, stimulation with antigen or superantigen activates T cells specific for the antigen, such that they divide for a few days and then die (1–4). They probably die for several reasons. For example, activation induces increased expression of Fas and tumor necrosis factor (TNF) receptors on T cells. Engagement of these receptors by their ligands can induce apoptosis in the target T cells (5–7). Also, as antigen disappears from the animal the activated T cells probably become deprived of growth factors such as interleukin (IL)-2 and die for lack of their support (8, 9). Events such as these probably help clear the animal of many of the T cells that are activated during an immune response and may also protect the animal against autoimmune attack by its own T cells.
That stimulated T cells are very likely to die has many useful consequences; however, it is incompatible with a productive immune response. Therefore, during an immune response to an infectious agent T cells must be protected against death caused by the phenomena described above. Several candidates for protective agents have been suggested. For example, immune responses often cause increases in the levels of B7–1 and B7–2 on macrophages, B cells, and dendritic cells (10–12). B7–1 and B7–2 interact with CD28 on T cells, thereby inducing expression of Bcl-XL in the T cells (13–18). Bcl-XL is thought to protect cells against apoptotic death (13–20). We have shown, however, that activated T cells can survive in animals in which reaction of CD28 with B7 proteins is blocked (21). Therefore, other mechanisms to protect T cells against activation-induced death must exist.
A few years ago we demonstrated that bacterial lipopolysaccharide (LPS) protected activated T cells against death in vivo (22). Action of the LPS depended on its induction of inflammatory cytokines such as TNFα and converted the activated T cells into a state in which they were resistant to Fas-induced death.
In addition, recent experiments have suggested that the cytokine, IL-7, may be intimately involved with T cell survival in vivo (23). For example, thymocyte development is severely compromised in animals that lack IL-7 or components of its receptor (24–28), and T cells that mature in such animals have shortened half lives and abnormal phenotypes (29, 30). These deficiencies can be overcome in IL-7 knockout mice by the introduction of a transgene driving expression of Bcl-2 (31, 32), a protein related to Bcl-XL that is also associated with inhibition of cell death (19, 20, 33, 34). It is therefore likely that the life and death of mature T cells might be affected by cytokines such as IL-7, and, in fact, recent experiments by ourselves and others have shown that this is so (9, 35).
With these points in mind, experiments were done to find out whether any cytokines might inhibit the death of antigen-activated T cells in vitro. Of the many cytokines tested, only four, IL-2, IL-4, IL-7, and IL-15 prevented the deaths of activated T cells in vitro. These cytokines are members of the same family in that their receptors share the IL-2 receptor (IL-2R) common gamma chain, γc (36–39). Therefore, it is likely that the four active cytokines rescue via a common pathway. The data in this paper show that inhibition of activated T cell death by one of these cytokines, IL-4, is accompanied by increased expression of the death-resisting protein, Bcl-2, and that for two of these cytokines, IL-4 and IL-7, resistance to death is not accompanied by massive proliferation. Hence these cytokines appear to act as true survival factors as well, in some cases, as active stimulators of proliferation. Finally, two of the cytokines, IL-2 and IL-4, were tested in vivo for their rescuing activities. One, IL-4, was particularly active, suggesting that this family may have some survival-stimulating abilities in whole animals as well as in vitro. Therefore, it is possible that these cytokines operate at particular times in immune responses not only to promote T cell division but also to protect T cells against death.
MATERIALS AND METHODS
Mice.
Breeding pairs of mice transgenic for a T cell receptor (TCR) bearing Vβ3 and specific for moth cytochrome c 88–104 bound to IEk were generously given to us by Stephen Hedrick. These animals were bred and maintained in the Biological Resource Center at National Jewish Medical and Research Center.
T Cell Activation, Culture, and Staining.
T cells were activated in vivo by intraperitoneal injection of AD10 mice with 0.1 μg of the Vβ3 reactive superantigen, staphylococcal enterotoxin A (SEA, Toxin Technology, Sarasota, FL). Two days later T cells were purified from the mice by passage of lymph node cells over sterile nylon wool columns, a procedure that does not affect the ratio of activated to resting T cells in the population (40). The cells were cultured in fortified culture medium containing 10% fetal bovine serum (FBS) at 106 cells per ml for 24 or 48 hr. Cells were then harvested and counted, and the percentages of apoptotic cells were measured by staining with propidium iodide as previously described.
Cells were analyzed for expression of surface proteins by fluorescent staining. Staining was performed in BSS, 5% FBS/0.1% sodium azide on ice with 30-min incubations and thorough washes to remove unbound stains. Reagents used were biotinylated anti-TCR Vβ3 with streptavidin CyChrome, phycoerythrin-conjugated anti-CD4, and fluorescein (FL)-conjugated anti-Fas or anti-CD69. All reagents were purchased from PharMingen. For each sample more than 10,000 cells were analyzed on a FACScan instrument (Becton Dickinson Immunocytometry Systems).
Immunoblots.
AD10 mice were injected intraperitoneally with 0.1 μg SEA. Two days later their lymph node T cells were purified on nylon wool columns. The cells were then cultured at 5 × 106/ml in the presence or absence of 1 μg/ml IL-4. At various times thereafter cells were harvested and lysed in 0.5% Nonidet P-40, 100 mM sodium chloride, 2 mM DTT, 1 mM EDTA, 20 mM Hepes (pH 7.2), 1.0 mM PMSF, and 1 μg/ml aprotinin. Lysates were cleared with a 12,000 × g spin at 4°C for 10 min. Volumes of lysate containing approximately 106 cell equivalents were fractionated on SDS/PAGE by using a Bio-Rad minigel apparatus. The fractionated proteins were transferred to Immobilon polyvinylidene difluoride membranes (Millipore) in 48 mM Tris, 39 mM glycine, and 20% methanol, pH 9.2, by using a semi-dry transblot apparatus (Bio-Rad). The blots were blocked overnight at 4°C with Tris-buffered saline containing 0.1% Tween-20 (Sigma) and 5% nonfat dried milk. The blots were probed with polyclonal rabbit anti-Bcl-2 or anti-Bcl-XL (Santa Cruz Biotechnology). Membranes were developed with alkaline phosphatase probes by using ECL Western blotting reagents (Amersham).
Superantigen and Cytokine Gene Expression Vectors.
The SEA gene was cloned into a eukaryotic expression vector, PCR 3.1 (Invitrogen), which contains a cytomegalovirus promoter. In vitro and in vivo expression of the gene was confirmed as previously described (41). The full-length cDNA for IL-4 was cloned by PCR from Con A-stimulated mouse spleen cells, and mouse IL-2 cDNA was obtained from the American Type Culture Collection. cDNAs for both cytokines were placed in the PCR3 eukaryotic expression vector. Expression of both cytokines was confirmed by in vitro transfection and IL-2 or IL-4 ELISA (PharMingen). The PCR3 expression vector without insert was used as the empty vector control.
In Vivo Gene Transfer and Expression.
Plasmids were propagated in E. coli, and their DNAs were isolated by modified alkaline lysis and purified by ultracentrifugation and CsCl banding. Purified plasmid DNA was resuspended in sterile PBS at 0.5 mg/ml. One hundred micrograms of each DNA was injected into each quadricep muscles of anaesthetized mice as described previously (41). Gene expression after such injections were confirmed as described in the Results section. In some cases, IL-4 produced after intramuscular injection in vivo was neutralized by daily intraperitoneal injections of 0.5 mg purified 11B11, a rat anti-mouse IL-4, beginning 12 hr after DNA injection and continuing for 5 days.
To measure cytokine expression in muscles, the quadricep muscles were placed in cell lysis buffer (Analytical Luminescence Laboratories, Ann Arbor, MI) and homogenized with a polytron. The supernatants of the homogenates were assayed for IL-2 and IL-4 by ELISAs (PharMingen). The ELISAs for both IL-2 and IL-4 were sensitive to 1.0 pg/ml cytokine. Protein concentration in the homogenates was determined with a BCA assay (Pierce).
RESULTS
Inhibition of Apoptosis of Activated T Cells.
Examination of T cell survival or death requires a uniform population of cells destined to die or live. Therefore, for many of the experiments described in this paper we used T cells from AD10 transgenic mice. Experiments showed that more than 90% of the T cells in young AD10 mice young mice bore Vβ3 and CD4 and were activated, as defined by increase in size and expression of CD69 and Fas, by injection of the Vβ3 specific superantigen, SEA.
Many experiments have shown that T cells activated in vivo by antigen or superantigen go on to die. We have previously shown that inflammatory cytokines can prevent this death in animals (22); however, it is difficult to establish in vivo that a given cytokine is acting directly on T cells rather than via some intermediate cell. Therefore, we decided to test various cytokines for their ability to act directly on activated T cells and prevent cell death with cultures of purified T cells isolated from animals 2 days after exposure to superantigen, at a time when they were on the verge of dying in the animal.
Purified T cells from AD10 mice given SEA 2 days previously died readily in vitro. Nearly half the cells were dead after 24 hr of culture, and their death could not be blocked by inhibiting the ligation of Fas or by ligating CD28 (data not shown). A number of cytokines, including many inflammatory cytokines such as TNFα, failed to prevent this death (data not shown). Therefore, the inflammatory cytokines that were previously shown to be effective in vivo probably did not affect T cells directly but rather via an indirect route with other cell types as intermediaries.
Of the many cytokines tested, only IL-2, IL-4, IL-7, and IL-15 prevented the in vitro death of T cells activated in vivo (Fig. 1). On a concentration basis, IL-4 and IL-15 were more efficient than IL-2 or IL-7 at inhibiting death. Two other members of the IL-2 family, IL-9 and IL-13, did not block death at all. This was probably for the following reasons. High-affinity receptors for IL-9 have not been found on T cells activated for short time periods (42), and therefore it is possible that they are also absent from SEA-activated T cells. Also, although the IL-13 receptor shares many activities with that of IL-4, unlike the IL-4 receptor, it does not operate via the γc and cannot activate Jak3 (43). This last result suggests that one of the crucial factors in the rescuing activities of IL-2, IL-4, IL-7, and IL-15 is their ability to act via the γc.
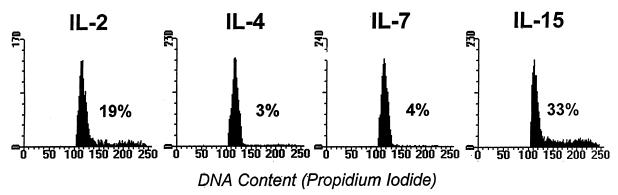
Figure 1.
IL-2, IL-4, IL-7, and IL-15 inhibit antigen-activated T cell death. T cells were activated in vivo with SEA and purified as described in Materials and Methods. They were cultured in triplicate wells of 96-well plates at 106 cells per ml. Cytokines were added to cultures at the indicated concentrations. Twenty-four hours later cells were harvested, stained with propidium iodide, and analyzed by flow cytometry. Shown are the means ± SEMs of the triplicate cultures. These data are from one experiment, representative of four. Different symbols represent the results of titrations of different cytokines as follows: □, IL-2; ⋄, IL-4; ▵, IL-7; ○, IL-15.
Some members of the IL-2 family stimulate activated T cells to divide. It therefore was possible that the cell survival observed in cultures of activated cells in the presence of these cytokines was due to overgrowth of the cultures by a few cells that were stimulated to divide, rather than inhibition of death of all the cells in the culture. To check this possibility, we evaluated the DNA content of the T cells after 24 and 48 hr of culture with IL-2, IL-4, IL-7, or IL-15. As expected, IL-2 stimulated activated T cells to proliferate, and after 48 hr culture with this cytokine, 19% of the activated T cells were in S/G2M (Fig. 2). IL-15 had the same effect and 33% of the activated T cells were in S/G2M after 48 hr of culture. In contrast, IL-4 and IL-7 induced much smaller percentages of the cells to enter S phase, with only 3 or 4% of the cells, respectively, containing greater than 2 N amounts of DNA after 48 hr of culture. In spite of this, IL-4 and IL-7 prevented activated T cell death as effectively as IL-2 and IL-15. Thus, members of this cytokine family did act as real survival factors. Experiments in which cell division was monitored by 3H thymidine incorporation led to similar conclusions. In 24-hr assays all four of these cytokines prevented cell death in the absence of detectable stimulation of 3H thymidine usage or cell division (Fig. 1 and data not shown).
Figure 2.
IL-4 and IL-7 prevent T cell death without promoting massive T cell proliferation. T cells were activated, isolated, and cultured as described in Materials and Methods, except that cells were cultured with 1 μg/ml of the indicated cytokines. After 48 hr culture cells were stained with propidium iodide to determine their DNA content. The percentages of cells in S/G2 were measured by gating on cells that contained more than 2 N amounts of DNA. These percentages are indicated on the figure. In cultures without cytokines more than 70% of the T cells had died and less than 5% of the cells were in cycle. Results shown are from one experiment of four similar experiments.
We have previously shown that members of this same family of cytokines prevent death of resting T cells in vitro (35). The effects described in this section were not due to the rescue of resting cells, however, because IL-2 and IL-15 have little effect on resting cells and because IL-6, a potent rescuer of resting T cells, had no effect on these activated cells (35, 44).
IL-4 Prevents the Decay of Bcl-2 in Activated T Cells.
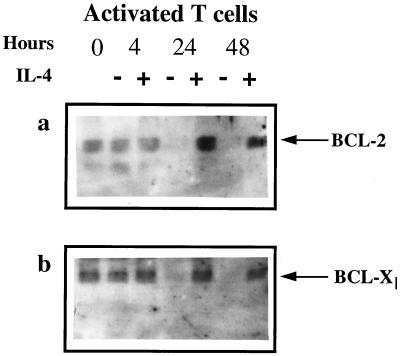
T cell survival is often associated with higher levels of Bcl-2 and/or Bcl-XL (31–34). We therefore measured the effect of IL-4, which prevented the death of activated T cells and caused relatively little proliferation, on the intracellular amounts of these proteins. AD10 T cells were activated in vivo with SEA and then cultured in the presence or absence of IL-4 for 0, 4, 24, or 48 hr. After culture, cell lysates were prepared, subjected to SDS/PAGE, and transferred onto Immobilon membranes. Blots were probed with antibodies specific for Bcl-2 and Bcl-XL (Fig. 3). Immediately after isolation from animals, activated T cells contained some Bcl-2 and Bcl-XL. These levels were unchanged after 4 hr of culture whether or not IL-4 was present. Culture of the activated cells for 24 or 48 hr in the absence of IL-4 led to a dramatic decrease in the amount of Bcl-2 and Bcl-XL detected, suggesting that the many dead cells in these cultures contained very little of either of these proteins. In contrast, the level of Bcl-2 in activated T cells cultured in the presence of IL-4 for 24 or 48 hr was considerably increased, suggesting that IL-4 induced Bcl-2 expression in the activated cells, a result that has previously been described by others. Levels of Bcl-XL, on the other hand, were essentially unchanged by comparison with those at the beginning of culture. IL-4 probably did not induce much extra Bcl-XL expression in the T cells, but rather, because culture with IL-4 increased the numbers of surviving cells in the cultures, it prevented loss of the protein as the cells died.
Figure 3.
Culture with IL-4 prevents decay of Bcl-2 and Bcl-XL in activated T cells. T cells were activated and purified as described in the legend to Fig. 2. Cells were then cultured at 5 × 106/ml for various lengths of time in the presence or absence of 1 μg/ml IL-4. Cells were harvested and lysed, and their lysates were run on SDS/PAGE and Western blotted for Bcl-2 and Bcl-XL expression as described in Materials and Methods. These data are from one experiment of four similar experiments.
A Role for IL-4 as a T Cell Survival Factor in Vivo.
Because IL-4 so effectively inhibited T cell death in vitro, we tested whether this cytokine would also be active in vivo. Preliminary experiments involved injection of animals with the superantigen SEA and simultaneous and/or subsequent injection of IL-4 or IL-2. In these experiments there was no evidence that either cytokine rescued the T cells from death (data not shown). These data were difficult to interpret, however, because it is impossible to know whether enough cytokine was injected and whether or not it was available at the right place and time to be effective.
We therefore tried a method that would allow release of the cytokine for a sustained period of time in the same location in the animal as the superantigen. Some of us have previously shown that injection of DNA coding for SEA into the quadricep muscles of mice leads to expression of SEA and substantial deletion of Vβ3+ T cells. To find out whether this method would allow local production of cytokines, DNA coding for IL-2 or IL-4 was injected into the quadricep muscles of B10.BR mice. This procedure led to the production, 24 hr later, of about 300 pg IL-2/mg muscle protein, or 2.5 pg IL-4/mg muscle protein with no detectable cytokine in the serum. Injection of the control plasmid did not cause cytokine production. Preliminary experiments suggest that this local production persisted for at least 14 days after injection of the DNA.
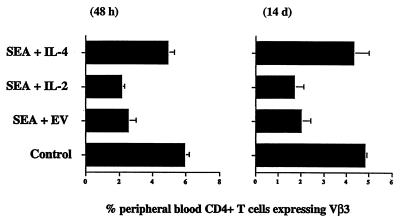
To find out if this local expression of cytokine affected activated T cell survival, B10.BR mice were injected in their quadricep muscles with plasmid DNA coding for SEA and for IL-2, IL-4, or an empty vector control. At various times thereafter the mice were bled and the percentage of CD4+ Vβ3+ peripheral blood T cells was determined by flow cytometry (Fig. 4). CD4 cells bearing Vβ3 disappeared very rapidly, within 2 days, from the peripheral blood of mice injected with SEA plus empty vector (Fig. 4). This disappearance was faster than that observed when SEA was given intravenously (22), indicating either that exposure to antigen in muscle, unlike exposure to intravenously injected antigen, caused target T cell death in excess of T cell division, or that many of the Vβ3 cells had temporarily migrated to the injected muscle. CD4 Vβ3+ T cells remained at a very low percentage in mice given SEA plus empty vector, indicating that they had died (Fig. 4). Similar results were seen in animals coinjected in their quadriceps with DNA coding for SEA and IL-2. In animals coinjected with DNA coding for SEA and IL-4, however, the percentages of CD4 Vβ3+ T cells did not fall nearly so dramatically.
Figure 4.
IL-4 promotes the survival of activated T cells in vivo. Mice were injected in their quadricep muscles with DNA coding for SEA together with DNA coding for IL-2, IL-4, or empty vector. Forty-eight hours or 14 days later peripheral blood was harvested from the animals and percentages of CD4+ T cells bearing Vβ3 were measured by flow cytometry. Similar results were obtained from the lymph nodes of similarly treated animals. Results shown are the means and SEMs of four identically treated mice and are representative of two similar experiments.
Fourteen days after injection, peripheral blood from these mice was analyzed for the presence of Vβ3 T cells (Fig. 4). The results showed that IL-4 increased the numbers of surviving, SEA-targeted T cells in blood whereas IL-2 did not. Similar results were observed in lymph nodes (data not shown). In a final test we examined the quadricep muscles into which the genes were injected to find out whether the differences seen in the periphery would be paralleled at the site of injection. The muscles of mice injected with SEA and empty vector contained many CD4+ T cells 4 days after injection but very few such cells 14 days after administration of the SEA gene (Fig. 5a and b). By contrast, muscles injected with the genes for SEA and IL-4 contained many CD4+ T cells 14 days later (Fig. 5c). Muscles injected with the DNAs for SEA and IL-2 contained intermediate numbers of CD4+ T cells (Fig. 5d). Preliminary experiments (data not shown) demonstrated that many of the CD4+ T cells in the injected muscles were responding to the SEA, because they bore Vβ3.
Figure 5.
IL-4 and IL-2 promote the survival of CD4+ T cells in muscle. Mice were injected in their quadricep muscles with DNA coding for SEA together with DNA coding for IL-2, IL-4, or empty vector. Fourteen days later the injected muscles were sectioned and stained for expression of CD4. (a) Muscles injected 4 days previously with DNA coding for SEA and empty vector. (b) Muscles injected 14 days previously with DNA coding for SEA and empty vector. (c) Muscles injected 14 days previously with DNA coding for SEA and IL-4. (d) Muscles injected 14 days previously with DNA coding for SEA and IL-2.
The effects of injection of IL-4 cDNA were blocked by chronic exposure of the injected animals to anti-IL-4 antibody (data not shown).
Collectively these data show that members of the IL-2 cytokine family, particularly IL-4, were able to sustain T cells after activation by superantigen in vivo. Although this result could have been due to the ability of these cytokines to promote T cell proliferation, it is also consistent with the idea that these cytokines promoted activated T cell survival. This conclusion is supported by IL-4 being more active in vivo than IL-2, in spite of the fact that IL-2 is a better inducer of T cell division and by the fact that IL-7, a cytokine with limited ability to stimulate division of activated T cells (45), has effects that are similar to those of IL-4 (S.D. and A.T.V., unpublished observations).
DISCUSSION
The clonal selection theory and common sense predicts that an encounter with antigen should make T cells divide and survive for some time so that they can participate in an effective immune response against invading organisms. Therefore, many scientists were surprised when experiments showed that T cells often die quickly after exposure to antigen in vivo (1–4). This phenomenon, known as activation-induced cell death, does explain how animals avoid overexpansion of their immune system due to exposure, in the wild, to many different invading organisms. It may also explain how animals avoid attack by autoreactive lymphocytes.
During productive immune responses in animals, T cells must somehow be protected against activation-induced cell death. The experiments in this paper represent our attempts to find cytokines that might participate in such rescue. Inflammatory cytokines such as TNFα, IL-1, and interferon γ were ineffective and did not prevent the death in vitro of T cells previously activated in vivo. This was surprising because we have previously shown that these inflammatory cytokines were effective in vivo (22). These results suggest that the inflammatory cytokines must be acting in vivo via some other factors that are not available in our in vitro cultures of purified T cells, for example, via induction of B7 family members on dendritic cells and macrophages.
Four cytokines were found to be effective in vitro. These were IL-2, IL-4, IL-7, and IL-15, four cytokines that share the γc as part of their receptors (38, 39). In vitro these four cytokines prevented activated T cell death and, to varying degrees, promoted the proliferation of these same cells. Measurements of the numbers of T cells in G2/M or that had undergone DNA synthesis suggested, however, that these cytokines did not act simply to promote T cell division but rather were genuine survival factors.
In vivo, IL-4 and, to a lesser extent, IL-2 sustained activated T cells. Preliminary experiments (not reported in this paper) showed that IL-7 had similar activity in vivo. These in vivo experiments did not distinguish between the ability of the cytokines to stimulate T cell proliferation or to promote activated T cell survival. However, the fact that IL-4 sustained activated T cell numbers in vivo better than IL-2, and that IL-4 is a better survival factor and worse proliferative factor than IL-2 in vitro, suggests that at least part of the action of these cytokines in vivo may be a result of their ability to promote activated T cell survival. Others have previously shown that the IL-2 group of cytokines protects long-term T cell lines from death (9); however, the results reported here demonstrate similar effects on short-term activated normal T cells, both in vitro and in vivo.
As shown in this paper and reported elsewhere, the ability of these cytokines to protect T cells from death probably depends on their ability to induce expression of Bcl-2, a protein that is known to protect T cells against death (33, 34). This protein is probably induced via a signal transduction pathway that involves the shared cytokine receptor chain, γc (38, 39). The γc associates with the tyrosine kinase, Jak3. Interestingly, recent experiments by Kawahara et al. showed that the kinase activity of Jak3 is required for its ability to promote cell proliferation but is not required for its ability to induce Bcl-2 (46). Likewise Ryan et al. have shown that although engagement of the IL-4R can induce cell proliferation as well as particular genes, different sites on the IL-4R are required for the two functions (47). These results indicate that the proliferative and rescuing activities of the IL-2 receptors may be separated from each other. This explains why IL-4 and IL-7 may prevent the deaths of activated T cells without causing substantial T cell proliferation, whereas rescue and proliferation are coinduced in activated T cells by IL-2 and IL-15.
We and others have previously shown that activation-induced death of T cells is prevented in vivo by reagents such as bacterial LPS or the heat-killed mycobacteria in Freund’s complete adjuvant (4, 22). These materials probably act via their ability to stimulate innate immune mechanisms and production of inflammatory cytokines. The ability of these adjuvants to protect T cells against death is probably crucial to the development of a productive immune response and hence to the ability of the host to reject invading organisms. Do adjuvants such as LPS and Gram-positive cell walls operate via the IL-2 family of cytokines identified in this paper and elsewhere as protective? On the whole, they probably do not, because most members of this family of cytokines are not induced by adjuvants such as LPS. Moreover, in preliminary experiments (not shown here), LPS protected T cells against activation-induced death in mice lacking IL-4 or saturated with anti-IL-7 antibody. Of the IL-2 family only IL-15 is known to be induced by materials such as LPS (48). However, in unpublished experiments we have not found increased levels of IL-15 expression in animals in which T cells are being protected from activation-induced death. Therefore, the IL-2 cytokine family is probably not crucial to the action of most adjuvants.
Arguments like these do not prove that the protective effects of the IL-2 cytokine family are irrelevant to immune responses in animals. Under certain circumstances this property may be very important. For example, very quickly after exposure to antigen, T cells increase the amounts of Fas on their surfaces and theoretically become sensitive to Fas-induced death (5, 6). Simultaneous production of IL-2 and, sometimes, IL-4 may protect recently activated T cells against Fas-induced death. Likewise production of IL-4 by, for example, mast cells may serve to protect bystander activated T cells. Other candidates as protective combinations, for example, CD28 engagement by B7 proteins, may also act in part via the IL-2 cytokine family because CD28 binding to B7 stimulates IL-2 production by activated T cells.
Acknowledgments
We thank Andrew Willson and Gary Shapiro for assistance with DNA preparation and in vivo cytokine assays and Leigh Landeskroner for assistance with photomicroscopy. This work was supported in part by U.S. Public Health Service Grants AI-17134, AI-18785, AI-22295, and AI-28115.
Footnotes
IL, interleukin; TNF, tumor necrosis factor; TCR, T cell receptor; SEA, staphylococcal enterotoxin A; LPS, lipopolysaccharide.
References
- 1.Kawabe Y, Ochi A. Nature (London) 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 2.Webb S, Morris C, Sprent J. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 3.McCormack J E, Callahan J E, Kappler J, Marrack P. J Immunol. 1993;150:3785–3792. [PubMed] [Google Scholar]
- 4.Kearney E R, Pape K A, Loh D H, Jenkins M K. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 5.Debatin K M, Goldmann C K, Bamford R, Waldmann T A, Krammer P H. Lancet. 1990;335:497–500. doi: 10.1016/0140-6736(90)90735-n. [DOI] [PubMed] [Google Scholar]
- 6.Van Parijs L, Ibraghimov A, Abbas A K. Immunity. 1996;4:321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 7.Sytwu H K, Liblau R S, McDevitt H O. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 8.Duke R C, Cohen J J. Lymphokine Res. 1986;5:289–299. [PubMed] [Google Scholar]
- 9.Akbar A N, Bothwick N J, Wickremasinghe R G, Panayiotidis P, Pilling D, Bofill M, Krajewski S, Reed J C, Salmon M. Eur J Immunol. 1996;26:294–299. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 10.Freedman A S, Freeman G J, Rhynhart K, Nadler L M. Cell Immunol. 1991;137:429–437. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy M K, Mohler K M, Shanebeck K D, Baum P R, Picha K S, Oten-Evans C A, Janeway C A, Jr, Grabstein K H. Eur J Immunol. 1994;24:116–123. doi: 10.1002/eji.1830240118. [DOI] [PubMed] [Google Scholar]
- 12.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. J Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- 13.Jenkins M K, Taylor P S, Norton S D, Urdahl K B. J Immunol. 1991;147:2461–2470. [PubMed] [Google Scholar]
- 14.Harding F A, McArthur J G, Gross J A, Raulet D H, Allison J P. Nature (London) 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 15.Sperling A I, Auger J A, Ehst B D, Rulifson I C, Thompson C B, Bluestone J A. J Immunol. 1996;157:3909–3917. [PubMed] [Google Scholar]
- 16.Shahinian A, Pfeffer K, Lee K P, Kundig T M, Kishihara K, Wakeham A, Kawai K, Ohashi P S, Thompson C B, Mak T W. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 17.Radvanzi L G, Shi Y, Vaziri H, Sharma A, Dhala R, Mills G B, Miller R G. J Immunol. 1996;156:1788–1798. [PubMed] [Google Scholar]
- 18.Noel P J, Boise L H, Green J M, Thompson C B. J Immunol. 1996;157:636–642. [PubMed] [Google Scholar]
- 19.Korsmeyer S J, Shutter J R, Veis D J, Merry D E, Oltvai Z N. Semin Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- 20.Chao D T, Linette G P, Boise L H, White L S, Thompson C B, Korsmeyer S J. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vella A T, Mitchell T, Groth B, Linsley P S, Green J M, Thompson C B, Kappler J W, Marrack P. J Immunol. 1997;158:4714–4720. [PubMed] [Google Scholar]
- 22.Vella A T, McCormack J E, Linsley P S, Kappler J W, Marrack P. Immunity. 1995;2:261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 23.Murray R, Suda T, Wrighton N, Lee F, Zlotnik A. Int Immunol. 1989;1:526–531. doi: 10.1093/intimm/1.5.526. [DOI] [PubMed] [Google Scholar]
- 24.Moore T A, von Freeden-Jeffry U, Murray R, Zlotnik A. J Immunol. 1996;157:2366–2373. [PubMed] [Google Scholar]
- 25.Peschon J J, Morrissey P J, Grabstein K H, Ramsdell F J, Maraskovsky E, Gliniak B C, Parks L S, Ziegler S F, Williams D E, Ware C B, et al. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao X, Shores E W, Hu-Li J, Anver M R, Kelsall B L, Russell S M, Drago J, Noguchi M, Grinberg A, Bloom E T, et al. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 27.Thomis D C, Gurniak C B, Tivol E, Sharpe A H, Berg L H. Science. 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 28.Nosaka M, van Deyrsen J M, Tripp R A, Thierfelder W E, Witthuhn B A, McMickle A P, Doherty P C, Grosveld G C, Ihle J N. Science. 1995;270:800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 29.Maraskovsky E, Teepe M, Morrissey P J, Braddy S, Miller R E, Lynch D H, Peschon J J. J Immunol. 1996;157:5315–5323. [PubMed] [Google Scholar]
- 30.Thomas D C, Berg L J. J Exp Med. 1997;185:197–206. doi: 10.1084/jem.185.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maraskovsky E, O’Reilly L A, Teepe M, Corcoran L M, Peschon J J, Strasser A. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 32.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman I L. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 33.Sentman C L, Shutter J R, Hockenberry D, Kanagawa O, Korsmeyer S J. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 34.Strasser A, Harris A W, Cory S. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 35.Vella A T, Teague K, Ihle J, Kappler J, Marrack P. J Exp Med. 1997;186:325–330. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima H, Shores E W, Noguchi M, Leonard W J. J Exp Med. 1997;185:189–196. doi: 10.1084/jem.185.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noguchi M, Yi H, Rosenblatt H M, Filipovich A H, Adelstein S, Modi W S, McBride O W, Leonard W J. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 38.Leonard W J, Noguchi M, Russell S M. Adv Exp Med Biol. 1994;365:225–232. doi: 10.1007/978-1-4899-0987-9_23. [DOI] [PubMed] [Google Scholar]
- 39.Giri J G, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki K, Namen A, Park L S, Cosman D, Anderson D. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Julius M H, Simpson E, Herzenberg L. Eur J Immunol. 1973;3:645–650. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 41.Dow S W, Potter T A. J Clin Invest. 1997;99:2616–2624. doi: 10.1172/JCI119450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renauld J C, Kermouni A, Vink A, Louahed J, van Snick J. J Leukocyte Biol. 1995;57:353–360. doi: 10.1002/jlb.57.3.353. [DOI] [PubMed] [Google Scholar]
- 43.Keegan A D, Johnston J A, Tortolani P J, McReynolds L J, Kinzer C, O’Shea J J, Paul W E. Proc Natl Acad Sci USA. 1995;92:7681–7685. doi: 10.1073/pnas.92.17.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teague T K, Marrack P, Kappler J W, Vella A T. J Immunol. 1997;158:5791–5796. [PubMed] [Google Scholar]
- 45.Grabstein K H, Namen A E, Shanebeck K, Voice R F, Reed S G, Widmer M B. J Immunol. 1990;144:3015–3020. [PubMed] [Google Scholar]
- 46.Kawahara A, Minami Y, Miyazaki T, Ihle J N, Taniguchi T. Proc Natl Acad Sci USA. 1995;92:8724–8728. doi: 10.1073/pnas.92.19.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan J J, McReynolds L J, Keegan A, Wang L H, Garfein E, Rothman P, Nelms K, Paul W E. Immunity. 1996;4:123–132. doi: 10.1016/s1074-7613(00)80677-9. [DOI] [PubMed] [Google Scholar]
- 48.Grabstein K H, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn M A, Ahdieh M, et al. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]